Highlights
-
•
Predominance of white matter impairment in DM1 is questioned.
-
•
Age poses vulnerability to grey matter loss in specific areas in DM1.
-
•
White matter alterations in DM1 may be developmental.
-
•
Muscular and genetic features are associated with brain abnormalities in DM1.
-
•
Neuropsychology is an unspecific but strong predictor of gray matter damage in DM1.
Keywords: Myotonic Dystrophy Type 1, MRI, VBM, DTI, neuropsychology
Abstract
Background
Myotonic Dystrophy type 1 (DM1) is a slowly progressive myopathy characterized by varying multisystemic involvement. Several cerebral features such as brain atrophy, ventricular enlargement, and white matter lesions (WMLs) have frequently been described. The aim of this study is to investigate the structural organization of the brain that defines the disease through multimodal imaging analysis, and to analyze the relation between structural cerebral changes and DM1 clinical and neuropsychological profiles.
Method
31 DM1 patients and 57 healthy controls underwent an MRI scan protocol, including T1, T2 and DTI. Global gray matter (GM), global white matter (WM), and voxel-level Voxel Based Morphometry (VBM) and voxel-level microstructural WM abnormalities through Diffusion Tensor Imaging (DTI) were assessed through group comparisons and linear regression analysis with age, degree of muscular impairment (MIRS score), CTG expansion size and neuropsychological outcomes from a comprehensive assessment.
Results
Compared with healthy controls, DM1 patients showed a reduction in both global GM and WM volume; and further regional GM decrease in specific primary sensory, multi-sensory and association cortical regions. Fractional anisotropy (FA) was reduced in both total brain and regional analysis, being most marked in frontal, paralimbic, temporal cortex, and subcortical regions. Higher ratings on muscular impairment and longer CTG expansion sizes predicted a greater volume decrease in GM and lower FA values. Age predicted global GM reduction, specifically in parietal regions. At the cognitive level, the DM1 group showed significant negative correlations between IQ estimate, visuoconstructive and executive neuropsychological scores and both global and regional volume decrease, mainly distributed in the frontal, parietal and subcortical regions.
Conclusions
In this study, we describe the structural brain signatures that delineate the involvement of the CNS in DM1. We show that specific sensory and multi-sensory — as well as frontal cortical areas — display potential vulnerability associated with the hypothesized neurodegenerative nature of DM1 brain abnormalities.
1. Introduction
Myotonic Dystrophy type 1 (DM1) is an autosomal dominant disease that is the most common adult-onset muscular dystrophy. The disease is characterized by severe neuromuscular defects, including myotonia and progressive muscle weakness and wasting (atrophy), leading to disability as the disease progresses, and respiratory distress either from primary muscle failure or from cardio-pulmonary complications. It is also characteristically multisystemic and degenerative, affecting the heart and the brain, among other body systems (reviewed in: Harper, 2001; Thornton, 2014). The molecular basis of the disease is the pathogenic expansion of an unstable CTG (cytosine-thymine-guanine) microsatellite in the 3’UTR of the DM protein kinase (DMPK) gene. Epidemiologically, it is the most frequent neuromuscular disorder with a reported prevalence of 1/7400 people worldwide (Harper, 2001), having a significantly higher prevalence in Gipuzkoa (North of Spain), reaching 300 cases per million inhabitants (López de Munain et al., 1993). Although CNS involvement in DM1 was reported a long time ago, implicated brain structures and the cognitive profile of DM1 is still a matter of debate.
Neuroimaging studies have recently emerged, reporting brain abnormalities in DM1 patients. On the whole, these studies have reported that a reduction in the volume of both gray and white matter and increased WM hyperintensities are of widespread occurrence in DM1 brains, along with other functional abnormalities such as abnormal connectivity patterns or reduced cerebral perfusion (Okkersen et al., 2017). However, results have so far failed to characterize the exact pattern of CNS involvement in DM1, and its association with clinical and neuropsychological features of the disease is still inconclusive.
Voxel based morphometry (VBM) is one of the most commonly employed techniques for measuring the loss of gray matter (GM) volume. In DM1, a widespread decrease has been reported across all cortical surface (with the frontal lobe being reduced in all cases) and in some subcortical structures (Antonini et al., 2004; Baldanzi et al., 2016; Caso et al., 2014; Minnerop et al., 2011; Ota et al., 2006; Schneider-Gold et al., 2015; Serra et al., 2015; Weber et al., 2010; Zanigni et al., 2016). Moreover, Diffusion Tensor Imaging (DTI) is been widely documented as a tool for quantifying WM damage. The structural integrity of all major association, projection, and commissural fibers has been reported to be altered in DM1 (Baldanzi et al., 2016; Caso et al., 2014; Fukuda et al., 2005; Minnerop et al., 2011; Ota et al., 2006; Serra et al., 2015; van Dorst et al., 2019; Wozniak et al., 2014, 2013, 2011; Zanigni et al., 2016). The study of the relationships between gray and white matter abnormalities in DM1 will help to elucidate the mechanisms of CNS involvement in the disease, which some have suggested to rely on a disconnection of cortical structures due to WM interruptions (Caso et al., 2014; Minnerop et al., 2011; Zanigni et al., 2016).
From a neuropsychological point of view, various patterns of cognitive impairment have been found across studies, possibly due to methodological discrepancies (such as assessment battery or representativeness of the DM1 population). Overall, studies have reported deficits in attention, memory, and language (Modoni et al., 2008; Rubinsztein et al., 1997), as well as in visuospatial/constructive and executive functions (Sistiaga et al., 2010; Winblad et al., 2006a) and facial emotion recognition (Kobayakawa et al., 2010; Labayru et al., 2018; Winblad et al., 2006b). Some authors have also suggested a pattern of frontal lobe degeneration, whilst others have found results consistent with a pattern of fronto-parietal impairment. Although clinical impressions as well as some recent findings suggest a cognitive decline over time (Gallais et al., 2017; Modoni et al., 2008; Sansone et al., 2007; Winblad et al., 2016), this issue still remains unclear.
The study of neuroimaging correlations with clinical and neuropsychological data is still scarce and the literature has yielded mixed results. Moreover, variations in cognitive assessment tests limit their comparison. The aim of this study is to determine the pattern of structural abnormalities in gray and white matter in juvenile and adult onset DM1 and to analyze the relationship between this pattern and muscular, molecular, and neuropsychological data.
2. Methods
2.1. Participants
The DM1 patients analyzed in this work were selected from those attending the outpatient consultancies at the Neurology Department of the Donostia Hospital (San Sebastian), a tertiary public hospital that serves a population of 650,000 inhabitants (almost all of the Guipuzcoa province). All patients were examined by a neurologist and underwent a neuropsychological assessment close to the MRI acquisition date.
Inclusion criteria for DM1 patients included being between 18 and 70 years old along with molecular confirmation of the clinical diagnosis. Patients were excluded on the basis of the following criteria: congenital or childhood form (considered to be qualitatively distinct phenotypes), history of major psychiatric or somatic disorder (in accordance with DSM-IV criteria), acquired brain damage or alcohol or drug abuse and the presence of corporal paramagnetic body devices (Pacemaker etc.) that could impede MRI studies.
35 DM1 patient with genetically confirmed juvenile (N = 10) and adult (N = 25) onset DM1 were recruited. Of the sample, 4 patients were excluded after MRI acquisition due to cerebral anomalies: 3 patients presented significant ventricular dilatation suggestive of hydrocephaly according to Evans´ index (44-year-old male with 333 CTG; 41-year-old female with 667 CTG; and 28-year-old female with 500 CTG) and 1 patient presented frontal hyperostosis (36-year-old female with 400 CTG). A final group of 31 DM1 patients (9 juvenile and 22 adult onset) was included for analysis. As a control group, 57 healthy controls (HC) were recruited including unaffected family members and healthy volunteers who met none of the exclusion criteria.
All participants were informed of the objectives and details of the study and gave informed written consent. The study was approved by the Ethics Committee of the Donostia University Hospital.
2.2. Clinical and neuropsychological assessment
Clinical data regarding CTG expansion size, Muscular Impairment Rating Scale (MIRS) score (Mathieu et al., 2001), disease form, and demographic data were extracted from medical records.
All patients underwent an assessment by an experienced neuropsychologist who was blind to the patient's clinical condition (CTG expansion size, clinical form, muscular impairment and MRI results). Neuropsychological assessment included several subtests from the Wechsler Adult Intelligence Scale III (WAIS III) (Wechsler, 1999), including: Digit span, Vocabulary, Block design, Object assembly, Arithmetic and Similarities; some of which were used for estimating IQ from a five-subtest short form based on López et al. (2003). Other cognitive tests used were: Stroop test (Golden, 2001), California Computerized Assessment Package (CALCAP) (Miller, 1990), Raven's progressive matrices (Raven et al., 2001), Rey Auditory Verbal Learning Test (RAVLT) (Lezak et al., 2004), Word Fluency (Casals-Coll et al., 2013; Peña-Casanova et al., 2009), Rey-Osterrieth Complex Figure test (ROCF) (Rey, 2009) and Benton's Judgement of Line Orientation (Benton et al., 1994). The patients’ raw scores were converted into standardized T values according to the Spanish population-based norms for each test.
2.3. MRI acquisition
MR scanning was performed on a 1.5 Tesla scanner (Achieva Nova, Philips). The current results are based on a high-resolution volumetric “turbo field echo” (TFE) series (sagital 3D T1 weighted acquisition, TR = 7.2, TE = 3.3, flip angle = 8, matrix = 256 × 232, slice thickness 1 mm, voxel dimensions of 1 mm x 1 mm x 1 mm, NSA = 1, no slices 160, gap = 0, total scan duration 5´34¨).
Diffusion-weighted images were acquired using a Single Shot SPIR: 1.75 × 1.75 × 2 mm voxels; 60 axial slices; b-value of 800 s/mm2; 32 direction diffusion-weighted and 1 baseline image; TR = 9967 ms, TE = 66 ms; angle 90, acquisition matrix size = 128 × 128.
All the scans were acquired on the same MR scanner and no hardware or software upgrades were carried out within the study period.
2.4. Data preprocessing
A detailed flowchart of the image-processing pipeline can be found in Supplementary Fig. 1. To study voxel based GM volume loss in DM1 patients and its association with different neuropsychological scales, FSL Voxel Based Morphometry (VBM) was used (Douaud et al., 2007), an optimized VBM protocol (Good et al., 2001) carried out with FSL tools (Smith et al., 2004). First, structural images were brain-extracted and GM-segmented before being registered to the MNI 152 standard space using non-linear registration (Andersson et al., 2007). The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific GM template. Second, all native GM images were non-linearly registered to this study-specific template and "modulated" to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. The modulated GM images were then smoothed with an isotropic Gaussian kernel with a sigma of 3.
To estimate global brain tissue volume, SIENAX tool was used (Smith et al., 2002). SIENAX is a FSL package for single-time-point analysis of brain atrophy (volumetric loss of brain tissue), which estimates total brain tissue volume from a single image, normalized for skull size. It first strips non-brain tissue, and then uses the brain and skull images to estimate the scaling between the subject's image and standard space. It then runs tissue segmentation to estimate the volume of brain tissue, and multiplies this by the estimated scaling factor to reduce head-size-related variability between subjects.
Additionally, anatomical T1 images were used to compute the cortical parcellation and volumetric segmentation of each subject's brain using FreeSurfer 6.0.0 (Dale et al., 1999). This pipeline included: removal of non-brain tissue using a hybrid watershed/surface deformation procedure; automated Talairach transformation; segmentation of the subcortical WM and deep GM structures (Fischl et al., 2002); intensity normalization; delineation of the GM/WM boundary; automated topology correction; and surface deformation following intensity gradients to optimally identify the gray/white and gray/cerebrospinal fluid boundaries. Surface based registration projected the Desikan-Killiany parcellation to individual subjects (Desikan et al., 2006).
FMRIB Software Library v5.0.7 (FSL) software was used for the preprocessing of Diffusion-weighted images. Following eddy current correction, gradient vectors were rotated to compensate for head motion. Local fitting of the diffusion tensor at each voxel was then computed and Fractional Anisotropy maps for each subject were generated. To use Desikan-Killiany parcellation and subcortical segmentation for the tractography analysis this atlas was projected from T1 space to diffusion space computing a non-linear transformation between the T1 and fractional anisotropy map. To model crossing fibers, the FSL BEDPOSTX (Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques) tool was used with default parameters, and probabilistic tractography was subsequently performed using the FSL PROBTRACKX2 (probabilistic tracking with crossing fibers) tool with 100 samples with loopcheck.
2.5. Statistical analysis
Demographic data were analyzed using the SPSS (IBM SPSS Statistics 24) statistical package. Intergroup comparisons were conducted to compare DM1 patients and HC, using contingency analysis (chi-square) for categorical data and parametric t test for interval data.
2.5.1. VBM between and within group statistical analysis
A general linear model was used to compute the between group statistical analysis using Matlab R2017a (see Supplementary File 1 for more detail). A two sample T-test was used, controlling for age and head size. All the results were corrected for multiple comparisons using Monte Carlo simulation cluster-wise correction from AFNI software (https://afni.nimh.nih.gov/) with 10,000 iterations to estimate the probability of false positive clusters with a p value<0.05.
To study the brain region implicated in the severity of the disease, we used a general linear model and evaluated the relationship between GM volume and CTG and MIRS clinical scales. Once again, the results were adjusted for age and head size and corrected for multiple comparisons using Monte Carlo simulation. Additionally, partial correlation was used to evaluate the relationship between global volume loss and disease severity, as measured by MIRS and CTG (adjusting for age and head size).
To study the effect of age in comparison with HC, a general linear model was used to compare the relationship between GM volume and age between the two groups. In this case, Monte Carlo simulation was used to correct for multiple comparison adjusting only for head size.
Global volume loss was also correlated with neuropsychological variables using a partial correlation analysis, controlling for age and head size. To control for the number of associations with different neuropsychological tests, a false discovery rate (FDR) correction was applied.
Finally, a within-group analysis was conducted to study the association between neuropsychological variables and GM volume, using a regression analysis. For each neuropsychological variable, the imaging results were controlled for age and head size and corrected for whole brain multiple comparisons using Monte Carlo simulation cluster-wise correction with 10,000 iterations to estimate the probability of false positive clusters with a p value < 0.05. After correcting for multiple comparisons, a mask with the results of the group difference was applied to look only for the associations in regions were DM1 patients show a significant GM volume loss compared with healthy controls. Only clusters with a spatial extent of 100 adjacent voxels are reported.
2.5.2. DTI between and within group statistical analysis
DTI data were used to examine the cortical and subcortical regions affected by the disintegration of the WM. First, the brain was divided into 86 regions: 68 cortical areas defined by Freesurfer Desikan parcellation and 18 subcortical regions obtained with Freesurfer segmentation. For each individual subject and brain region probabilistic tractography was used with the probtrackx2 tool, taking 100 samples from the range of possible principal diffusion directions within each voxel. The probabilistic tracking output was a density map defining the number of fibers passing through each voxel when tracking from a given seed. To remove false positives from these maps, voxels with fewer fibers than 0.1% of all the tracked fibers were thresholded. The thresholded density maps of each region were used to compute the weighted FA values as a proxy of WM integrity throughout different areas (high values of FA representing high integrity). The FA values of the voxels in the path of the fibers starting in each region, defined in the density map, were extracted and weighted based on the probability of fibers passing through each voxel; the weighted mean FA gives more importance to voxels with higher probability. DTI images were available in 23 patients and 44 HC.
To examine between-group differences of the mean weighted FA values of the 86 regions in each individual, a general linear model was performed adjusting for age using Matlab (see Supplementary File 1 for more detail). This analysis identified cortical and subcortical brain areas showing differential fiber integrity in patients with DM1 compared with healthy controls. To correct for multiple comparisons, both FDR with q=0.05 and Bonferroni with α=0.05 corrections were applied.
To study the relationship between fiber integrity and both disease severity and neuropsychological variables, a general linear model was used, controlling for age. The results were corrected for multiple comparison using FDR, with q=0.05. The resulting ROI that were not present in the group difference correcting for FDR were removed, to focus only on those regions showing decreased diffusivity compared to HC.
Using the same approach as in GM volume, the effect of age was compared between DM1 and HC, using FDR to correct for multiple comparisons.
3. Results
3.1. Demographic, clinical, and neuropsychological outcomes
The main demographic characteristics of the sample are shown in Table 1. No statistical differences were found between the patient group and the HC group in terms of mean age and sex ratio. The neuropsychological outcomes of the DM1 patients are summarized in Supplementary Table 1. The profile is characterized by slow processing speed, mild executive impairment (abstract reasoning, planning and flexibility) and visuoconstructive impairment, and a mean IQ of 84.29 (standard deviation, SD: 12.67). The patients as a group scored below the normative mean on every cognitive subtest and obtained a mean score below -1 SD on nine measures and below -2 SD on one subtest (ROCF copy).
Table 1.
Socio-demographic, clinical, and molecular characteristics according to group.
| DM1 | HC | DM1 vs HC | ||
|---|---|---|---|---|
| N = 31 | N = 57 | Statistic | p | |
| Sex N (%) | ||||
| Male | 13 (41.9%) | 27 (47.4%) | X² = .24 | .625 |
| Female | 18 (58.1%) | 30 (52.6%) | ||
| Age | ||||
| Mean (SD) | 43.94 (11.59) | 45.14 (12.96) | t = −.43 | .667 |
| Min-max | 22–61 | 18–70 | ||
| Muscular weakness (MIRS) | ||||
| Mean (SD) | 2.8 (1.22) | - | ||
| Min-max | 1–5 | - | ||
| Molecular defect (CTG) | ||||
| Mean (SD) | 667.77 (473.97) | - | ||
| Min-max | 63–1833 | - | ||
| Gray matter volume (mm3) | ||||
| Mean (SD) | 750765.77 | 792954.13 | F = 14.84 | .000 |
| (54664.48) | (45787.84) | |||
| White matter volume (mm3) | ||||
| Mean (SD) | 686896.86 | 711533.98 | F = 6.64 | .011 |
| (43162.82) | (42684.27) | |||
Note: DM1, Myotonic Dystrophy Type 1; HC, Healthy Controls; SD, Standard deviation; MIRS, Muscular Impairment Rating Scale; CTG, triplet expansion size.
3.2. VBM and DTI– between group comparison
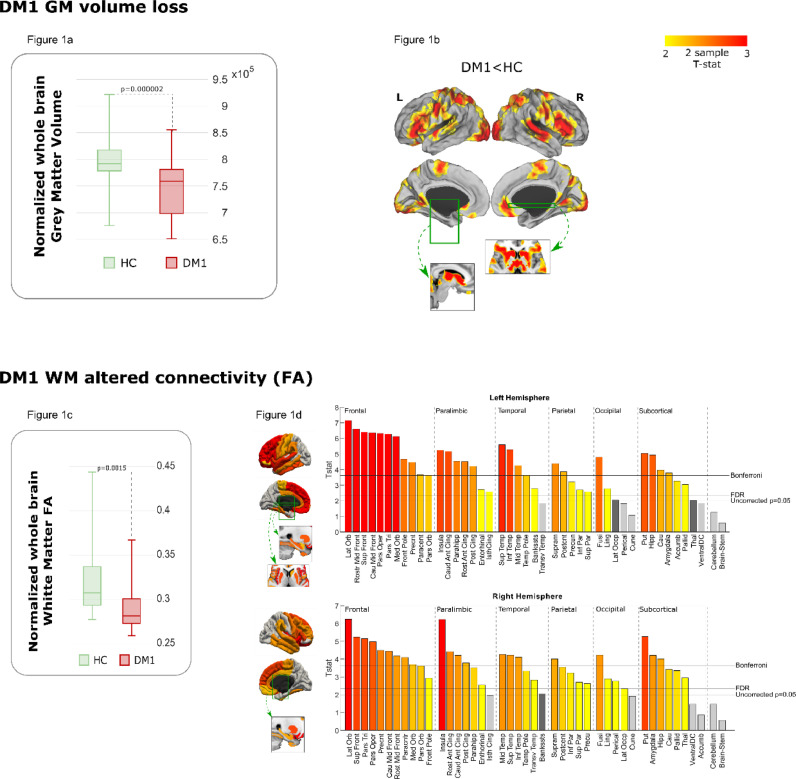
VBM analysis revealed statistically significant differences in both total gray and white matter volume between DM1 and HC subjects. (Table 1 and box plot Fig. 1a for GM).
Fig. 1.
Gray matter (GM) volume loss and decrease in white matter (WM) integrity (FA, fractional anisotropy) in DM1 patients. T statistic maps with the results of two sample T test comparing DM1 vs healthy controls (HC) are displayed. Only results surviving multiple comparison adjusting for age and brain size are shown for GM volume. The box plot in Fig. 1a shows the comparison of whole brain GM volume between the DM1 and HC group adjusting for age and head size. Fig. 1b shows areas of greater decrease in DM1 compared with HC. The cortical results were projected on a brain surface and were complemented with subcortical slices; the green boxes and arrows represent the position of these slices in the brain surface. The box plot in Fig. 1c shows the comparison of whole brain WM FA between the DM1 and the HC group adjusting for age. Fig. 1d shows areas of major FA decrease in DM1 vs healthy subjects according to brain region. The bar plots display the T statistics results of the group difference (the magnitude of the difference between the two groups) for all the brain regions.
When analyzing the location of GM volume differences between groups in VBM corrected for age and head size, specific areas emerged as showing a significant decrease in DM1 compared with the HC group. The brains in Fig. 1b show a widespread decrease in volume in comparison with healthy subjects in primary regions (visual, somatomotor, and auditive), multimodal integration regions, the ventromedial and dorsolateral prefrontal cortex, intraparietal sulcus (IPS), striatum, thalamus, hypothalamus, periaqueductal gray and cerebellum.
DTI group comparison between DM1 patients and HC revealed a significant decrease in total FA (Fig. 1c). Locally, using Bonferroni correction, a significantly decreased FA was found in 54.02% of all studied tracts (going to both cortical and subcortical areas, Fig. 1d). Areas with affected connectivity were bilaterally but non-symmetrically (higher number of affected connectivity areas in the left hemisphere) distributed across the entire cortical surface, with the exception of the cerebellum and brainstem. The regions with the highest percentage of affected connectivity areas were the frontal cortex (90.9% of the frontal areas with affected fibers), paralimbic cortex (64.2%), temporal cortex (50%) and subcortical regions (43.75%).
3.3. VBM and DTI – association with clinical and neuropsychological data
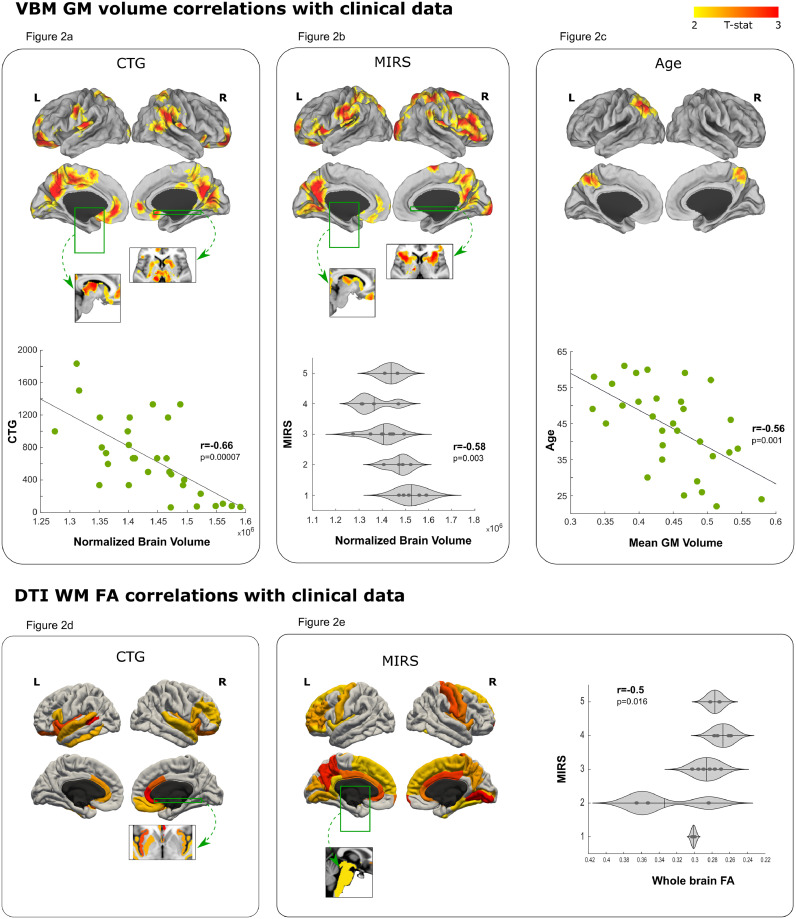
Associations between GM volumes and CTG, MIRS, and age are shown in Fig. 2a–c. All the three statistical analyses were corrected for head size, and additionally corrected for age in the case of MIRS and CTG correlations. Scatter plots in the lower part of the figure indicate that total brain volume is significantly correlated with MIRS outcome, CTG expansion size, and age (higher ratings on MIRS, CTG repeat size and older age correlate with lower volume). Areas with atrophies that correlate with increased CTG expansion size are the orbitofrontal area, anterior and posterior cingulate cortex, left sensorimotor areas, right temporoparietal junction and precuneus, visual association areas, thalamus, striatum and subcallosal cortex (Fig. 2a). Areas with atrophies correlated with increased MIRS are primary visual and sensorimotor regions, prefrontal ventromedial and orbitofrontal areas, anterior cingulate cortex, IPS and precuneus, left thalamus and bilateral striatum (Fig. 2b). Finally, the correlation between GM volume and age was compared between DM1 patients and HC. The areas in which volume loss related to age was greater in DM1 patients than healthy controls are the precuneus, left latero-occipital and superior parietal areas (Fig. 2c).
Fig. 2.
Gray matter (GM) volume loss and decrease in white matter (WM) integrity (FA, fractional anisotropy) associated with severity of DM1 patients. The T-statistic showing the relationship between GM volume and WM integrity in DM1 patients and various disease severity markers is displayed. Only results surviving multiple comparisons are shown, correcting for age and head size in GM volume and for age in WM integrity. Fig. 2a shows the areas significantly decreased in relation to CTG in DM1 patients. The scatter plot shows the association between whole brain volume and CTG value. Fig. 2b shows the areas significantly decreased in relation to MIRS scale in DM1 patients. The violin plot shows the association between whole brain volume and MIRS scale. Fig. 2c shows the areas where greater volume loss occurs in DM1 patients with age in comparison with healthy controls (HC). The scatter plots show the effect of age and volume loss in this specific region. Fig. 2d shows the areas with significantly disrupted connectivity in relation to CTG in DM1 patients. Fig. 2e shows the areas with significantly disrupted connectivity in relation to MIRS scale in DM1 patients. The violin plot shows the association between whole brain WM FA and MIRS scale. The cortical results were projected on a brain surface and were complemented with subcortical slices; the green boxes and arrows represent the position of this slices in the brain surface.
Further, DTI analysis showed that whole brain FA correlated only with MIRS (higher MIRS was associated with lower FA). Regionally, patients with higher CTG showed decreased FA in the fibers starting in bilateral prefrontal areas, anterior cingulate, temporal cortex (superior, inferior and banks of the superior temporal sulculs), insula and putamen (Fig. 2d). Patients with higher MIRS showed altered FA in the fibers starting in the frontal cortex (except the orbitofrontal areas), cingulate cortex, primary sensory cortex, insula and precuneus, ventral temporal areas (Fusiform gyrus, lingual cortex, and para-hippocampus), brainstem and ventral diencephalon (Fig. 2e). No correlations were found between FA measures and age.
The correlation analysis between neuropsychological performance and total volume loss in GM is presented in Table 2. Only those correlations that remained statistically significant after correcting for multiple comparisons (FDR) are presented. Lower scores on neuropsychological tests predicted lower total GM volume. Only the color subtest of the Stroop test correlated with total FA, showing an inverse correlation (r = −=.72, p = .000).
Table 2.
Association between total GM atrophy and neuropsychological outcomes in DM1
| Total GM atrophy |
||
|---|---|---|
| Neuropsychological measure | r | p |
| Block design | .49 | .006 |
| Raven's progressive matrices | .52 | .006 |
| ROCF copy | .55 | .001 |
| IQ estimate | .46 | .010 |
Note: GM, Gray matter; ROCF, Rey-Osterrieth Complex Figure test; IQ, Intelligence Quotient. All the correlations are performed with standardized T scores or standard scores (IQ estimate only) of the neuropsychological outcome measures.
To obtain an association between neuropsychological outcomes and affected brain areas after correcting for whole brain multiple comparisons, we focused on regions defined by the mask of group differences between DM1 and HC. Associations between different neuropsychological tests and the GM intensity are shown in Table 3 (and in Supplementary Figure 2). All associations except one were negative (lower score on cognitive tests correlated with greater GM volume loss). Regarding WM integrity, an isolated negative association was found between regional FA (parietal lobe, precuneus, postcentral gyrus and supramarginal cortex) and one subtest of the Stroop (Supplementary Figure 2).
Table 3.
Gray matter volume loss associated with neuropsychological outcome of DM1 patients.
| Cluster |
MNI coordinates |
||||||
|---|---|---|---|---|---|---|---|
| Size | Mean T | p | Peak ROI Structure | X | Y | Z | |
| IQ estimate | 133 | 3 | .005 | Left Frontal Pole | −40 | 40 | −8 |
| Arithmetic | 181 | 2.28 | .030 | Right Cuneal Cortex | 4 | −84 | 24 |
| Block design | 132 | 2.66 | .013 | Left Frontal Pole | −46 | 44 | −8 |
| 257 | 2.47 | .020 | Right Precuneus Cortex | 10 | −64 | 52 | |
| ROCF copy | 413 | 2.65 | .013 | Left Frontal Orbital Cortex | −20 | 20 | −22 |
| 860 | 2.64 | .013 | Left Caudate | −16 | −8 | 20 | |
| 180 | 2.66 | .013 | Left Precentral Gyrus | −52 | 6 | 16 | |
| 427 | 2.62 | .014 | Left Precentral Gyrus | −52 | 0 | 34 | |
| 597 | 2.59 | .015 | Left Superior Frontal Gyrus | −8 | −6 | 72 | |
| ROCF memory | 705 | 2.9 | .007 | Right Subcallosal Cortex | 4 | 30 | −30 |
| 250 | 2.39 | .024 | Left Insular Cortex | −30 | 0 | 14 | |
| 136 | 2.52 | .018 | Left Precentral Gyrus | −54 | 8 | 38 | |
| 116 | 2.49 | .019 | Left Inferior Frontal Gyrus | −38 | 16 | 26 | |
| 328 | 2.96 | .006 | Left Precentral Gyrus | −16 | −12 | 76 | |
| Object assembly | 543 | 2.47 | .020 | Right Lingual Gyrus | 6 | −74 | −8 |
| 337 | 2.67 | .012 | Right Precentral Gyrus | 34 | −18 | 56 | |
| 269 | 2.78 | .009 | Right Superior Frontal Gyrus | 18 | −6 | 68 | |
| Stroop Interference | 521 | 2.54 | .018 | Left Frontal Medial Cortex | −2 | 32 | −28 |
| 197 | 2.41 | .024 | Right Putamen | 24 | 8 | −6 | |
| 232 | −2.57 | .017 | Right Precentral Gyrus | 50 | 4 | 40 | |
| Stroop Word | 992 | 2.63 | .015 | Left Putamen | −30 | 6 | −8 |
| 872 | 3.08 | .005 | Right Caudate | 8 | 16 | 10 | |
| Stroop Color-Word | 694 | 2.61 | .015 | Left Frontal Orbital Cortex | −18 | 12 | −12 |
| 241 | 2.52 | .019 | Right Putamen | 24 | 10 | −8 | |
| Raven's progressive | 147 | 2.5 | .019 | Left Inferior Frontal Gyrus | −52 | 34 | −16 |
| matrices | 142 | 2.62 | .015 | Left Middle Frontal Gyrus | −50 | 22 | 32 |
| 390 | 2.67 | .013 | Left Precentral Gyrus | −48 | −8 | 46 | |
| 251 | 2.52 | .019 | Left Superior Parietal Lobule | −40 | −40 | 54 | |
| 154 | 2.58 | .016 | Left Precuneus Cortex | −10 | −54 | 52 | |
| 163 | 2.74 | .011 | Right Precuneus Cortex | 12 | −50 | 60 | |
| Election RT | 352 | 2.58 | .017 | Left Occipital Pole | −16 | −94 | 0 |
| Sequence 2 RT | 469 | 2.72 | .012 | Right Frontal Orbital Cortex | 10 | 24 | −28 |
| 272 | 2.37 | .026 | Left Middle Frontal Gyrus | −46 | 30 | 24 | |
| 107 | 2.39 | .026 | Left Parietal Operculum Cortex | −48 | −24 | 16 | |
| 196 | 2.32 | .029 | Left Postcentral Gyrus | −44 | −16 | 30 | |
Note. DM1: Myotonic Dystrophy Type 1; MNI: Montreal Neurosciences Institute; ROI: Regions of Interest; IQ: Intelligence Quotient; ROCF: Rey-Osterrieth Complex Figure; RT: Reaction Time. Only results surviving multiple comparisons are shown, correcting for age and head size. The results were then masked to display only those results in the regions where DM1 patients showed a significant decrease in volume compared with healthy controls. For each neuropsychological measure the table shows the cluster size representing the total number of voxels that comprise the cluster; the mean T represents the magnitude of the association between the mean grey matter measure in the cluster and the p-value of this association (Higher T indicates a stronger association).
4. Discussion
The current paper presents a thorough analysis of structural abnormalities in both gray and white matter in a juvenile and adult onset DM1 sample compared with an extensive healthy control group. GM VBM analysis was performed using the highest standards of statistical control, and correlation analysis was conducted to explore the relationships with clinical parameters. Volume reduction was not limited to the whole brain volume, but was extended to specific regions. In this work WM integrity was assessed with a statistical threshold corrected for multiple comparisons. In addition, while many of the DTI studies have focused on Tract-Based Spatial Statistics (TBSS, analyzing the diffusion parameters in the skeleton of the WM tracts), the present study used probabilistic tractography to assess structural connectivity alterations in DM1 patients. Whereas some studies used tractography to focus on a small number of hypothesized and predefined tracts, in this work we conducted whole brain tractography and studied connectivity to all cortical areas, obtaining new and valuable information regarding the cortical signature of the disease due to the disruption of WM connections.
Further, this study comprises a comprehensive neuropsychological assessment of DM1 patients, and the most relevant clinical features were correlated with MRI findings. The DM1 sample in this study showed a neuropsychological profile that is representative of the DM1 population according to the current literature (Jean et al., 2014).
4.1. VBM and DTI – group differences
Consistent with the findings of the most recent studies (Okkersen et al., 2017), overall the MRI analysis showed widespread bilateral alteration in both gray and white matter, which could partially be explained by the heterogeneous effect of genetics upon a variety of tissues in DM1.
Cortical GM atrophy was found to be more pronounced in certain areas of every cerebral lobe. The involvement of subcortical structures has been confirmed in previous work, reporting lower volumes in striatum, thalamus, hypothalamus and periaqueductal gray, with a bilateral and symmetric involvement. Both cortical and subcortical atrophy fits well with the histopathological findings in DM1, including RNA nuclear inclusions or neurofibrillary tangles found in the cortex and several deep GM structures (Caillet-Boudin et al., 2014).
When analyzing WM FA alteration related to connectivity areas, anterior cortical areas and again subcortical regions appeared to be the most affected. Interestingly, all the anterior GM areas with decreased volume resulted in altered WM integrity. Conversely, there was no correspondence between gray-white matter impairment in the case of more posterior and deep gray structures (i.e. IPS, thalamus, periaqueductal gray, and cerebellum). DM1 has been proposed to be a predominantly WM disease. However, based on the extent to which our data on GM damage exceeds that of WM integrity loss, this idea could be questioned (Schneider-Gold et al., 2015). So far, DM1 should be considered a disease with both gray and white matter involvement, without any clearly established trend towards a greater involvement of either type of tissue.
4.2. VBM and DTI – correlation with clinical data
Although not consistently reported in the literature, there is evidence in support of the possibility that longer CTG could be a predictor of structural abnormalities. Similar to other studies (Park et al., 2018), we found a significant correlation between muscular and genetic impairment and GM volume loss.
CTG expansion size determined in peripheral blood cells has been questioned for genotype-phenotype correlation analysis due to tissue and temporal variations (Martorell et al., 1998). However, in this study, patients with greater CTG expansion sizes showed reduced GM volumes, primarily in fronto-parietal areas and subcortical regions, according to the functional cognitive profile described in some neuropsychological studies (Peric et al., 2017, 2014; Sistiaga et al., 2010). WM abnormalities related to longer CTG also appeared in both cortical and subcortical regions. Taken together, CTG-related associations, along with brain imaging findings, encourage us to maintain the idea that genetic features of DM1 are target variables in the study of possible mediators between CNS involvement and actual symptoms.
Muscular impairment also appeared to be associated with total FA reduction, regional FA reduction and with GM volume loss, but more widely dispersed throughout the cortical tissue than genetic features, which is suggestive of this being a good global severity marker of the disease. Consistent with this proposal is the fact that previous studies have reported not only evidence of regional atrophy in motor-related structures, but also correlations between MIRS and global brain atrophy (Schneider-Gold et al., 2015) and with altered integrity in the majority of WM tracts (Wozniak et al., 2014). This reinforces the notion of central, and not merely muscular, motor dysfunction in adult DM1.
In DM1, an association between age and MRI findings has been reported (Minnerop et al., 2018). Although studies assessing disease progression are still scarce, the results so far indicate a premature aging effect in DM1. Accordingly, in this study, age predicted global GM volume loss and was associated with particular vulnerability to parietal GM regions, suggesting an age-related higher vulnerability of that specific cortical lobe. Follow up studies including both neuropsychological and neuroimaging data are strongly needed in order to clarify the neurodegenerative hypothesis that has repeatedly been suggested. In view of the parietal vulnerability to age indicated in this study, the inclusion of visuoconstructive/visuospatial tasks in longitudinal assessments of cognition should be considered for characterization of the patients.
Neither total FA reduction nor FA decrease in specific connectivity areas was found to be related to age, which could reflect the neurodevelopmental nature of WM abnormalities in DM1, rather than a degenerative process. Indeed, diffuse WM integrity disruption has been reported in early stages in young DM1 patients (Wozniak et al., 2013). However, only longitudinal data with a baseline set at disease onset could clarify this hypothesis.
4.3. VBM and DTI – correlation with neuropsychological data
The lack of consistent results on neuropsychological correlates with neuroimaging results have been reported to date in the DM1 literature. Our findings, however, suggest that the selected neuropsychological measures are highly sensitive for detecting structural changes in the brain, as quantified in the numerous correlations found primarily in cortical areas. Nevertheless, a lack of specificity could still be an issue, since anatomo-functional correspondence of the results found might not be those that were expected. The network-wise organization for cognitive functions (as opposed to a greater localizationist approach) could be behind this lack of correspondence. Moreover, considering the large global atrophy consistently reported in DM1, functional reorganization could be playing a role.
Overall, visuoconstructive and executive performance correlated with both total GM atrophy and with some of the specific areas of decreased volume in DM1 compared with healthy controls. Taken together, the results point to the possibility that these cognitive domains are good markers of brain abnormalities in the DM1 population.
This study is not without limitations. Considering the clinical heterogeneity of the disease, the sample size is relatively small in this study. The inclusion of larger sample sizes will allow for a more in-depth analysis of MRI abnormalities, and could provide accurate and evidence-based information to suggest alternative classifications of DM1 patients. Another limitation in the present study is that congenital and childhood forms were not included, and therefore, the results cannot be generalized to the whole DM1 population. Additionally, there are other measures of DTI apart from FA that were not analyzed in the present work in an effort to simplify the quantity of results presented. Whilst other measures correlate more strongly with features such as cellularity or membrane diameter, FA is more sensitive to tissue integrity, which represents a main focus of the present work. Finally, in DTI, tractography algorithms are not able to reconstruct single axons. While deterministic tractography does not account for crossing fibers, probabilistic tracking generates a distribution of fiber directions in each voxel to deal with this problem, although this produces a large number of false positive fibers. To minimize this error, less probable fibers were removed and the weighted mean FA was calculated. In the future, the inclusion of higher resolution protocols and new probabilistic tractography algorithms, including anatomical information, could lead to more accurate and reproducible fiber estimation.
4.4. Conclusion
Global GM atrophy and WM integrity compromise is confirmed in DM1. A pattern of greater fronto-temporo-parietal and subcortical impairment is proposed, with a gray-related WM integrity loss. On balance, our results, together with those previously reported, suggest the existence of a complex neuronal network damage-related disease as opposed to the idea that focal structural degeneration is responsible for CNS symptoms in DM1.
Neuropsychological assessment emerges as an unspecific but strong predictor of GM abnormalities. Age-related vulnerability of certain regions suggests a possible neurodegenerative process in DM1 that remains to be thoroughly studied. Longitudinal studies including both GM and WM structural data in cognitive and clinically well characterized patients are needed. This will allow for a more complete characterization of the interrelations between gray and white matter in DM1, and will also allow us to elucidate whether a progression over time is confirmed in relation to cognitive decline.
Declaration of Competing Interest
Authors declare that this work was supported by the Institute of Health Carlos III co-founded by Fondo Europeo de Desarrollo Regional-FEDER (grant numbers PI17/01231 and PI17/01841), CIBERNED (grant number: 609), the Basque Government (grant numbers SAIO08-PE08BF01 and PRE_2016_1_0187) and the postdoctoral fellowship program from the Basque country Government.
Acknowledgements
We are grateful to all the patients, their families and the healthy volunteers who participated in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102078.
Appendix. Supplementary materials
References
- Andersson, J.L.R., Jenkinson, M., Smith, S., 2007. Non-linear registration, aka spatial normalisation. FMRIB Technial Report TR07JA2. Oxford Cent. Funct. Magn. Reson. Imaging Brain, Dep. Clin. Neurol. Oxford Univ. Oxford, UK 22. 10.1016/j.neuroimage.2008.10.055 [DOI]
- Antonini G., Mainero C., Romano A., Giubilei F., Ceschin V., Gragnani F., Morino S., Fiorelli M., Soscia F., Di Pasquale A., Caramia F. Cerebral atrophy in myotonic dystrophy: a voxel based morphometric study. J. Neurol. Neurosurg. Psychiatry. 2004;75:1611–1613. doi: 10.1136/jnnp.2003.032417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldanzi S., Cecchi P., Fabbri S., Pesaresi I., Simoncini C., Angelini C., Bonuccelli U., Cosottini M., Siciliano G. Relationship between neuropsychological impairment and grey and white matter changes in adult-onset myotonic dystrophy type 1. NeuroImage Clin. 2016;12:190–197. doi: 10.1016/j.nicl.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton A., Sivan A., Hamsher K., Varney N.R., Spreen O. 2nd ed. Oxford University Press; New York: 1994. Contributions to Neuropsychological Assessment. [Google Scholar]
- Caillet-Boudin M.L., Fernandez-Gomez F.J., Tran H., Dhaenens C.M., Buee L., Sergeant N. Brain pathology in myotonic dystrophy: when tauopathy meets spliceopathy and RNAopathy. Front. Mol. Neurosci. 2014;6:1–20. doi: 10.3389/fnmol.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Coll M., Sánchez-Benavides G., Quintana M., Manero R.M., Rognoni T., Calvo L., Palomo R., Aranciva F., Tamayo F., Peña-Casanova J. Estudios normativos españoles en población adulta joven (proyecto NEURONORMA jóvenes): normas para los test de fluencia verbal. Neurología. 2013;28:33–40. doi: 10.1016/j.nrl.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Caso F., Agosta F., Peric S., Rakočević-Stojanović V., Copetti M., Kostic V.S., Filippi M. Cognitive impairment in myotonic dystrophy type 1 is associated with white matter damage. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J., James S., Voets N., Watkins K., Matthews P.M., James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Van Der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Horiguchi J., Ono C., Ohshita T., Takaba J., Ito K. Diffusion tensor imaging of cerebral white matter in patients with myotonic dystrophy. Acta Radiol. 2005;46:104–109. doi: 10.1080/02841850510015974. [DOI] [PubMed] [Google Scholar]
- Gallais B., Gagnon C., Mathieu J., Richer L. Cognitive decline over time in adults with myotonic dystrophy type 1: a 9-year longitudinal study. Neuromuscul. Disord. 2017;27:61–72. doi: 10.1016/j.nmd.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Golden C.J. 3rd ed. TEA Ediciones; Madrid: 2001. STROOP. Test de Colores y Palabras. [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N.A., Friston K.J., Frackowiak R.S.J. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Harper P.S. 3rd ed. Saunders; London: 2001. Myotonic Dystrophy. [Google Scholar]
- Jean S., Richer L., Laberge L., Mathieu J. Comparisons of intellectual capacities between mild and classic adult-onset phenotypes of myotonic dystrophy type 1 (DM1) Orphanet J. Rare Dis. 2014;9:1–10. doi: 10.1186/s13023-014-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa M., Tsuruya N., Takeda A., Suzuki A., Kawamura M. Facial emotion recognition and cerebral white matter lesions in myotonic dystrophy type 1. J. Neurol. Sci. 2010;290:48–51. doi: 10.1016/j.jns.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Labayru G., Arenzana I., Aliri J., Zulaica M., López de Munain A., Sistiaga A. Social cognition in Myotonic Dystrophy Type 1 : specific or secondary impairment? PLoS One. 2018;13:1–11. doi: 10.1371/journal.pone.0204227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M., Howieson D.B., Loring D. 4th ed. Oxford University Press; New York: 2004. Neuropsychological Assessment. [Google Scholar]
- López J.M., Rodríguez J.M., Santín C., Torrico E. Utilidad de las formas cortas de la Escala de Inteligencia de Wechsler para Adultos (WAIS) An. Psicol. 2003;19:53–63. [Google Scholar]
- López de Munain A., Blanco A., Emparanza J.I., Poza J.J., Martí-Massó J.F., Cobo A.M., Martorell L., Baiget M., Martínez-Lage J. Prevalence of myotonic dystrophy in Guipúzcoa (Basque Country, Spain) Neurology. 1993;43:1573–1576. doi: 10.1212/wnl.43.8.1573. [DOI] [PubMed] [Google Scholar]
- Martorell L., Monckton D.G., Gamez J., Johnson K.J., Gich I., Lopez De Munain A., Baiget M. Progression of somatic CTG repeat length heterogeneity in the blood cells of myotonic dystrophy patients. Hum. Mol. Genet. 1998;7:307–312. doi: 10.1093/hmg/7.2.307. [DOI] [PubMed] [Google Scholar]
- Mathieu J., Boivin H., Meunier D., Gaudreault M., Bégin P. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology. 2001;56:336–340. doi: 10.1212/WNL.56.3.336. [DOI] [PubMed] [Google Scholar]
- Miller, E.N., 1990. CalCAP: California Computerized Assessment Package.
- Minnerop M., Gliem C., Kornblum C. Current progress in CNS imaging of myotonic dystrophy. Front. Neurol. 2018;9:1–21. doi: 10.3389/fneur.2018.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnerop M., Weber B., Schoene-Bake J.C., Roeske S., Mirbach S., Anspach C., Schneider-Gold C., Betz R.C., Helmstaedter C., Tittgemeyer M., Klockgether T., Kornblum C. The brain in myotonic dystrophy 1 and 2: evidence for a predominant white matter disease. Brain. 2011;134:3527–3543. doi: 10.1093/brain/awr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modoni A., Silvestri G., Vita M.G., Quaranta D., Tonali P.A., Marra C. Cognitive impairment in myotonic dystrophy type 1 (DM1): a longitudinal follow-up study. J. Neurol. 2008;255:1737–1742. doi: 10.1007/s00415-008-0017-5. [DOI] [PubMed] [Google Scholar]
- Okkersen K., Monckton D.G., Le N., Tuladhar A.M., Raaphorst J., van Engelen B.G.M. Brain imaging in myotonic dystrophy type 1. A systematic review. Am. Acad. Neurol. 2017;89:960–969. doi: 10.1212/WNL.0000000000004300. [DOI] [PubMed] [Google Scholar]
- Ota M., Sato N., Ohya Y., Aoki Y., Mizukami K., Mori T., Asada T. Relationship between diffusion tensor imaging and brain morphology in patients with myotonic dystrophy. Neurosci. Lett. 2006;407:234–239. doi: 10.1016/j.neulet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Park J.S., Song H., Jang K.E., Cha H., Lee S.H., Hwang S.K., Park D., Lee H.J., Kim J.Y., Chang Y. Diffusion tensor imaging and voxel-based morphometry reveal corticospinal tract involvement in the motor dysfunction of adult-onset myotonic dystrophy type 1. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-34048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Casanova J., Quiñones-Úbeda S., Gramunt-Fombuena N., Quintana-Aparicio M., Aguilar M., Badenes D., Cerulla N., Molinuevo J.L., Ruiz E., Robles A., Barquero M.S., Antúnez C., Martínez-Parra C., Frank-García A., Fernández M., Alfonso V., Sol J.M., Blesa R. Spanish multicenter normative studies (NEURONORMA Project): norms for verbal fluency tests. Arch. Clin. Neuropsychol. 2009;24:395–411. doi: 10.1093/arclin/acp042. [DOI] [PubMed] [Google Scholar]
- Peric S., Mandic-Stojmenovic G., Stefanova E., Savic-Paviceciv D., Pesovic J., Ilic V., Dobricic V., Basta I., Lavrnic D., Rakocevic-Stojanovic V. Frontostriatal dysexecutive syndrome: a core cognitive feature of myotonic dystrophy type 2. J. Neurol. 2014;262:142–148. doi: 10.1007/s00415-014-7545-y. [DOI] [PubMed] [Google Scholar]
- Peric S., Racoceviz-Stojanovic V., Mandic-Stojmenovic G., Ilic V., Kovacevic M., Parojcic A., Pesovic J., Mijajlovic M., Savic-Pavicevic D., Meola G. Clusters of cognitive impairment among different phenotypes of myotonic dystrophy type 1 and type 2. Neurol. Sci. 2017;38:415–423. doi: 10.1007/s10072-016-2778-4. [DOI] [PubMed] [Google Scholar]
- Raven J.C., Court J.H., Raven J. TEA Ediciones; Madrid: 2001. Raven: Standard Progressive Matrices. [Google Scholar]
- Rey A. TEA Ediciones; Madrid: 2009. Test de copia y de reproducción de memoria de figuras geométricas complejas. [Google Scholar]
- Rubinsztein S., Rubinsztein D.C., Mckenna P.J., Goodburn S., Holland A.J. Mild myotonic dystrophy is associated with memory impairment in the context of normal general intelligence. J. Med. Genet. 1997;34:229–233. doi: 10.1136/jmg.34.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone V., Gandossini S., Cotelli M., Calabria M., Zanetti O., Meola G. Cognitive impairment in adult myotonic dystrophies: a longitudinal study. Neurol. Sci. 2007;28:9–15. doi: 10.1007/s10072-007-0742-z. [DOI] [PubMed] [Google Scholar]
- Schneider-Gold C., Bellenberg B., Prehn C., Krogias C., Schneider R., Klein J., Gold R., Lukas C. Cortical and subcortical grey and white matter atrophy in myotonic dystrophies type 1 and 2 is associated with cognitive impairment, depression and daytime sleepiness. PLoS One. 2015;10:1–20. doi: 10.1371/journal.pone.0130352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L., Petrucci A., Spanò B., Torso M., Olivito G., Lispi L., Costanzi-Porrini S., Giulietti G., Koch G., Giacanelli M., Caltagirone C., Cercignani M., Bozzali M. How genetics affects the brain to produce higher-level dysfunctions in myotonic dystrophy type 1. Funct. Neurol. 2015;30:21–31. doi: 10.11138/FNeur/2015.30.1.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistiaga A., Urreta I., Jodar M., Cobo A.M., Emparanza J., Otaegui D., Poza J.J., Merino J.J., Imaz H., Martí-Massó J.F., López de Munain A. Cognitive/personality pattern and triplet expansion size in adult myotonic dystrophy type 1 (DM1): CTG repeats, cognition and personality in DM1. Psychol. Med. 2010;40:487–495. doi: 10.1017/S0033291709990602. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Zhang Y., Jenkinson M., Chen J., Matthews P.M., Federico A., De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Thornton C.A. Myotonic dystrophy. Neurol. Clin. 2014;32:705–719. doi: 10.1016/j.ncl.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorst M., Okkersen K., Kessels R.P.C., Meijer F.J.A., Monckton D.G., Engelen B.G.M.Van, Tuladhar A.M., Raaphorst J. Structural white matter networks in myotonic dystrophy type 1. NeuroImage Clin. 2019;21 doi: 10.1016/j.nicl.2018.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber Y.G., Roebling R., Kassubek J., Hoffmann S., Rosenbohm A., Wolf M., Steinbach P., Jurkat-Rott K., Walter H., Reske S.N., Lehmann-Horn F., Mottaghy F.M., Lerche H. Comparative analysis of brain structure, metabolism, and cognition in myotonic dystrophy 1 and 2. Neurology. 2010;74:1108–1117. doi: 10.1212/WNL.0b013e3181d8c35f. [DOI] [PubMed] [Google Scholar]
- Wechsler D. TEA Ediciones; Madrid: 1999. WAIS-III: Escala de Inteligencia de Wechsler para Adultos III. [Google Scholar]
- Winblad S., Hellstrom P., Lindberg C., Hansen S. Facial emotion recognition in myotonic dystrophy type 1 correlates with CTG repeat expansion. J. Neurol. Neurosurg. Psychiatry. 2006;77:219–223. doi: 10.1136/jnnp.2005.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad S., Lindberg C., Hansen S. Cognitive deficits and CTG repeat expansion size in classical myotonic dystrophy type 1 (DM1) Behav. brain Funct. 2006;2:16. doi: 10.1186/1744-9081-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad S., Samuelsson L., Lindberg C., Meola G. Cognition in myotonic dystrophy type 1: a 5-year follow-up study. Eur. J. Neurol. 2016;23:1471–1476. doi: 10.1111/ene.13062. [DOI] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Lim K.O., Hemmy L.S., Day J.W. Tractography reveals diffuse white matter abnormalities in Myotonic Dystrophy Type 1. J. Neurol. Sci. 2014;341:73–78. doi: 10.1016/j.jns.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Ward E.E., Lim K.O., Day J.W. White matter abnormalities and neurocognitive correlates in children and adolescents with myotonic dystrophy type 1: A diffusion tensor imaging study. Neuromuscul. Disord. 2011;21:89–96. doi: 10.1016/j.nmd.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.aA, Bell C.J.B., Muetzel R.L., Lim K.O., Day J.W. Diffusion tensor imaging reveals widespread white matter abnormalities in children and adolescents with myotonic dystrophy type 1. J. Neurol. 2013;260:1122–1131. doi: 10.1038/cdd.2010.172.MicroRNAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanigni S., Evangelisti S., Giannoccaro M.P., Oppi F., Poda R., Giorgio A., Testa C., Manners D.N., Avoni P., Gramegna L.L., De Stefano N., Lodi R., Tonon C., Liguori R. Relationship of white and gray matter abnormalities to clinical and genetic features in myotonic dystrophy type 1. NeuroImage Clin. 2016;11:678–685. doi: 10.1016/j.nicl.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.