Abstract
Objective
Frozen–thawed embryo transfer enables surplus embryos derived from IVF or IVF-ICSI treatment to be stored and transferred in subsequent cycles into a more “physiologic environment”. This study aimed to investigate the clinical effect of letrozole use or hMG stimulation on pregnancy and neonatal outcomes in ovulatory patients undergoing FET.
Methods
This study includes a total of 5901 FET cycles with letrozole use (n = 1569), HMG (n =1827) or letrozole + HMG (n = 2505). In the letrozole group, 2.5 mg of letrozole was administered on menstrual cycle day 3 to 5 for 3 days for patients, and then follicle growth was monitored beginning on day 10. If the follicular diameter was ≥14 mm on the 10th day, no other ovarian stimulation drugs were needed. If the follicular diameter was <14 mm on the 10th day, 150 IU human menopausal gonadotropin (hMG) was added to stimulate follicle growth every two days (hMG + letrozole group). In hMG stimulation group, a total of 150 IU of hMG was injected every two days to stimulate development of follicles from cycle day 10 to 12.
Results
Compared with the patients undergoing hMG stimulation, the group receiving letrozole or letrozole+HMG stimulation exhibits significantly higher clinical pregnancy rates per transfer (hMG: 47.02% vs letrozole: 52.07% vs letrozole+HMG: 52.26%) and implantation rates (hMG: 31.76% vs letrozole: 34.36% vs letrozole+HMG: 34.24%). In addition, the letrozole group was associated with a statistically significantly lower incidence of miscarriage (hMG: 14.78% vs letrozole: 10.53% vs letrozole+HMG: 14.13%) and ectopic pregnancies (hMG: 1.83% vs letrozole: 0.97% vs letrozole+HMG: 1.58%) than the letrozole + HMG and HMG groups. Neonatal outcomes are similar among the three groups.
Conclusion
Our data demonstrate that the letrozole use may improve clinical pregnancy outcomes and decrease the risk of ectopic pregnancies and miscarriage in ovulatory patients who receive FET cycles.
Keywords: frozen–thawed embryo transfer, Letrozole, ovulation induction, hMG, clinical efficacy
Introduction
Frozen–thawed embryo transfer (FET) enables the excess embryos generated by IVF and ICSI to be stored and utilized at a later date. This has been widely used in in-vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) because it can effectively improve the cumulative pregnancy rate and avoid successive procedures for oocyte retrieval. FET serves to prevent ovarian hyperstimulation syndrome or delay transfer of embryos when no optimal endometrial preparation is available.1 A recently published meta-analysis study showed that pregnancies from FET are associated with decreased risks of preterm birth, low birth weights, and perinatal deaths, as compared with pregnancies from fresh-embryo transfer.2 However, one of the limitations of FET is that ovulation timing may present challenges for women who have irregular cycles, which may result in higher cancellation rates. Menstrual cycles can be influenced by a wide range of factors, including BMI, smoking, alcohol intake and physical activity, as well as pathologic conditions, including polycystic ovary syndrome (PCOS).3–6 In these patients, the use of mild ovarian stimulation with gonadotropins or aromatase inhibitors to lessen the stimulation of follicular development is an effective approach to reduce cancellation rates and to diminish the hypoestrogenic effects of GnRH agonists.
One of the most crucial steps in assisted reproductive technology (ART) is implantation of the embryo, which mainly relies on three factors: quality of embryo, receptivity of endometrium, and optimal synchronization between the growth of endometrium and development of the embryo.7 Thus, effective preparation of the endometrium prior to FET is indispensable. The most common endometrial preparation strategies for FET include natural cycle, ovarian stimulation, and artificial or stimulated preparation (hormonal substitution) with estrogen and progesterone. Stimulation of the ovaries with exogenous gonadotropins has been suggested to correct defects in the follicular and luteal phase, which may result in an improved endometrial preparation for the implantation of an embryo.8 Additionally, a pilot study has also shown that endometrial preparation for FET patients with PCOS using letrozole (an aromatase inhibitor) stimulation exhibits improved clinical effects, as compared with human menopausal gonadotropin (hMG) stimulation in the initial follicular phase.9 However, the effect of letrozole vs hMG on the pregnancy and neonatal outcomes of ovulatory women is uncertain.
Thus, in this study, we aimed to compare the reproductive outcomes after FET cycles stimulated with letrozole use, HMG or letrozole + HMG in ovulatory patients. Our findings may offer important insights into identifying the ideal endometrial preparation conditions prior to FET.
Materials And Methods
Patients
This was a retrospective and non-interventional study. A cohort of 5901 patients who underwent treatment with FET were enrolled into this study at the Department of Assisted Reproduction of Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, from October 2007 to July 2016. Inclusion criteria included 1) age 20–40 years; 2) regular menstrual cycles (a spontaneous cycle length of ≥30 days and ≤35 days); 3) basal serum FSH concentration <10 IU/L. Exclusion criteria included: 1) documented ovarian failure including basal FSH ≥10 IU/L or no antral follicles according to ultrasound examination; 2) diagnosis of polycystic ovarian syndrome; The assignment of ovarian stimulation protocols was not randomized but was based on physicians’ habitual practice and/or patients’ preference. Couples enrolled in this study were evaluated for infertility prior to the treatment of ART. The medical history, physical examinations, pelvic ultrasound, hysteroscopy, endometrial biopsy, and semen analysis were also performed. Tubal patency or lack of patency was recorded through either hysterosalpingography or methylene blue tubal testing during laparoscopy. The protocol of the study was approved by the Ethics Committee (Institutional Review Board) of Shanghai Ninth People’s Hospital. Informed written consent in accordance with the ethics committee protocol was obtained from all patients. The study was conducted according to the Declaration of Helsinki for medical research.
Letrozole Group
In the letrozole group, 2.5 mg of letrozole (Jiangsu Hengrui Medicine Co.) was administered on menstrual cycle day 3 to 5 for 3 days for patients, and then follicle growth was monitored beginning on day 10. If the follicular diameter was ≥14 mm on the 10th day, no other ovarian stimulation drugs were needed until follicle maturation and hCG triggering. If the follicular diameter was <14 mm on the 10th day, 150 IU human menopausal gonadotropin (hMG) (Maanshan Pharmaceutical Trading Co., Anhui, China) was added to stimulate follicle growth every two days (hMG + letrozole group). If the follicular diameter was >16 mm and endometrial thickness was ≥8 mm, and then 5000 IU of hCG was provided at different times; consequently, FET times were also adjusted in accordance with the serum LH levels on the day of hCG injection. The details are described below.
hMG Stimulation Group
In this group, patients had a pelvic ultrasound examination and blood analysis at cycle day 10 to 12. A total of 150 IU of hMG was injected every two days to stimulate development of follicles as well as for endometrial proliferation. Follicular monitoring, measurement of serum levels of hormones and triggering final oocyte maturation had been carried out in the same way as the letrozole group.
Synchronization Of The Embryo And Endometrium
Follicle growth was monitored on the 10th day of the cycle. If the diameter of the prevailing follicle was >16 mm and endometrial thickness was ≥8 mm, serum levels of E2 are >150 pg/mL and serum P levels are <1.0 ng/mL, one of the following two procedures was conducted depending on the serum levels of LH. When LH was <20 IU/L, hCG 5000 IU was given at night (21:00) to trigger ovulation, and the transfer of 3-day-old embryos was also performed 5 days later. On the contrary, when the LH value was >20 IU/L, hCG 5000 IU was administered in the afternoon (14:00), and then the transfer was carried out after 4 days. The transfer of blastocysts was arranged on the sixth or seventh day depending on the serum levels of hormones and the results of ultrasound exams. A total of 40 mg/day of exogenous progesterone (Duphaston; Abbott Biologicals) was given for luteal support starting on the third day after hCG injection.
Transfer Of Frozen–Thawed Embryos
The embryos were examined to determine the number and morphology of blastomeres as well as the level of embryonic disintegration according to the Cummins’s criteria.10 All high-quality embryos (such as grade 1 and grade 2, 8-cell embryos) were frozen by vitrification on day three after oocyte retrieval. Embryos that were of low quality were placed in extensive culture up to the blastocyst phase. On day 5 or 6 of this phase, the blastocysts with normal morphology were frozen. The freezing and thawing procedure of cleavage-stage embryos and blastocysts is described below.11
Embryo Vitrification And Thawing Procedure
High-quality day 3 embryos were selected for cryopreservation, and spare embryos were cultured for an extended period until the blastocyst phase. The protocol for freezing and thawing cleavage-stage embryos and blastocysts using MediCult Vitrification Cooling (Origio, Denmark) has previously been described.11 Briefly, vitrification was performed at room temperature, and embryos were first suspended in pre-warmed Equilibration Medium for 5 mins. Subsequently, embryos were transferred into the Vitrification Medium for no more than 1 mins. Finally, embryos were loaded on the tip of the vitrification carrier and plunged immediately into liquid nitrogen for storage. Thawing was performed using MediCult Vitrification Warming (Origio, Denmark). First, vitrified embryos on the tip of the carrier were quickly transferred into the Warming Medium, which has been warmed up to 37 °C, and kept there for 1 mins. Second, the embryos were suspended in Dilution Medium 1 for 3 mins and Dilution Medium 2 for 5 mins at room temperature. Finally, they were kept in Washing Medium for 3 mins, and the thawed embryos were transferred into equilibrated culture medium and allowed to rest in an incubator until the transfer.
Outcome Assessment
The transfer of embryos was conducted under the guidance of a transabdominal ultrasound. The main outcome measure was the live birth which was defined as an infant born alive after 24 weeks of gestation who survived more than 28 days. Clinical pregnancy was regarded as the presence of gestational sac and fetal heart activity, evaluated by ultrasound at 7 weeks of gestation. Secondary outcomes included miscarriage rates, embryological details and hormonal profile at time of hCG injection and on the day of embryo transfer. The failure to grow or the discontinuation of a pregnancy before the 24 weeks of gestation is considered as miscarriage. The embryological parameters comprised the mean number of high-quality transformed thawed embryos and endometrial thickness on the transfer day of embryo.
Hormone Measurement And Biochemical Assays
Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and progestogen (P) were measured on menstrual cycle day 3 and on the day of trigger and embryo transfer. Hormone levels were determined using chemiluminescence (Abbott Biologicals B.V.). The lower limits of sensitivity were as follows: FSH, 0.06 mIU/mL; LH, 0.09 mIU/mL; E2, 10 pg/mL and P, 0.1 ng/mL. The upper limit of the E2 measurement was 5000 pg/mL. The E2 values were recorded as 5000 pg/mL if the E2 level on the trigger day or the day after trigger was higher than the upper limit.
Statistical Analysis
Patients were separated into Letrozole, HMG, and Letrozole + HMG groups. For continuous variables, the normality was tested by the graphical use of histograms and Q–Q plots as well as the Shapiro–Wilk test. If data are normally distributed, then they were presented as mean with standard deviation (SD); otherwise, they were presented as median (min–max).
Continuous variables were compared via one way ANOVA (Bonferroni’s post hoc test was used when p < 0.05), while categorical variables were compared via chi-squared tests. Odds ratios (OR) with corresponding 95% confidence intervals (CIs) were generated via logistic regression models and were used when assessing the relationship between variables and live birth following adjustment for potential confounds such as maternal age, maternal BMI, cause of infertility, means of fertilization, numbers of transferred embryos, and quality of embryos. SPSS v22.0 (IBM, NY, USA) was used for all statistical testing, with a P<0.05 significance threshold.
Results
Patient Characteristics
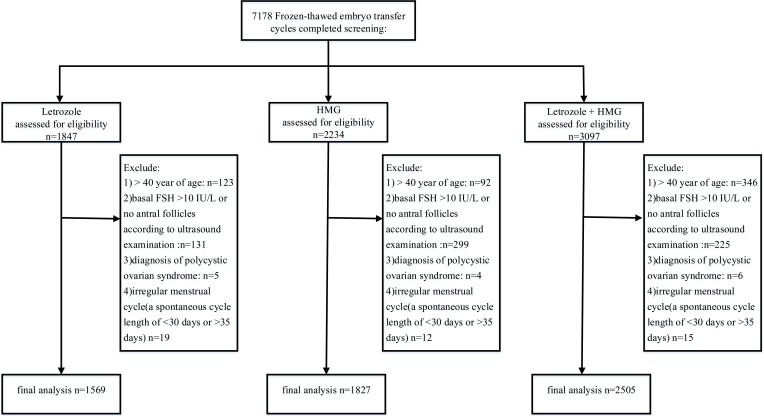
As illustrated in Table 1, the three groups exhibit similar characteristics, including BMI, duration of infertility, basal endocrine characteristics, and fertilization method (P > 0.05) in the cycles. However, the patients in letrozole + hMG group were significantly older than the patients in the hMG group and the patients in the letrozole group (Table 1). Figure 1 outlines the flow of the study participants.
Table 1.
Baseline Characteristics At Cycle Level
| Characteristic | Letrozole (n=1569) | HMG (n=1827) | Letrozole + HMG (n=2505) | P-Valuea | P-Valueb | P-Valuec |
|---|---|---|---|---|---|---|
| Maternal age (year) | 35.03±3.88 | 34.46±4.24 | 35.48±4.59 | 0.002 | <0.001 | 0.048 |
| Duration of infertility (years) | 3.73±2.85 | 3.62±2.87 | 3.69±2.82 | 0.846 | 0.693 | 0.865 |
| BMI of women | 23.51±4.21 | 23.39±4.83 | 23.67±4.93 | 0.529 | 0.167 | 0.873 |
| Day 3 FSH (mIU/mL) | 4.09(0.17–9.97) | 4.13(0.84–9.98) | 4.17(0.32–9.99) | 0.482 | 0.851 | 0.186 |
| Day 3 LH (mIU/mL) | 4.98(0.11–18.94) | 4.92(0.17–19.01) | 4.96(0.94–17.32) | 0.239 | 0.694 | 0.481 |
| Day 3 E2 (pg/mL) | 34.00(10.00–85.00) | 33.00(10.00–90.00) | 33.00(10.00–99.00) | 0.461 | 0.861 | 0.336 |
| Day 3 P (ng/mL) | 0.30(0.10–0.80) | 0.30(0.10–0.90) | 0.30(0.10–0.90) | 0.151 | 0.197 | 0.751 |
| Infertility causes, n (%) | ||||||
| Male factor | 193(12.28%) | 215(11.75%) | 309(12.35%) | 0.206 | 0.455 | 0.799 |
| Tubal factor | 736(46.89%) | 825(45.15%) | 1145(45.69%) | |||
| Endometriosis | 18(1.17%) | 36(1.99%) | 39(1.56%) | |||
| Mixed | 577(36.80%) | 685(37.48%) | 942(37.61%) | |||
| Unexplained | 45(2.86%) | 66(3.63%) | 70(2.79%) | |||
| Fertilization method | ||||||
| IVF (%) | 1007(64.18%) | 1104(60.43%) | 1487(59.36%) | 0.077 | 0.183 | 0.023 |
| ICSI (%) | 377(24.03%) | 480(26.27%) | 715(28.54%) | |||
| Half IVF +half ICSI (%) | 185(11.79%) | 243(13.30%) | 303(12.10%) | |||
| Year of treatment (%) | ||||||
| 2007–2010 | 50(3.19%) | 76(4.16%) | 72(2.87%) | <0.001 | <0.001 | <0.001 |
| 2011–2012 | 228(14.53%) | 247(13.52%) | 290(11.58%) | |||
| 2013–2014 | 645(41.11%) | 1044(57.14%) | 918(36.65%) | |||
| 2015–2016 | 646(41.17%) | 460(25.18%) | 1225(48.90%) | |||
Notes: aLetrozole vs HMG; bHMG vs letrozole+HMG; cLetrozole vs letrozole+HMG. Bold values represent statistical significance.
Figure 1.
Diagram of inclusion criteria.
Hormonal Profiles
The mean serum levels of FSH and E2 on the day of hCG administration in the hMG group were significantly higher than those in the letrozole group (P < 0.001) and in the letrozole + hMG group (P <0.001) (Table 2). Nonetheless, we found out that the mean serum P level on the trigger day in the hMG group was significantly lower than those in the letrozole group (P < 0.001) and in the letrozole + hMG group (P < 0.001). No significant differences in the mean LH level were observed on the trigger day between the three groups. Subsequently, on the day of embryo transfer, the hMG group displayed higher E2 and lower P levels than the letrozole group and the letrozole + hMG group.
Table 2.
Ovarian Stimulation Characteristics And Hormonal Data On The Trigger Day In FET Cycle
| Characteristics | Letrozole (n=1569) | HMG (n=1827) | Letrozole+HMG (n=2505) | P-Valuea | P-Valueb | P-Valuec |
|---|---|---|---|---|---|---|
| Hormones at time of hCG injection | ||||||
| FSH (mIU/mL) | 8.47(1.67–26.17) | 9.98(1.52–28.39) | 9.30(1.03–30.86) | <0.001 | <0.001 | <0.001 |
| LH (mIU/mL) | 19.05(0.72–74.64) | 17.99(0.42–77.75) | 18.64(0.89–80.76) | 0.054 | 0.091 | 0.658 |
| E2 (pg/mL) | 284.00(135.00–1058.00) | 483.00(128.00–2811.00) | 327.00(101.00–2443.00) | <0.001 | <0.001 | <0.001 |
| P (ng/mL) | 0.60(0.10–3.40) | 0.40(0.10–3.90) | 0.60(0.10–4.1) | <0.001 | <0.001 | 0.396 |
| Hormones on day of transfer | ||||||
| E2 (pg/mL) | 107.00(10.00–403.00) | 298.00(25.00–849.00) | 122.00(12.00–772.00) | <0.001 | <0.001 | 0.026 |
| P (ng/mL) | 18.30(1.80–56.20) | 15.00(0.50–57.50) | 19.00(0.90–60.70) | <0.001 | <0.001 | 0.008 |
| Endometrial thickness (mm) | 11.07±2.11 | 11.15±2.02 | 11.12±2.45 | 0.099 | 0.991 | 0.929 |
Notes: aLetrozole vs HMG; bHMG vs letrozole+HMGL; cLetrozole vs letrozole+HMG. Bold values represent statistical significance.
Pregnancy And Neonatal Outcomes Between The hMG And Letrozole Groups
The comparison of the pregnancy outcomes between the hMG, letrozole, and letrozole + hMG groups is presented in Table 3. A total of 11,213 embryos were thawed, and the rate of viable embryos after thawing was 99.12% (11114/11213). The implantation, clinical pregnancy, and live birth rate were significantly higher in the letrozole and letrozole + hMG group than in the hMG group (Table 3). Moreover, the miscarriage rate and the ectopic pregnancy rate were significantly lower in letrozole groups than in hMG and letrozole + hMG groups. Since the majority of the cycles were from the years of 2013 to 2016, a sub-analysis of the pregnancy outcome for those three years is presented in Supplementary Table 1 and showed consistent trends.
Table 3.
Reproductive Outcomes After FET According To The Type Of Endometrial Preparation Treatments
| Characteristics | Letrozole (n=1569) | HMG (n=1827) | Letrozole+HMG (n=2505) | P-Valuea | P-Valueb | P-Valuec |
|---|---|---|---|---|---|---|
| Number of FET | 1569 | 1827 | 2505 | |||
| Number of thawed embryos | 3022 | 3476 | 4715 | |||
| Number of viable embryos after thawed | 2986 | 3441 | 4687 | |||
| Developmental stage at cryopreservation | ||||||
| Cleavage stage (Day 3 or 4) | 2619(87.71%) | 3043(88.42%) | 2203(87.93%) | 0.196 | 0.295 | 0.412 |
| Blastocyst stage (Day 5) | 367(12.29%) | 398(11.58%) | 302(12.07%) | |||
| Embryo quality | ||||||
| Grade I | 649(21.73%) | 690(20.06%) | 523(20.86%) | 0.245 | 0.076 | 0.091 |
| Grade II | 2332(78.09%) | 2746(79.79%) | 1970(78.66%) | |||
| Grade III | 5(0.18%) | 5(0.15%) | 12(0.48%) | |||
| Number of embryos transferred | ||||||
| 1 | 194(6.49%) | 221(6.42%) | 285(6.08%) | 0.472 | 0.279 | 0.246 |
| 2 | 2792(93.51%) | 3220(93.58) | 4402(99.92%) | |||
| Clinical pregnancy rate per transfer (%) | 52.07% (817/1569) | 47.02% (859/1827) | 52.26% (1309/2505) | 0.047 | 0.037 | 0.486 |
| Implantation rate (%) | 34.36% (1026/2986) | 31.76% (1086/3441) | 34.24% (1605/4687) | 0.048 | 0.036 | 0.481 |
| Miscarriage rate (%) | 10.53% (86/817) | 14.78% (127/859) | 14.13% (185/1309) | 0.013 | 0.381 | 0.018 |
| Multiple pregnancy rate (%) | 25.58% (209/817) | 26.43% (227/859) | 22.61% (296/1309) | 0.402 | 0.063 | 0.121 |
| Ectopic pregnancy rate (%) | 0.97% (5/833) | 1.83% (16/875) | 1.58% (21/1330) | 0.018 | 0.392 | 0.031 |
| Intrauterine and ectopic pregnancy rate (%) | 0.36% (3/833) | 0.80%(7/875) | 0.38% (5/1330) | 0.194 | 0.161 | 0.629 |
| Ongoing pregnant rate per transfer (%) | 47.61% (747/1569) | 41.32%(755/1827) | 47.27% (1184/2505) | 0.012 | 0.009 | 0.461 |
| Live birth rate (%) | 46.59% (731/1569) | 39.96%(730/1827) | 44.87% (1124/2505) | 0.008 | 0.022 | 0.265 |
Notes: aLetrozole vs HMG; bHMG vs letrozole+HMG; cLetrozole vs letrozole+HMG. Bold values represent statistical significance.
The details of the neonatal outcomes regarding gestational week, mode of delivery, birth weight, and sex are summarized in Table 4. Data are provided separately for singleton and multiple births. As the results show, no notable differences in these characteristics were evident between these three groups. The overall incidence of neonatal death in live-born infants was also similar between the 3 groups (P>0.05). These results indicated that comparable neonatal outcomes were achieved for three groups.
Table 4.
Neonatal Outcomes After FET According To The Type Of Endometrial Preparation Treatments
| Characteristics | Letrozole (n=931) | HMG (n=952) | Letrozole+HMG (n=1363) | P-Valuea | P-Valueb | P-Valuec | P-Vlued | P-Valuee | P-Valuef | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Singleton Births (n=531) | Multiple Births (n=400) | Singleton Births (n=508) | Multiple Births (n=444) | Singleton Births (n=885) | Multiple Births (n=478) | |||||||
| Stillborn- no (%) | 0(0) | 2(0) | 0(0) | 0(0) | 1(0.11) | 0(0) | ||||||
| Live born- no (%) | 531(100) | 398(99.5) | 508(100) | 444(100) | 884(0.89) | 478(0100) | ||||||
| Gestational weeks at delivery (weeks) |
||||||||||||
| <32 | 6(1.12%) | 4(2.00%) | 6(1.18%) | 8(1.80%) | 5(0.56%) | 10(2.09%) | 0.892 | 0.346 | 0.491 | 0.341 | 0.056 | 0.925 |
| 32–36 | 33(6.22%) | 92(46.00%) | 28(5.51%) | 232(52.25%) | 58(6.55%) | 212(44.35%) | ||||||
| ≥37 | 492(92.66%) | 104(52.00%) | 474(93.31%) | 204(45.95%) | 822(92.89%) | 256(53.56%) | ||||||
| Birth weight (g) | ||||||||||||
| <1500 | 5(0.94%) | 8(2.00%) | 5(0.98%) | 6(1.35%) | 4(0.45%) | 8(1.67%) | 0.831 | 0.108 | 0.291 | 0.154 | 0.857 | 0.086 |
| 1500–2499 | 22(4.14%) | 115(28.75%) | 25(4.92%) | 154(34.68%) | 27(3.05%) | 171(35.77%) | ||||||
| ≥2500 | 504(94.92%) | 277(69.25%) | 478(94.10%) | 284(63.97%) | 853(96.38%) | 299(62.56%) | ||||||
| Sex of neonates | ||||||||||||
| Male | 258(48.59%) | 208(52.00%) | 253(49.81%) | 231(52.03%) | 438(49.49%) | 236(49.37%) | 0.371 | 0.478 | 0.392 | 0.524 | 0.231 | 0.241 |
| Female | 273(51.41%) | 192(48.00%) | 255(50.19%) | 213(47.97%) | 447(50.51%) | 242(50.63%) | ||||||
| Mode of delivery | ||||||||||||
| Vaginal | 122(22.98%) | 14(3.50%) | 134(26.38%) | 10(2.26%) | 243(27.46%) | 14(2.93%) | 0.115 | 0.355 | 0.069 | 0.189 | 0.332 | 0.386 |
| Cesarean section | 409(77.02%) | 386(96.50%) | 374(73.62%) | 434(97.74%) | 642(72.54%) | 464(97.07%) | ||||||
Notes: aLetrozole singleton births vs HMG singleton births; bHMG singleton births vs letrozole+HMG singleton births. cLetrozole singleton births vs letrozole+HMG singleton births; dLetrozole multiple births vs HMG multiple births. eHMG multiple births vs letrozole+HMG multiple births. fLetrozole multiple births vs letrozole+HMG multiple births.
Logistic Regression Of Pregnancy Outcome In FET Cycles
Table 5 presents the results of a logistic regression model for the factors that may have influenced the live birth rate. The final results indicated significant positive (favorable) effects of the endometrial thickness (OR = 1.12, 95% CI: 1.03–1.16), the use of letrozole (OR = 1.56, 95% CI: 1.21–2.02), and letrozole+HMG (OR = 1.42, 95% CI: 1.24–1.62); however, negative (adverse) effects of maternal age (OR = 0.83, 95% CI: 0.71–0.93), previous IVF attempts (OR = 0.79, 95% CI: 0.67–0.96), and serum levels of E2 (OR = 0.72, 95% CI: 0.45–0.82) were identified on the outcome.
Table 5.
Logistic Regression Of Pregnancy Outcome In FET Cycles
| Baseline Parameter | Category | aOR (95% CI) | P-Value |
|---|---|---|---|
| Age (years) | per year increased | 0.83(0.71–0.93) | <0.001 |
| BMI (kg/m2) | per kg/m2 increased | 0.75(0.22–2.26) | 0.61 |
| Number of previous attempts | per time increased | 0.79(0.67–0.96) | <0.001 |
| Hormones at time of hCG injection | |||
| FSH (IU/L) | per IU/L increased | 0.97(0.82–1.83) | 0.63 |
| LH (IU/L) | per IU/L increased | 1.01(0.83–1.79) | 0.56 |
| E2 (pg/mL) | per pg/mL increased | 0.72(0.45–0.82) | 0.03 |
| Progesterone (ng/mL) | per ng/mL increased | 0.97(0.78–1.54) | 0.94 |
| Endometrial thickness (mm) | per mm increased | 1.12(1.03–1.16) | <0.001 |
| Type of stimulation protocol | |||
| letrozole vs HMG | 1.56(1.21–2.02) | <0.001 | |
| HMG vs letrozole+HMG | 1.42(1.24–1.62) | <0.001 | |
| letrozole vs letrozole+HMG | 1.16(0.83–1.34) | 0.45 | |
Notes: Analyses were adjusted for maternal age, maternal BMI, infertility causes, fertilization method, number of embryo transferred, and embryo quality. Bold values represent statistical significance.
Abbreviation: aOR, adjusted odd ratio
Discussion
In this study, we investigated the effects of letrozole, letrozole + HMG, and HMG stimulation on the pregnancy and neonatal outcomes using a large sample size of ovulatory patients undergoing FET. Our study showed that the letrozole and letrozole + HMG group were associated with significantly greater success rates of implantation, clinical pregnancy, ongoing pregnancy, and live birth, compared with the patients receiving hMG stimulation. The miscarriage rate was significantly lower in the letrozole group, compared with letrozole + HMG and HMG groups, although there is no significant difference in neonatal outcomes between the three groups.
Notably, our findings are in agreement with previous clinical trial studies in patients with ovulation defects.9,12–14 Hu et al demonstrated that patients with letrozole stimulation in FET had a significantly higher success rates of implantation (38.3%), ongoing pregnancy (60.0%), and clinical pregnancy (65.0%), compared with the artificial (23.3%, 36.8%, and 40.8%, respectively) and hMG stimulation groups (34.4%, 25.0%, and 15.3%, respectively) in 120 patients with PCOS (10). Tatsumi et al also revealed significantly higher rates of clinical pregnancy (56.5%) and live birth (51.3%) following letrozole use, compared with natural-cycle group (32.5%, 26.4%, respectively) and HRT (28.8%, 23.3%, respectively) cycles, while the miscarriage rate significantly decreases in the letrozole group (16.1%), compared with the natural (27.0%) and HRT groups (29.0%) in women receiving FET, single-embryo transfer.14
Consistent with earlier studies,13,14 our results demonstrated similar neonatal outcomes between letrozole, letrozole + HMG, and HMG group. Tatsumi et al showed that letrozole use results in mostly similar outcomes, compared with the natural and HRT cycles in fresh or FET cycles.13,14 Additionally, a randomized controlled trial study suggests that patients with letrozole and gonadotropin cycles display comparable pregnancy outcomes, including baby gender, gestational age at delivery, birth weight, and neonatal complications, thus further confirming the validity of our study.15 The half-life of letrozole ranges from 30 to 60 hrs with a mean of 45 hrs, and hence it should have been eliminated from the body by the time of implantation, thereby producing no effect on the gestational duration and the development of fetus.16
Letrozole improves the outcome of FET probably by increasing the expression of receptivity marker, integrin, among ART patients with low integrin expression. Ganesh et al demonstrated that the use of letrozole is associated with significantly increased expression of integrin in the uterine endometrium, compared with natural cycles in patients with unexplained infertility.17 Furthermore, Miller et al also reported that letrozole upregulates the expression levels of integrin and significantly increases the pregnancy and implantation rates after IVF among women who lacked normal expression levels of integrin.18 Integrin is thought to be involved in the initial attachment of the embryo to the endometrium, and its reduced expression in the luteal phase of the cycle is reported to have an adverse effect on blastocyst implantation.19–22
In addition, we found that women in the letrozole and letrozole + HMG group had significantly decreased E2 levels on the day of hCG administration and embryo transfer, compared with those in the HMG stimulation group. Moreover, the logistic-regression analyses demonstrated that estradiol levels were significantly associated with pregnancy outcomes in FET cycles. Interestingly, several studies have been conducted to assess the impact of estradiol levels on IVF–ICSI outcomes.19,23 The findings of those studies were not consistent with each other. Some studies have reported that higher estradiol values adversely affected endometrial receptivity,23,24 whereas others showed no significant effect.25,26 Conversely, high estradiol levels on the day of hCG administration were found to produce a positive effect on embryo quality.19 High levels of estradiol were found to affect embryo quality, implantation, and pregnancy by modulating the leptin levels in the follicular fluid.25,27 Significantly higher successful pregnancy rates after FET, compared with fresh-embryo transfer, have been attributed to exposure to low steroid hormones, such as E2.28 Moreover, low serum levels of estrogen result in upregulation of the expression of estrogen receptors and subsequent increase in the sensitivity to high estrogen levels, which increases endometrial proliferation and blood flow in the uterus and endometrium. Implantation failure is thought to arise for reasons similar to those resulting in miscarriage, with disrupted endometrial and placental blood flow disrupting endometrial receptivity or inducing miscarriage.29 We therefore speculate that reduced estrogen levels may be one of the mechanisms underlying the beneficial effects of letrozole stimulation in FET leading to higher rates of implantation and decrease rates of miscarriage.9,14
In addition, we observed a significantly higher P level on trigger day in the letrozole group, likely as a result of a larger number of follicles having undergone induction, thereby leading to corpus lutea.30 Another prospective randomized controlled study has similarly found that patients administered letrozole exhibited significantly higher mid-luteal progesterone levels relative to those administered clomiphene citrate.31 Adequate luteal function and sufficient circulating P4 levels are necessary in order to yield a uterine setting capable of promoting continued embryo elongation during the critical period of attachment.
Moreover, our study also indicated that the letrozole group (14.78%) exhibits a statistically significantly lower incidence of ectopic pregnancies than the letrozole + HMG (14.13%) and HMG groups (14.78%). Previous studies reported that women with high estradiol levels on the day of hCG administration were found to have a higher risk of ectopic pregnancies than those with low estradiol level in fresh embryo transfer and high estradiol levels may have played a critical role in the development of ectopic pregnancies.32,33 Thus, it is possible that optimization of estradiol levels resulting from letrozole treatment may serve to decrease the incidence of ectopic pregnancies.
The main limitation is that the data were drawn from a retrospective analysis. The protocols, methods, and results of treatment with medically assisted reproduction may have differed with time, since this study was carried out over a long duration of more than 10 years. An alternative option to the single-center study with extended duration would be to conduct a multi-center clinical trial over a shorter duration.
Conclusion
Our data demonstrated that FET after ovarian stimulation with letrozole significantly improves the clinical pregnancy outcome, reduces the risks of miscarriage and ectopic pregnancies, and produces no effect of the neonatal outcomes in ovulatory patients, although further randomized controlled trials with larger sample size are warranted to validate these findings.
Acknowledgements
We would like to appreciate Dr Qianqian Zhu‘s generous help with statistical analysis.
Funding Statement
This research was supported by grants from National Natural Science Foundation of China (81801526, 81801527 and 31770989), Shanghai ninth hospital (JYLJ030).
Abbreviations
FET, frozen–thawed embryo transfer; PCOS, polycystic ovary syndrome; ART, assisted reproductive technology; hMG, human menopausal gonadotropin.
Ethics Approval And Consent To Participate
This study was approved by the Ethics Committee (Institutional Review Board) of Shanghai Ninth People’s Hospital.
Availability Of Data And Material
The corresponding authors can be contacted regarding data requests.
Disclosure
The authors declare that they do not have any commercial or associative interest that represents a conflict of interest in connection with the published work.
References
- 1.De Neubourg D, Peeraer K, Debrock S, D’Hooghe T. Belgium model of coupling reimbursement of ART costs to restriction in number of embryos transferred. BMJ. 2014;348:g1559. doi: 10.1136/bmj.g1559 [DOI] [PubMed] [Google Scholar]
- 2.Roque M, Lattes K, Serra S, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–162. doi: 10.1016/j.fertnstert.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 3.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;422–428. doi: 10.1097/00001648-199907000-00014 [DOI] [PubMed] [Google Scholar]
- 5.Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13:668–674. doi: 10.1097/00001648-200211000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Lyngsø J, Toft G, Høyer BB, Guldbrandsen K, Olsen J, Ramlau-Hansen C. Moderate alcohol intake and menstrual cycle characteristics. Hum Reprod. 2014;29:351–358. doi: 10.1093/humrep/det417 [DOI] [PubMed] [Google Scholar]
- 7.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004 [DOI] [PubMed] [Google Scholar]
- 8.Peeraer K, Couck I, Debrock S, et al. Frozen–thawed embryo transfer in a natural or mildly hormonally stimulated cycle in women with regular ovulatory cycles: a RCT. Hum Reprod. 2015;dev224. [DOI] [PubMed] [Google Scholar]
- 9.Hu YJ, Chen YZ, Zhu YM, Huang HF. Letrozole stimulation in endometrial preparation for cryopreserved–thawed embryo transfer in women with polycystic ovarian syndrome: a pilot study. Clin Endocrinol. 2014;80:283–289. doi: 10.1111/cen.12280 [DOI] [PubMed] [Google Scholar]
- 10.Cummins J, Breen T, Harrison K, Shaw J, Wilson L, Hennessey J. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transfer. 1986;3:284–295. doi: 10.1007/BF01133388 [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Xue S, Jin W, Lv Q, Peng Q, Cao S. Impact of incubation time of vitrification-warming embryos on frozen-thawed embryo transfer outcomes. J Reprod Med. 2010;19:104–107. [Google Scholar]
- 12.Li S-J, Zhang Y-J, Chai X-S, et al. Letrozole ovulation induction: an effective option in endometrial preparation for frozen–thawed embryo transfer. Arch Gynecol Obstet. 2014;289:687–693. doi: 10.1007/s00404-013-3044-0 [DOI] [PubMed] [Google Scholar]
- 13.Tatsumi T, Jwa SC, Kuwahara A, Irahara M, Kubota T, Saito H. Pregnancy and neonatal outcomes following letrozole use in frozen-thawed single embryo transfer cycles. Hum Reprod. 2017;32:1244–1248. doi: 10.1093/humrep/dex066 [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi T, Jwa S, Kuwahara A, Irahara M, Kubota T, Saito H. Pregnancy and neonatal outcomes following letrozole use in frozen-thawedsingle embryo transfer cycles. Hum Reprod. 2017;32:1244‐1248. doi: 10.1093/humrep/dex066 [DOI] [PubMed] [Google Scholar]
- 15.Diamond MP, Legro RS, Coutifaris C, et al. Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373:1230–1240. doi: 10.1056/NEJMoa1414827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Requena A, Herrero J, Landeras J, et al. Use of letrozole in assisted reproduction: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:571–582. doi: 10.1093/humupd/dmn033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesh A, Chauhan N, Das S, Chakravarty B, Chaudhury K. Endometrial receptivity markers in infertile women stimulated with letrozole compared with clomiphene citrate and natural cycles. Syst Biol Reprod Med. 2014;60:105–111. doi: 10.3109/19396368.2013.862316 [DOI] [PubMed] [Google Scholar]
- 18.Miller PB, Parnell BA, Bushnell G, et al. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum Reprod. 2012;27:881–888. doi: 10.1093/humrep/der452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddhartha N, Reddy NS, Pandurangi M, Tamizharasi M, Radha V, Kanimozhi K. Correlation of serum estradiol level on the day of ovulation trigger with the reproductive outcome of intracytoplasmic sperm injection. J Hum Reprod Sci. 2016;9:23. doi: 10.4103/0974-1208.178631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceydeli N, Kaleli S, Calay Z, Erel CT, Akbas F, Ertungealp E. Difference in αvβ3 integrin expression in endometrial stromal cell in subgroups of women with unexplained infertility. Eur J Obstetrics Gynecol Reprod Biol. 2006;126:206–211. doi: 10.1016/j.ejogrb.2005.11.034 [DOI] [PubMed] [Google Scholar]
- 21.Nardo LG, Bartoloni G, Di Mercurio S, Nardo F. Expression of αvβ3 and α4β1 integrins throughout the putative window of implantation in a cohort of healthy fertile women. Acta Obstet Gynecol Scand. 2002;81:753–758. [PubMed] [Google Scholar]
- 22.Tei C, Maruyama T, Kuji N, Miyazaki T, Mikami M, Yoshimura Y. Reduced expression of αvβ3 integrin in the endometrium of unexplained infertility patients with recurrent IVF-ET failures: improvement by danazol treatment. J Assist Reprod Genet. 2003;20:13–20. doi: 10.1023/A:1021254620888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahyaoglu S, Yumusak OH, Ozgu-Erdinc AS, et al. Can serum estradiol levels on the fourth day of IVF/ICSI cycle predict outcome in poor responder women? Syst Biol Reprod Med. 2015;61:233–237. doi: 10.3109/19396368.2015.1013645 [DOI] [PubMed] [Google Scholar]
- 24.Melnick AP, Pereira N, Murphy EM, Rosenwaks Z, Spandorfer SD. How low is too low? Cycle day 28 estradiol levels and pregnancy outcomes. Fertil Steril. 2016;105:905–909. doi: 10.1016/j.fertnstert.2015.11.046 [DOI] [PubMed] [Google Scholar]
- 25.Kyrou D, Popovic-Todorovic B, Fatemi H, et al. Does the estradiol level on the day of human chorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/GnRH antagonist? Hum Reprod. 2009;dep290. [DOI] [PubMed] [Google Scholar]
- 26.Zavy MT, Craig LB, Wild RA, Kahn SN, O’Leary D, Hansen KR. In high responding patients undergoing an initial IVF cycle, elevated estradiol on the day of hCG has no effect on live birth rate. Reprod Biol Endocrinol. 2014;12:1. doi: 10.1186/1477-7827-12-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anifandis G, Koutselini E, Louridas K, et al. Estradiol and leptin as conditional prognostic IVF markers. Reproduction. 2005;129:531–534. doi: 10.1530/rep.1.00567 [DOI] [PubMed] [Google Scholar]
- 28.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen–thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–348. doi: 10.1016/j.fertnstert.2011.05.050 [DOI] [PubMed] [Google Scholar]
- 29.Fatemi H, Popovic-Todorovic B. Implantation in assisted reproduction: a look at endometrial receptivity. Reprod biomed. 2013;27:530–538. doi: 10.1016/j.rbmo.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 30.Montville CP, Khabbaz M, Aubuchon M, Williams DB, Thomas MAJ. Luteal support with intravaginal progesterone increases clinical pregnancy rates in women with polycystic ovary syndrome using letrozole for ovulation induction. Fertil Steril. 2010;94:678–683. doi: 10.1016/j.fertnstert.2009.03.088 [DOI] [PubMed] [Google Scholar]
- 31.Elsedeek MS-E-A, Elmaghraby HA. Predictors and characteristics of letrozole induced ovulation in comparison with clomiphene induced ovulation in anovulatory PCOS women. Middle East Fert Soc J. 2011;16:125–130. [Google Scholar]
- 32.Wang J, Wei Y, Diao F, et al. The association between polycystic ovary syndrome and ectopic pregnancy after in vitro fertilization and embryo transfer. Am J Obstetrics Gynecol. 2013;209:139.e131–139.e139. doi: 10.1016/j.ajog.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 33.Shao R, Feng Y, Zou S, et al. The role of estrogen in the pathophysiology of tubal ectopic pregnancy. Am J Transl Res. 2012;4:269–278. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding authors can be contacted regarding data requests.