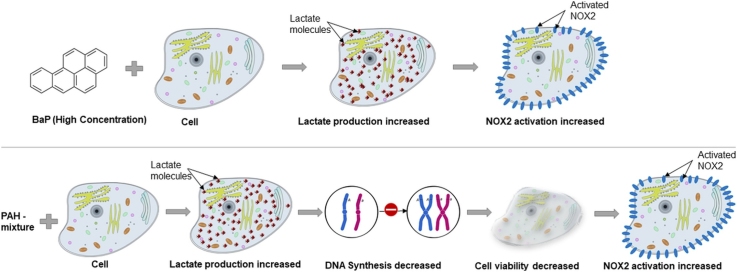
Graphical abstract
Keywords: Polycyclic aromatic hydrocarbons, Benzo[a]pyrene, Toxicity, NOX2, Flow cytometry
Highlights
-
•
PAHs cause decrease in cell viability and increase in lactate levels.
-
•
The mixture of PAHs suppress S phase.
-
•
Toxicity is accompanied by NOX2 activation.
Abstract
Polycyclic Aromatic Hydrocarbons (PAHs) are environmental pollutants.
The present study compares the toxic effects of BaP alone and a mixture of PAHs on human breast cancer cells. We hypothesize that PAH mixture is more toxic than BaP alone, and an increased NOX2 activation is related to PAH-induced oxidative stress. Initially, we exposed cultured human breast cancer cells to BaP alone (125 ng/mL and 500 ng/mL) and a mixture of PAHs (125 ng/mL and 500 ng/mL). After 24 h of exposure, the PAH mixture demonstrated a significant (P < 0.05) reduction in cell viability. The higher concentration of BaP alone (500 ng/mL) and both 125 ng/mL and 500 ng/mL PAH mixture significantly (P < 0.05) increased lactate production by MDA-MB-231 cells. We had observed an identical level of increased lactate levels when the cells were exposed to PAHs for 48 h. Flow cytometric analysis revealed that only PAHs mixture (both 125 ng/mL and 500 ng/mL) suppressed S phase significantly (P < 0.05). Finally, immunofluorescence microscopy was undertaken to examine the role of NOX2 due to PAHs toxicity. Colocalization of GP91phox and P47phox, a hallmark of NOX2 activation in the cell membrane of macrophage Kupffer cells demonstrated that higher concentration of BaP or PAH mixture showed increased colocalization events. These data suggest that the mixture of PAHs is more toxic and perturbing to DNA synthesis than BaP alone in cultured cells, and the toxicity is accompanied by NOX2 activation. Thus PAHs can lead to the increased burden of oxidative stress and alter the cellular redox status.
1. Introduction
Polycyclic Aromatic Hydrocarbons (PAHs), a type of organic environmental pollutant that are toxic to cells, are found in abundance in our atmosphere [1]. According to the International Agency for Research on Cancer, PAHs are grouped as probable or possibly carcinogens to humans [2]; and many of them are considered to be potential endocrine disruptors. PAHs are products of incomplete combustion, and along with their metabolites, they damage cellular DNA [3,4], contaminate the environment, and eventually contribute to dilemma of environmentally induced inflammatory diseases [5].
In the environment, humans are exposed to a variety of PAHs and a mixture of PAHs may have either additive or antagonistic effects. A group of researchers in Nigeria found higher levels of PAHs in cosmetics and personal care products than in plastic products. The concentrations of most of the PAHs in consumer products exceeded the recommended level by WHO [6]. Several factors, including the relative proportion of PAHs present in the mixture and the length of exposure to the PAH are critical to the level of toxicity or carcinogenesis. Based on a particular study, it was found that the exposure of benzo(a)pyrene, a type of PAH, on rat testicular Sertoli cells resulted in cellular changes characteristic of apoptotic conditions [7]. A recent study in fish reported that a short-term exposure to sub-lethal dose of BaP impaired thyroid function that included decrease in T3, T4 and increase in TSH level in blood plasma [8]. Based on these findings, our focus in this study is to assess the effects of exposure to an individual PAH compound or mixture.
Our current study addresses the hypothetical toxic effects of BaP alone, as well as a mixture of PAHs, on human breast cancer MDA-MB-231 cells. In the cell viability study, we included tamoxifen because it acts as an anti-estrogen [[9], [10], [11]]. Though this breast cancer cell type is triple negative and does not possess estrogen receptors, tamoxifen may act as an anti-proliferative agent, and therefore, the direct effect of tamoxifen on this cell line could not be overruled. The results suggest that BaP alone can produce toxic effects in the cells, but the mixture of PAHs exerts toxic and growth inhibitory effects by inhibiting DNA synthesis.
The NADPH oxidase (NOX) is commonly expressed in both phagocytic and non-phagocytic cells [12]. Environmental toxicity to organ systems has been found to induce NOX2, as shown in advanced chronic liver and kidney diseases [13,14]. PAH effects in the liver and subsequent exacerbations to chronic liver injury are poorly understood. Apart from the established toxicity of PAHs and predictive carcinogenicity, recent advancements in toxicity studies in the liver show that environmental exposures of toxins can aid in the pathology of liver diseases. Importantly, the western world has seen an upsurge in nonalcoholic fatty liver disease that can lead to hepatocellular carcinoma. Also, the incidences of cancers including hepatocellular carcinoma are on the rise [[15], [16], [17], [18]]. Thus it is crucial that mechanisms of toxicity of PAHs be studied in the resident macrophages in the liver that are critical to the immune microenvironment, tolerance, and pathogenicity. The NOX2 is primarily composed of several subunits, mainly GP91phox (membrane subunits) and P47phox (cytosolic subunit) [12]. When the proper signal stimulates NOX2 activation, it leads to the alignment of the cytosolic subunit (P47phox) to the membrane subunit (GP91phox). Localized NOX2 is involved in superoxide generation and can lead to the increased burden of oxidative stress and inflammation to other organs [19]. NOX2 has also been shown to activate the TLR4 system, which includes recruitment of TLR4 into lipid rafts, advance receptor dimerization, and activation in several inflammatory responses [13,20]. Considering these facts, we hypothesize that increased NOX2 activation is related to PAH-induced oxidative stress in Kupffer cells. Interestingly, for the present study, we have used only immortalized Kupffer cells. But it is important to note that both breast cancer cells [21] and Kupffer cells [13] share NOX2 regulation through lipid rafts.
2. Materials and methods
2.1. Cell culture
We purchased the MDA-MB-231 human breast cancer cell line from the American Type Culture Collection (ATCC, Rockville, MD). The growth medium contained Dulbecco’s Modified Eagle’s/ F-12 media (DMEM; Sigma Chemical Co., St. Louis, MO), with 10% Fetal Bovine Serum (FBS; Atlanta Biologicals, Norcross, GA). Cells were cultured at 37 °C in 5% CO2 atmosphere (ATCC). 1.0 × 106 cells were cultured on 35 mm culture dishes or 20,000 cells/well onto 96 well plates for 24 or 48 h in 10% FBS at 37 °C in 5% CO2. Each treatment had three replicates.
For the first set of experiments, cultured cells were incubated for 24 h in either growth medium alone or growth medium containing 0.5% acetonitrile (both as vehicle controls), BaP (125 ng/mL and 500 ng/mL) or PAH mixture (125 ng/mL and 500 ng/mL).
The Polycyclic Aromatic Hydrocarbons (PAH) mixture was composed of acenaphthylene, benz[a]anthracene, benzo[b]fluoranthene, benzo[ghi]perylene, BaP, dibenz[a,h]anthracene, fluoranthene, and phenanthrene. The concentration of each PAH in the mixture is 15.7 or 62.5 ng/mL to accomplish the final concentrations (125 ng/mL or 500 ng/mL), respectively. This mixture dissolved in acetonitrile was obtained from Ultra Scientific, North Kingstown, RI.
In the second set of experiments, cultured cells (1.0 × 106 cells) were incubated for 48 h in growth medium containing 0.025% dimethylsulfoxide (DMSO) or 0.5% acetonitrile (both as vehicle controls), BaP alone (125 ng/mL and 500 ng/mL), a mixture of PAHs (125 ng/mL or 500 ng/mL), or tamoxifen alone (125 ng/mL or 500 ng/mL). Each treatment had three replicates, and there was a total of 24 wells used in the experiment with 48 h exposure time.
2.2. MTT assay
The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay was used to determine cell viability [7]. This assay was performed following instructions in the MTT assay kit purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.3. Lactate assay
In the second experiment, a lactate assay kit from Sigma-Aldrich, St. Louis, MO was used to quantify and enzymatically determine presence of lactate in media [20,22]. In brief, an aliquot of each sample was deproteinized with 10% trichloroacetic acid. The acid solution was then centrifuged, with the resulting supernatant assayed for lactate using lactate dehydrogenase. To provide a measure of the initial lactate concentration in a sample, the amount of reduced nicotinamide adenine dinucleotide (NADH) formed from NAD was measured as an absorbance at 340 nm. Then, 1 mL aliquots of spent media were prepared for lactate measurement. Lactate concentrations were determined using comparison with a standard curve to yield values in terms of mg lactate per mL solution. These values were then compared to the total mg cell protein to produce final determinations expressed as mg lactate per mL solution per mg cellular protein (mg/mL/mg).
2.4. Protein assay
Cells were removed from the culture dishes, which were then rinsed with PBS and collected into plastic tubes. The pooled cells underwent sonication and then collected in PBS by centrifugation (@ 200 x g for 5 min). Protein samples were stored at −70 °C until assayed. The BCA protein assay kit was purchased from Pierce Chemical Company (Rockford, Ill) and used to determine cell protein concentrations as described earlier [7].
2.5. Flow cytometry
For flow cytometry, 1.0 × 106 cells were plated on 35 mm dishes in duplicate. After 24 h of the incubation period, cells were trypsinized (trypsin-EDTA) briefly and centrifuged at 3500 rpm for five minutes. The cell pellet from each treatment group was fixed in cold ethanol for 15 min and stained with 1 mL Propidium Iodide (PI) solution (10 mM Tris-HCl, 10 μg/mL RNase, 10 mM NaCl, and 0.1 mg PI/mL and 2.0% NP-40) before analyzing the DNA ploidy distributions at 488 nm wavelength using a Beckman Coulter FACScan flow-cytometer equipped with a 15 mW air-cool laser. Nuclear DNA content was examined based on the procedure, as described earlier [22,23].
2.6. Immunofluorescence
2.6.1. Culture of macrophage cells
Rat Kupffer macrophages cell line was a generous gift from the Environmental Health and Disease Laboratory, University of South Carolina. These cells were maintained in DMEM, Corning (Tewksbury, MA). The media was supplemented with 10% FBS purchased from Atlanta Biologicals (Norcross, GA), and 2 mM glutamine, 100U/mL penicillin, and 100 μg/mL streptomycin obtained from Fisher Scientific (Hanover Park, IL).
Coverslips were added to 35 mm tissue culture dishes and were sterilized for 24 h. Then, 20,000 Kupffer Macrophage cells were placed on each one, in duplicate and were cultured for three days at 37 °C in 5% CO2. At the beginning of the third day, the cells were exposed for 24 h to medium containing 0.025% DMSO and 0.5% acetonitrile (both as vehicle controls), BaP alone (125 ng/mL and 500 ng/mL), or a mixture of PAHs (125 ng/mL and 500 ng/mL). After completion of the treatments as mentioned above in the cell culture section, macrophage cells attached on coverslips were fixed with 10% neutral buffered saline.
2.6.2. Co-localization of GP91phox and P47phox
After the fixed cells were washed with PBS containing 0.1% Triton X 100, the cells were blocked with 5% BSA, 0.2% Tween-20, 10% FBS in PBS. Cells were incubated with primary antibodies; anti-GP-91phox and anti-P47phox, both at a dilution of 1:250 for dual labeling. Species-specific anti-IgG secondary antibodies conjugated with Alexa Fluor 633 – red (Invitrogen, California, USA) was used against anti-GP91phox, and Alexa Fluor 488 - green (Invitrogen) was used against anti-P47phox for immunofluorescence staining. The stained cells attached to the coverslips were mounted on slides with ProLong Gold Antifade reagent with DAPI (Life Technologies) as counterstain and viewed under 40X objectives with an Olympus BX43 microscope.
2.7. Statistical analysis
Data were analyzed using a software program Prism 3.02 (GraphPad Inc., San Diego, CA). Data were presented as mean ± SE. Each experiment was conducted over three times, with 2–3 replicates per sample. Multiple comparisons among the various treatment groups were achieved by one-way analysis of variance (ANOVA) followed by Tukey test as a post-ANOVA test, and P < 0.05 was the level of significance.
3. Results
3.1. Effects of PAHs on cell viability (24 h)
MTT assay was performed after 24 h of exposure of PAHs and MDA-MB-231 cell viability was only decreased to 10–15% with both BaP treated groups (Table 1A) when compared to the DMSO control. Cells exposed to both 125 ng/ml or 500 ng/mL PAH mixture demonstrated significant (P < 0.05) reductions in viability (over 20–35%) as compared to the acetonitrile control (Table 1A).
Table 1.
Effects of a single exposure of BaP and PAH mixture on the viability (A) and lactate production (B–C) by MDA-MB-231 cells.
| Treatments | % of viable cells | Lactate (mg/mL/mg) |
|
|---|---|---|---|
| (A) | (B) | (C) | |
| 0.025% DMSO | 97.2+4.3 | 0.28+0.004 | 0.30+0.007 |
| 125 ng/mL BAP | 86.7+4.7 | 0.30+0.005 | 0.45+0.004 |
| 500 ng/mL BAP | 81.8+4.8 | 0.43+0.007* | 0.64+0.006* |
| 0.5% Acetonitrile | 96.5+4.2 | 0.26+0.005 | 0.31+0.006 |
| 125 ng/mL PAH | 75.5+5.4* | 0.48+0.007* | 0.77+0.007* |
| 500 ng/mL PAH | 61.9+6.1* | 0.56+0.009* | 0.91+0.009* |
A. Data represent the mean and SEM from 3 separate experiments. The cell viability assays were performed after 24 h of treatment. Cells exposed to both 125 ng/ml or 500 ng/mL PAH mixture demonstrated significant (P < 0.05) reductions in viability as compared to the acetonitrile control.
B. Lactate assays were done after 24 h (B) of exposure. Only 500 ng/mL BAP increased lactate level significantly (P < 0.05) when compared to DMSO control. However, both125 ng/mL or 500 ng/mL PAH mixture demonstrated significant (P < 0.05) increased lactate levels as compared to the acetonitrile control.
C. Lactate production was measured after 48 h © of exposure. Again, the 500 ng/mL BaP alone significantly (P < 0.05) increased lactate level when compared to the DMSO control. Both 125 ng/mL and 500 ng/mL PAH mixture significantly (P < 0.05) increased lactate production by MDA-MB-231 cells as compared to the acetonitrile control.
3.2. Effect of PAHs on lactate production
Table 1B presents lactate production by MDA-MB-231 cells exposed to PAHs for 24 h. The 500 ng/mL BaP alone significantly (P < 0.05) increased lactate level when compared to the DMSO control. In contrast, both 125 ng/mL and 500 ng/mL PAH mixture significantly (P < 0.05) increased lactate production by MDA-MB-231 cells as compared to the acetonitrile control.
Table 1C shows lactate production by MDA-MB-231 cells exposed to PAHs for 48 h. We have observed increased lactate levels with both BaP and PAH mixture groups when compared between 24 and 48 h exposure times. Again, the 500 ng/mL BaP alone significantly (P < 0.05) increased lactate level when compared to the DMSO control. Both 125 ng/mL and 500 ng/mL PAH mixture significantly (P < 0.05) increased lactate production by MDA-MB-231 cells as compared to the acetonitrile control.
3.3. Effects of PAHs and Tamoxifen on cell viability (48 h)
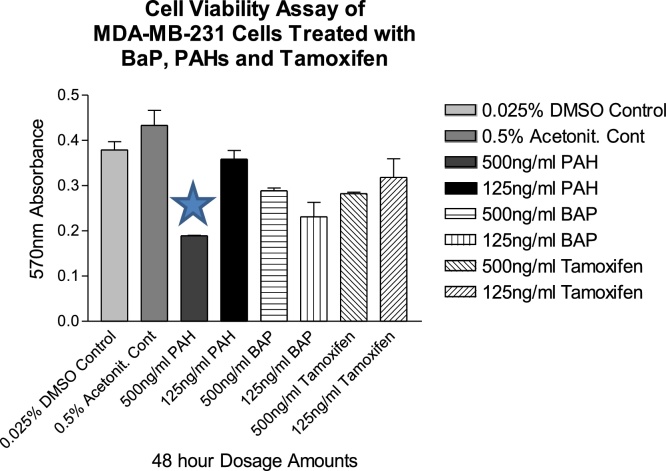
Fig. 1 represents results from the cell viability assay of MDA-MB-231 cells exposed to PAH mixture, BaP, or tamoxifen alone for 48 h. Cell viability was only weakly affected by both BaP and tamoxifen-treated groups when compared to DMSO control. A statistically significant decreased cell viability was observed with 500 ng/mL PAH mixture (P < 0.05) as compared to the acetonitrile control.
Fig. 1.
Effects of BaP, PAH mixture, and Tamoxifen on the cell viability of MDA-MB-231 cells as determined by MTT assay. After 48 h exposure, the 500 ng/mL PAH mixture significantly (P < 0.05) decreased cell viability when compared to the acetonitrile control group. Data represent mean ± SEM from three separate experiments. *indicates significantly different (P < 0.05) from the acetonitrile control group.
3.4. Effect of PAHs on cell cycle
Flow cytometric analysis of the cell cycle data is presented in Table 2. Both 500 ng/mL and 125 ng/ml BaP showed a minor decrease in S phase DNA but were not statistically significant when compared to the DMSO control. In contrast, both 125 ng/mL and 500 ng/mL PAH mixture significantly (P < 0.05) reduced S-phase DNA in MDA-MB-231 cells as compared to the acetonitrile control. The G0/G1 and G2/M DNA remained unaffected in all exposure groups.
Table 2.
Analysis of the effects of BaP and PAH mixture on the cell cycle in MDA-MB-231 cells as determined by flow cytometry.
| Treatments | Phases of cell cycle |
||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| 0.025% DMSO | 73.2+4.3 | 8.5+0.7 | 15.7+1.1 |
| 125 ng/mL BAP | 76.7+4.7 | 6.6+0.8 | 13.2+1.6 |
| 500 ng/mL BAP | 75.5+4.8 | 6.4+0.6 | 14.1+1.4 |
| 0.5% Acetonitrile | 73.5+4.2 | 8.7+0.5 | 15.8+1.0 |
| 125 ng/mL PAH | 81.8+5.4 | 4.1+0.6* | 10.8+1.3 |
| 500 ng/mL PAH | 81.9+6.1 | 3.3+0.3* | 11.1+1.3 |
Data represent the mean and SEM from 3 separate experiments. Flow cytometry was performed after 24 h of exposure. Both 125 ng/mL and 500 ng/mL PAH mixture significantly (*P < 0.05) reduced S-phase DNA in MDA-MB-231 cells as compared to the acetonitrile control.
3.5. Effect of PAHs on co-localization of GP91phox and P47phox
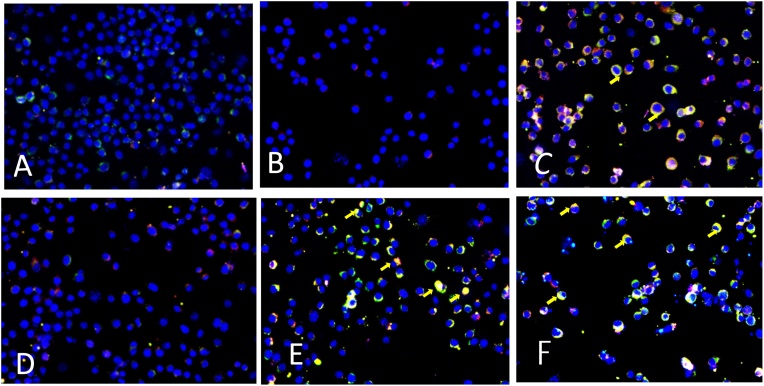
We have examined NOX2 activation in Kupffer cells by dual-labeled GP91phox-red and P47phox-green immunofluorescence microscopy. The immunofluorescence results showed that there was a significant increase in GP91phox-P47phox colocalization (yellow) events in the PAHs exposed groups as compared to their respective vehicle controls (Fig. 2). Cells exposed to 125 ng/mL BaP (Fig. 2B) alone or vehicle (Fig. 2A) did not show any colocalization of both subunits. Whereas, cells exposed to 500 ng/mL BaP alone (Fig. 2C), or the PAH mixture at both 125 ng/mL (Fig. 2E) or 500 ng/mL (Fig. 2F) concentrations showed increased colocalization events in these cells.
Fig. 2.
NOX2 activation in macrophages Kupffer cells as examined under the fluorescence microscope. Yellow small arrow refers to colocalization of GP91phox and P47phox in the cell membrane. Cells exposed to 0.025% DMSO as vehicle control (Fig. 2A) or 125 ng/mL BaP (Fig. 2B) alone did not show any colocalization of both subunits. Whereas, cells exposed to 500 ng/mL BaP alone (Fig. 2C), or the PAH mixture at both 125 ng/mL (Fig. 2E) or 500 ng/mL (Fig. 2F) concentrations showed increased colocalization events in Kupffer cells. Fig. 2D represents 0.5% acetonitrile as vehicle control for PAH mixture.
4. Discussion
Our experiments have demonstrated that BaP and the mixture of PAHs, at lower concentrations (nanogram levels) are toxic to MDA-MB-231 cell lines. The toxic effects of the combination of PAHs are exerted through the S phase of the cell cycle. Thus, the mixture of PAHs decreased cell proliferation capacity by reducing DNA synthesis. We have reported earlier [24] that BaP at10 μg/mL concentrations suppressed the G0/G1 phase of the MCF-7 cell cycle. However, there was no significant reduction of G0/G1 or G2/M of the MDA-MB-231 cells in any one of the treatments used in this experiment. Therefore, the mixture of PAHs was found to have only suppressed the S phase of the cell cycle. The significant blockade or arrest at S phase observed in our study is similar to that seen in other human cell lines [25] including MCF-7 cells [24]. Since the mixture of PAHs includes BaP, fluoranthene and other members of the PAH family, biotransformation of some of the PAHs including fluoranthene is possible, and that may imply cancer risks as suggested by others [25].
In our study we have seen synergistic responses in toxicity and DNA synthesis/binding when PAH mixture of acenaphthylene, benz[a]anthracene, benzo[b]fluoranthene, benzo[ghi]perylene, benzo[a]pyrene (BaP), dibenz[a,h]anthracene, fluoranthene, and phenanthrene were used at a physiologically relevant doses. According to the IARC report benz[a]anthracene, benzo[b]fluoranthene are classified as 2 B, and BaP is in class 1. These are either possible human carcinogens (class 2 B) or carcinogens (class 1). On the other hand, fluoranthene, and phenanthrene are in class 3, implying that these two are not classified as human carcinogens [26]. In a study conducted in some part of Nigeria, higher levels of the low molecular weight PAHs in groundwater exceeded the allowable safety limit as recommended by WHO [27]. Another study conducted in Southern Nigeria reported varying levels of different PAHs in four species of smoked fish [28]. This research also raised a serious concern with potential cancer risks in human from consumption of smoked fish. In smoked fish, fluoranthene, and phenanthrene levels are significantly higher than BaP [29]. Humans are exposed to a mixture of PAHs than a single PAH compound through dietary or air intake. As it is a common phenomenon in mixture toxicity, some PAHs may have either synergistic or antagonistic effects. The severity of toxicity is dependent on the combination of PAHs present in the mixture, their relative proportions and how they are metabolized. Polycyclic aromatic hydrocarbon-induced carcinogenesis requires long term exposure to an individual PAH compound [[30], [31], [32]] or mixtures [33]. This issue brings to light the strong interactions between various PAHs and cytotoxicity.
An earlier study from this laboratory [24] has demonstrated that more prolonged exposure to BaP and tamoxifen decreased viability, proliferative ability, and induced apoptosis in MCF-7 cells. Benzo[a]pyrene is among the PAHs that has been reported to be carcinogenic [2,4] and estrogenic [34,35]. PAHs resemble structural similarity to estrogen and estrogen-like compounds [36,37], which enables them to interact with the estrogen receptors (ER) and aryl hydrocarbon receptors (AhR) to induce estrogenic and anti-estrogenic properties in vitro [[38], [39], [40]]. Tamoxifen, indeed, significantly reduced the amount of DNA in all phases of the MCF-7 cell cycle [24]. We did not observe similar effects in MDA-MB-231 cells, presumably because the exposure time was different.
Moreover, MDA-MB-231 cells are not estrogen-responsive, whereas MCF-7 cells are estrogen-sensitive [40]. Tamoxifen blocks the ER-mediated proliferative effects in MCF-7 cells and thus is used as an anti-cancer agent. It may also suggest that MDA-MB-231 cells are more resistant to the damaging effects of these compounds when used alone as a single exposure. So far, we did not report the studies to test toxicity on MCF-7 cells using the mixture of PAHs. However, we are comparing both human breast cancer cell lines to bridge the knowledge gap in studying the toxicity of PAH mixtures. Since both BaP and fluoranthene are present in the mixtures, metabolic activation or biotransformation induce production of reactive oxygen species, which may result in inflammatory disease of the breast as it is reported in intestinal inflammation [41].
In the current study, the PAHs, including BaP, significantly killed many viable cells after 48 h of exposure. Consequently, the mitochondrial reductase enzymes were not active in these non-viable cells and thereby demonstrated stress put on MDA-MB-231 cells following treatment with PAHs. In addition, we have observed an increase in lactate production (released into the media) by MDA-MB-231 cells. Others have reported energy and lipid metabolite alternations in HaCaT cells by AhR binding PAHs that included BaP, which in turn, would affect cellular oxidation process [42]. It would be interesting to see the regulation of a stress-related family of proteins. The NADPH oxidase isoform 2 (NOX2) is one of the several isoforms of the GP91-phox catalytic subunit of NADPH oxidase [43]. Our co-localization results showed increased NOX2 activation in Kupffer cells due to exposure to PAHs. The results suggested an enhanced NADPH oxidative activation in cells exposed to higher concentrations of BaP or both lower and higher concentrations of PAH mixture. The toxicity of PAHs is accompanied by NOX2 activation. The future research of NOX2 induced redox signaling will advance our understanding in this field by including breast cancer cells.
In summary, the mixture of PAHs is more toxic and perturbing to DNA synthesis than BaP alone in cultured cells, and the toxicity is accompanied by NOX2 activation.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
This research and student training program was supported by a grant # HRD-1436222 from the National Science Foundation. Part of the work was presented in the Ernest E. Just Scientific Symposium, Medical University of South Carolina (MUSC), USA. The authors gratefully acknowledge the expert review of the MS by Dr. Ed Krug and Parag Raychoudhury at MUSC.
References
- 1.Arcaro K.F., O’Keefe P.W., Yang Y., Clayton W., Gierthy J.F. Antiestrogenicity of environmental polycyclic aromatic hydrocarbons in human breast cancer cells. Toxicology. 1999;133:115–127. doi: 10.1016/s0300-483x(99)00018-9. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research in Cancer . Volume 32. Part I: WHO Press IARC; Lyon, France: 1983. (IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans). [Google Scholar]
- 3.Mendelez-Colon V.J., Luch A., Seidel A., Baird W.M. Cancer initiation by polycyclic aromatic hydrocarbons results from the formation of stable DNA adducts rather than apurinic sites. Carcinogenesis. 1999;20:1885–1891. doi: 10.1093/carcin/20.10.1885. [DOI] [PubMed] [Google Scholar]
- 4.Marston C.P., Pereira C., Ferguson J., Fischer K., Hedstrom O., Dashwood W., Baird W.M. Effects of a complex environmental mixture from coal tar containing polycyclic aromatic hydrocarbons (PAH) on the tumor initiation, PAH-DNA binding and metabolic activation of carcinogenic PAH in mouse epidermis. Carcinogenesis. 2001;22:1077–1086. doi: 10.1093/carcin/22.7.1077. [DOI] [PubMed] [Google Scholar]
- 5.Ramesh A., Archibong A.E., Hood D.B., Guo Z., Loganathan B.G. Global environmental distribution and human health effects of polycyclic aromatic hydrocarbons. In: Loganathan B.G., Lam P.K.S., editors. Global Contamination Trends of Persistent Organic Chemicals. CRC Press; Boca Raton, FL: 2011. pp. 95–124. [Google Scholar]
- 6.Adekunle A.S., Oyekunle J.A.O., Ola I.J., Obisesan O.R., Maxakato N.W. Determination of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in some personal care products in Nigeria. Toxicol. Rep. 2018;5:994–1001. doi: 10.1016/j.toxrep.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raychoudhury S.S., Kubinski D. Polycyclic aromatic hydrocarbon-induced cytotoxicity in cultured rat Sertoli cells involves differential apoptotic response. Environ. Health Perspect. 2003;111:33–38. doi: 10.1289/ehp.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Movahedinia A., Salamat N., Kheradmand P. Effects of the environmental endocrine disrupting compound benzo[a]pyrene on thyroidal status of abu mullet (Liza abu) during short-term exposure. Toxicol. Rep. 2018:377–382. doi: 10.1016/j.toxrep.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy B.J. Massive estrogen administration in premenopausal women with breast cancer. Cancer. 1962;15:641–648. doi: 10.1002/1097-0142(196205/06)15:3<641::aid-cncr2820150330>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Heel R.C., Brogden R.N., Speight T.M., Avery G.S. Tamoxifen: a review of its pharmacological properties and therapeutic use in the treatment of breast cancer. Drugs. 1978:16. doi: 10.2165/00003495-197816010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Forbes J.F. The control of breast cancer: the role of tamoxifen. Semin. Oncol. 1997;24(Suppl. 1):S1–5-S1-19. [PubMed] [Google Scholar]
- 12.Lambeth J.D., Kawahara T., Diebold B. Regulation of Nox and Duox enzymatic activity expression. Free Radic. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S., Alhasson F., Dattaroy D., Pourhoseini S., Seth R.K., Nagarkatti M., Nagarkatti P.S., Michelotti G.A., Diehl A.M., Kalyanaraman B., Chatterjee S. NADPH oxidase-derived peroxynitrite drives inflammation in mice and human nonalcoholic steatohepatitis via TLR4-lipid raft recruitment. Am. J. Pathol. 2015;185:1944–1957. doi: 10.1016/j.ajpath.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhasson F., Seth R.K., Sarkar S., Kimonoa D.A., Albadrania M.S., Dattaroy D., Chandrashekaran V., Scott G.I., Raychoudhury S., Nagarkatti M., Nagarkatti P., Diehl A.M., Chatterjee S. High circulatory leptin mediated NOX-2-peroxynitrite-miR21 axis activate mesangial cells and promotes renal inflammatory pathology in nonalcoholic fatty liver disease. Redox Biol. 2018;17:1–15. doi: 10.1016/j.redox.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer; 2018. Available from: https://gco.iarc.fr/tomorrow (accessed: 29.09.2018) [Google Scholar].
- 16.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. PMID: 25220842. [DOI] [PubMed] [Google Scholar]
- 17.Antoni S., Soerjomataram I., Møller B., Braya F., Ferlay J. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull. World Health Organ. 2016;94:174–184. doi: 10.2471/BLT.15.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawla P., Sunkara T., Muralidharan P., Raj J.P. Update in global trends and etiology of hepatocellular carcinoma. Contemp. Oncol. (Pozn) 2018;22:141–150. doi: 10.5114/wo.2018.78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee S., Lardinois O., Bonini M.G., Bhattacharjee S., Stadler K., Corbett J., Deterding L.J., Tomer K.B., Kadiiska M., Mason R.P. Site-specific carboxypeptidase B1 tyrosine nitration and pathophysiological implications following its physical association with nitric oxide synthase-3 in experimental sepsis. J. Immunol. 2009;183:4055–4066. doi: 10.4049/jimmunol.0900593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C.S., Shin D.M., Kim K.H., Lee Z.W., Lee C.H., Park S.G., Bae Y.S., Jo E.K. NADPH oxidase 2 interaction with TLR2 Is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J. Immunol. 2009;182:3696–3705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 21.Malla R.R., Raghu H., Rao J.S. Regulation of NADPH oxidase (Nox2) by lipid rafts in breast carcinoma cells. Int. J. Oncol. 2010;37:1478–1493. doi: 10.3892/ijo_00000801. [DOI] [PubMed] [Google Scholar]
- 22.Raychoudhury S.S., Flowers A.F., Millette C.F., Finlay M.F. Toxic effects of polychlorinated biphenyls on cultured rat Sertoli cells. J. Androl. 2000;21:964–973. [PubMed] [Google Scholar]
- 23.Raychoudhury S.S., Blake C., Millette C.F. Toxic effects of octylphenol on cultured rat spermatogenic cells and Sertoli cells. Toxicol. Appl. Pharmacol. 1999:192–202. doi: 10.1006/taap.1999.8664. [DOI] [PubMed] [Google Scholar]
- 24.Ogba N., Wang C., Raychoudhury S. Differential effects of fluoranthene and benzo[a]pyrene in MCF-7 cells. J. Environ. Sci. Health Part A. 2005;40:927–936. doi: 10.1081/ese-200056110. [DOI] [PubMed] [Google Scholar]
- 25.Wei W., Li X.F., Li X.N., Chen X.M., Liu A.L., Lu W.Q. Oxidative stress and cell cycle change induced by coexposed PCB126 and benzo(a)pyrene to human hepatoma (HepG2) cells. Environ. Toxicol. 2010;27:316–320. doi: 10.1002/tox.20649. [DOI] [PubMed] [Google Scholar]
- 26.WHO Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposure IARC monographs on the evaluation of carcinogenic risks to humans. IARC Monogr. 2010;92:1–819. [PMC free article] [PubMed] [Google Scholar]
- 27.Adekunle A.S., Oyekunle J.A., Ojo O.S., Maxakato N.W., Olutona G.O., Obisesan O.R. Determination of polycyclic aromatic hydrocarbon levels of groundwater in Ife north local government area of Osun state. Nigeria. Toxicol. Rep. 2017;4:39–48. doi: 10.1016/j.toxrep.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tongo I., Ogbeide O., Ezemonye L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 2017;4:55–61. doi: 10.1016/j.toxrep.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson B.K. Polycyclic aromatic hydrocarbons in smoked herring. Z. Lebensm. Forsch. 1982;174:101–107. [Google Scholar]
- 30.Jeffy B.D., Chen E.J., Gudas J.M., Romagnolo D.F. Disruption of cell cycle kinetics by benzo[a]pyrene: inverse expression patterns of BRCA-1 and p53 in MCF-7 cells arrested in S and G2. Neoplasia. 2010;2:460–470. doi: 10.1038/sj.neo.7900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris K.L., Myers J.N., Ramesh A. Benzo(a)pyrene modulates fluoranthene-induced cellular responses in HT-29 colon cells in a dual exposure system. Environ. Toxicol. Pharmacol. 2013;36:358–367. doi: 10.1016/j.etap.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris K.L., Washington M.K., Hood D.B., Roberts L.J., II, Ramesh A. Dietary fat-influenced development of colon neoplasia in ApcMin mouse exposed to benzo(a)pyrene. Toxicol. Pathol. 2009;37:938–946. doi: 10.1177/0192623309351722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakura A., Seki Y., Sonoda J., Hosokawa S., Aoki T., Suganuma A., Kerna W.D., Tsukidate K. Rapid induction of colonic adenocarcinoma in mice exposed to benzo[a]pyrene and dextran sulfate sodium. Food Chem. Toxicol. 2011;49:2997–3001. doi: 10.1016/j.fct.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 34.Wester P.W., Muller J.J., Slob W., Mohn G.R., Dortant P.M., Kroese E.D. Carcinogenic activity of benzo[a]pyrene in a two-year oral study in Wistar rats. Food Chem. Toxicol. 2012;50:927–935. doi: 10.1016/j.fct.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Culp S.J., Gaylor D.W., Sheldon W.G., Goldstein L.S., Beland F.A. A comparison tar and benzo(a)pyrene in a 2-year bioassay. Carcinogenesis. 1998;19:117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 36.Charles D.G., Bartels M.J., Zacharewski T.R., Gollapudi B.B., Freshour N.L., Carney E.W. Activity of benzo[a]pyrene and its hydroxylated metabolites in an estrogen receptor-α reported gene assay. Toxicol. Sci. 2000;55:320–326. doi: 10.1093/toxsci/55.2.320. [DOI] [PubMed] [Google Scholar]
- 37.Fertuck K.C., Kumar S., Sikka H.C., Matthews J.B., Zacharewski T.R. Interaction of PAH-related compounds with the α and β isoforms of the estrogen receptor. Toxicol. Lett. 2001;121:167–177. doi: 10.1016/s0378-4274(01)00344-7. [DOI] [PubMed] [Google Scholar]
- 38.Tran D.Q., Ide C.F., McLachlan J.A., Arnold S.F. The anti-estrogenic activity of selected polynuclear aromatic hydrocarbons in yeast expressing human estrogen receptor. Biochem. Biophys. Res. Commun. 1996;229:102–108. doi: 10.1006/bbrc.1996.1764. [DOI] [PubMed] [Google Scholar]
- 39.Clemons J.H., Allan L.M., Marvin C.H., Wu Z., McCarry B.E., Bryant D.W., Zacharewski T.R. Evidence of estrogen- and TCDD-like activities in crude and fractionated extracts of PM10 air particulate material using in vitro gene assays. Environ. Sci. Technol. 1998;32:1853–1860. [Google Scholar]
- 40.DePasquale J.A., Samsonoff W.A., Gierthy J.F. 17-beta-estradiol induces alternations of cell matrix and intercellular adhesions in a human mammary carcinoma cell line. J. Cell Sci. 1994;107:1241–1254. doi: 10.1242/jcs.107.5.1241. [DOI] [PubMed] [Google Scholar]
- 41.Khalil A., Villard P.H., Dao M.A., Brucelin R., Champion S., Fouchier F., Savouret J.F., Barra Y., Seree E. Polycyclic aromatic hydrocarbons potentiate high-fat diet effects on intestinal inflammation. Toxicol. Lett. 2010;196:161–167. doi: 10.1016/j.toxlet.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Potratz S., Jungnickel H., Grabiger S., Tarnow P., Otto W., Fritsche E., von Bergen M., Luch A. Differential cellular metabolite alternations in HaCaT cells caused by exposure to the aryl hydrocarbon receptor-binding polycyclic aromatic hydrocarbons chrysene, benzo[a]pyrene and dibenzo[a,l]pyrene. Toxicol. Rep. 2016;3:763–773. doi: 10.1016/j.toxrep.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braunersreuther V., Montecucco F., Asrih M., Pelli G., Galan K., Frias M., Burger F., Quinderé A.L., Montessuit C., Krause K.H., Mach F., Jaquet V. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2013;64:99–107. doi: 10.1016/j.yjmcc.2013.09.007. [DOI] [PubMed] [Google Scholar]