Abstract
Background
Within the Australian public hospital setting, no studies have previously reported total hospital utilisation and costs (pre/postoperatively) and costed patient-level pathways for primary bariatric surgery and surgical sequelae (including secondary surgery) informed by Australia’s Independent Hospital Pricing Authority’s activity-based funding (ABF) model.
Objective
We aimed to provide our Tasmanian state government partner with information regarding key evidence gaps about the resource use and costs of bariatric surgery (including pre- and postoperatively, types of surgery and comorbidities), the costs of surgical sequelae and policy direction regarding the types of bariatric surgery offered within the Tasmanian public hospital system.
Methods
Hospital inpatient length of stay (days), episodes of care (number) and aggregated cost data were extracted for people who were waiting for and subsequently received bariatric surgery (for the fiscal years 2007–2008 to 2015–2016) from administrative sources routinely collected, clinically coded/costed according to ABF. Aggregated ABF costs were expressed in 2016–2017 Australian dollars ($A). Sensitivity (cost outliers) and subgroup analyses were conducted.
Results
A total of 105 patients entered the study. Total costs (pre/postoperative over 8 years) for all inpatient episodes of care (n = 779 episodes of care) were $A6,018,349. When the ten cost outliers were omitted from the total cost, this cost reduced to $A4,749,265. Mean costs for primary laparoscopic adjustable gastric band (LAGB) and sleeve gastrectomy (SG) bariatric surgery were $A14,622 and $A15,014, respectively. The average cost/episode of care for people with diabetes decreased in the first year postoperatively, from $A7258 to $A5830/episode of care. In total, 27 LAGB patients (30%) required surgery due to surgical sequelae (including revisional/secondary surgery; n = 58 episodes of care) and 56% of these episodes of care were secondary LAGB device related (mostly port/reservoir related), with a mean cost of $A6267.
Conclusions
Taking into account our small SG sample size and the short time horizon for investigating surgical sequalae for SG, costs may be mitigated in the Tasmanian public hospital system by substituting LAGB with SG when clinically appropriate due to costs associated with the LAGB device for some patients. At 3 years postoperatively versus preoperatively, episodes of care and costs reduced substantially, particularly for people with diabetes/cardiovascular disease. We recommend that a larger confirmatory study of bariatric surgery including LAGB and SG be undertaken of disaggregated ABF costs in the Tasmanian public hospital system.
Key Points for Decision Makers
| This is the first Australian study to investigate patient-level journeys of resource use and costs for publicly waitlisted patients before and after bariatric surgery over a lengthy time horizon. |
| Public hospital inpatient costs of providing bariatric surgery decrease from year 3 after surgery, with costs peaking at year 2. |
| Laparoscopic adjustable gastric band appliance-related costs are prevalent. |
Introduction
Obesity and Bariatric Surgery: The Australian Context
The obesity epidemic is a complex public health, economic and strategic policy problem [1–4].
The most recent estimate in the published literature of the total annual direct cost of overweight and obesity in Australia in 2005 was 21 billion Australian dollars ($A), and this estimate was substantially higher than previous estimates [5]. An international study has established that costs rise rapidly in the range of severe obesity [6].
In Australia, over 60% of adults are overweight or obese and, in line with global trends, the rate of severe obesity (body mass index (BMI) ≥ 35 kg/m2) is increasing more rapidly than overweight and obesity (overweight BMI 25–29.9 kg/m2; obesity BMI 30–34.9 kg/m2) [7–9]. Given this increasing trend of severe obesity, recent clinical literature also describes a fourth class of obesity, known as ‘super-obesity’ defined as a BMI of ≥ 50 kg/m2 [10]. The Australian National Health and Medical Research Council (NHMRC) clinical guidelines regarding the management of overweight and obesity recommend bariatric (obesity, weight loss, metabolic [11]) surgery as a treatment option for severe obesity where lifestyle modifications have been ineffective in reducing the person’s weight [12]. The NHMRC guidelines state that, for adults with BMI > 40 kg/m2 or adults with BMI > 35 kg/m2 and comorbidities that may improve with weight loss, bariatric surgery may be considered, taking into account the individual situation. The guidelines also state that bariatric surgery may also be a consideration for people with a BMI > 30 kg/m2 who have poorly controlled type 2 diabetes mellitus and are at increased cardiovascular risk, taking into account the individual situation. The Australian guidelines also state that bariatric surgery is not generally an immediate consideration unless other interventions have not been successful, or other interventions are contraindicated, or a person’s BMI is > 50 kg/m2.
Bariatric surgical procedures involve gastric restriction to augment early satiety and limit meal portions, or intestinal diversion designed to reduce caloric absorption. Some bariatric procedures contain elements of restriction and diversion. Sleeve gastrectomy (SG) is the most prevalent procedure in Australia and has been described as being less technically demanding than the gastric bypass or biliopancreatic diversion with duodenal switch, has minimal morbidity, involves no implanted device and is without marginal ulcers, dumping syndrome, internal hernias, or nutritional deficiencies [13]. Complications are mainly staple line leaks and strictures, but the leak rate has decreased with improved surgical techniques [13].
The goal of the adjustable gastric band bariatric surgical procedure [including the laparoscopic adjustable gastric band (LAGB)] was to develop a gastric band that could be adjusted to the individual needs of the patient; however, the procedure has longer-term potential risks of band slippage, erosion and foreign body infection [13]. Some studies have called for a reconsideration of adjustable gastric band surgery (compared with other bariatric procedures such as SG), particularly for Medicare beneficiaries in the USA [14–16].
The Australian healthcare system presents a complex and fragmented set of arrangements between the public (two tiers of government) and private sectors [17]. Australia’s Commonwealth government holds the major revenue-raising power. Australian state governments operate public hospitals, which account for about two-thirds of all hospitalisations and provide emergency department visits without charge. Australia’s National Health Reform Agreement established the new basis for the Commonwealth’s contribution to public hospital funding based on a hospital’s casemix and defined as activity-based funding (ABF) [18, 19]. Constrained public sector budgets contribute to the inability of the Australian public health system to address the problems of severe obesity increasing more rapidly than obesity [1, 20]. This problem is reflected internationally [17, 21–23].
The Australian Institute for Health and Welfare (AIHW) report regarding weight loss surgery in Australia reported that, in 2014–2015, more than 22,700 weight loss surgery separations were conducted in Australia, most of which involved a primary procedure (79.4%) [7]. The majority of these bariatric surgery separations (88.0%; 20,000 separations) occurred in private hospitals, and the most common procedure for both public and private hospitals in Australia was laparoscopic SG (52.5%) [7].
A recent Australian study determined that the potential demand for publicly and privately funded bariatric surgery in Australia was 882,441 adults aged 18–65 years [24]. Importantly, 45.8% of these potential bariatric surgery candidates had no private health insurance [24]. Our recent qualitative health economics study found that many of the people who partially or fully self-funded their bariatric surgery experienced economic burden to do so [25]. This qualitative study also found that some people were accessing their superannuation to fund their surgery because they either had no private health insurance or they had to pay the concomitant health insurance gap [25].
The AIHW report regarding weight loss surgery in Australia used Medicare data to analyse the costs of bariatric surgery. The report suggested further research could report on ‘typical patient journeys’, incorporating a broad range of direct medical costs (rather than just analysing Medicare-linked data). The report stated that the ability to track and analyse patient journeys from primary through to surgical sequelae (including secondary surgery) would greatly assist in understanding the broader relationships between primary surgical procedures and subsequent adjustments and revisions and their associated costs [7]. Notably this AIHW report only provided aggregate figures for the number of bariatric surgery separations for some Australian jurisdictions, including Tasmania, suggesting a key evidence gap [7].
Previously Published Reviews
Our comprehensive systematic review of the health economic evaluations of bariatric surgery found that bariatric surgery is potentially cost effective or even cost saving for severely obese patients (BMI ≥ 35 kg/m2) with concomitant diabetes mellitus [1]. However, the review also found that costs due to complications and reoperations of bariatric surgery were only incorporated in one-third of the included studies [1]. Additionally, when these costs were included, the estimates of the costs of complications or reoperations were conservative or low probabilities of these events were assumed [1]. Another recent systematic review regarding reoperations after secondary bariatric surgery found that, despite being poorly reported, risks of reoperations and long-term complications and tertiary bariatric surgery are higher than the usually reported risks of short-term complications [26]. The most recently published Australian cost–utility study of bariatric surgery adopted rates of complications and reoperations from the literature, and the base case for the cost–utility study assumed a severely obese female cohort aged 30 years with no comorbidity at the time of the operation [27].
Objectives of this Study
An Australian NHMRC partnership project between the Tasmanian state government and the University of Tasmania was developed, in part to investigate and identify direct medical costs for a retrospective cohort of bariatric surgery patients in Tasmania, based on the Australian Independent Hospital Pricing Authority’s ABF model.
Our state government project partner does not know the hospital utilisation and costs for people with severe obesity (mostly with concomitant comorbidity) who receive bariatric surgery in the Tasmanian public hospital system. Nor does our project partner know the relative costs of the two types of bariatric surgery offered in the Tasmanian public hospital system. Therefore, we aimed to estimate the health service resource use and direct costs for (1) patients waiting for bariatric surgery, (2) the index of surgery, and (3) up to 3 years post-surgery. We particularly aimed to investigate health service use and costs over a longer timeframe and to track individual patient journeys, including surgical sequalae.
Methods
Study Design
Validated Guidelines
This study was conducted in accordance with validated guidelines. These included the Consolidated Health Economic Evaluation Standards used to inform the quality of our study [28], the Independent Hospital Pricing Authority Patient Costing Standards version 3.1, the Tasmanian Department of Health and Human Services (DHHS; now Department of Health [DoH]) Patient-Level Costing Policy and Manual (October 2016) [19] used to inform the definitions of episodes of care and costing of those episodes under ABF and the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement that informed the flow of patients into the study [29]. We also reported in accordance with the ISPOR (International Society for Pharmacoeconomics and Outcomes Research) Real-World Data Taskforce Report [30], which states that real-world data are essential for sound coverage and reimbursement decisions: Context matters greatly in determining the value of a particular type of intervention in any circumstance [30].
Study Setting and Perspective
This retrospective health economics study was part of a much broader mixed-methods partnership project between the Tasmanian State Government and the University of Tasmania. The partnership adopted the economic concepts of heterogeneity of human capital [31], division of labour [32] and comparative advantage [33] to drive a clinical costing research team between government and university researchers regarding bariatric surgery in the Tasmanian public hospital system. The team comprised state government officials who were experts in the department’s administrative databases, clinical coding and costing (under the auspices of the Australian Independent Hospital Pricing Authority’s ABF model that informed the costs extracted for analyses), database construction and raw cost data extraction; university researchers expert in clinical research, health economics and epidemiology; and a state government official who was a health economist, policy leader and decision maker within the department.
The costing was performed primarily from the Tasmanian Government’s perspective and, to a lesser extent, the Commonwealth Government’s perspective (under the National Health Reform Agreement [18]). Relevant ethics approvals were obtained from the University of Tasmania’s Health and Medical Human Research Ethics Committee (approval number H0013845).
Study Population
From the patients who were enrolled on the Tasmanian public hospital waiting list for bariatric surgery between 1 January 2008 and 31 December 2013 (and in line with our NHMRC partnership project and ethics approvals), the study population was defined as all patients who had received primary bariatric surgery in a Tasmanian public hospital (i.e. excluding patients contracted out into the private hospital sector for their bariatric surgery and patients treated elsewhere privately using their own funds) and subsequently ABF costed for the primary procedure for the fiscal years 2007–2008 to 2015–2016.
Patients who were then identified from the waiting list (1 January 2008 to 31 December 2013) as having received primary bariatric surgery in the Tasmanian public hospital system over this time horizon were then classified by surgery type: primary LAGB and primary SG. Importantly, these were the only forms of bariatric surgery performed in the public hospital system in Tasmania during this interval.
Activity-Based Funding Model and the Extraction of Hospital Resource Use and Cost Data
A bottom-up costing methodology (in relation to the DoH predefined ABF cost buckets) for resource use and costing was used [34]. Within the ABF model, the DoH focused on costs at the patient level. The DoH states that a consistent approach to identifying how individual patient costs are built up can help organisations understand where variations arise within a patient pathway, for example, in theatres, wards or diagnostics [34]. The DoH’s development of patient-level costs builds costs from the bottom up, identifying where possible the resources used in treating individual patients—for example, prosthetic devices (such as an LAGB appliance), the intensity of nursing resources and indirect or overhead costs such as the costs of the payroll or finance team through appropriate allocation and apportionment methods [34].
More specifically, the DoH costing methodology for all patients admitted within the acute care system [34] adheres to six steps for ABF patient-level costing [19]:
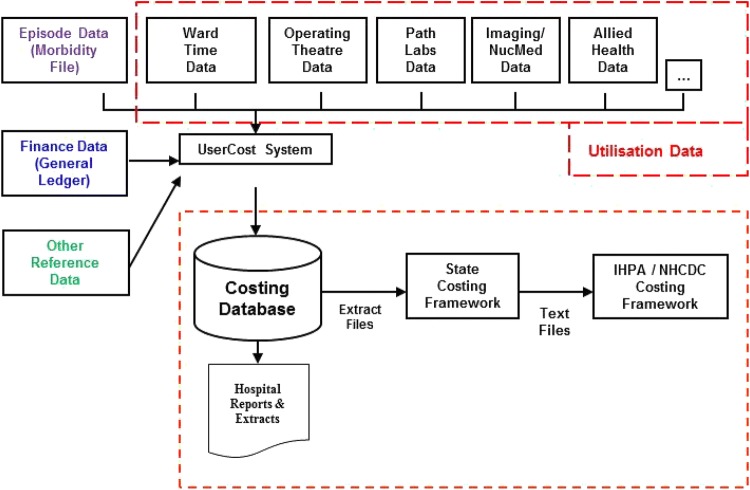
‘Define the patient care to be costed’—the first step is to identify the elements of patient care that need to be costed, for example operating theatre time, ward time, pharmaceuticals, theatre appliances (such as an adjustable gastric band). Figure 1 provides an outline of the DoH process to identify the patient care to be costed.
‘Identify the activities’—to accurately assign costs to a defined element of patient care, the activities associated with delivering that care need to be accurately identified.
‘Identify the relevant costs’—once the element of patient care to be costed has been defined, and associated activities and resources identified, the next step is to determine the relevant costs incurred in delivering the patient care.
‘Classify costs’—after identifying costs for an element of patient care, the next step is to analyse and classify these costs. Under ABF, the Independent Hospital Pricing Authority’s costing standards classify costs based on direct and overhead costs and fixed, semi-fixed and variable costs.
‘Assign costs’—once the resource costs and activities underpinning the element of patient care to be costed have been fully analysed and understood, the next step is to assign the resource costs to the respective elements of patient care (costs can be attributed using the following methodologies: actual use, weighted costs, apportionment based on relevant statistics such as floor area).
‘Validate the outputs’—basis checks are undertaken to ensure the costing is accurate [34].
Fig. 1.
The Tasmanian Department of Health and Human Services patient-level costing process mapping. Source: Tasmanian Department of Health and Human Services Patient-Level Costing Guidance Manual (October 2016). IHPA Independent Hospital Pricing Authority, NHCDC National Hospital Cost Data Collection
This study investigated the aggregated ABF costs that were generated from this costing method.
An episode of care is defined by the Australian Independent Hospital Pricing Authority as “A phase of treatment from admission to separation. An admission may be ‘statistical’ in that the patient changed from one type of admitted patient category to another (between any two of acute, rehabilitation, palliation or non-acute) without being separated from the hospital. It follows that there must be a ‘statistical separation’ before every statistical admission” [19]. Our study population’s primary bariatric surgery inpatient hospital admissions and all their preoperative and postoperative inpatient hospital admissions (for the predefined time horizon of 2007–2008 to 2015–2016) were extracted to generate a unique episode of care number that was costed according to ABF [19, 35].
Table 1 (supported by Appendix 1A and B) provides examples of International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Clinical Modification (ICD-10-CM), Australian Refined Diagnostic Related Group (AR-DRG) and procedure codes of interest for the primary LAGB and SG surgery [36, 37].
Table 1.
Examples of International Classification of Diseases, Tenth Revision, Clinical Modification, Australian Refined Diagnostic Related Group codes and procedure codes of interest for primary bariatric surgery
| Principal diagnosis | Description |
|---|---|
| ICD-10-CM codes | |
| E65 | Localised adiposity |
| E66.8 | Other obesity |
| E66.9 | Obesity, unspecified |
| E10 | Type 1 diabetes mellitus |
| E11 | Type 2 diabetes mellitus |
| AR-DRG codes | |
| G02A | Major small and large bowel procedures, major complexity |
| G02B | Major small and large bowel procedures, intermediate complexity |
| G05C | Minor small and large bowel procedures |
| K04A | Major procedures for obesity |
| K04B | Major procedures for obesity |
| K04Z | Major procedures for obesity |
| K12Z | Other bariatric procedures |
| K60 | Diabetes |
| K60A | Diabetes minor complexity |
| Procedure codes | |
| 30511-02 | Laparoscopic adjustable gastric banding |
| 30511-04 | Adjustable gastric banding |
| 30511-09 | Laparoscopic sleeve gastrectomy |
| 30511-10 | Sleeve gastrectomy |
AR-DRG Australian Refined Diagnostic-Related Group, ICD-10-CM International Classification of Diseases, Tenth Revision, Clinical Modification
Patient sociodemographic (age, sex, smoking history and occupational status), clinical [BMI and comorbidity (diabetes mellitus, cardiovascular disease)], resource use (length of stay, in days) and cost data were extracted from the DoH’s Patient Management System and Clinical Cost databases. These variables and outcomes of interest are described in detail in Sect. 2.2.
Data Analyses: Key Variables and Outcomes of Interest
Patients’ sociodemographic and clinical characteristics and resource use and cost data were described as summary statistics of mean ± standard deviation (SD) and median [interquartile range (IQR)] for continuous variables and frequencies for categorical variables. It is noted that median and IQR were reported for cost data that are generally skewed.
Sociodemographic and clinical variables and their outcomes of interest included age (at primary bariatric surgery), sex, history of smoking (yes, no) and occupational status (employed, home duties, retired, pensioner, student or unemployed), BMI [before primary bariatric surgery BMI was calculated as weight (kg)/height (m2)] and comorbidities (including documented history of diabetes mellitus and cardiovascular disease).
Key outcomes estimated from the patient-level resource utilisation and aggregated cost data were (1) the length of stay expressed in days (to ascertain the resource utilisation within the acute hospital setting) and direct medical costs of the inpatient episodes of care for primary bariatric surgery in Tasmanian public hospitals for the predefined study population; (2) the number and aggregated costs of all inpatient episodes of care before and after primary bariatric surgery for the predefined study population from 2007–2008 to 2015–2016 (also expressed as mean cost per patient and mean cost per episode of care expressed in $A, year 2017 values); (3) the number and costs of all episodes of care for 3 years before and 3 years after primary LAGB bariatric surgery (on a year-by-year basis and totals) and calculated from the date of the primary surgical procedure, and the pre- and postoperative total costs for both LAGB and SG; and the relative costs of the primary surgical procedure for LAGB versus SG in the Tasmanian public hospital system.
Sensitivity analyses of the total inpatient costs for all surgical procedures for the study population included the investigation of cost outliers (defined as an episode of care > $A50,000). Subgroup analyses were conducted for patients with a reported history of diabetes mellitus or cardiovascular disease (inclusive of hypertensive diagnosis) sourced from patient diagnoses tables contained within the Patient Management System. Subgroup analyses were also conducted for patients with a BMI ≥ and < the median cut-point of the sample.
Surgical sequelae procedures’ key outcomes of interest included the inpatient episode of care’s length of stay and direct medical costs for surgical sequelae. Secondary surgical sequelae for the predefined study population included any LAGB device/implant-related procedures such as LAGB revisions or reversals, and LAGB port/reservoir-related procedures (e.g. port revision, port re-suturing, change of port, infection and/or wound or sinus debridement). Other surgical sequelae (i.e. directly related to the primary surgery) that generated an inpatient episode of care included hernia repair, cholecystectomy, complex gastrointestinal procedures that were LAGB related (e.g. leaks, bleeding and subsequent corrective surgery), any body-contouring surgery or body-lifting procedures [38] (e.g. abdominoplasty or panniculectomy) and colonoscopy and gastroscopy. Length of stay and cost outliers for secondary surgical sequelae were identified and assumed as a length of stay of > 6 days per episode of care and a cost of ≥ $A25,000 per episode of care.
For surgical sequelae, individual patients could be represented more than once, and this was reflected in the sociodemographic, clinical and cost analyses of the surgical sequelae and secondary procedures.
Costs were expressed in constant $A with 2016–2017 as the reference year (Appendix 2). There are a wide variety of price indexes (deflators) for the Australian health sector, and these may be distinguished by the scope of the index or the technical manner in which the indexes are constructed. Our study’s costs were adjusted for inflation using the price index for government final consumption expenditure (GFCE) on hospitals and nursing homes index ([39]; Appendix 2). The GFCE on hospitals and nursing homes index is the one that most appropriately reflects the scope of the health services being analysed in this study.
Statistical analyses were conducted with ‘R’, version 3.0.2.
Results
Patient Eligibility and Characteristics
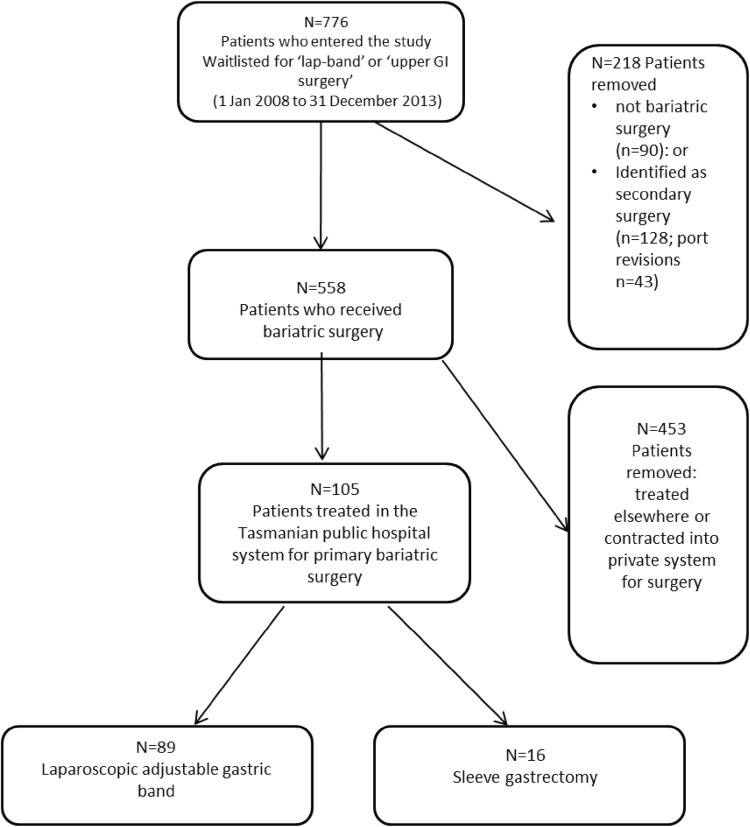
Figure 2 provides an outline of the flow of patients into the study. In total, 776 patients were waitlisted for ‘lap-band’ or ‘upper gastrointestinal surgery’ on the DoH’s Patient Management System between 1 January 2008 and 31 December 2013. Of these, 218 were removed, with 90 identified as not bariatric surgery (e.g. there were other gastrointestinal procedures such as bowel resection for a cancer diagnosis) and 128 identified as secondary surgery (e.g. 43 port (reservoir) revisions). Therefore, 558 patients were identified as receiving primary bariatric surgery; of these, 89 received LAGB surgery and 16 received SG in the public hospital system. The number of SGs performed in the Tasmanian public hospital system was low over the interval, with more SGs performed at the end of our study’s time horizon (2014).
Fig. 2.
Flow of patients into the study. GI gastrointestinal
Detailed patient-level cost data were available for this study group of Tasmanian public hospital-treated patients but not for patients contracted to private hospitals or treated elsewhere.
Clinical sociodemographic characteristics of the 105 included patients are reported in Table 2. For the LAGB bariatric surgery patient group, the mean ± SD age at the time of surgery was 47.9 ± 11.3 years and the SG bariatric surgery patient group was a decade older; 74% of the LAGB patient group and 56% of the SG patient group were female. The LAGB patient group’s obesity classification and comorbidity load revealed severe obesity (BMI 50.2 ± 10.5 kg/m2) with comorbidity, namely 51% (diabetes mellitus) and 53% (cardiovascular disease); 61% of these patients also had a history of smoking (previous or current smokers), and the recorded BMI was in the super-obesity classification of > 50 kg/m2. Only one BMI reading was available for the SG patient group. The comorbidity load for the SG patient group was higher, with 69% of patients reporting a history of diabetes mellitus and 94% a history of cardiovascular disease. Additionally, 88% of the SG patients reported a history of smoking. Most of the primary bariatric surgery patients were retired, receiving a government pension or unemployed (Table 2).
Table 2.
Participant characteristics for patients who had primary LAGB surgery (n = 89) and sleeve gastrectomy (n = 16) surgery, and patients who had LAGB surgical sequelae (n = 27)
| Patient characteristics (n = 105) | Primary surgery | Surgical sequelae | |
|---|---|---|---|
| LAGB (n = 89) | SG (n = 16) | LAGB only (n = 27) | |
| Number of inpatient episodes of care | 89 | 16 | 58 |
| Age years (at surgery)a | |||
| Mean ± SD | 47.9 ± 11.3 | 57.2 ± 8.1 | 51.5 ± 10.7b |
| Median (IQR) | 47 (40–57) | 59 (50–62.3) | 48.5 (44.0–60.5) |
| Sex | |||
| Male | 23 (26) | 7 (44) | 7 (26) |
| Female | 66 (74) | 9 (56) | 20 (74) |
| Comorbidity status | |||
| Diabetes mellitus | 45 (51) | 11 (69) | 12 (44) |
| Cardiovascular disease | 47 (53) | 15 (94) | 19 (70) |
| Smoker (reported history) | 54 (61) | 14 (88) | 14 (52) |
| Occupation statusc | |||
| Employed | 11 (12)c | 1 (6)d | 3 (11)c |
| Home duties | 21 (24) | 3 (19) | 7 (26) |
| Retired | 17 (19) | 3 (19) | 6 (22) |
| Pensioner | 18 (20) | 7 (44) | 7 (26) |
| Student | 0 | 0 | 1 (4) |
| Unemployed | 5 (6) | 0 | 1 (4) |
| BMI before surgery | |||
| Mean ± SD | 50.2 ± 10.5e | NA | 52.2 ± 9.2 |
| Median (IQR) | 48.6 (45.8–54.1) | 52.7 (48.8–58.1) | |
| Min; max | 32.4; 97.9 | 32.4; 66.4 | |
Data are presented as n (%) or mean ± standard deviation unless otherwise indicated
BMI body mass index, IQR interquartile range, LAGB laparoscopic adjustable gastric band, NA not applicable, SD standard deviation, SG sleeve gastrectomy
aAge in years at surgery identified at the inpatient episode for the primary surgical procedure
bAge in years at surgery identified at the surgical sequelae procedure (note that the same patient may be a different age for a different episode)
cAvailable data for occupational status reflected in the n
dAvailable data for the occupational status reflected in the n
en = 52
Of the 89 primary LAGB bariatric surgery patients, 27 (30%) underwent surgical sequelae LAGB-related surgery. Compared with the primary surgical group, this patient group had a higher mean age at the time of surgery sequelae (including secondary/revisional surgery) and an increased prevalence of cardiovascular disease. The prevalence of diabetes mellitus was similar. The general trend of mean ± SD BMI was marginally higher: 52.2 ± 9.2 kg/m2 (Table 2). One of the 16 SG patients recorded a surgical sequelae event (outlined in section 3.2.4).
Tasmanian Public Hospital Utilisation and Cost Analyses
Total Episodes of Care and Costs
Table 3 describes the total costs of all inpatient episodes of care for the study population (n = 105 patients) from the fiscal years 2007–2008 to 2015–2016 expressed in $A, year 2017 values.
Table 3.
Total costs of all inpatient episodes of care for patients who were waitlisted (1 January 2008 to 31 December 2013) and then underwent primary bariatric surgery in the Tasmanian public hospital system over 8 years for the fiscal years 2007–2008 to 2015–2016 expressed in constant dollars (reference case 2016–2017 = 100), and sensitivity analyses (cost outliers removed)
| Costs | Totals | Totalsa (cost outliers removed) | Totals before surgeryb | Totals after surgeryb |
|---|---|---|---|---|
| Global (n = 105 patients) | ||||
| Episodes of care (n) | 779c | 768c | 278b | 397b |
| Total costs ($) | 6,018,349c,d | 4,749,265 | 1,589,101 | 2,902,043 |
| Cost per episode | 7725 | 6184 | 5716 | 7309 |
| Costs per patient | 57,317 | 45,231 | 15,133 | 27,639 |
| LAGB (n = 89) | ||||
| Episodes of care (n) | 692 | 682a | 219b | 384b |
| Total costs ($) | 5,462,275c,d | 4,266,017a | 1,329,873 | 2,831,034 |
| Cost per episode | 7892 | 6255 | 6072 | 7372 |
| Cost per patient | 61,382 | 47,933 | NA | NA |
| Total cost of primary surgery | 1,301,367d | |||
| Total cost of secondary surgery | 503,234 | |||
| SG (n = 16) | ||||
| Episodes of care (n) | 87c | 86a | 59b | 13b |
| Total costs ($) | 556,074c | 483,249 | 259,228b | 71,008b |
| Cost per episode | 6392 | 5619 | 4394 | 5462 |
| Cost per patient | 34,754 | 30,202 | 16,202 | 4438 |
| Total cost of primary SG surgery (n = 15)c | 225,838 | NA | ||
Primary SG recorded from 2013 to 2014
LAGB laparoscopic adjustable gastric band, LoS length of stay (days), NA not applicable, SG sleeve gastrectomy
Includes all costs (including the cost of primary bariatric surgery)
aTen cost outliers removed for LAGB > $50,000 (namely, $52,749 primary LAGB surgery; $58,143 orthopaedic admission; $57,532 congestive cardiac failure admission LoS 44 days; $62,822 neurology admission LoS 7 days; $62,994 primary LAGB surgery; $95,180 multiple morbidity admission LoS 16 days; $114,878 brain tumour admission LoS 42 days; $136,967 surgical sequelae from primary LAGB surgery; $178,253 multiple morbidity LoS 43 days; and $376,930 multiple morbidity admission LoS 291 days)
bTotals before and after surgery do not include the cost of the primary surgical procedure but do include the cost of the secondary and tertiary surgical sequelae procedures after surgery. Note that SG = 15 for costed primary surgery
cCosts available for 15 SG only (not available for one primary bariatric surgery in 2016–17)
dOne major LAGB cost outlier $376,930 included i.e. multiple morbidity admission LoS 291 days
The total number of episodes of care for the study population over the 8-year time horizon was 779, at a total cost to the Tasmanian (and to some extent Australian) healthcare system of $A6,018,349. This total cost included the cost of the primary bariatric surgery and the surgical sequelae (including secondary and tertiary revisional surgery).
For the entire study population (and excluding the episodes of care for the primary bariatric surgical procedures), the total number of inpatient episodes of care before surgery was 278, at a cost of $A1,589,101. After surgery (including the inpatient admission costs for surgical sequelae, and excluding the costed inpatient episodes of care for the primary bariatric surgery), the number of episodes of care was 397, at a cost of $A2,902,043 (Table 3). The relative costs per episode of care for SG patients were lower than those for LAGB, both before and after surgery (Table 3). Additionally, for the LAGB group (n = 89 patients), total costs for the 692 episodes of care for the study population both pre- and postoperatively was $A5,462,275. For the SG patient group (n = 16 patients), total costs for the 87 episodes of care both pre- and postoperatively was $A556,074 (Table 3).
Regarding the total costs for the bariatric surgical procedures, the total cost for primary LAGB (n = 89 patients) was $A1,301,367 and for surgical sequelae (including secondary revisional surgery) $A503,234, or an additional 39% of the cost of the primary procedure. The total cost for primary SG bariatric surgery was $A225,838 (n = 16 patients) (Table 3).
For the SG patient group (n = 16 patients), total costs for the 87 episodes of care both pre- and postoperatively was $A556,074. The relative costs per episode of care were $A7892 (LAGB) and $A6392 (SG), and the costs per patient were $A61,382 (LAGB) and $A34,754 (SG). Therefore, the total episodes of care pre- and postoperative costs for LAGB patients were almost twice those for SG patients (Table 3). One LAGB patient of the 105 patients recorded a catastrophic event (major gastrointestinal secondary surgery), with a 291-day length of stay, at a total cost of $A376,930. A further nine cost outliers were identified for the LAGB patient group, with costs ranging from $A52,749 to $A178,253 for an episode of care (Table 3). When all cost outliers were omitted, total costs reduced from $A5,462,275 to $A4,266,017 (Table 3). Sensitivity analyses also revealed that the relative total costs for an episode of care reduced by over $A1600 to $A6255 for LAGB patients and by over $A600 to $A5619 for SG (Table 3).
Appendices 1A and B also provide the ICD-10-CM and AR-DRG coding for the total episodes of care identified from the administrative databases for the study cohort of 105 people who received bariatric surgery.
Analyses of Pre and Postoperative Public Hospital Utilisation and Costs
Table 4 provides analyses of all inpatient episodes of care and costs pre- and postoperatively for the LAGB surgical group (n = 89 patients) 3 years before (defined as − 3 years from the date of the primary procedure, − 2 years, − 1 year) and for the 3 years after surgery (described as + 1 year from the date of the primary procedure, + 2 years and + 3 years) as a subset of the 8-year time horizon.
Table 4.
Total costs and inpatient episodes of care for patients who were waitlisted (1 January 2008 to 31 December 2013) and then underwent primary bariatric surgery in the Tasmanian public hospital system expressed in 2016–2017 constant dollars for the 3 years before and after surgery, sensitivity (cost outliers omitted) and subgroup analyses (patients with diabetes mellitus and cardiovascular disease)
| LAGB (n = 89) | Before surgery | After surgery | ||||||
|---|---|---|---|---|---|---|---|---|
| − 3 years | − 2 years | − 1 years | Total/average (before surgery) | + 1 year | + 2 years | + 3 years | Total/average (after surgery) | |
| Episodes of care | 38 | 60 | 82 | 180 | 72 | 88 | 34 | 194 |
| Total costs | 214,451 | 292,832 | 451,735 | 959,019 | 556,260 | 1,151,073 | 127,630 | 1,834,963 |
| Average cost per episode of care | 5644 | 4881 | 5508 | 5344 (average) | 7726 | 13,080 | 3754 | 9548 (average) |
| Sensitivity analyses (cost outliers removed) | ||||||||
| Episodes of care | 37 | 60 | 81 | 178 | 72 | 85 | 34 | 191 |
| Total costs | 151,630 | 292,832 | 356,554 | 801,017 | 556,260 | 522,298 | 127,630 | 1,206,189 |
| Average costs per episode of care | 4099 | 4881 | 4402 | 4500 | 7726 | 6145 | 3753 | 6315 |
| Total cost outliers removed | 62,822a | No cost outliers | 95,180b | 158,002 | No cost outliers | 628,774c | No cost outliers | 628,774 |
| Subgroup analysis: patients with diabetes (n = 45) | ||||||||
| Episodes of care | 15 | 19 | 26 | 60 | 36 | 49 | 20 | 105 |
| Total costs | 51,390 | 130,572 | 188,691 | 370,653 | 209,879 | 261,059 | 87,856 | 558,894 |
| Average cost per episode of care | 3426 | 6871 | 7258 | 6177 | 5830 | 5328 | 4398 | 5322 |
| Cost outliers removed | No cost outliers | No cost outliers | No cost outliers | No cost outliers | No cost outliers | No cost outliers | ||
| Subgroup analysis: Patients with CVD (n = 47) | ||||||||
| Episodes of care | 23 | 45 | 55 | 123 | 56 | 57 | 23 | 136 |
| Total costs | 159,697 | 244,209 | 385,074 | 788,981 | 464,750 | 494,283 | 82,919 | 1,041,951 |
| Average cost per episode of care | 6943 | 5426 | 7001 | 6414 | 8299 | 8671 | 3605 | 7661 |
| Cost outliers removed | 1a | No cost outliers | 1b | 2 | No cost outliers | 1 | No cost outliers | 1 |
| Episodes of cared | 22 | 45 | 54 | 121 | 56 | 56 | 23 | 135 |
| Total costs | 98,815 | 244,209 | 294,571 | 640,562 | 464,750 | 357,270 | 82,920 | 904,938 |
| Average cost per episode of care | 4491 | 5493 | 5455 | 5294 | 8299 | 6381 | 3605 | 6703 |
CVD cardiovascular disease, LAGB laparoscopic adjustable gastric band, LoS length of stay
aCost outlier > $50,000, namely $62,822 neurology
bCost outlier > $50,000, namely $95,180 multiple medical issues not surgical sequelae
cCost outlier > $50,000, namely $376,930 multiple issues LoS 291 days; $114,878 brain tumour LoS 42 days; $136,967 bariatric surgery cost outlier surgical sequelae
dCost outliers removed
Table 4 particularly highlights that the number of episodes of care and costs increased from − 3 years to − 1 year before surgery and for the 2 years after surgery, and then decreased at year + 3 after surgery. More specifically, episodes of care were as follows: − 3 year, 38 episodes of care; − 2 year, 60 episodes of care; − 1 year, 82 episodes of care; + 1 year, 72 episodes of care; + 2 year, 88 episodes of care; + 3 year, 34 episodes of care. Costs per episode of care were as follows: − 3 year $A5644, − 2 year $A4881, − 1 year $A5508 and + 1 year $A7726; + 2 year, $A13,080 and + 3 year $A3754. Interestingly, this result revealed that the number of inpatient episodes of care reduced from 82 in the year − 1 before surgery to 34 episodes of care year + 3 after surgery, at an average cost of $A5508 and $A3754, respectively. The table also revealed that the number of episodes of care and total and average costs maximised at year + 2 after surgery.
The subgroup analyses presented in Table 4 revealed that the general trend of a decrease of costs per episode of care at year + 3 after surgery was lower than for the entire study population. To illustrate, for patients with cardiovascular disease and diabetes mellitus, the difference from year − 1 before surgery to year + 3 after surgery was $A3396 and $A2865, respectively, compared with $A1754 for the entire study population. The total cost and episodes of care maximised at year + 2 after surgery for the entire cohort and people with cardiovascular disease. Interestingly, for people with diabetes mellitus, average costs maximised before surgery, and a decrease in average costs was revealed from the year + 1 after surgery (compared with year + 3 for the entire cohort and for people with cardiovascular disease).
Primary Bariatric Surgery
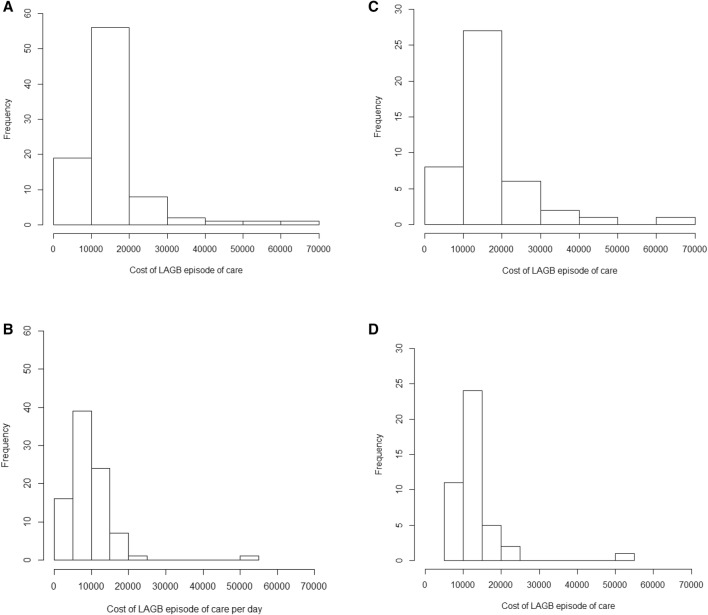
Table 5 describes total hospital utilisation and summary inpatient episode of care costs and subgroup analyses (diabetes mellitus and BMI) for primary LAGB and primary SG bariatric surgery. Figure 3a, b provide the frequency distributions for inpatient LAGB episode of care costs and episode of care costs per day.
Table 5.
Inpatient episode of care length of stay (days) and public hospital activity-based funding costs expressed in constant dollars (reference case: 2016–2017 = 100) for patients who underwent primary laparoscopic adjustable gastric band (n = 89) and sleeve gastrectomy (n = 16) bariatric surgery in the Tasmanian public hospital system, and subgroup analyses
| Primary surgery (n = 105) | Episode of care for primary surgery (n) | LoS | LoS (sensitivity analyses) | Cost of episode of care | Cost of episode of care (sensitivity analysis) | Cost of episode of care per day |
|---|---|---|---|---|---|---|
| LAGB (n = 89) | ||||||
| Entire cohort | 89 |
(n = 89) 2.4 ± 3.6 |
(n = 83) 1.7 ± 0.9 |
(n = 89) 14,622 ± 9142 12,293 (10,198–15,452) 5343; 62,994 |
(n = 83)a 12,595 ± 4180 12,085 (9971–14,590) 5343; 23,838 |
(n = 89) 9050 ± 6332 7698 (5542–11,119) 1107; 52,748 |
| Subgroup analyses | ||||||
| Diabetes | 45 | 3.1 ± 4.8 |
(n = 40)b 1.8 ± 0.8 |
(n = 45) 16,502 ± 10,496 13,073 (10,787–18,757) 5682; 62,994 |
(n = 40) 13,488 ± 4605 12,657 (10,382–15,608) |
NA |
| ToS WR | p = 0.01* | p = 0.06 | p = 0.02* | p = 0.07 | ||
| No diabetes | 44 | 1.7 ± 1.3 |
(n = 43)c 1.6 ± 0.9 |
(n = 44) 12,699 ± 7129 11,223 (9889–13,021) 5343; 52,748 |
(n = 43) 11,763 ± 3599 11,119 (9887–12,875) 5343; 22,519 |
NA |
| BMI (high) ≥ 48.6 | 26 | 3.7 ± 6.1 | NA |
17,271 ± 14,083 12,782 (10,807–15,847) 5343; 62,994 |
NA | NA |
|
ToS WR BMI (low) < 48.6 |
26 |
p = 0.24 1.9 ± 1.3 |
NA |
p = 0.15 12,428 ± 6471 11,348 (9118–12,449) 5532; 38,896 |
NA | |
| SG (n = 16) | ||||||
| Entire cohort | 16 | 4.0 ± 1.1 | 3.8 ± 1.1 |
15,014 ± 5900d 13,290 (10,995–18,747) 6559; 26,158 p = 0.80 |
NA |
4030 ± 6364 3322 (3085–4583) 2186; 8040 |
| Diabetes | 11 | NA | 3.9 ± 1.3e |
15,899 ± 6491 14,559 (9092–21,480) 9024; 26,156 |
NA | NA |
Data are presented as mean ± standard deviation, median (interquartile range) or minimum; maximum unless otherwise indicated
BMI body mass index, LAGB laparoscopic adjustable gastric band, LoS length of stay, NA not applicable, SG sleeve gastrectomy, ToS WR test of significance Wilcoxon rank sum test
aReference case 2016–2017 = 100
bFive LoS outliers removed in diabetes subgroup (range 6–30 days)
cOne outlier removed in not diabetes subgroup 8 days
dCost data for primary SG available for 15 patients (also see Table 3). Cost outliers removed > $25,000
en = 10
*p < 0.05 level of significance
Fig. 3.
Frequency distribution of an episode of care of inpatient costs expressed in constant dollars (reference 2016–2017 = 100) for all patients (n = 89) who received laparoscopic adjustable gastric band surgery (a) and cost/day for an episode of care (b). Frequency distribution of inpatient episode of care costs expressed in constant dollars (reference 2016–2017 = 100) for all patients c with diabetes (n = 46) and d without diabetes (n = 45) who underwent laparoscopic adjustable gastric band surgery in the Tasmanian public hospital system. LAGB laparoscopic adjustable gastric band
The mean ± SD length of stay for SG was 1.6 days longer than for LAGB (2.4 ± 3.6 days), but this result was not tested for significance. When length of stay outliers were omitted for LAGB sensitivity analyses (n = 6; range 6–30 days), this reduced to 1.7 ± 0.9 days per episode of care for primary LAGB bariatric surgery (Table 5).
Mean ± SD costs for an inpatient episode of care for primary LAGB and SG surgeries (that included the costs of the surgery) were $A14,622 ± 9142 and $A15,014 ± 5900, respectively. The mean costs per day for SG were half those for LAGB (Table 5). When cost outliers were omitted for the LAGB primary surgery sensitivity analyses (i.e. n = 6; range $A28,895–65,409), the inpatient cost per episode of care for primary surgery reduced to $A12,595 ± 4180. There were no reported cost outliers for SG primary bariatric surgery (Tables 3, 5; Fig. 2a). The base-case costs per day for an episode of care revealed that the mean ± SD costs per day for SG were half those for LAGB (Table 5).
Subgroup analyses for the LAGB primary surgery group revealed that most cost outliers were people with a reported history of diabetes mellitus (n = 45) (Tables 3, 5; Fig. 3c, d).
Overall, LAGB subgroup analyses for patients with or without diabetes mellitus revealed that the mean cost per inpatient episode of care for surgically treating people with diabetes was $A3803 higher (p = 0.02) than for people without diabetes mellitus. When cost outliers were omitted from both samples (patients with a history of diabetes, n = 5; patients without a history of diabetes, n = 1), this difference reduced to only $A1725 (p = 0.07) (Table 5). Inpatient length-of-stay analyses revealed similar trends. Table 4 also revealed that the average cost per episode of care for people with diabetes mellitus decreased substantially from 1 year before surgery to 3 years after surgery and that the total cost was reduced by almost half.
The SG subgroup with a reported history of diabetes mellitus (n = 11) revealed similar costs to those of the LAGB subgroup with diabetes mellitus (SG mean ± SD; $A15,899 ± 6491) (Table 5). Nevertheless, Table 4 showed that costs and episodes of care for people with diabetes mellitus decreased from + 1 year after surgery (compared with year + 3 for the entire cohort and for people with cardiovascular disease).
Subgroup analyses of people with a BMI classified above and below the median cut-point of 48.6 kg/m2 for LAGB primary bariatric surgery revealed that the mean cost per inpatient episode of care for the primary surgical procedure for people with a BMI > 48.6 kg/m2 (n = 26) was $A4956 higher than for people with a BMI ≤ 48.6 kg/m2 (n = 26). This result was not statistically significant. Similar trends were revealed for length of stay (Table 5).
Surgical Sequelae: Laparoscopic Adjustable Gastric Band and Sleeve Gastrectomy
Table 6 describes the inpatient hospital utilisation and direct medical costs of LAGB-related surgical sequelae and secondary LAGB surgery. The classifications of secondary/tertiary LAGB surgery for analyses included LAGB device/implant-related procedures (e.g. revision or reversals of the LAGB system, change of tubing, re-suturing of port, flipped port, change of port, wound or sinus debridement related to an infected port) and surgical sequelae of colonoscopy/gastroscopy, hernia repair, cholecystectomy and body-contouring surgery.
Table 6.
Length of stay and costs for surgical sequelae (including secondary/tertiary revisional surgery) after laparoscopic adjustable gastric band surgery, and subgroup analyses
| LAGB secondary surgery (n = 27) | Episodes of care (n) | Patients (n) | LoS (days) | Costs |
|---|---|---|---|---|
| Total |
51 (cost)a 58 (LoS) |
27 |
3.5 ± 6.6 1 (1–3.8) 1; 46 |
9867 ± 19,313b 4979 (3326–11,015) 155; 136,962 |
| Sensitivity analysis 1c (cost and LoS outliers removed) |
50 (cost) 57 (LoS) |
27 |
2.7 ± 3.3 1 (1–3) 1; 20 |
7325 ± 6659 4942 (3320–10,589) 155; 33,118 |
| Sensitivity analysis 2d (cost and LoS outliers removed) |
48 (cost) 51 (LoS) |
27 |
1.8 ± 1.5 1 (1–2) 1; 6 |
6399 ± 4877 4799 (3093–9683) 155; 18,975 |
| Subgroup analyses | ||||
| Device related (including port related) | 33 | 16 |
2.2 ± 2.4 1 (1–2) 1; 11 |
6267 ± 4711 4906 (3388–9400) 155; 16,273 Costs 29 |
| Port-related | 27 | 12 |
1.8 ± 2.3 1 (1–1) 1; 11 |
5296 ± 3856 4618 (3375–6298) 155; 15,546 Costs 25 |
| Revision or reversal | 6 |
3.5 ± 2.1 3.5 (2–5) 1; 6 |
11,753 ± 6423 14,091 (12,314–15,504) 582; 16,273 |
|
| Colonoscopy/gastroscopy | 10 | 9 | 1 day |
2525 ± 1104 2285 (1729–3093) 1378; 4295 |
| Cholecystectomy | 4 | 4 | 3 ± 1.2 |
9985 ± 4236c 10,610 (8041–12,241) 5470; 13,873 |
| Hernia repair | 4 | 4 |
3.3 ± 3.9 1.5 (1–3.8) 1; 9 |
10,317 ± 8871 6777 (5337–11,753) 4272; 23,537 |
| Body-contouring surgery | 1 | 1 | 4 | NA |
Data are presented as mean ± standard deviation, median (interquartile range) or minimum; maximum unless otherwise indicated
LAGB laparoscopic adjustable gastric band, LoS length of stay, NA not applicable
an = 51 costs, i.e. n = 7 costs not available
bNote that total costs for the 51 episodes of care were $503,233 (reference case: 2016–2017 = 100)
cSensitivity analyses 1: one major cost and LoS outlier for major and complicated gastrointestinal surgery LoS 46 days (including an intensive care unit admission of 3199 min) and cost $136,967
dSensitivity analyses 2: LoS outliers > 6 days (n = 6) and cost outliers ≥ $25,000 (n = 2; $136,967 and $33,119)
In total, 27 patients (30%) recorded an LAGB surgical sequelae (including secondary/tertiary revisional surgery) and (n = 58) associated inpatient episodes of care. Of these (n = 27) patients, eight required three or more secondary surgical procedures and an associated inpatient episode of care. Over half of the surgical sequelae procedures were secondary surgery LAGB device/implant related [33 of the 58 episodes of care (57%)], and most of these procedures were LAGB port/reservoir-related surgical procedures (Table 6).
A total of 16 patients required 33 episodes of care for LAGB device-related procedures, and 12 patients required 27 episodes of care for LAGB port-related procedures. The remaining six device-related procedures were LAGB revisions or reversals. Colonoscopy and gastroscopy accounted for ten of the 58 episodes of care of the surgical sequelae procedures. There were only two major LAGB-related secondary gastrointestinal surgical procedures, related to two patients. Only one of these procedures could be classified as a postoperative catastrophic event and was a major cost outlier of the cohort (Table 6).
The mean ± SD inpatient length of stay and costs for the total episodes of care for LAGB-related surgical sequelae were 3.5 ± 6.6 days and $A9867 ± 19,313, respectively. Removal of length of stay and cost outliers for sensitivity analyses revealed a substantial reduction in both length of stay and costs to 1.8 ± 1.5 days and $A6399 ± 4877 per inpatient episode of care (Table 6). Mean ± SD LAGB port/reservoir-related costs per inpatient episode of care was $A5296 ± 3856. Revisions or reversals of the LAGB device (not specifically described or classified as a device/port-related procedure) accounted for 12% of the total episodes of care, and the mean ± SD costs were $A11,753 ± 6423. Colonoscopy and gastroscopy accounted for 17% of the episodes of care, and mean ± SD costs were $A2525 ± 1104 (Table 6). Body-contouring surgery (abdominoplasty and panniculectomy) was provided to one patient at a length of stay of 4 days (cost data not available as the procedure was undertaken in the current fiscal year) (Table 6).
Of the 16 primary SG procedures, 14 were performed in 2015 and 2016. There were 13 episodes of care recorded after the primary SG procedures, and one of these procedures could be attributed as surgical sequelae of the primary SG procedure. This inpatient episode of care was a laparoscopic cholecystectomy performed 10 months after the SG procedure, at a cost of $A7759.
Discussion
The Costs of Obesity and Bariatric Surgery in Tasmania
Our study provided much-needed information regarding the inpatient episodes of care, resource use and costs of obesity and bariatric surgery in the Tasmanian public hospital system. We extracted resource use and aggregated cost data on an individual patient basis to track the primary ABF episodes of care and the aggregated costs attributed to each patient pathway before and after primary bariatric surgery.
We found that total costs of public hospital inpatient care to the Tasmanian public healthcare system for the 105 patients who were waitlisted for and subsequently received bariatric surgery in the Tasmanian public healthcare system over an 8-year time horizon was almost $A6.0 million, of which $A1.6 million was for the primary bariatric surgery procedures.
Another key finding was that the average cost of providing primary bariatric surgery in the Tasmanian public hospital system was lower than the most recent Australian estimate, which was derived from a sample of Queensland data and then extrapolated in a cost-effectiveness model. The base-case scenario of a severely obese 30-year-old female with no comorbidity was estimated to cost $A24,167 for a primary adjustable gastric band and $A52,440 for an SG [27]. We also found that our average cost of providing primary bariatric surgery in the Tasmanian public hospital system was lower than recent comparable international estimates [1].
Other findings included that cost outliers mostly involved patients with a reported history of cardiovascular disease and diabetes mellitus, and that those for LAGB surgery device/implant-related surgical sequelae accounted for half of the secondary and tertiary surgery after the primary bariatric procedure. From a patient journey/pathway perspective, we also found that device-related procedures were concentrated to a subgroup of patients.
Importantly, we also found that, for people with diabetes mellitus, the costs for inpatient public hospital care from a total cost and cost per episode of care perspective were substantially reduced 3 years after surgery compared with 1 year before surgery. Another interesting finding from our subgroup analysis was that the average cost for an episode of care decreased for people with diabetes mellitus from the first year after surgery.
A recent AIHW study estimated the cost of primary bariatric surgery by adopting a narrower cost base (Australian Medicare-linked data) than our study or the recent Australian cost–utility study [7]. The AIHW report showed that the costs of primary bariatric surgery were marginally lower than our base-case analyses. Nevertheless, this report did not include the broader costs captured in our study using ABF data linked with patient records.
Overall, our findings suggest that bariatric surgery in the Tasmanian public hospital system may be an attractive value-based proposition in the longer term: bariatric surgery realised health benefits (reduced inpatient episodes of care) and savings (reduced costs) at year 3 postoperatively. At 1 year preoperatively, the study population recorded the highest number of inpatient episodes of care and costs, suggesting that the severely obese study population with multimorbidity was experiencing substantially reduced health 1 year before surgery. We also found that the cost of bariatric surgical sequelae (including secondary and tertiary revisional surgery) maximised at year 2. The maximisation of inpatient episodes of care and costs at year 2 suggests that postoperative care should be ongoing during this critical time horizon and could potentially mitigate some of these inpatient costs.
Surgical Sequelae (Including Secondary/Tertiary Surgery)
Our investigation of patient-level data regarding surgical sequelae after primary LAGB surgery is novel. We tracked each publicly treated LAGB and SG patient’s individual inpatient episode of care and cost pathway to provide our project partners with a comprehensive understanding of the patient pathway, the prevalence of surgical sequelae and secondary and tertiary surgery resource use and costs.
Importantly, our study found that over half of the costs of complications and reoperations for publicly treated LAGB patients were device related and that these episodes of care and costs were mostly LAGB port/reservoir related and concentrated to a further subgroup of patients. Nevertheless, the overall costs of bariatric surgery (including the total costs of reoperations and complications) for our older and sicker cohort of bariatric surgery patients were less than the direct medical costs reported in the most recent Australian cost–utility study.
We also found that, for SG, one in 16 inpatient episodes of care could be attributed to surgical sequelae. However, the time horizon for this small sample of patients who had received an SG was also short.
A recent comprehensive systematic review regarding the health economic evaluation of bariatric surgery found that one-third of the 77 included studies either ignored the costs and/or consequences of complications and reoperations or, for the studies that accounted for reoperations and complications, commonly only assumed short-term events, considered an incomplete list of complications or assumed relatively low probabilities of adverse events occurring. The review also found that the longer-term costs of bariatric surgery have therefore probably been underestimated and the value for money of bariatric surgery subsequently overestimated. Additionally, the most recent Australian cost–utility study estimated the prevalence of surgical sequelae and secondary surgery from the literature (notwithstanding estimating the surgical costs from an administrative database).
In direct comparison, our study suggests that the most recent health economics studies have underestimated the prevalence and real costs of surgical sequelae and secondary/tertiary bariatric surgery.
Resource Allocation: Type of Surgery and Patient Prioritisation
Contemporary debate regarding the provision of bariatric surgery has, to a certain degree, shifted beyond the cost per quality-adjusted life-year health economic metric to the economic barriers to entry in public healthcare systems and the associated issue of supply not meeting ever-increasing demand for publicly provided bariatric surgery [24, 40].
Other authors have called for a reconsideration of the use and role of adjustable gastric band surgery (compared with other procedures such as SG), particularly for Medicare beneficiaries in the USA [14–16]. A recent key epidemiological study that investigated reoperations and Medicare (USA) expenditures after LAGB surgery found that device-related reoperation was common and costly and varied widely across hospital referral regions [41]. On the other hand, it has been suggested that no single bariatric procedure is appropriate for all patients and that the regional variation in outcomes observed is important [15].
Given our study’s reported rates (and costs) of secondary/tertiary LAGB device-related surgery, policy makers could reconsider the type of surgery provided to certain patient groups to mitigate LAGB device-related issues.
A common theme that emerged from a review of the health economics reporting of bariatric surgery was that it is highly cost effective (and even cost saving) for severely obese patients with type 2 diabetes mellitus [1]. Our subgroup analyses revealed that half of our severely obese cohort had a history of diabetes mellitus and that the cost of providing them with the primary surgical procedure was only marginally higher than that for people without diabetes mellitus. More importantly, our subgroup analyses for people with diabetes mellitus also found that total costs of publicly funded inpatient care were substantially reduced 3 years after bariatric surgery.
Our previously published work also found that long-waiting public hospital system bariatric surgery patients should not be ‘written-off’ by healthcare planners—they can still realise significant improvements in health-related quality-of-life outcomes when ultimately treated, and this should be factored into patient prioritisation decisions [42]. Our previously published work also suggested that addressing this issue given the large gap between the demand for and supply of publicly funded bariatric surgery in many countries, would require significant commitment and investment [42].
Strategic Research Alliance in an Applied Health Economics Study
Our study harnessed the comparative advantages of a strategic alliance between university researchers and government policy makers that comprised heterogeneous human capital. The team identified key gaps for the state government partner regarding the resource use and costs of publicly provided bariatric surgery, including a comparison between LAGB and SG surgery. Additionally, this study has enabled university researchers to build on a collaborative relationship with our health partner.
Limitations and Strengths
The main limitation of our study was the small sample size and shortened time horizon for SG and the single-centre nature of our study (the Tasmanian hospital system), therefore limiting the overall generalisability of the study. Nevertheless, our Tasmanian State Government partner indicated that this study and the investigation of the small yet pragmatic sample of SG patients was important and that we should track each patient journey for both LAGB and SG. We recommend that a larger confirmatory study of disaggregated costs for later years be conducted given that SG as a treatment option in the Tasmanian public hospital system from 2014 may have increased.
A further limitation was that our study focused on the inpatient hospitalisation and direct medical costs (compared with, for example, primary care). On the other hand, this focus was also a key strength of our paper because we provided our policy decision makers with important information about the study population that was previously unknown. Two further strengths were the overall sample size of bariatric surgery patients and their associated before and after surgery episodes of care individually tracked for a long time horizon and that we collected sufficient data to enable sensitivity and subgroup analyses over a long timeframe.
A final limitation concerns our assumption that secondary surgical sequelae included colonoscopies. However, only a few colonoscopies were identified in the sample (combined gastroscopy and colonoscopy, ten episodes of care), and a proportion of this small sample would be directly related to LAGB surgery. We suggest that a larger confirmatory study also investigate the rate of colonoscopy and gastroscopy for the Tasmanian public hospital system.
Conclusions
The costs of providing bariatric surgery in Tasmania are lower than comparable national and international published estimates, even after Tasmanian costs for surgical sequelae and secondary/tertiary surgery are included. A robust cost-effectiveness study could be the subject of further research for the retrospective cohort and our planned prospective cohort.
Targeting appropriately prioritised patients with SG in preference to LAGB surgery in Tasmania could mitigate LAGB implant-related costs.
Patients with diabetes mellitus and cardiovascular disease incur lower costs in the longer term after bariatric surgery.
This study and our health-related quality-of-life work provide the building blocks for our project team to conduct a robust real-world cost-effectiveness analysis for our DoH project partner. We also recommend that a larger confirmatory cost study be conducted to investigate disaggregated ABF costs about SG versus LAGB (and other forms of bariatric surgery that could now be offered) in the Tasmanian public hospital system from 2013–2014 to 2018–2019.
Acknowledgements
The authors thank Dr Virginia Mumford for providing discussant comments to an early draft of the paper presented at the Australian Health Economics Society Doctoral Workshop on 20 September 2017 and for valuable written comments after the workshop. Thanks also to Kevin Ratcliffe and Julie Turtle (DoH) for providing advice regarding the DoH’s clinical coding policies and processes.
Appendix 1A
The principal and additional diagnoses for all International Classification of Diseases—Tenth Edition—Clinical Modifications (ICD-10-CM) codes for people who received primary and surgical sequelae (including secondary and tertiary) laparoscopic adjustable gastric band surgery in the Tasmanian public hospital system.
| Principal and secondary diagnosis ICD-10-CM codes | Description |
|---|---|
| B95.6 | Staphylococcus aureus |
| D64.9 | Anaemia unspecified |
| E65 | Localized adiposity |
| E66.8 | Other obesity |
| E66.9 | Obesity not elsewhere classified |
| E10.61 | Type 1 diabetes mellitus with specified diabetic musculoskeletal and connective tissue complication |
| E10.64 | Type 1 diabetes mellitus with hypoglycaemia |
| E11.21 | Type 2 diabetes mellitus with incipient diabetic nephropathy |
| E11.22 | Type 2 diabetes mellitus with established diabetic nephropathy |
| E11.31 | Type 2 diabetes mellitus with background retinopathy |
| E11.4 | Type 2 diabetes mellitus with neurological complication |
| E11.65 | Type 2 diabetes mellitus with poor control |
| E11.71 | Type 2 diabetes mellitus with multiple microvascular and other specified nonvascular complications |
| E11.72 | Type 2 diabetes mellitus with features of insulin resistance |
| E87.6 | Hypokalaemia |
| G47.32 | Obstructive sleep apnoea syndrome |
| G62.9 | Polyneuropathy, unspecified |
| H35.0 | Background retinopathy and retinal vascular changes |
| I10.0 | Essentially (primary) hypertension |
| I20.0 | Incisional hernia without obstruction or gangrene |
| I95.5 | Hypotension |
| I97.8 | Other intraoperative and post procedural complications and disorders |
| I99.59 | Chronic embolism and thrombosis of other specified deep vein of lower extremity |
| K31.88 | Other specified diseases of stomach and duodenum |
| K42.9 | Umbilical hernia without obstruction or gangrene |
| K43.2 | Incisional hernia without obstruction or gangrene |
| K43.9 | Other unspecified ventral hernia without obstruction or gangrene |
| K44.9 | Diaphragmatic hernia without obstruction or gangrene |
| K55.8 | Other vascular disorders of intestine |
| K56.5 | Intestinal adhesions [bands] with obstruction |
| K66.0 | Peritoneal adhesions |
| K80.1 | Calculus of gallbladder with other cholycystitis |
| K92.2 | Gastrointestinal haemorrhage, unspecified |
| M79.58 | Residual foreign body in soft tissue, other site |
| M79.61 | Achilles tendonitis, right leg |
| M79.62 | Pain in limb shoulder region |
| N18.2 | Chronic kidney disease stage 2 |
| N18.9 | Chronic kidney disease unspecified |
| N99.0 | Post procedural kidney failure. |
| R00.0 | Tachycardia, unspecified |
| R00.1 | Bradycardia unspecified |
| R07.4 | Chest pain, unspecified |
| R10.4 | Other unspecified abdominal pain |
| R19.5 | Other faecal abnormalities |
| R51 | Headache |
| R52 | Headache |
| S35.2 | Injury of celiac or mesenteric artery and branches |
| S36.52 | Injury of transverse colon |
| T43.0 | Tricyclic and tetracyclic antidepressants |
| T81.0 | Haemorrhage or haematoma complicating a procedure, not elsewhere classified |
| T81.1 | Post procedural shock |
| T81.2 | Post-procedural shock |
| T81.4 | Infection following a procedure |
| T81.2 | Accidental puncture and laceration during a procedure, not elsewhere classified |
| T85.6 | Mechanical complication of internal prosthetic devices implants and grafts not elsewhere classified |
| T85.78 | Infection and inflammatory reaction to other internal prosthetic devices, implants and grafts |
| U79.3 | Depression |
| U82.3 | Hypertension |
| U86.2 | Arthritis and osteoarthritis |
| Y60.0 | Unintentional cut, puncture, perforation or haemorrhage during surgical operation |
| Y60.8 | Unintentional cut, puncture, perforation or haemorrhageduring other surgical and medical care |
| Y83.1 | Surgical operation with implant of artificial internal device |
| Y92.22 | Place of occurrence health service area |
| Z41.1 | Other plastic surgery for unacceptable cosmetic appearance |
| Z72.0 | Tobacco use current |
| Z80.0 | Family history of malignant neoplasm of digestive organs |
| Z86.43 | Personal history of tobacco use disorder |
| Z92.21 | Personal history of long-term (current) use of medicaments aspirin |
| Z92.22 | Personal history of monoclonal drug therapy |
| Z95.5 | Presence of coronary angioplasty and graft |
| Z96.8 | Presence of other specified functional implants |
Appendix 1B
Australian Refined Diagnostic Related Groups for people who received primary laparoscopic adjustable gastric band primary and surgical sequelae in the Tasmanian public hospital system.
| AR-DRG | Description |
|---|---|
| F21B | Other circulatory system general interventions, intermediate complexity |
| G02A | Major small and large bowel procedures, major complexity |
| G02B | Major small and large bowel procedures, intermediate complexity |
| G04C | Peritoneal adhesiolysis, minor complexity |
| G05C | Minor small and large bowel procedures |
| G10B | Hernia procedures, minor complexity |
| G11Z | Anal and stomal procedures |
| G47C | Gastroscopy, minor complexity |
| G48A | Colonoscopy, major complexity |
| G48B | Colonoscopy, minor complexity |
| G48C | Colonoscopy, same day |
| H07A | Open cholecystectomy, major complexity |
| H08A | Laparoscopic cholecystectomy, major complexity |
| H06B | Other hepatobiliary and pancreas general interventions, intermediate complexity |
| H08B | Laparoscopic cholecystectomy, minor complexity |
| K04A | Major procedures for obesity |
| K04B | Major procedures for obesity |
| K04Z | Major procedures for obesity |
| K10A | Revisional and open bariatric procedures, major complexity |
| K12Z | Other bariatric procedures |
| T01C | Infectious and parasitic diseases with general interventions, minor complexity |
| X06B | Other procedures for other injuries, intermediate complexity |
| X63B | Sequelae of treatment, minor complexity |
| Z01B | Other contacts with health services with GIs, minor complexity |
| Z40Z | Other contacts with health services with endoscopy |
Source: Independent Hospital Pricing Authority, Australian Refined Diagnosis Related Groups Version (6.0-9.0)
Appendix 2
Health indices—general final consumption expenditure (GFCE) on hospitals and nursing homes (reference year 2016–2017 = 100).
| 2006–2007 | 2007–2008 | 2008–2009 | 2009–2010 | 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GFCE on hospitals and nursing homes | 77.7 | 80.1 | 82.6 | 85.7 | 86.9 | 89.0 | 91.6 | 94.1 | 96.4 | 98.2 | 100.0 |
Sourced from Australian Institute of Health and Welfare Health Expenditure Australia Report 2016–2017
Author Contributions
JC conceptualised the study and developed and coordinated the strategic research alliance for the study with the project partner; coordinated the initial ethics approvals; developed and undertook the study design; coordinated and managed the data extracted for analysis; analysed and interpreted the data; and compiled the manuscript for submission. AP assisted with the conceptualisation of the study and the study design and with the interpretation of the findings and critically reviewed the manuscript. DD contributed to the study design, extracted the raw cost data, validated the final cohort and data for analysis and developed the cost database for data management. MG assisted with the initial data capture for analysis, assisted with the extraction of cost data and the development of the cost database for data management. BH assisted with the extraction of cost data and the development of the cost database for data management. IJ contributed to the study design and reviewed the manuscript. AV assisted with the conceptualisation of the study and the study design, assisted with the interpretation of the findings and critically examined the manuscript. AK assisted with the conceptualisation of the study, collected the BMI data from medical records, validated the surgical sequelae from medical records as requested by JC, and reviewed the manuscript. AN assisted with the conceptualisation of the study and the study design and critically reviewed the manuscript. MH assisted with the conceptualisation of the study and the strategic research alliance, assisted with the interpretation of the findings and critically examined the manuscript. SW assisted with the study design and critically examined the revised manuscript.
Compliance with Ethical Standards
Funding
This work was supported by a NHMRC Partnership Project Grant (APP1076899).
Conflict of interest
Julie Campbell, Martin Hensher, Daniel Davies, Matthew Green, Barry Hagan, Ian Jordan, Alison Venn, Alexandr Kuzminov, Amanda Neil, Stephen Wilkinson and Andrew Palmer have no conflicts of interest that are directly relevant to the content of this article.
Data Availability
The dataset for this study contains the participant clinical and sociodemographic data and ABF costs. The corresponding author will provide a deidentified dataset upon reasonable request.
References
- 1.Campbell J, Venn A, Neil A, Hensher M, Sharman M, Palmer A. Diverse approaches to the health economic evaluation of bariatric surgery: a comprehensive systematic review. Obes Rev. 2016;17(9):850–894. doi: 10.1111/obr.12424. [DOI] [PubMed] [Google Scholar]
- 2.Clarke B, Swinburn B, Sacks G. The application of theories of the policy process to obesity prevention: a systematic review and meta-synthesis. BMC Public Health. 2016;16(1):1084. doi: 10.1186/s12889-016-3639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gortmaker SL, Swinburn BA, Levy D, Carter R, Mabry PL, Finegood DT, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011;378(9793):838–847. doi: 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Colagiuri S, Lee C, Colagiuri R, Magliano D, Shaw JE, Zimmet PZ, et al. The cost of overweight and obesity in Australia. Med J Aust. 2010;192(5):260–264. doi: 10.5694/j.1326-5377.2010.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 6.Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US adults with obesity, by diabetes status. PharmacoEconomics. 2015;33(7):707–722. doi: 10.1007/s40273-014-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AIHW. Weight Loss Surgery in Australia 2014–15: Australian Hospital Statistics (Australian Institute of Health and Welfare); 2017.
- 8.Cawley J, Maclean JC, Hammer M, Wintfeld N. Reporting error in weight and its implications for bias in economic models. Econ Hum Biol. 2015;19:27–44. doi: 10.1016/j.ehb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO). Obesity Fact Sheet October 2017. Accessed October 2017.
- 10.Gould JC, Garren MJ, Boll V, Starling JR. Laparoscopic gastric bypass: risks vs. benefits up to two years following surgery in super-super obese patients. Surgery. 2006;140(4):524–529. doi: 10.1016/j.surg.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822–1832. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 12.National Health and Medical Research Council (NHMRC) Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. Melbourne: National Health and Medical Research Council; 2013. [Google Scholar]
- 13.Celio AC, Pories WJ. A history of bariatric surgery: the maturation of a medical discipline. Surg Clin N Am. 2016;96(4):655–667. doi: 10.1016/j.suc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim AM, Thumma JR, Dimick JB. Reoperation and medicare expenditures after laparoscopic gastric band surgery. JAMA Surg. 2017;152(9):835–842. doi: 10.1001/jamasurg.2017.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould JC. Considering the role of the laparoscopic adjustable gastric band: do not throw the baby out with the bathwater. JAMA Surg. 2017;152(9):842. doi: 10.1001/jamasurg.2017.1082. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257(1):87–94. doi: 10.1097/SLA.0b013e31827b6c02. [DOI] [PubMed] [Google Scholar]
- 17.Hall J. Australian health care—the challenge of reform in a fragmented system. N Engl J Med. 2015;373(6):493–497. doi: 10.1056/NEJMp1410737. [DOI] [PubMed] [Google Scholar]
- 18.National Partnership Agreement on Improving Health Services in Tasmania. Australian Council on Federal Financial Relations; 2014.
- 19.Independent Hospital Pricing Authority (IHPA). https://www.ihpa.gov.au/who-we-are. Accessed Nov 2016.
- 20.Willis EM, Reynolds L, Keleher H. Understanding the Australian Health Care System. Chatswood New South Wales: Churchill Livingston Elsevier; 2009. [Google Scholar]
- 21.Owen-Smith A, Kipping R, Donovan J, Hine C, Maslen C, Coast J. A NICE example? Variation in provision of bariatric surgery in England. BMJ (Online) 2013;346(7909):f2453. doi: 10.1136/bmj.f2453. [DOI] [PubMed] [Google Scholar]
- 22.Gill RS, Majumdar SR, Wang X, Tuepah R, Klarenbach SW, Birch DW, et al. Prioritization and willingness to pay for bariatric surgery: the patient perspective. Can J Surg. 2014;57(1):33–39. doi: 10.1503/cjs.021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagner M. Bariatric surgery tourism hidden costs? How Canada is not doing its part in covering bariatric surgery under the Canada Health Act. Can J Surg. 2017;60(4):222–223. doi: 10.1503/cjs.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharman MJ, Breslin MC, Kuzminov A, Palmer AJ, Blizzard L, Hensher M, et al. Population estimates and characteristics of Australians potentially eligible for bariatric surgery: findings from the 2011–13 Australian Health Survey. Aust Health Rev. 2017 doi: 10.1071/AH16255. [DOI] [PubMed] [Google Scholar]
- 25.Campbell JA, Ezzy D, Neil A, Hensher M, Venn A, Sharman MJ, Palmer AJ. A qualitative investigation of the health economic impacts of bariatric surgery for obesity, and implications for improved practice in health economics. Health Econ. 2018;27(8):1300–1318. doi: 10.1002/hec.3776. [DOI] [PubMed] [Google Scholar]
- 26.Kuzminov A, Palmer AJ, Wilkinson S, Khatsiev B, Venn AJ. Re-operations after secondary bariatric surgery: a systematic review. Obes Surg. 2016;26(9):2237–2247. doi: 10.1007/s11695-016-2252-7. [DOI] [PubMed] [Google Scholar]
- 27.James R, Salton R, Byrnes J, Scuffham P. Cost–utility analysis for bariatric surgery compared with usual care for the treatment of obesity in Australia. Surg Obes Relat Dis. 2017;13(12):2012–2020. doi: 10.1016/j.soard.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Garrison LP, Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real-world data for coverage and payment decisions: the ISPOR real-world data task force report. Value Health. 2007;10(5):326–335. doi: 10.1111/j.1524-4733.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 31.Porac JF, Wade JB, Fischer HM, Brown J, Kanfer A, Bowker G. Human capital heterogeneity, collaborative relationships, and publication patterns in a multidisciplinary scientific alliance: a comparative case study of two scientific teams. Res Policy. 2004;33(4):661–678. [Google Scholar]
- 32.Wang J, Hicks D. Scientific teams: self-assembly, fluidness, and interdependence. J Informetr. 2015;9(1):197–207. [Google Scholar]
- 33.Culyer AJ. The dictionary of health economics. Cheltenham: Edward Elgar Publishing Limited; 2010. [Google Scholar]
- 34.Department of Health and Human Services (DHHS), Tasmania. Patient Level Costing Policy and Manual (Draft v1.0); 2016.
- 35.Independent Hospital Pricing Authority (IHPA). Hospital Patient Costing Standards. Version 3.1; 2014.
- 36.Independent Hospital Pricing Authority (IHPA). AR-DRG codes. Versions 6.0 to 9.0. https://www.ihpa.gov.au/publications.
- 37.Independent Hospital Pricing Authority (IHPA). Development of the AR-DRG V9.0 Final Report May 2017.
- 38.Soldin M, Bain CJ, Mughal M. Body contouring surgery after bariatric surgery. Obesity, bariatric and metabolic surgery. Berlin: Springer; 2016. pp. 713–721. [Google Scholar]
- 39.Australian Institute of Health and Welfare (AIHW) 2016. Health Expenditure Australia 2016–17. Health and welfare expenditure series no. 57. Cat. no. HWE 67. Canberra: AIHW.
- 40.Owen-Smith A, Kipping R, Donovan J, Hine C, Maslen C, Coast J. A NICE example? Variation in provision of bariatric surgery in England. BMJ. 2013;1(346):f2453. doi: 10.1136/bmj.f2453. [DOI] [PubMed] [Google Scholar]
- 41.Lewis KH, Zhang F, Arterburn DE, Ross-Degnan D, Gillman MW, Wharam JF. Comparing medical costs and use after laparoscopic adjustable gastric banding and Roux-en-Y gastric bypass. JAMA Surg. 2015;150(8):787–794. doi: 10.1001/jamasurg.2015.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell J, Hensher M, Neil A, Venn A, Wilkinson S, Palmer AJ. An exploratory study of long-term publicly waitlisted bariatric surgery patients’ quality-of-life before and 1 year after bariatric surgery, and considerations for healthcare planners. PharmacoEconomics Open. 2017 doi: 10.1007/s41669-017-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset for this study contains the participant clinical and sociodemographic data and ABF costs. The corresponding author will provide a deidentified dataset upon reasonable request.