Abstract
This review presents an overview on the recent progress in the synthesis, crosslinking, interpenetrating networks, and applications of poly(aspartic acid) (PASP)-based hydrogels. PASP is a synthetic acidic polypeptide that has drawn a great deal of attention in diverse applications due particularly to its biocompatibility and biodegradability. Facile modification of its precursor, poly(succinimide) (PSI), by primary amines has opened a wide window for the design of state-of-the-art hydrogels. Apart from pH-sensitivity, PASP hydrogels can be modified with suitable species in order to respond to the other desired stimuli such as temperature and reducing/oxidizing media as well. Strategies for fabrication of nanostructured PASP-based hydrogels in the form of particle and fiber are also discussed. Different cross-linking agents for PSI/PASP such as diamines, dopamine, cysteamine, and aminosilanes are also introduced. Finally, applications of PASP-based hydrogels in diverse areas particularly in biomedical are reviewed.
Keywords: hydrogels, poly(aspartic acid), poly(succinimide), crosslinking, nanoparticles, interpenetrating polymer networks (IPNs)
Introduction
Hydrogels are 3-D networks composed of water-soluble polymer chains linked together by chemical or physical bonds. They are employed as carriers for delivery of bioactive agents, as wound healing films, bio-sensing materials, implants, and scaffolds in tissue engineering, etc. (Ullah et al., 2015; Wang L. et al., 2016; Al Harthi et al., 2019). In the presence of water, instead of dissolution, hydrogels are swollen from a few to several times of their own dry weight depending on the crosslinking degree. Similar to water-soluble polymers, based on their chemical structures, hydrogels can be divided into anionic (Ullah et al., 2015), cationic (Qi et al., 2018), non-ionic (Golabdar et al., 2019), and zwitterionic (Vatankhah-Varnosfaderani et al., 2018).
Anionic polymers, which are essentially poly(acid)s, show globule to coil transition upon pH increment and/or reduction of ionic strength (Abu-Thabit and Hamdy, 2016; Meka et al., 2017). This behavior is reflected in hydrogels as swelling when the polymer is cross-linked (Varaprasad et al., 2017). Aside from polysaccharide-based anionic polymers, most of anionic hydrogels are not biodegradable, thereby posing environmental problems and creating pollution challenges in the long-term (Guilherme et al., 2015; Pakdel and Peighambardoust, 2018). Therefore, seeking a suitable substitute that is biodegradable and non-toxic is of outmost importance.
Poly(aspartic acid) (PASP), a synthetic poly(amino acid) with a protein-like amide bond in its backbone, and a carboxylic acid as a pendant group in each repeating unit, has drawn a great deal of attention and so the demand for its production has significantly grown. The former bond provides PASP with degradability (Nakato et al., 1998; Tabata et al., 2000, 2001), while the latter groups gives the polymer acidic properties and negative charge (Yang et al., 2011; Sattari et al., 2018). Various enzymes such as trypsin (Zhang C. et al., 2017), chymotrypsin (Wei et al., 2015), dispase and collagenase I (Juriga et al., 2016), as well as different media such as activated sludge (Alford et al., 1994) and river water (Tabata et al., 2000) have been examined for biodegradation of PASP-based hydrogels and polymers. Depending on the condition (e.g., enzyme concentration and temperature), complete degradation varies from a few days to one month.
PASP hydrogels typically exhibit the same response to pH and ionic strength as other anionic ones, such that higher swelling ratio can be achieved by increasing pH or lowering ionic strength (Zhao et al., 2005; Sharma et al., 2014). This property, which is called polyelectrolyte effect, stems from ionization of carboxylic groups. Ionization (or de-protonation) creates negative charges along the chain/network, causing extended chain conformation and globule to coil transition. On the other hand, high ion concentration shields the network charge and lowers swelling (Yang et al., 2006). Additionally, by changing crosslinking density, mechanical properties and swelling could be tuned (Vatankhah-Varnoosfaderani et al., 2016). More importantly, PASP hydrogels can be readily modified with a wide variety of species to meet the need of any given application. This feature arises from highly reactive imide rings in the intermediate poly(succinimide) (PSI), which allow grafting of different molecules bearing primary amine group under mild conditions without using any catalyst. Therefore, considering facile modification, biocompatibility, and biodegradability, PASP-based hydrogels offers potential advantages over conventional anionic hydrogels [e.g., poly(acrylic acid)] and can be considered as a promising choice for hydrogel preparation in diverse applications.
In the light of the aforementioned features, in this paper we provide a review on the synthesis, gelation process, cross-linking agents, and recent applications of hydrogels based on PASP.
Synthesis of Polyaspartic Acid
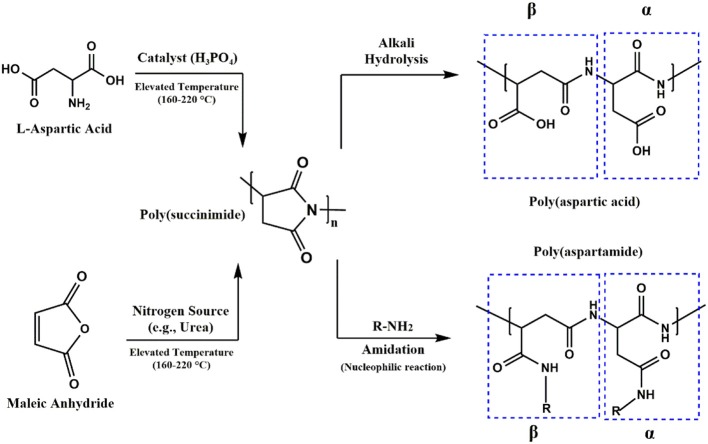
PASP homopolymer is generally synthesized through poly-condensation of aspartic acid (ASP) monomer or polymerization of maleamic acid which is produced from maleic anhydride and a nitrogen source like ammonia or urea as shown in Figure 1. Whatever the method is, the reaction yields the intermediate poly(anhydroaspartic acid), i.e., poly(succinimide) (PSI). The subsequent alkaline hydrolysis of PSI leads to imide ring opening through either carbonyl groups, resulting in a mixture of α and β. Also, as mentioned, PSI can easily undergo a nucleophilic reaction with primary and secondary amines without catalyst even at room temperature to yield poly(aspartamide) derivatives, allowing one to tailor-make PASP to be exploited as a versatile and multi-functional hydrogels (Feng et al., 2014; Nayunigari et al., 2014; Zhang S. et al., 2017).
Figure 1.
Synthesis procedure of PSI, PASP, and poly(aspartamide). PSI can either be obtained through poly-condensation (usually at elevated temperature >160°C using an acid as the catalyst) of ASP or malic acid (synthesized by maleic anhydride and a nitrogen source such as urea or ammonia). PSI can be hydrolysed under alkali media to yield PASP or reacted with primary amines (without catalyst at room temperature) to yield poly(aspartamide) derivatives. The ring opening of succinimde groups occurs both at α and β sites in amidation and hydrolysis reaction.
Poly-Condensation of Aspartic Acid
Thermal poly-condensation of ASP at elevated temperatures (typically higher than 160°C) can either be conducted in bulk (Nakato et al., 2000; Zrinyi et al., 2013) or in solution (Low et al., 1996; Tomida et al., 1997a) in the presence or absence of a catalyst. The reaction by-product, i.e., water, should be eliminated during the course of polymerization. The most effective solvent and catalyst have been found to be the mixture of mesitylene/sulfolane (7/3, w/w) and phosphoric acid, respectively (Tomida et al., 1997a). The reactions catalyzed by phosphoric acid yield linear chain whereas uncatalyzed reactions lead to branching (Wolk et al., 1994). High temperature, high catalyst, and aspartic acid monomer concentration can significantly increase molecular weight (Mw) (Jalalvandi and Shavandi, 2018; Yavvari et al., 2019). Recent studies have also shown when phosphoric acid is utilized as both catalyst and polymerization media (aspartic acid monomer: phosphoric acid, 1:1), PASP with high Mw and reaction yield is achieved (Zakharchenko et al., 2011; Moon et al., 2013; Szilágyi et al., 2017). It is noteworthy to mention that the use of solvent though improves heat transfer, it may reduce the reaction rate, as the availability of functional groups (NH2 and COOH) is reduced (Stevens, 1990). Additionally, solvent should be removed after the reaction by washing polymer. Therefore, bulk reaction under batch and continuous (through extruder) conditions is preferable in industry (Kokufuta et al., 1978; Nakato et al., 2000; Zrinyi et al., 2013).
Polymerization of Maleamic Acid/Ammonium Salt of Maleic Acid
The second method involves polymerization of maleamic acid without catalyst for 6–8 h at high temperature (>160°C), during which period water is removed by distillation (shown in Figure 1) (Koskan and Meah, 1993; Wood, 1994; Boehmke and Schmitz, 1995; Ni et al., 2006; Shi et al., 2016). Maleamic acid is prepared by reacting maleic anhydride (MA) (or maleic acid) with anhydrous ammonia or urea as a nitrogen source or heating the monoammonium salt of maleic acid. This method was first introduced as a patent by Boehmke, where PASP was synthesized with a relatively low degree of polymerization 15–20%, using ammonia (AN) and MA which was heated in water (at 75°C) to change to maleic acid (Boehmke, 1989). The reaction is typically carried out without solvent in a reactor, oven (Freeman et al., 1995), or under microwave irradiation (Huang et al., 2007). Although this method employs industrially inexpensive and available raw materials such as maleic anhydride and ammonia, it gives low yields and low molecular weight (Boehmke, 1989; Koskan and Meah, 1993; Wood, 1994; Boehmke and Schmitz, 1995; Freeman et al., 1995; Ni et al., 2006; Huang et al., 2007; Shi et al., 2016).
Crosslinking
Commonly, hydrogels based on PASP are prepared either via crosslinking of PSI followed by alkali hydrolysis, or by crosslinking of PASP itself. Various types of crosslinking agents can be used. Because of the simplicity, the agent is generally introduced by PSI modification or the gelation process itself is carried out on PSI followed by alkali hydrolysis.
Hydrogels Based on Diamines
Simple succinimide ring opening by primary amines allows the use of various diamines for the synthesis of a PASP hydrogel (Jalalvandi and Shavandi, 2018). This reaction occurs at room temperature without requiring any catalyst (Fang et al., 2006a,b). Gyenes et al. (2008) employed different natural amines and amino acid derivatives such as putrescin, spermine, spermidine, lysine, and cystamine for crosslinking. They indicated that the cystamine-based hydrogels dissolve above pH 8.5 as the disulfide linkage breaks under alkaline media. Gyarmati et al. (2015) reported the synthesis of super-macroporous PASP hydrogels using 1,4-diaminobutane as a cross-linker under cryogenic condition of DMSO. Phase separation was induced by freezing DMSO as the solvent of PSI. As a result, highly porous interconnective hydrogels (pore size 9–259 μm) was fabricated, which is useful for in vitro cell seeding with pH-induced detachment of the grown cells. In a similar study, aside from chemical crosslinking with hexamethylenediamine (HMDA), freeze/thaw technique was also applied to induce phase separation and physical crosslinking (Zhao and Tan, 2006). Swelling behavior was highly affected by changing freeze/thaw cycle number, time, and temperature. Chen et al. (2016) also prepared PASP superabsorbent cross-linked by HMDA in the presence of organic bentonite (OB) with high swelling capacity (491 g/g in water). It was shown that OB can serve as a crosslinker due to its surface amine groups since high OB content (above 3%) led to lower swelling.
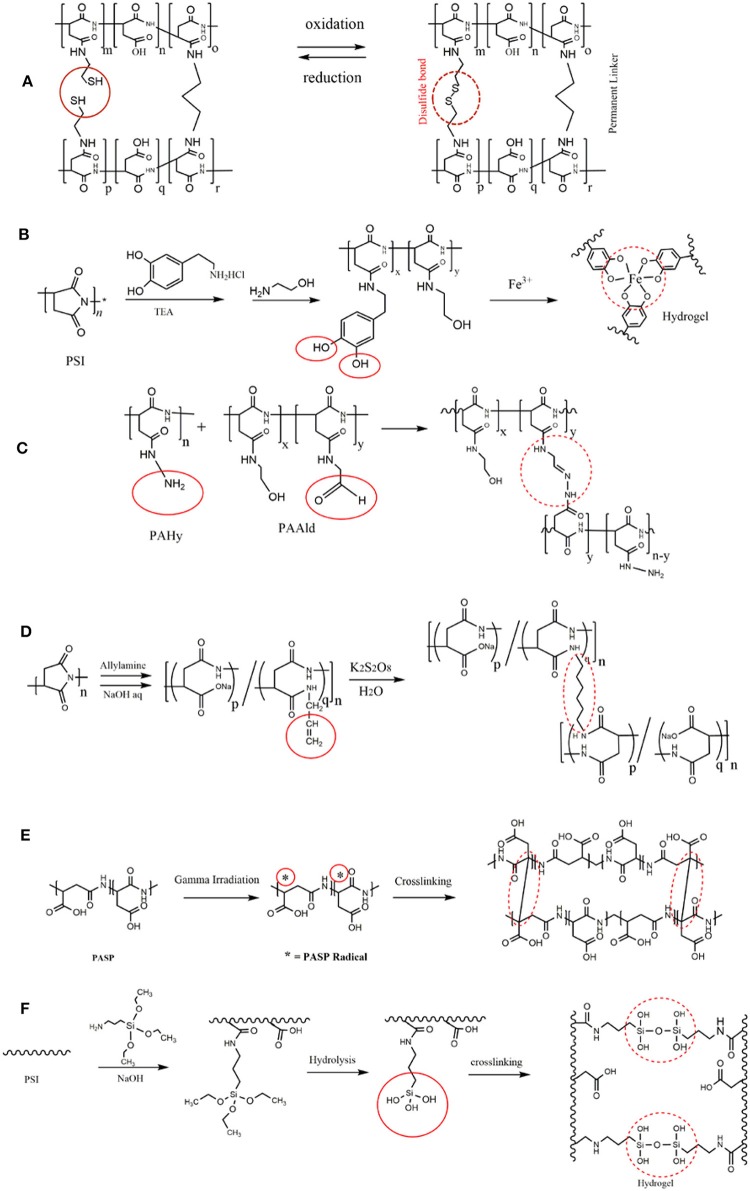
Hydrogels Based on Disulfide Bond
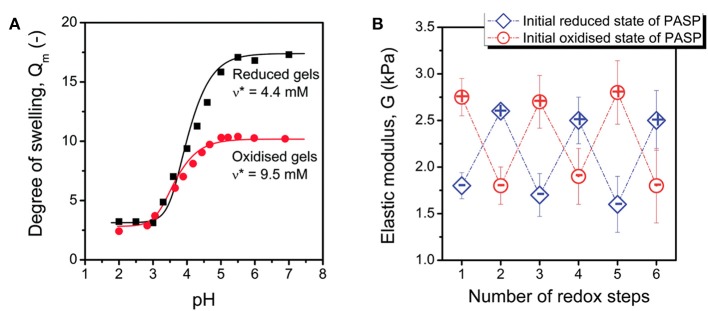
Crosslinking through disulfide or thiol containing agents endows an interesting feature to the PASP-based hydrogels. The reaction of thiol to disulfide can be carried out under application of a reducing agent. This reaction can be reversed in the presence of an oxidizing agent. Therefore, PSI is generally modified with thiol groups (cysteamine or cystamine) for the preparation of reducing/oxidizing-responsive PASP hydrogels (Molnar et al., 2014). In order to maintain structural integrity in different media, a permanent linker such as a diamine can be employed (Figure 3A; Zrinyi et al., 2013; Krisch et al., 2018). Recently, such dual cross-linked hydrogels have drawn a great deal of attention due to swelling under reductive state. For instance, Zrinyi et al. (2013) synthesized PASP with diaminobutane (DAB), and cystamine (CYS) as permanent and cleavable crosslinkers, respectively. They showed that disulfide bonds arising from the latter is broken by the addition of a reducing agent, leading to an increase in swelling and a decrease in modulus. Likewise, redox- and pH-responsive PASP hydrogels were prepared by dual crosslinking using cysteamine, and 1,4-diaminobutane which creates reversible and irreversible bonds, respectively (Gyarmati et al., 2014). It was indicated that swelling degree of hydrogel and elastic modulus can be tuned by reducing/oxidizing agents without hydrogel disintegration/dissolution. Swelling increased as pH increased both under oxidized and reduced states. However, under the latter condition, swelling was higher. The hydrogels maintained their mechanical stability under repeated redox cycles for at least three cycles and the reversibility was shown to be independent of initial redox state of PASP (reduced or oxidized) (Figures 2A,B). Krisch et al. (2018) employed poly(ethylene glycol) diglycidyl ether (PEGDGE) for crosslinking thiolated PASP in order to secure structural integrity of the hydrogels in reducing media. A part of thiol groups were reacted with the former to establish a non-cleavable gel junction while the remaining ones were oxidized into breakable disulfide bonds. It should be noted that the epoxide groups with thiol groups form unbreakable S-C bonds.
Figure 2.
PASP hydrogels based on disulfide bonds. (A) Swelling of PASP hydrogels cross-linked with cysteamine in reduced and oxidized states as a function of pH shows conventional behavior of anionic hydrogels, swelling under reduced condition is higher. (B) Elastic modulus of the corresponding hydrogels shows reversible increase and decrease upon oxidation/reduction. Reproduced from Gyarmati et al. (2014) with permission from The Royal Society of Chemistry.
Hydrogels Based on Dopamine
Catechol moieties in dopamine exhibit a multifunctional characteristic for the design of mussel-inspired coatings (Ryu et al., 2015, 2018; Saiz-Poseu et al., 2019). Complex formation of catechol with boron and/or iron ions (Fe3+) can be employed for hydrogel preparation (Vatankhah-Varnoosfaderani et al., 2014; Krogsgaard et al., 2016). Injectable dopamine modified PASP hydrogels with superior adhesive character were synthesized by complexation with Fe3+ ions (gelation time around 1 min; Figure 3B; Gong et al., 2017). It was suggested that the resulting crosslinking are composed of both Fe3+ coordination as well as covalent quinone-quinone bonds. Boric acid was also shown to crosslink dopamine-modified PASP and yield hydrogels due to boron–catechol coordination (Wang B. et al., 2016). The prepared hydrogels had autonomous self-healing feature due to such a coordination.
Figure 3.
PASP hydrogels prepared by different cross-linkers. (A) PASP hydrogel with a permanent and a cleavable cysteamine cross-linker exhibited response to oxidation/reduction without the gel dissolution. (B) Modification of PSI with dopamine led to PASP with catechol pendant groups which can establish complex with Fe3+ ions, and form hydrogel as a results; adapted from Gong et al. (2017) with permission from Wiley. (C) Synthesis of injectable PASP hydrogels by introduction of aldehyde and amine groups on PASP. Reproduced from Lu et al. (2014) with permission from Wiley. (D) Allyl amine as a monomer that is polymerized via radical polymerization is grafted onto PASP; the subsequent polymerization of allyl amine gives rise to PASP-based hydrogel. (E) Application of gamma-irradiation for crosslinking of PASP. (F) Grafting of APTS as an aminosilane on PSI backbone followed by its hydrolysis.
Other Hydrogels
Apart from diamine and disulfide crosslinking, other strategies have also been developed for fabrication of PASP hydrogels. The reaction of hydrazine and aldehyde, radical polymerization of pendant double bond, sol-gel reaction of aminosilane, and application of gamma-irradiation are some examples that will be discussed in this section for preparation of PASP hydrogels.
Lu et al. (2014) prepared injectable PASP-based hydrogel through introduction of hydrazine and aldehyde to PSI backbone by hydrazine hydrate and 3-amino-1,2-propanediol, respectively. Therefore, hydrazine and aldehyde modified PASPs were used as two gel precursors as shown in Figure 3C. The same strategy was used for crosslinking of oxidized alginate and polyaspartamide conjugated with RGD peptide (Jang and Cha, 2018).
Conventional radical polymerization of allyl amine monomer grafted onto PSI can also lead to crosslinking (Figure 3D; Umeda et al., 2011; Némethy et al., 2013). Minimum value of allyl amine content was found to be 5% for gel formation (Umeda et al., 2011).
Gamma-irradiation can be typically utilized for crosslinking of polymers as it delivers high amount of energy and is capable of forming free radical on polymer backbone. Using such radiation (dosage of 32–100 kGy), Tomida et al. (1997b) prepared PASP hydrogel (Figure 3E). It was shown that the reaction should be conducted under N2 atmosphere as oxygen scavenges free radicals. It was also found that low polymer concentration, as well as low Mw does not lead to gelation and also acidic conditions destabilize the generated radicals.
γ-aminopropyltriethoxysilane (APTS) (an aminosilane) is generally used for attachment of organic/inorganic materials, and surface modification (Adelnia et al., 2015; Bidsorkhi et al., 2017). Its amine and hydroxyl groups make it an excellent candidate as a linker. Meng et al. (2016) introduced APTS on PSI backbone and used it as a crosslinker for PASP gel formation (Figure 3F).
Ethylene glycol diglycidyl ether (EGDGE) can also react with PASP to yield hydrogels (at 180°C for 30 min, dry state, pH before drying 5–6.5) (Chang and Swift, 1999). As the degree of ionization of PASP as well as the protonation of epoxide ring is highly dependent on pH, crosslinking occurs at optimum pH of 5–6.5. Acidic media hydrolyse epoxide group whereas alkaline media reduce the protonated acid group concentration required for nucleophilic attack on the epoxide ring. Meng et al. (2015) however, utilized EGDGE for PSI crosslinking and compared it with hydrazine as a diamine. The produced bonds of the former and the latter are ester and amide, respectively. The PASP hydrogels with the latter had faster swelling kinetic, while lower stability in terms of maintaining the absorbed water.
PASP Hydrogel Nanostructures
PASP Hydrogel Particles (i.e., Nanogels and Microgels)
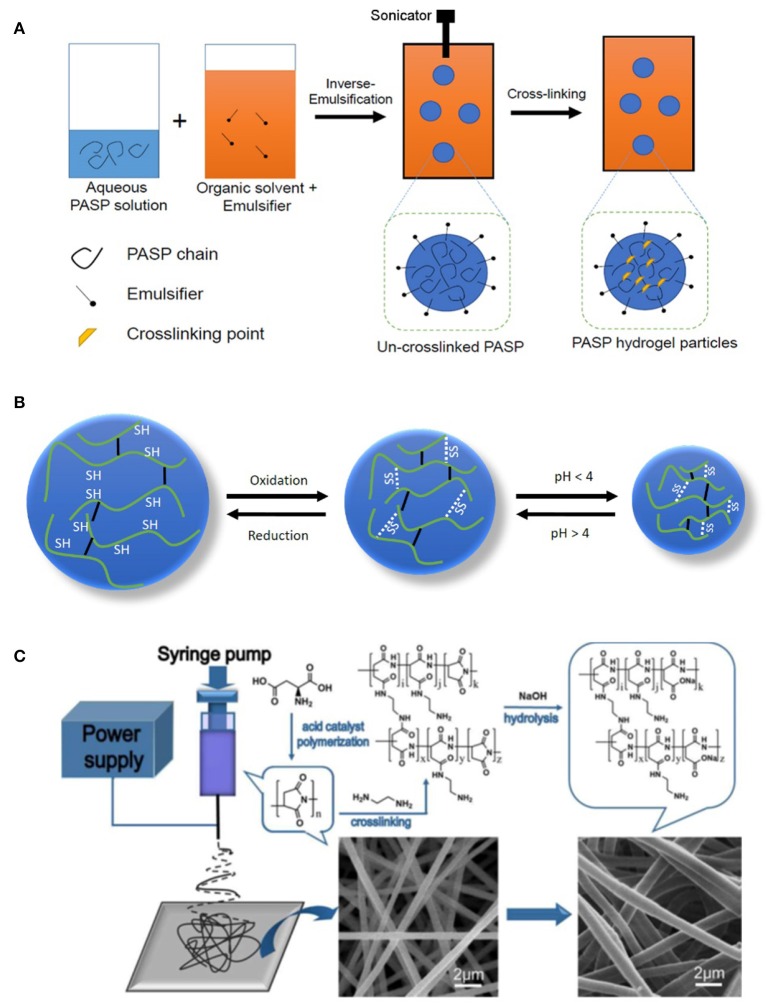
Hydrogel nanoparticles (i.e., nanogels) can find much more applications compared to their own bulk counterpart especially as a carrier for delivery of bioactive agents (Cuggino et al., 2019; Molaei et al., 2019). Preparation of such structures is generally carried out via inverse type emulsion techniques where aqueous phase containing hydrophilic polymer is dispersed in an organic solvent (typically hydrocarbons such as hexane) containing emulsifier (e.g., span-80; Krisch et al., 2017). Formation of small droplets/particles requires high shear stress which can be applied through high speed homogenizer or sonication. Schematic representation of typical emulsification is drawn in Figure 4A. Network formation should be conducted after particle formation as premature crosslinking leads to bulk gelation and does not allow emulsification. For example, Krisch et al. (2016) prepared nanogels of thiolated PASP by inverse miniemulsion (water in n-hexane). After particle formation, the thiolated groups of PASP were oxidized by sodium bromate NaBrO3, giving rise to gel formation. To maintain structural integrity of nanogels in reducing media, the same group utilized poly(ethylene glycol) diglycidyl ether for crosslinking of a part of S-H groups. The nanogels diameter increased in reducing media due to disulfide bond breakage and thus swelling while maintaining nanogel integrity (Figure 4B; Krisch et al., 2018).
Figure 4.
Preparation of nanostructured PASP hydrogels. (A) Schematic representation of the preparation of PASP-based nanogels in inverse emulsion technique. (B) Schematic representing volume change of pH- and redox/reductive-responsive nanogels, white dashed lines represent cleavable disulfide bonds while solid black lines stand for permanent linker; oxidation leads to shrinkage of nanogels as crosslinking density increases, while acidic pH values protonate COOH groups of PASP, lowering the swelling. (C) Schematic representation of the method for PASP fiber hydrogel formation. Reproduced from Zhang et al. (2015) with permission from Elsevier.
Inverse emulsion method, though effective in preparation of small size and uniform particles, is accompanied with several problems such as using toxic organic solvent, relatively high amount of emulsifier, and the need for medium substitution to water. Therefore, alternative methods need to be adopted. Since PSI is hydrophobic, its particles can be formed in aqueous media (Hill et al., 2015). PASP-g-PEG hydrogel nanoparticles were fabricated via self-association of hydrophobic PSI units in water (i.e., micelle formation), followed by their hydrolysis (Park et al., 2017). The particles were crosslinked with HMDA or cystamine. PASP particles can also be formed via self-assembly with cationic polymers such as chitosan, as it is an anionic polyelectrolyte. Such an electrostatic self-assembly (also referred to as ionic gelatification) can yield composite nanoparticles in water through polyelectrolyte complexation (i.e., electrostatic charge attraction of the two polymer; Zheng et al., 2007; Zhang et al., 2008; Wei Wang et al., 2009). PASP/chitosan particles were prepared by drop-wise addition of chitosan to PASP solution. When chitosan/PASP ratio increased from 0.75 to 2.5, the size increased from 84 to 1,364 nm (Hong et al., 2014).
PASP Hydrogel Nanofibers
Regarding the fiber formation, since gels cannot flow, network formation should be conducted after fiber formation similar to the emulsification mentioned above. A typical PASP-based fiber preparation is exhibited in Figure 4C. In this method, PSI is dissolved in its solvent (commonly DMF or DMSO) and electrospun into nanofibers. The resulting PSI nanofibers are cross-linked in this step, employing a suitable agent (e.g., ethylenediamine) and converted to PASP by alkali hydrolysis (Zhang et al., 2015; Zhang C. et al., 2017). Interestingly, Zhang C. et al. (2017) found that inter-fiber crosslinking can also occur, resulting in hydrogels with interconnected microporous structure. This causes higher deformation, swelling kinetic, and swelling ratio compared to hydrogel films. Another study revealed that crosslinking can also be carried out during electrospinning for cysteamine-grafted PASP (diameter 80–500 nm; Molnar et al., 2014). However, relatively lower polymer concentration (15 wt.%) compared to conventional electrospinning process should be used to avoid premature gelation.
Interpenetrating Polymer Network (IPN) Based on PASP
Interpenetrating polymer networks (IPN) are a class of materials composed of two chemically distinct, but highly compatible polymers that are uniformly mixed in each other in microscopic scales without any phase separation. IPNs are divided into semi-IPNs and full IPNs, in which one or both components are cross-linked, respectively (Roland, 2015). IPNs are generally fabricated to take advantage of the features of both components.
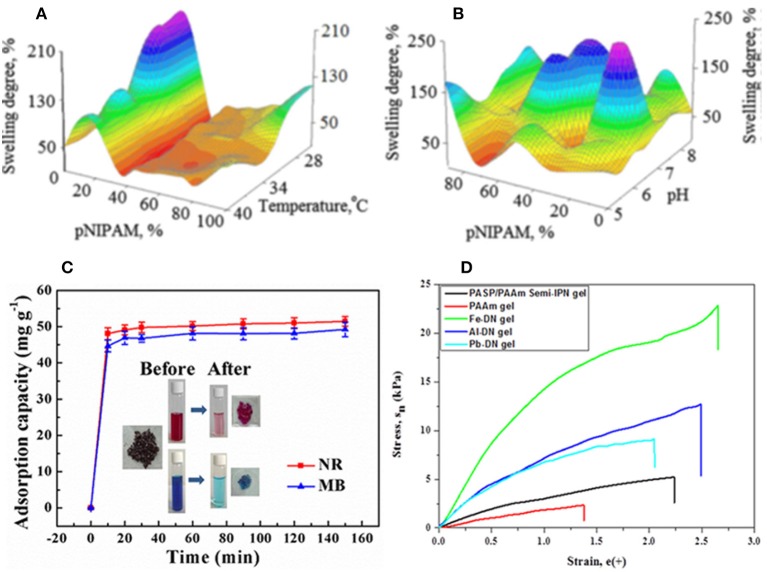
For instance, poly(N-isopropylacrylamide) [poly(NIPAAm)] which is a well-known polymer with LCST at around physiological temperature, can be introduced for providing the IPN with temperature sensitivity. Liu et al. (2012) prepared NIPAAm/PASP IPN hydrogels that show response both to pH and temperature. They first cross-linked PSI with a diamine, followed by its hydrolysis to PASP hydrogel. The hydrogel was then swelled with NIPAAm monomer/N,N′-methylene bisacrylamide (MBA) crosslinker followed by their polymerization. Némethy et al. (2013) synthesized NIPAAm/PASP co-network hydrogels by grafting allyl amine monomer onto PSI backbone followed by its radical polymerization with NIPAAm, and PSI hydrolysis. Nistor et al. (2013) evaluated swelling degree of PASP/PNIPAAm semi-IPN as a function of pH, temperature and NIPAAm content (Figures 5A,B). Other polymers including poly(vinyl alcohol), poly(acrylic acid), and poly(acrylamide) have also been employed for PASP-based IPN preparation as summarized in Table 1. Zhao et al. (2006) introduced PAA to PASP-based semi-IPN hydrogels by polymerization of acrylic acid and MBA as a cross-linker in the PASP solution. It was found that the swelling ratio increases with increasing PASP content as well as temperature (range of 40–60°C). The incorporation of high Mw PASP also inhibited gel formation due to steric hindrance. Jv et al. (2019) indicated that semi-IPNs based on PASP/PAA possess excellent ability for removal of methylene blue and neutral red with maximum adsorption of 357.14 and 370.37 mg/g, respectively (Figure 5C). Magnetic nanoparticles of Fe3O4, were incorporated into the hydrogels for facile separation of the dye-containing solid. Lee et al. (2018) exhibited that PASP improves mechanical properties of brittle PAAm significantly. They suggested that the addition of multivalent cations such as Fe3+, Al3+, Pb2+, Cu2+ results in ionic coordination, and thus creation of second network. Iron cation (Fe3+) had the highest impact on improving mechanical properties (Figure 5D).
Figure 5.
Properties of IPN hydrogels based on PASP. (A,B) 3D swelling degree dependence on the network components and test parameters: (A) Temperature and (B) pH. Reproduced from Nistor et al. (2013) with permission from Wiley. (C) Adsorption capacity of methylene blue (MB) and neutral red (NR) by PASP/PAA semi-IPN hydrogels. Reproduced from Jv et al. (2019) with permission from American Chemical Society. (D) Stress-strain curve of double networks of PASP/PAAm hydrogels in the presence and absence of metallic cations. Reproduced from Lee et al. (2018) with permission from Wiley.
Table 1.
PASP-based IPN and semi-IPN with different polymers (denoted as polymer 2).
| Polymer 2 | X-linker1 | X-linker2 | Type | Characteristic | References | |
|---|---|---|---|---|---|---|
| 1 | N-isopropylacrylamide | 1,4-diaminobutane (DAB) | N,N′-methylene bisacrylamide (MBA) | IPN | Excellent pH-responsiveness | Némethy et al., 2013 |
| Hexamethylenediamine (HMDA) | MBA | IPN | Dual pH- and temperature- sensitive hydrogel. Large porous structure; fast shrinking and re-swelling | Liu et al., 2012 | ||
| Diethylene Glycol diacrylate (DEGDA) | Semi-IPN | Dual pH- and temperature-sensitive hydrogel | Nistor et al., 2013 | |||
| 2 | Acrylic acid | MBA | Semi-IPN | The freezing temperature resulted in a more porous hydrogel and faster swelling/deswelling rates | Lim et al., 2016 | |
| MBA | Semi-IPN | Improving responsive behavior of the hydrogel to alternating changes in inorganic salt, pH, and temperature | Zhao et al., 2006 | |||
| MBA | Semi-IPN | The hydrogels had dye pollutant removal ability. Magnetic NPs were added for separation of the solid after dye removal | Jv et al., 2019 | |||
| MBA | Semi-IPN | Polygorskite clay caused higher swelling rate than that of pure hydrogels of PAA/PASP | Ma et al., 2015 | |||
| MBA and Ethylene glycol dimethylacrylate (EGDMA) | Semi-IPN | MBA and EGDMA resulted in higher swelling behavior in acidic and basic medium, respectively | Sharma et al., 2016 | |||
| 3 | Acrylamide | MBA | Semi-IPN | Metal cations (Fe3+, Al3+, Pb2+, Cu2+) led to creation of second network, and increased mechanical strength and decreased swelling ratio of the gel | Lee et al., 2018 | |
| 4 | poly(vinyl alcohol) | γ-aminopropyltriethoxysilane (APTS) | Semi-IPN and IPN | Interpenetrating PASP/PVA hydrogel resulted in higher, faster swelling ratio, and higher drug releasing | Lu et al., 2015 |
PASP Hydrogel Applications
Apart from conventional and common applications of hydrogels such as hygiene, and agricultural products, PASP hydrogels can be utilized in a wide variety of biomedical engineering areas such as development of scaffolds for tissue engineering, and carriers for sustained or targeted drug delivery systems (DDS). This is mainly due to its biocompatibility, biodegradability, as well as stimuli-responsive characteristic. Regarding the latter, in particular pH- and redox/oxidation-sensitivity of PASP has been exploited for DDS (Horvát et al., 2015; Sim et al., 2018). In this section, some recent studies in these regards are presented.
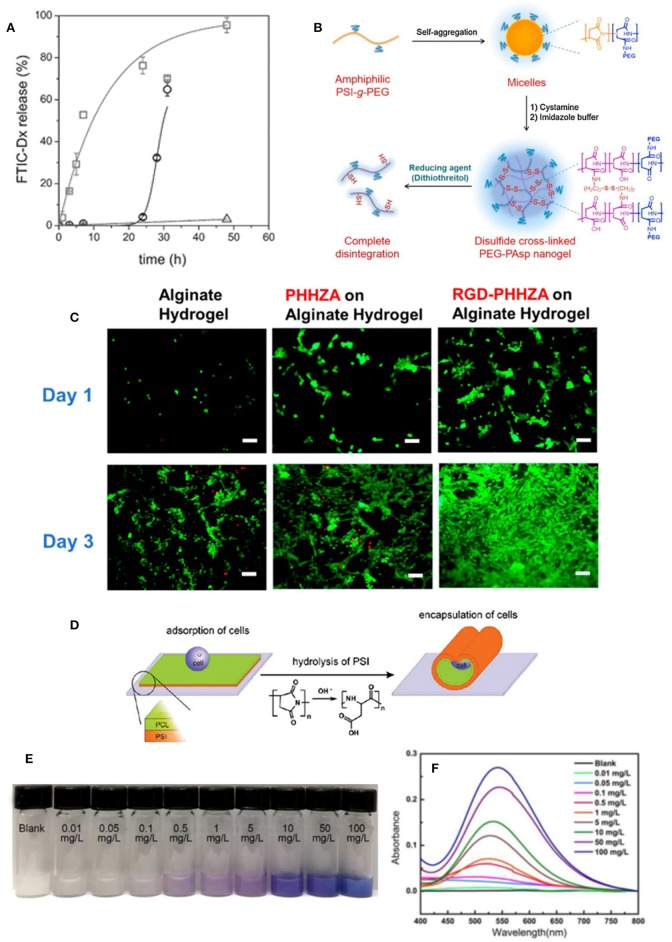
PASP/PNIPAAm co-network hydrogels loaded with sodium diclofenac (DFS) showed pH sensitivity such that the release of DFS increased when the gel is delivered from stomach (pH 1.2) into the bowels (pH 7.6) (Némethy et al., 2013). Such a conventional pH sensitivity feature can protect both the stomach from the side effects of DFS and the drug itself from acidity of stomach. However, in another study, unusual pH-response was observed in PASP hydrogels cross-linked with hydrazine and aldehyde (Lu et al., 2014). The release rate of DOX was accelerated by decreasing pH from 7 to a weak acidic condition (ca. pH 5). This behavior was attributed to instability of the hydrazone bond in acidic media, resulting in loosening of gel network. DFS was also employed as an ocular drugs and loaded in in-situ gelling thiolated PASP for its sustainable delivery (Horvát et al., 2015). The polymer due to its negative charge showed strong mucoadhesion, as well as high resistance against lachrymation of the eye. This is attributed to mucin glycoproteins role for crosslinking (i.e., disulfide linkage). The drug release showed a burst-like profile in the first hour followed by sustained release up to 24 h. In another work, fluorescent dextran (FTIC-Dx) was loaded into thiolated PASP nanogels prepared by inverse emulsion (Krisch et al., 2016). Disulfide bonds were cleaved by a reducing agent for gel disintegration, and release of the loaded drug. As seen in Figure 6A, the release profile dramatically increased by the addition of DTT as a reducing agent. The same redox-response and DOX release was seen in thiolated PASP-g-PEG nanogels (Park et al., 2017). Under reductive intracellular conditions, the prepared nanogels were shown to have the ability to release DOX and efficiently translocated to the nucleus of cancer cells (Figure 6B). Epigallocatechin Gallate (EGCG) which is the main bioactive element of green tea and is unstable in vitro was encapsulated in PASP/chitosan particles (Hong et al., 2014). The release of EGCG was investigated by simulation of food ingestion pH condition. It was demonstrated that EGCG is much more effective against rabbit atherosclerosis when encapsulated into PASP/chitosan.
Figure 6.
Biomedical and industrial applications of PASP-based hydrogels. (A) FITC-Dx release from PASP nanogels as a function of time and DTT concentration (squares: 100 × 10−3 M, spheres: 10 × 10−3 M, and triangles: 0 × 10−3 M DTT). Reproduced from Krisch et al. (2016) with permission from Wiley. (B) Synthesis of the disulfide cross-linked PASP nanogel and its complete disintegration by reducing agent. Reproduced from Park et al. (2017) from Elsevier. (C) The live (green) and dead (red) cells cultured on alginate, alginate/poly(2-hydroxyethyl-co-hydrazidoadipoyl aspartamide) (PHHZA), and alginate/RGD-PHHZA hydrogels after 1 and 3 days (scale bar: 100 μm). Reproduced from Jang and Cha (2018) with permission from American Chemical Society. (D) The schematic illustration of cell encapsulation by bilayer PSI/PCL tubes. Reproduced from Zakharchenko et al. (2011) with permission from American Chemical Society. (E) Color change and (F) absorption spectra of PASP in different Cu2+ ions solution. Reproduced from Zhang et al. (2015) from with permission Elsevier.
Jang and Cha (2018) incorporated RGD peptide to PSI for improving 3T3 fibroblast cell adhesion. PSI was further modified with hydrazide, and subsequently reacted with oxidized alginate, bearing aldehyde groups. The Schiff reaction (i.e., aldehyde-hydrazide, yielding hydrazone bond) leads to in-situ gelation of poly(aspartamide)/alginate. As shown in Figure 6C, RGD-modified hydrogels possessed much better cell viability, adhesion and proliferation compared to un-modified hydrogels. Juriga et al. (2016) also modified thiolated PASP hydrogels with RGD and utilized them as scaffolds for MG-63 osteoblast-like cells. It was shown that RGD introduction leads to compacted cluster formation of the cells. The prepared scaffolds provided the osteoblast-like cells with excellent condition for adhesion, viability, and proliferation.
Zakharchenko et al. (2011) fabricated polymer tubes and encapsulated yeast cells within them. The tubes composed of bilayer cross-linked films of PSI/polycaprolactone. Upon the hydrolysis of PSI in physiological buffer environment, and conversion into PASP gels, the films self-rolled due to the produced internal stress as a result of swelling of the lower layer, i.e., PASP (Figure 6D). Such a self-rolling was exploited for cell encapsulation. Hydrolysis of PSI was shown to be step like process and initiates after nearly 8 h in PBS buffer.
PASP due to its negative charge can endow electrostatic stability to colloidal systems. For example, iron oxide (Fe3O4) nanoparticles coated with a thin layer of PASP hydrogels had improved colloidal stability (Vega-Chacón et al., 2017). The composite magnetic particles did not show any adverse effect on cell viability of L929 fibroblast. Also, particles exhibited response to pH, presenting them as promising candidate for magnetic drug delivery. Iron oxide nanoparticles as negative contrast agents for magnetic resonance imaging (MRI) have been employed widely for detection of diseases (Ta et al., 2011, 2017a,b, 2018; Gaston et al., 2018; Wu et al., 2018; Yusof et al., 2019; Zhang et al., 2019). Multifunctional PASP nanoparticles containing iron oxide nanocrystals and doxorubicin was also developed for simultaneous diagnosis and treatment of cancer by Yang et al. (2011). Iron oxide nanocrystals were loaded in PASP nanoparticles through an emulsion method using octadecyl grafted PASP, then doxorubicin (DOX), was incorporated in the magnetic PASP nanoparticles. It was shown that the DOX loaded nanoparticles exhibited high T2 relaxivity and strong cytotoxicity for cancer cells.
Due to its strong ability for chelation, PAPS nanofiber hydrogels were utilized as chemosensor for Cu2+ ions detection (Zhang et al., 2015). The hydrogels showed high sensitivity and selectivity to Cu2+ ions compared with other ions such as Ag+, and Ca2+ where no color change was observed (Figures 6E,F). The detection limit of as low as 0.01 mg/L was reported.
Because of its bio-degradation and water uptake, PASP hydrogels could be regarded as a promising candidate for ecological restoration and plant survival especially in arid area. Wei et al. (2016) employed PASP hydrogel to transplant Xanthoceras sorbifolia seedlings. The survival rate and the leaf water content were improved in soils containing PASP hydrogels.
Conclusion and Prospects
Although synthesis of PASP-based hydrogels is relatively more complex than other anionic-based hydrogels such as PAA-based ones, its biocompatibility and biodegradability make it attractive particularly in biomedical applications. Though pH-responsive, PASP is further modified with other moieties to provide sensitivity to the desired stimuli as well including temperature and reducing/oxidizing media. Incorporation of other water-soluble polymers into the PASP network may also provide the final hydrogel with superior properties. Scrutinizing the literature, it is found that PASP hydrogels has not yet been employed for inhibition of scale formation in which PASP solution has exhibited promising results (Hasson et al., 2011). Moreover, PASP hydrogel fibers may potentially be a good candidate as scaffold for cell culture as well as tissue engineering. Additionally, due to its anionic nature, PASP-based hydrogels can be used for preparation of electrically-responsive materials (Murdan, 2003).
Author Contributions
HA wrote and revised the manuscript. IB and PL supervised the work. HT supervised and revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- APTS
γ-aminopropyltriethoxysilane
- AN
ammonia
- ASP
Aspartic acid
- CYS
cystamine
- DAB
1,4-diaminobutane
- DEGDA
diethylene glycol diacrylate
- DTT
dithiothreitol
- DDS
drug delivery system
- EGDGE
ethylene glycol diglycidyl ether
- EGDMA
ethylene glycol dimethylacrylate
- FTIC-Dx
fluorescent dextran
- HMDA
hexamethylenediamine
- MA
maleic anhydride
- MBA
N,N′-methylene bisacrylamide
- MB
methylene blue
- NR
neutral red
- PAAm
poly(acrylamide)
- PASP
poly(aspartic acid)
- PEGDGE
poly(ethylene glycol) diglycidyl ether
- poly(NIPAAm)
poly(N-isopropylacrylamide)
- PSI
poly(succinimde)
- TMEDA
tetramethylenediamine (TMEDA).
Footnotes
Funding. This work was funded by National Health and Medical Research Council (HT: APP1146694).
References
- Abu-Thabit N. Y., Hamdy A. S. (2016). Stimuli-responsive polyelectrolyte multilayers for fabrication of self-healing coatings–a review. Surf. Coat. Technol. 303, 406–424. 10.1016/j.surfcoat.2015.11.020 [DOI] [Google Scholar]
- Adelnia H., Bidsorkhi H. C., Ismail A., Matsuura T. (2015). Gas permeability and permselectivity properties of ethylene vinyl acetate/sepiolite mixed matrix membranes. Sep. Purif. Technol. 146, 351–357. 10.1016/j.seppur.2015.03.060 [DOI] [Google Scholar]
- Al Harthi S., Alavi S. E., Radwan M. A., El Khatib M. M., Alsarra I. A. (2019). Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer's disease. Sci. Rep. 9:9563. 10.1038/s41598-019-46032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford D. D., Wheeler A., Pettigrew C. A. (1994). Biodegradation of thermally synthesized polyaspartate. J. Environ. Polymer Degrad. 2, 225–236. 10.1007/BF02071970 [DOI] [Google Scholar]
- Bidsorkhi H. C., Adelnia H., Naderi N., Moazeni N., Mohamad Z. (2017). Ethylene vinyl acetate copolymer nanocomposites based on (un) modified sepiolite: Flame retardancy, thermal, and mechanical properties. Polymer Comp. 38, 1302–1310. 10.1002/pc.23695 [DOI] [Google Scholar]
- Boehmke G. (1989). Polyaspartic Acid From Maleic Acid and Ammonia. United States Patent US 4,839,461, Bayer AG.
- Boehmke G., Schmitz G. (1995). Process for the Preparation of Polysuccinimide, Polyaspartic Acid and Their Salts. United States Patent US 5,468,838, Bayer AG.
- Chang C., Swift G. (1999). Poly(aspartic acid) hydrogel. J. Macromol. Sci. Pure Appl. Chem. 36, 963–970. 10.1081/MA-100101575 [DOI] [Google Scholar]
- Chen X., Jia Z., Shi H., Mao C., Gu H., Liu Y., et al. (2016). Synthesis and characterization of hydroxyl poly(aspartic acid)/organic bentonite superabsorbent composite. Iran. Polymer J. 25, 539–548. 10.1007/s13726-016-0445-5 [DOI] [Google Scholar]
- Cuggino J. C., Blanco E. R. O., Gugliotta L. M., Igarzabal C. I. A., Calderón M. (2019). Crossing biological barriers with nanogels to improve drug delivery performance. J. Control. Release 307, 221–246. 10.1016/j.jconrel.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Fang L., Yang J., Tan T. W. (2006a). Effect of drying process on structure and property of polyaspartic acid resin. J. Sol-Gel Sci. Technol. 40, 89–99. 10.1007/s10971-006-9084-1 [DOI] [Google Scholar]
- Fang L., Zhao Y., Tan T. W. (2006b). Preparation and water absorbent behavior of superabsorbent polyaspartic acid resin. J. Polymer Res. 13, 145–152. 10.1007/s10965-005-9022-x [DOI] [Google Scholar]
- Feng J., Gao L., Wen R., Deng Y., Wu X., Deng S. (2014). Fluorescent polyaspartic acid with an enhanced inhibition performance against calcium phosphate. Desalination 345, 72–76. 10.1016/j.desal.2014.04.019 [DOI] [Google Scholar]
- Freeman M. B., Paik Y. H., Simon E. S., Swift G. (1995). Production of Polysuccinimide by Thermal Polymerization of Maleamic Acid. United States Patent US 5,393,868, Rohm, Haas Co.
- Gaston E., Fraser J. F., Xu Z. P., Ta H. T. (2018). Nano- and micro-materials in the treatment of internal bleeding and uncontrolled hemorrhage. Nanomedicine 14, 507–519. 10.1016/j.nano.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Golabdar A., Adelnia H., Moshtzan N., Nasrollah Gavgani J., Izadi-Vasafi H. (2019). Anti-bacterial poly(vinyl alcohol) nanocomposite hydrogels reinforced with in situ synthesized silver nanoparticles. Polymer Comp. 40, 1322–1328. 10.1002/pc.24859 [DOI] [Google Scholar]
- Gong C., Lu C., Li B., Shan M., Wu G. (2017). Injectable dopamine-modified poly(α, β-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system. J. Biomed. Mater. Res. A 105, 1000–1008. 10.1002/jbm.a.35931 [DOI] [PubMed] [Google Scholar]
- Guilherme M. R., Aouada F. A., Fajardo A. R., Martins A. F., Paulino A. T., Davi M. F., et al. (2015). Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: a review. Euro. Polymer J. 72, 365–385. 10.1016/j.eurpolymj.2015.04.017 [DOI] [Google Scholar]
- Gyarmati B., Mészár E. Z., Kiss L., Deli M. A., László K., Szilágyi A. (2015). Supermacroporous chemically cross-linked poly(aspartic acid) hydrogels. Acta Biomater. 22, 32–38. 10.1016/j.actbio.2015.04.033 [DOI] [PubMed] [Google Scholar]
- Gyarmati B., Némethy Á., Szilágyi A. (2014). Reversible response of poly(aspartic acid) hydrogels to external redox and pH stimuli. RSC Adv. 4, 8764–8771. 10.1039/c3ra47530a [DOI] [Google Scholar]
- Gyenes T., Torma V., Gyarmati B., Zrínyi M. (2008). Synthesis and swelling properties of novel pH-sensitive poly(aspartic acid) gels. Acta Biomater. 4, 733–744. 10.1016/j.actbio.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Hasson D., Shemer H., Sher A. (2011). State of the art of friendly “green” scale control inhibitors: a review article. Ind. Eng. Chem. Res. 50, 7601–7607. 10.1021/ie200370v [DOI] [Google Scholar]
- Hill M. R., Mackrell E. J., Forsthoefel C. P., Jensen S. P., Chen M., Moore G. A., et al. (2015). Biodegradable and pH-responsive nanoparticles designed for site-specific delivery in agriculture. Biomacromolecules 16, 1276–1282. 10.1021/acs.biomac.5b00069 [DOI] [PubMed] [Google Scholar]
- Hong Z., Xu Y., Yin J.-F., Jin J., Jiang Y., Du Q. (2014). Improving the effectiveness of (–)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J. Agric. Food Chem. 62, 12603–12609. 10.1021/jf504603n [DOI] [PubMed] [Google Scholar]
- Horvát G., Gyarmati B., Berkó S., Szabó-Révész P., Szilágyi B. Á., Szilágyi A., et al. (2015). Thiolated poly(aspartic acid) as potential in situ gelling, ocular mucoadhesive drug delivery system. Euro. J. Pharm. Sci. 67, 1–11. 10.1016/j.ejps.2014.10.013 [DOI] [PubMed] [Google Scholar]
- Huang J. L., Zhang Y. L., Cheng Z. H., Tao H. C. (2007). Microwave-assisted synthesis of polyaspartic acid and its effect on calcium carbonate precipitate. J. Appl. Polymer Sci. 103, 358–364. 10.1002/app.24437 [DOI] [Google Scholar]
- Jalalvandi E., Shavandi A. (2018). Polysuccinimide and its derivatives: degradable and water soluble polymers. Euro. Polymer J. 109, 43–54. 10.1016/j.eurpolymj.2018.08.056 [DOI] [Google Scholar]
- Jang J., Cha C. (2018). Multivalent polyaspartamide cross-linker for engineering cell-responsive hydrogels with degradation behavior and tunable physical properties. Biomacromolecules 19, 691–700. 10.1021/acs.biomac.8b00068 [DOI] [PubMed] [Google Scholar]
- Juriga D. V., Nagy K., Jedlovszky-Hajd,ú A. L., Perczel-KováCh K., Chen Y. M., Varga G. B., et al. (2016). Biodegradation and osteosarcoma cell cultivation on poly(aspartic acid) based hydrogels. ACS Appl. Mater. Interfaces 8, 23463–23476. 10.1021/acsami.6b06489 [DOI] [PubMed] [Google Scholar]
- Jv X., Zhao X., Ge H., Sun J., Li H., Wang Q., et al. (2019). Fabrication of a magnetic poly(aspartic acid)- poly(acrylic acid) hydrogel: application for the adsorptive removal of organic dyes from aqueous solution. J. Chem. Eng. Data. 64, 1228–1236. 10.1021/acs.jced.8b01117 [DOI] [Google Scholar]
- Kokufuta E., Suzuki S., Harada K. (1978). Temperature effect on the molecular weight and the optical purity of anhydropolyaspartic acid prepared by thermal polycondensation. Bullet. Chem. Soc. Japan 51, 1555–1556. 10.1246/bcsj.51.1555 [DOI] [Google Scholar]
- Koskan L. P., Meah A. R. (1993). Production of High Molecular Weight Polysuccinimide and High Molecular Weight Polyaspartic Acid From Maleic Anhydride and Ammonia. United States Patent US 5,219,952, Donlar Corp.
- Krisch E., Gyarmati B., Barczikai D., Lapeyre V., Szilágyi B. Á., Ravaine V., et al. (2018). Poly(aspartic acid) hydrogels showing reversible volume change upon redox stimulus. Euro. Polymer J. 105, 459–468. 10.1016/j.eurpolymj.2018.06.011 [DOI] [Google Scholar]
- Krisch E., Gyarmati B., Szilágyi A. (2017). Preparation of pH-responsive poly(aspartic acid) nanogels in inverse emulsion. Period. Polytech. Chem. Eng. 61, 19–26. 10.3311/PPch.9788 [DOI] [Google Scholar]
- Krisch E., Messager L., Gyarmati B., Ravaine V., Szilágyi A. (2016). Redox-and pH-responsive nanogels based on thiolated poly(aspartic acid). Macromol. Mater. Eng. 301, 260–266. 10.1002/mame.201500119 [DOI] [Google Scholar]
- Krogsgaard M., Nue V., Birkedal H. (2016). Mussel-inspired materials: self-healing through coordination chemistry. Chem. Euro. J. 22, 844–857. 10.1002/chem.201503380 [DOI] [PubMed] [Google Scholar]
- Lee J. S., Park H. S., Kim Y. J., Kim J. H. (2018). Hybrid double-network hydrogel based on poly(aspartic acid) and poly(acryl amide) with improved mechanical properties. J. Appl. Polymer Sci. 135:45925 10.1002/app.45925 [DOI] [Google Scholar]
- Lim S. L., Tang W. N. H., Ooi C. W., Chan E. S., Tey B. T. (2016). Rapid swelling and deswelling of semi-interpenetrating network poly(acrylic acid)/poly(aspartic acid) hydrogels prepared by freezing polymerization. J. Appl. Polymer Sci. 133:43515 10.1002/app.43515 [DOI] [Google Scholar]
- Liu M., Su H., Tan T. (2012). Synthesis and properties of thermo-and pH-sensitive poly(N-isopropylacrylamide)/polyaspartic acid IPN hydrogels. Carbohydr. Polymers 87, 2425–2431. 10.1016/j.carbpol.2011.11.010 [DOI] [Google Scholar]
- Low K. C., Wheeler A., Koskan L. P. (1996). Commercial poly(aspartic acid) and its uses. Adv. Chem. Series 248, 99–112. 10.1021/ba-1996-0248.ch006 [DOI] [Google Scholar]
- Lu C., Wang X., Wu G., Wang J., Wang Y., Gao H., et al. (2014). An injectable and biodegradable hydrogel based on poly(α, β-aspartic acid) derivatives for localized drug delivery. J. Biomed. Mater. Res. A 102, 628–638. 10.1002/jbm.a.34725 [DOI] [PubMed] [Google Scholar]
- Lu J., Li Y., Hu D., Chen X., Liu Y., Wang L., et al. (2015). Synthesis and properties of pH-, thermo-, and salt-sensitive modified poly(aspartic acid)/poly(vinyl alcohol) IPN hydrogel and its drug controlled release. BioMed. Res. Int. 2015:236745. 10.1155/2015/236745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Yang Q., Ran F., Dong Z., Lei Z. (2015). High performance and low cost composite superabsorbent based on polyaspartic acid and palygorskite clay. Appl. Clay Sci. 118, 21–28. 10.1016/j.clay.2015.09.001 [DOI] [Google Scholar]
- Meka V. S., Sing M. K., Pichika M. R., Nali S. R., Kolapalli V. R., Kesharwani P. (2017). A comprehensive review on polyelectrolyte complexes. Drug Disc. Today 22, 1697–1706. 10.1016/j.drudis.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Meng H., Zhang X., Chen Q., Wei J., Wang Y., Dong A., et al. (2015). Preparation of poly(aspartic acid) superabsorbent hydrogels by solvent-free processes. J. Polymer Eng. 35, 647–655. 10.1515/polyeng-2014-0275 [DOI] [Google Scholar]
- Meng H., Zhang X., Sun S., Tan T., Cao H. (2016). Preparation of γ-aminopropyltriethoxysilane cross-linked poly(aspartic acid) superabsorbent hydrogels without organic solvent. J. Biomater. Sci. Polymer Ed. 27, 133–143. 10.1080/09205063.2015.1112497 [DOI] [PubMed] [Google Scholar]
- Molaei S. M., Adelnia H., Seif A. M., Gavgani J. N. (2019). Sulfonate-functionalized polyacrylonitrile-based nanoparticles; synthesis, and conversion to pH-sensitive nanogels. Colloid Polymer Sci. 297, 1–9. 10.1007/s00396-019-04543-0 [DOI] [Google Scholar]
- Molnar K., Juriga D., Nagy P. M., Sinko K., Jedlovszky-Hajdu A., Zrinyi M. (2014). Electrospun poly(aspartic acid) gel scaffolds for artificial extracellular matrix. Polymer Int. 63, 1608–1615. 10.1002/pi.4720 [DOI] [Google Scholar]
- Moon J. R., Jeon Y. S., Zrinyi M., Kim J. H. (2013). pH-Responsive PEGylated nanoparticles based on amphiphilic polyaspartamide: preparation, physicochemical characterization and in vitro evaluation. Polymer Int. 62, 1218–1224. 10.1002/pi.4412 [DOI] [Google Scholar]
- Murdan S. (2003). Electro-responsive drug delivery from hydrogels. J. Control. Release 92, 1–17. 10.1016/S0168-3659(03)00303-1 [DOI] [PubMed] [Google Scholar]
- Nakato T., Kusuno A., Kakuchi T. (2000). Synthesis of poly(succinimide) by bulk polycondensation of L-aspartic acid with an acid catalyst. J. Polymer Sci. A 38, 117–122. [DOI] [Google Scholar]
- Nakato T., Yoshitake M., Matsubara K., Tomida M., Kakuchi T. (1998). Relationships between structure and properties of poly(aspartic acid)s. Macromolecules 31, 2107–2113. 10.1021/ma971629y [DOI] [Google Scholar]
- Nayunigari M. K., Gupta S. K., Kokkarachedu V., Kanny K., Bux F. (2014). Development of anti-scale poly(aspartic acid–citric acid) dual polymer systems for water treatment. Environ. Technol. 35, 2903–2909. 10.1080/09593330.2014.925510 [DOI] [PubMed] [Google Scholar]
- Némethy Á., Solti K., Kiss L., Gyarmati B., Deli M. A., Csányi E., et al. (2013). pH-and temperature-responsive poly(aspartic acid)-l- poly(N-isopropylacrylamide) conetwork hydrogel. Euro. Polymer J. 49, 2392–2403. 10.1016/j.eurpolymj.2013.02.015 [DOI] [Google Scholar]
- Ni L., Chiriac A., Popescu C., Neam I. (2006). Possibilities for poly(aspartic acid) preparation as biodegradable compound. J. Optoelectr. Adv Mater. 8, 663–666. [Google Scholar]
- Nistor M. T., Chiriac A. P., Nita L. E., Neamtu I., Vasile C. (2013). Semi-interpenetrated network with improved sensitivity based on poly(N-isopropylacrylamide) and poly(aspartic acid). Polymer Eng. Sci. 53, 2345–2352. 10.1002/pen.23488 [DOI] [Google Scholar]
- Pakdel P. M., Peighambardoust S. J. (2018). A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manag. 217, 123–143. 10.1016/j.jenvman.2018.03.076 [DOI] [PubMed] [Google Scholar]
- Park C. W., Yang H.-M., Woo M.-A., Lee K. S., Kim J.-D. (2017). Completely disintegrable redox-responsive poly(amino acid) nanogels for intracellular drug delivery. J. Ind. Eng. Chem. 45, 182–188. 10.1016/j.jiec.2016.09.021 [DOI] [Google Scholar]
- Qi X., Wu L., Su T., Zhang J., Dong W. (2018). Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surfaces B 170, 364–372. 10.1016/j.colsurfb.2018.06.036 [DOI] [PubMed] [Google Scholar]
- Roland C. (2015). Interpenetrating polymer networks (IPN): structure and mechanical behavior, Encyclopedia of Polymeric Nanomaterials, eds Kobayashi S., Müllen K. (Berlin: Springer; ), 1004–1011. [Google Scholar]
- Ryu J. H., Hong S., Lee H. (2015). Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: a mini review. Acta Biomater. 27, 101–115. 10.1016/j.actbio.2015.08.043 [DOI] [PubMed] [Google Scholar]
- Ryu J. H., Messersmith P. B., Lee H. (2018). Polydopamine surface chemistry: a decade of discovery. ACS Appl. Mater. Interfaces 10, 7523–7540. 10.1021/acsami.7b19865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz-Poseu J., Mancebo-Aracil J., Nador F., Busqu,é F., Ruiz-Molina D. (2019). The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. 58, 696–714. 10.1002/anie.201801063 [DOI] [PubMed] [Google Scholar]
- Sattari S., Tehrani A. D., Adeli M., Azarbani F. (2018). Development of new nanostructure based on poly(aspartic acid)-g-amylose for targeted curcumin delivery using helical inclusion complex. J. Mol. Liquids 258, 18–26. 10.1016/j.molliq.2018.02.116 [DOI] [Google Scholar]
- Sharma S., Dua A., Malik A. (2014). Polyaspartic acid based superabsorbent polymers. Euro. Polymer J. 59, 363–376. 10.1016/j.eurpolymj.2014.07.043 [DOI] [Google Scholar]
- Sharma S., Dua A., Malik A. (2016). Polyaspartic acid based superabsorbent gels with different cross-linkers-a comparative study. J. Appl. Chem. 9, 56–66. 10.9790/5736-0905015666 [DOI] [Google Scholar]
- Shi S., Zhao X., Wang Q., Shan H., Xu Y. (2016). Synthesis and evaluation of polyaspartic acid/furfurylamine graft copolymer as scale and corrosion inhibitor. RSC Adv. 6, 102406–102412. 10.1039/C6RA22048G [DOI] [Google Scholar]
- Sim T., Lim C., Cho Y. H., Lee E. S., Youn Y. S., Oh K. T. (2018). Development of pH-sensitive nanogels for cancer treatment using crosslinked poly(aspartic acid-graft-imidazole)-block-poly(ethylene glycol). J. Appl. Polymer Sci. 135:46268 10.1002/app.46268 [DOI] [Google Scholar]
- Stevens M. P. (1990). Polymer Chemistry. New York, NY: Oxford University Press. [Google Scholar]
- Szilágyi B. Á., Gyarmati B., Horvát G., Laki Á., Budai-Szucs M., Csányi E., et al. (2017). The effect of thiol content on the gelation and mucoadhesion of thiolated poly(aspartic acid). Polymer Int. 66, 1538–1545. 10.1002/pi.5411 [DOI] [Google Scholar]
- Ta H. T., Arndt N., Wu Y., Lim H. J., Landeen S., Zhang R., et al. (2018). Activatable magnetic resonance nanosensor as a potential imaging agent for detecting and discriminating thrombosis. Nanoscale 10, 15103–15115. 10.1039/C8NR05095C [DOI] [PubMed] [Google Scholar]
- Ta H. T., Li Z., Hagemeyer C. E., Cowin G., Zhang S., Palasubramaniam J., et al. (2017a). Molecular imaging of activated platelets via antibody-targeted ultra-small iron oxide nanoparticles displaying unique dual MRI contrast. Biomaterials 134, 31–42. 10.1016/j.biomaterials.2017.04.037 [DOI] [PubMed] [Google Scholar]
- Ta H. T., Li Z., Wu Y., Cowin G., Zhang S., Yago A., et al. (2017b). Effects of magnetic field strength and particle aggregation on relaxivity of ultra-small dual contrast iron oxide nanoparticles. Mater. Res. Express 4:116105 10.1088/2053-1591/aa96e3 [DOI] [Google Scholar]
- Ta H. T., Prabhu S., Leitner E., Jia F., Putnam K., Bassler N., et al. (2011). Antibody-sortagging: a universal approach towards targeted molecular imaging and cell homing in cardiovascular disease. Circ. Res. 107, e37–e38. 10.1161/CIRCRESAHA.111.249375 [DOI] [PubMed] [Google Scholar]
- Tabata K., Abe H., Doi Y. (2000). Microbial degradation of poly(aspartic acid) by two isolated strains of Pedobacter sp. and Sphingomonas sp. Biomacromolecules 1, 157–161. 10.1021/bm9900038 [DOI] [PubMed] [Google Scholar]
- Tabata K., Kajiyama M., Hiraishi T., Abe H., Yamato I., Doi Y. (2001). Purification and characterization of poly(aspartic acid) hydrolase from Sphingomonas sp. KT-1. Biomacromolecules 2, 1155–1160. 10.1021/bm0155468 [DOI] [PubMed] [Google Scholar]
- Tomida M., Nakato T., Matsunami S., Kakuchi T. (1997a). Convenient synthesis of high molecular weight poly(succinimide) by acid-catalysed polycondensation of L-aspartic acid. Polymer 38, 4733–4736. 10.1016/S0032-3861(96)01079-8 [DOI] [Google Scholar]
- Tomida M., Yabe M., Arakawa Y., Kunioka M. (1997b). Preparation conditions and properties of biodegradable hydrogels prepared by γ-irradiation of poly(aspartic acid) s synthesized by thermal polycondensation. Polymer 38, 2791–2795. 10.1016/S0032-3861(97)85616-9 [DOI] [Google Scholar]
- Ullah F., Othman M. B. H., Javed F., Ahmad Z., Akil H. M. (2015). Classification, processing and application of hydrogels: a review. Mater. Sci. Eng: C 57, 414–433. 10.1016/j.msec.2015.07.053 [DOI] [PubMed] [Google Scholar]
- Umeda S., Nakade H., Kakuchi T. (2011). Preparation of superabsorbent hydrogels from poly(aspartic acid) by chemical crosslinking. Polymer Bullet. 67, 1285–1292. 10.1007/s00289-011-0493-0 [DOI] [Google Scholar]
- Varaprasad K., Raghavendra G. M., Jayaramudu T., Yallapu M. M., Sadiku R. (2017). A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 79, 958–971. 10.1016/j.msec.2017.05.096 [DOI] [PubMed] [Google Scholar]
- Vatankhah-Varnoosfaderani M., Hashmi S., Ghavaminejad A., Stadler F. J. (2014). Rapid self-healing and triple stimuli responsiveness of a supramolecular polymer gel based on boron–catechol interactions in a novel water-soluble mussel-inspired copolymer. Polymer Chem. 5, 512–523. 10.1039/C3PY00788J [DOI] [Google Scholar]
- Vatankhah-Varnoosfaderani M., Ina M., Adelnia H., Li Q., Zhushma A. P., Hall L. J., et al. (2016). Well-defined zwitterionic microgels: synthesis and application as acid-resistant microreactors. Macromolecules 49, 7204–7210. 10.1021/acs.macromol.6b01713 [DOI] [Google Scholar]
- Vatankhah-Varnosfaderani M., Hu X., Li Q., Adelnia H., Ina M., Sheiko S. S. (2018). Universal coatings based on zwitterionic–dopamine copolymer microgels. ACS Appl. Mater. Interfaces 10, 20869–20875. 10.1021/acsami.8b05570 [DOI] [PubMed] [Google Scholar]
- Vega-Chacón J., Arbeláez M. I. A., Jorge J. H., Marques R. F. C., Jafelicci M., Jr. (2017). pH-responsive poly(aspartic acid) hydrogel-coated magnetite nanoparticles for biomedical applications. Mater. Sci. Eng. C 77, 366–373. 10.1016/j.msec.2017.03.244 [DOI] [PubMed] [Google Scholar]
- Wang B., Jeon Y. S., Park H. S., Kim J.-H. (2016a). Self-healable mussel-mimetic nanocomposite hydrogel based on catechol-containing polyaspartamide and graphene oxide. Mater. Sci. Eng. C 69, 160–170. 10.1016/j.msec.2016.06.065 [DOI] [PubMed] [Google Scholar]
- Wang L., Jiang J., Hua W., Darabi A., Song X., Song C., et al. (2016b). Mussel-inspired conductive cryogel as cardiac tissue patch to repair myocardial infarction by migration of conductive nanoparticles. Adv. Funct. Mater. 26, 4293–4305. 10.1002/adfm.201505372 [DOI] [Google Scholar]
- Wei Wang T., Xu Q., Wu Y., Jun Zeng A., Li M., Gao H. (2009). Quaternized chitosan (QCS)/poly(aspartic acid) nanoparticles as a protein drug-delivery system. Carbohydr. Res. 344, 908–914. 10.1016/j.carres.2009.02.018 [DOI] [PubMed] [Google Scholar]
- Wei J., Xue M., Li C., Cao H., Tan T. (2015). Effect of enzyme and mechanical stirring on the degradation of polyaspartic acid hydro-gel. Progr. Nat. Sci. 25, 425–429. 10.1016/j.pnsc.2015.10.005 [DOI] [Google Scholar]
- Wei J., Yang H., Cao H., Tan T. (2016). Using polyaspartic acid hydro-gel as water retaining agent and its effect on plants under drought stress. Saudi J. Biol. Sci. 23, 654–659. 10.1016/j.sjbs.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk S. K., Swift G., Paik Y. H., Yocom K. M., Smith R. L., Simon E. S. (1994). One-and two-dimensional nuclear magnetic resonance characterization of poly(aspartic acid) prepared by thermal polymerization of L-aspartic acid. Macromolecules 27, 7613–7620. 10.1021/ma00104a016 [DOI] [Google Scholar]
- Wood L. L. (1994). Preparation of Salt of Polyaspartic Acid by High Temperature Reaction. United States Patent US 5,288,783, SRCHEM Inc.
- Wu Y., Yang Y., Zhao W., Xu Z. P., Little P. J., Whittaker A. K., et al. (2018). Novel iron oxide–cerium oxide core–shell nanoparticles as a potential theranostic material for ROS related inflammatory diseases. J. Mater. Chem. B 6, 4937–4951. 10.1039/C8TB00022K [DOI] [PubMed] [Google Scholar]
- Yang H.-M., Oh B. C., Kim J. H., Ahn T., Nam H.-S., Park C. W., et al. (2011). Multifunctional poly(aspartic acid) nanoparticles containing iron oxide nanocrystals and doxorubicin for simultaneous cancer diagnosis and therapy. Colloids Surf. A 391, 208–215. 10.1016/j.colsurfa.2011.04.032 [DOI] [Google Scholar]
- Yang J., Fang L., Tan T. (2006). Synthesis and characterization of superabsorbent hydrogels composites based on polysuccinimide. J. Appl. Polymer Sci. 102, 550–557. 10.1002/app.24282 [DOI] [Google Scholar]
- Yavvari P. S., Awasthi A. K., Sharma A., Bajaj A., Srivastava A. (2019). Emerging biomedical applications of polyaspartic acid-derived biodegradable polyelectrolytes and polyelectrolyte complexes. J. Mater. Chem. B 7, 2102–2122. 10.1039/C8TB02962H [DOI] [PubMed] [Google Scholar]
- Yusof N. N. M., Mccann A., Little P. J., Ta H. T. (2019). Non-invasive imaging techniques for the differentiation of acute and chronic thrombosis. Thromb. Res. 177, 161–171. 10.1016/j.thromres.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Zakharchenko S., Sperling E., Ionov L. (2011). Fully biodegradable self-rolled polymer tubes: a candidate for tissue engineering scaffolds. Biomacromolecules 12, 2211–2215. 10.1021/bm2002945 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wan L. Y., Wu S., Wu D., Qin X., Ko F. (2015). A reversible colorimetric chemosensor for naked-eye detection of copper ions using poly(aspartic acid) nanofibrous hydrogel. Dyes Pigments 123, 380–385. 10.1016/j.dyepig.2015.07.028 [DOI] [Google Scholar]
- Zhang C., Wu S., Wu J., Wu D., Qin X. (2017a). Preparation and characterization of microporous sodium poly(aspartic acid) nanofibrous hydrogel. J. Porous Mater. 24, 75–84. 10.1007/s10934-016-0239-3 [DOI] [Google Scholar]
- Zhang D.-Y., Shen X.-Z., Wang J.-Y., Dong L., Zheng Y.-L., Wu L.-L. (2008). Preparation of chitosan-polyaspartic acid-5-fluorouracil nanoparticles and its anti-carcinoma effect on tumor growth in nude mice. World J. Gastroenterol. 14:3554. 10.3748/wjg.14.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Qu H., Yang Z., Fu C.-E., Tian Z., Yang W. (2017b). Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination 419, 152–159. 10.1016/j.desal.2017.06.016 [DOI] [Google Scholar]
- Zhang Y., Koradia A., Kamato D., Popat A., Little P. J., Ta H. T. (2019). Treatment of atherosclerotic plaque: perspectives on theranostics. J. Pharm. Pharmacol. 71, 1029–1043. 10.1111/jphp.13092 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Kang J., Tan T. (2006). Salt-, pH-and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly(aspartic acid) and poly acrylic acid. Polymer 47, 7702–7710. 10.1016/j.polymer.2006.08.056 [DOI] [Google Scholar]
- Zhao Y., Su H., Fang L., Tan T. (2005). Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature-and pH-responsiveness properties. Polymer 46, 5368–5376. 10.1016/j.polymer.2005.04.015 [DOI] [Google Scholar]
- Zhao Y., Tan T. (2006). poly(aspartic acid) super-absorbent resin produced by chemical crosslinking and physical freeze/thawing. Macromol. Chem. Phys. 207, 1297–1305. 10.1002/macp.200600168 [DOI] [Google Scholar]
- Zheng Y., Yang W., Wang C., Hu J., Fu S., Dong L., et al. (2007). Nanoparticles based on the complex of chitosan and polyaspartic acid sodium salt: preparation, characterization and the use for 5-fluorouracil delivery. Euro. J. Pharm. Biopharm. 67, 621–631. 10.1016/j.ejpb.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Zrinyi M., Gyenes T., Juriga D., Kim J.-H. (2013). Volume change of double cross-linked poly(aspartic acid) hydrogels induced by cleavage of one of the crosslinks. Acta Biomater. 9, 5122–5131. 10.1016/j.actbio.2012.08.046 [DOI] [PubMed] [Google Scholar]