Highlights
-
•
Interaction of negative affect and hippocampal food-image response predicts BMI gain.
-
•
Interaction of negative affect, vermis and precuneus food response predicts BMI gain.
-
•
Interaction of stress and middle occipital gyrus milkshake response predicts BMI gain.
-
•
Weight gain associated with restrained eating and eating-disorder related behavior.
Keywords: Obesity, Adolescents, Reward, fMRI, Negative affect, Stress
Abstract
Obesity is a major public health concern that is associated with disruption in food reward-related brain function. This study examined if negative affect and stressful events enhance the relation between the food reward-related neural response and future weight gain. Initially healthy weight adolescents (N = 135) completed fMRI paradigms in which they tasted milkshakes and viewed palatable food images, and reported on negative affect and stressful events at baseline; BMI was measured annually over 3-year follow-up. Whole-brain analyses revealed that among participants with higher negative affect, weight gain over 3-year follow-up was predicted by elevated response to appetitive versus unappetitive food images in the left hippocampus, and elevated response in the vermis and the bilateral precuneus to tastes of milkshake versus tasteless solution. Among participants who experienced more stressful events, elevated right middle occipital gyrus response to milkshakes predicted future weight gain. Profiling analyses suggested that participants with higher negative affect or more stressful events who later gained weight reported engaging in more restrained eating and eating disorder-related behaviors. Results suggest that negative affect or stressful events may amplify the relation of neural response to food and the risk for future weight gain.
Obesity is a debilitating health condition that is becoming increasingly common worldwide. There is emerging evidence that obesity is strongly associated with disruptions in food-related reward response. For example, in individuals with obesity, self-report and behavioral data suggest hyper-responsivity to palatable food cues (Stice et al., 2009), and neuroimaging studies suggest hyperresponsivity in corticolimbic-striatal pathways during anticipation of food cues (Martin et al., 2010). Prospective longitudinal studies indicate that future weight gain is predicted by elevated responsivity of reward regions (striatum, amygdala, orbitofrontal cortex) to high-calorie food images (Demos et al., 2012), high-calorie food commercials (Yokum et al., 2014), cues that predict palatable high-calorie food image presentation (Yokum et al., 2011), and cues that signal impending high-calorie food tastes (Stice et al., 2015). These data dovetail with evidence that individuals with obesity who show greater response to high-calorie food images in reward- and attention-related regions exhibit worse response to behavioral weight loss treatment (Murdaugh et al., 2012).
Obesity interventions often target eating behavior, which is influenced by individual differences in reward-related neural circuits that are involved in food-related pleasure (liking) and food-motivated behavior (wanting; Berridge et al., 2010). Unfortunately, few existing obesity prevention (Stice et al., 2006) or behavior weight loss treatments achieve substantial and sustained weight loss (Jeffery et al., 2000). An improved understanding of obesity-related risk factors (Romain, 2018), especially factors that moderate the relationship between food-related reward responses and future weight gain, is critical (Stice, 2016).
Negative affect and stress are two contextual factors that may enhance the relation between food-related reward response and weight gain. High stress predicts future weight gain among adolescents (Ruttle et al., 2013) and adults (Block et al., 2009; Chao et al., 2017; Masih et al., 2017) with relatively small effect sizes (Wardle et al., 2011). Stress may influence eating behavior (Araiza and Lobel, 2018; Sinha, 2018; Sominsky and Spencer, 2014), which can lead to weight gain via poor dietary habits such as stress-eating (Rabasa and Dickson, 2016), preference for high fat and high sugar foods over nutritious foods (Yau and Potenza, 2013), and uncontrolled eating (Hilbert et al., 2011; Sulkowski et al., 2011). For example, high cortisol-reactive women consume more calories in response to stress and negative affect than low cortisol-reactive women (Epel et al., 2001), and animal models demonstrate that restrained rats develop a stress-induced preference for calorically dense lard and sucrose (Pecoraro et al., 2004). Negative affect and stress also disrupt food reward-related response. Negative mood induction increases parahippocampus gyrus and anterior cingulate gyrus response during food anticipation and consumption in female emotional eaters (Bohon et al., 2009), and obese relative to lean individuals report stronger food craving and increased corticolimbic-striatal activation in response to stress cues (Jastreboff et al., 2013). These data provide evidence for a biopsychosocial model in which negative affect and stress increase risk for obesity by enhancing the reward-value of high fat and/or high sugar foods.
Negative affect and stressful life events may be particularly impactful during adolescence, as reward-related brain circuitry continues to develop (Andersen and Teicher, 2008; Davey et al., 2008; Spear, 2013) and an over-emphasis on body weight grows (Voelker et al., 2015). Compared to preadolescents and adults, adolescents exhibit more negative affect (Ge et al., 2006) and experience more stressors (Larson and Ham, 1993), including decline in parent-child relationship quality (Loeber et al., 2000; McGue et al., 2005) and stressful peer interactions (Nelson et al., 2005). Life stress in adolescents predicts reward-related neural responses and pathological symptoms (Casement et al., 2014; Casement et al., 2015). Given the high prevalence of obesity in youth (between 17% and 19%) (Hales, Fryar, Carroll, Freedman, & Ogden, 2018), adolescents are a key population for studies of reward-related neural functioning, negative affect/life stress, and obesity.
1. Present study
Although studies have provided evidence that elevated reward region response to food stimuli increases risk for future weight gain, and that negative affect and stress increase risk for weight gain and disrupted neural response to food stimuli, to our knowledge, no studies have tested the hypothesis that negative affect or stress moderate the relation of neural response to food reward and future weight gain. In the present study, we evaluate whether negative affect or stress enhance the association between reward-related brain functioning and weight gain in adolescents over 3-year follow-up. Participants completed paradigms assessing neural response to palatable food images and palatable food tastes. We hypothesized that higher negative affect and more stressful events at the initial assessment would enhance food reward value, thereby strengthening the association between elevated reward-related neural responses to rewarding foods and later weight gain. We also performed exploratory profile analyses to highlight distinct factors that may characterize participants who gain weight in the context of negative affect or stressful events.
2. Methods
2.1. Participants
Data were derived from a community sample of 135 adolescents [73 female; mean age = 15.01, SD = 0.87; mean baseline body mass index (BMI) = 21.16, SD = 2.25 (a healthy weight range)]. Participants completed a 3-year prospective study investigating eating behaviors, neural indexes, and related health outcomes. The racial and ethnic backgrounds were 9% Hispanic, 3% American Indian/Alaska Native, 6% Asian-American, 12% African-American, 2% Pacific Islander, and 74% European-American. Exclusion criteria included BMI 〈 18 or 〉 25, reported binge eating or compensatory behavior in the past 3 months, and a head injury with loss of consciousness. All participants were free from current use of psychotropic medications or illicit drugs, or Axis I psychiatric disorder in the past year. More than half of the sample's highest parental education achieved was college graduate (mode). This study was approved by the Oregon Research Institute Institutional Review Board. All adolescent participants provided assent and parents provided written informed consent.
2.2. Primary analyses measures
Affect, stress. Measures of negative affect and stressful life events were collected from participants at the initial assessment. The negative affect subscale of the Positive Affect and Negative Affect Scale was used to assess the negative affect felt by the participants during the past week (Watson et al., 1988). The subscale consists of 20 items, and each item is rated on a frequency scale of not at all (1) to extremely (5). Average scores were computed. This scale has high construct validity and reliability (Watson et al., 1988). The internal consistency for the negative affect subscale was 0.94.
A modified 11-item Negative Life Events Scale1 for adolescents was used to assess stressful life events that affected the self or significant others in the past year (Lewinsohn et al., 1994). The 11 items evaluated the incidence of negative life events of different categories: an important possession stolen, hospitalization from an illness or an accident, being physically harmed, being arrested by the police, a car/bike accident, family financial stress, parental divorce, parents living with a new partner, breaking up with a serious dating partner, ending a close friendship, and being rejected from a group. The items were reported on a frequency scale of none (0), once (1) or at least twice (2). Count scores were computed. The internal consistency was 0.52, indicating that the incidence of individual stressful life events is relatively independent from each other.
Body mass index (BMI). The BMI (kg/m2) was used to measure height-adjusted weight. After participants’ removal of shoes and coats, we collected two measures of height (measured to the nearest millimeter) and weight (measured to the nearest 0.1 kg) at each wave of data collection (baseline, and 1-, 2-, and 3-year follow-up; Stice and Yokum, 2018). The average of the two height and weight measurements within each wave was used in analyses. BMI correlates highly with direct measures of total body fat and other health indicators (Dietz & Robinson, 1998; Steinberger et al., 2006). We chose raw BMI scores over age- and sex-adjusted percentiles or BMI z-scores to improve the modeling of within-subject change in longitudinal analyses, following the recommendation of Berkey and Colditz (2007).
Sensory and hedonic measures. Participants sampled a small amount of each milkshake (order counterbalanced) and rated pleasantness and wanting ratings on 20-cm cross modal visual analog scales (VASs) immediately before the MRI scan. Participants also reported hunger and fullness on VASs scales prior to the scan. VAS ratings ranged from not at all (0) to highest level (20). For details on hunger standardization during the scan and milkshake ratings, see Stice et al. (2013).
2.3. Secondary exploratory measures
Block food frequency questionnaire (Block FFQ). Block FFQ measures habitual consumption of 78 types of food (e.g., fruits, beans, and cheese) in the last two weeks from never in last two weeks (1) to daily or more in last two weeks (6) (Block et al., 1990). In addition, because qualitative interviews with adolescents revealed that they often consume foods from fast food restaurants and mini-markets, we added four new items to this scale assessing the number of fast food restaurants and mini-markets within a 15-minute walking distance (e.g., about 1 mile) from participants’ homes and schools.
Food craving inventory. This inventory measures the craving for 28 hard-to-resist, high fat and high carbohydrate foods (e.g., cake, French Fries) (White et al., 2002). It was adapted to also measure liking of the listed foods. Participants reported within the past month the frequency of a craving (an intense desire to consume a particular food) on a scale of never (1) to always (5) and the degree that they like each food type on a scale of dislike (1) to love (4). In this sample, the internal consistency for the craving subscale was 0.92 and liking was 0.83
Dutch Eating Behavior Questionnaire (DEBQ) restraint subscale. The 10-item restraint subscale from DEBQ is a questionnaire that measures the concern about dieting and weight from never (1) to always (5) (van Strien et al., 1986). It has good internal consistency (α = 0.95), 2-week test–retest reliability (r = 0.82), convergent validity with self-reported (but not objectively measured) caloric intake, predictive validity for bulimic symptoms, and sensitivity to intervention effects (Stice et al., 2008; van Strien et al., 1986). In this sample, the internal consistency was 0.88.
The Eating Disorder Diagnostic Interview. A semi-structured interview was conducted to assess DSM-IV eating disorders including anorexia nervosa, bulimia nervosa, and binge eating disorder. The interview also measures global eating disorder symptoms, binge eating frequency, and weight loss behaviors, for each month in the past year. It has high internal consistency (α = 0.86), 1-week test-retest reliability (r = 0.81), inter-rater agreement (κ = 0.86), and predictive validity (Stice et al., 2008).
2.4. Neuroimaging tasks, acquisition parameters, preprocessing, and analysis
fMRI food picture paradigm. A food picture paradigm was adopted to test neural responses to imagined consumption in response to pictures of appetizing foods, unappetizing foods, and glasses of water. Food pictures were individualized based on the participants’ previous appetizing ratings [see earlier papers for details on this paradigm (Shearrer et al., 2018; Stice and Yokum, 2018)]. During the task, each picture was presented for five seconds and participants were instructed to imagine tasting and eating the pictured food. The order of the events was randomized and separated by a fixation cross with jittered duration between two and four seconds. Each image type was presented for 32 trials. This paradigm elicits robust food reward-related neural response (Shearrer et al., 2018; Stice and Yokum, 2018).
fMRI food receipt paradigm. A food receipt paradigm (Supplementary Materials Fig. 1) was used to examine the differential impact from sugar and fat in recruiting reward circuitry (Stice et al., 2013). Stimuli included four types of chocolate milkshakes and a tasteless solution. Each type of milkshake consisted of the same ice cream base and chocolate syrup without fat substitutes/thickeners or artificial sweeteners. With varied milkfat and syrup components, four combinations of chocolate milkshakes of distinguishable fat and sweetness levels were created and delivered to participants through a tube during the task: high fat, high sugar (HFHS); high fat, low sugar (HFLS); low fat, high sugar (LFHS); and low fat, low sugar (LFLS). An image of a milkshake preceded the delivery of each milkshake, and an image of water preceded the delivery of tasteless solution. All milkshakes were preceded by the same image of a milkshake to not confound the neural response to tastes with expectations. The delivery of milkshake or tasteless solution occurred in unpredictable blocks with varied duration (one block presented four, five, or seven events in each of the two runs). An event was considered when a tastant was delivered over five seconds. Two runs (13 min each) were performed with each run randomly presenting three blocks of each of the four milkshake types and the tasteless solution. This food reward paradigm consistently elicits response in brain regions implicated in reward (dorsal striatum), attention (anterior cingulate cortex), and gustatory processing (insula, Rolandic operculum; Stice et al., 2013).
fMRI data acquisition. BOLD echo-planar images were acquired on a Siemens Tim Trio 3 MRI scanner. Functional scans were collected using a T2*-weighted gradient echo sequence (TE = 30 ms, TR = 2000 ms, flip angle = 80°) with an in-plane resolution of 3.0 × 3.0 mm (64 × 64 matrix; 192 × 192 mm field of view) that included 32, 4 mm slices (interleaved acquisition, no skip) along the AC-PC transverse, oblique plane (Stice and Yokum, 2018). Structural scans were collected using an inversion recovery T1-weighted sequence (MP-RAGE) in the same orientation. High-resolution structural MRI scans were performed for coregistration and normalization of functional imaging data (FOV = 256 × 256 mm2, 256 × 256 matrix, thickness = 1.0 mm, slice number ≈ 160). Prospective acquisition correction (PACE) was used during scanning to correct for slice position and orientation, as well as to re-grid residual volume-to-volume motion during data acquisition for the purpose of reducing motion-induced effects (Thesen et al., 2000). fMRI data of three participants were discarded due to an acquisition error and two were unusable, leaving a total sample size of 130.
fMRI data preprocessing. DICOM images were converted to NIfTI format and realigned to the AC-PC line in SPM (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Skull-stripped images were created with the Brain Extraction Tool in FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET/UserGuide). Functional data preprocessing included: (1) slice timing correction; (2) adjustment for variation in magnetic field distortion using field maps (Poldrack et al., 2011); (3) realignment to the mean functional image; (4) coregistration to the anatomical image; (5) normalization to Montreal Neurological Institute (MNI) space using a sample-specific DARTEL template and individual-level deformation fields, which allows more precise alignment (Klein et al., 2009); and (6) smoothing using a Gaussian filter set to 6 mm full-width-at-half-maximum (FWHM). Artifact Detection Toolbox (ART; https://www.nitrc.org/projects/artifact_detect) was used to detect global mean response spikes and motion outliers. Motion parameters were used as regressors, and extreme outlier image volumes were de-weighted during individual-level model estimation. Neural response to milkshake intake was estimated using four separate contrasts of BOLD signal during the delivery of milkshake (three trials of each milkshake type in each run) against tasteless solution (three trials in each run). Neural response related to viewing palatable food images was estimated using contrasts of BOLD signal during viewing appetizing versus unappetizing foods or pictures of water.
2.5. Statistical analysis
Hypothesis tests. BMI gain was estimated with random intercept, mixed effects growth curve analyses of BMI from baseline, and 1-, 2-, and 3-year follow-ups [see Stice and Yokum (2018) for details on the model fitting]. Upon examining BMI data over the study course, one participant's BMI slope (weight gain) was 2.51, which was four SD (0.49) higher than the rest of the sample. This extreme outlier was excluded for data analysis.2
Regression analyses were conducted in SPM to evaluate whether stressful event count or negative affect moderate the relation between the food-related BOLD response and future BMI gain. Individual contrasts of BOLD response during four types of milkshake > tasteless solution, and appetizing > unappetizing food images, were entered into second-level regression models. Each milkshake type was evaluated separately to maintain the same number of trials for milkshake versus tasteless solution. In the second-level regression models, the following covariates were added: initial BMI (BMI intercept), change in BMI during follow-up (BMI slope), one of the two moderators (negative affect scores and stressful event scores), the cross-product terms between BMI slopes and one of the two moderators, and hunger (a covariate of no interest). BMI slopes, BMI intercepts and moderators were mean-centered. We chose whole-brain analyses over region of interest analyses because: (a) there is limited prior research on similar moderation analyses, (b) our study has a relatively large sample size, and (c) effective whole-brain error control methods exist (Poldrack, 2007). We thus applied the binarized DARTEL-derived sample-specific gray matter mask to the whole-brain (Stice and Yokum, 2018). To correct for multiple comparisons, we first estimated the intrinsic smoothness of the masked functional data with the spatial autocorrelation function (acf) option in the 3D FWHM module in AFNI (Version AFNI_17.0.03; https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dFWHMx.html). We then applied the acf parameters to 10,000 Monte Carlo simulations of random noise at 3 mm3 through the gray matter masked data with the 3DClustSim module of AFNI. Simulation results indicated that neural activity that survived multiple comparison corrections needed to meet a threshold of p < .001 with a cluster size of k ≥ 32 (Stice and Yokum, 2018). Within R (version 3.4.3; R Core Team, 2018), the ggplot2 package (version 3.2.1; Wickham, 2016) was used for data visualization.
Imputation of missing data to assess reliability. The same hypothesis tests were then conducted with multiply imputed datasets to mitigate the impact from missing questionnaire values. We started collecting data on negative affect and stressful events after recruitment began, resulting in missing data for these two measures (negative affect and stressful events data were unavailable for 22 participants; two participants missed only stressful events data; 18% of the sample missed data from either scale). Given the assumption that the negative affect and stressful events values were missing at random, multiple imputation was used to substitute missing values (Sinharay et al., 2001; Sterne et al., 2009). Multiple imputation is more desirable than single imputation because the incomplete consideration of uncertainty about the missing data in the latter approach can result in bias with underestimated standard errors (Sterne et al., 2009). The mice package (version 3.3.0; van Buuren and Groothuis-Oudshoorn, 2011) was used to complete multiple imputation with 10 iterations for the missing data. A complete set of relevant key variables [age, sex, baseline BMI (intercept), BMI change from baseline through follow-up (slope), food liking and craving scores, and restrained eating scores] were included as reference variables during imputation. Including variables that correlate highly with missing data improves the accuracy of the imputed datasets (van Buuren et al., 1999).
In each iteration, 22 missing values for negative affect, and 24 for stressful events, were computed. Normalizing transformations were used because of high positive skewness in negative affect and stressful events data. In multiple imputation, after each imputed dataset is analyzed with statistical tests, Rubin's rules suggest that individual estimates and standard errors are combined to test for statistical significance (Rubin, 2004). In our case, given that the multiple imputed datasets were used to detect significant activations in neural regions, we present a ratio metric for the 10 iterations that is akin to the practice of bootstrapping. This was intended to evaluate the reproducibility of fMRI findings (Stice and Yokum, 2018).
Exploratory analyses. Descriptive profiles were conducted with the self-report measures to highlight distinct features in the participants who experienced negative affect or stressful events and later gained weight. This approach is intended to use external (behavioral) data to assist in the interpretation of BOLD peaks (Smeets et al., 2019).
3. Results
3.1. Descriptive and bivariate correlation results
Negative affect (M = 1.51, SD = 0.52, n = 108) and stressful events (M = 2.58, SD = 2.22, n = 106) were positively correlated in this sample, r(104) = 0.28, p = .003. In addition, participants with higher baseline BMI tended to have greater increases in weight during the 3-year follow-up period, r(128) = 0.24, p = .006. Negative affect positively correlated with BMI gain, r(106) = 0.28, p = .004, whereas stressful events had no significant association with BMI gain, r(104) = 0.14, p = .140. Table 1 in Supplementary Materials provides further descriptive results of the self-report measures at baseline in this sample.
3.2. Primary hypothesis analyses
3.2.1. Baseline negative affect as a moderator of the relation of reward-related brain function and future weight gain
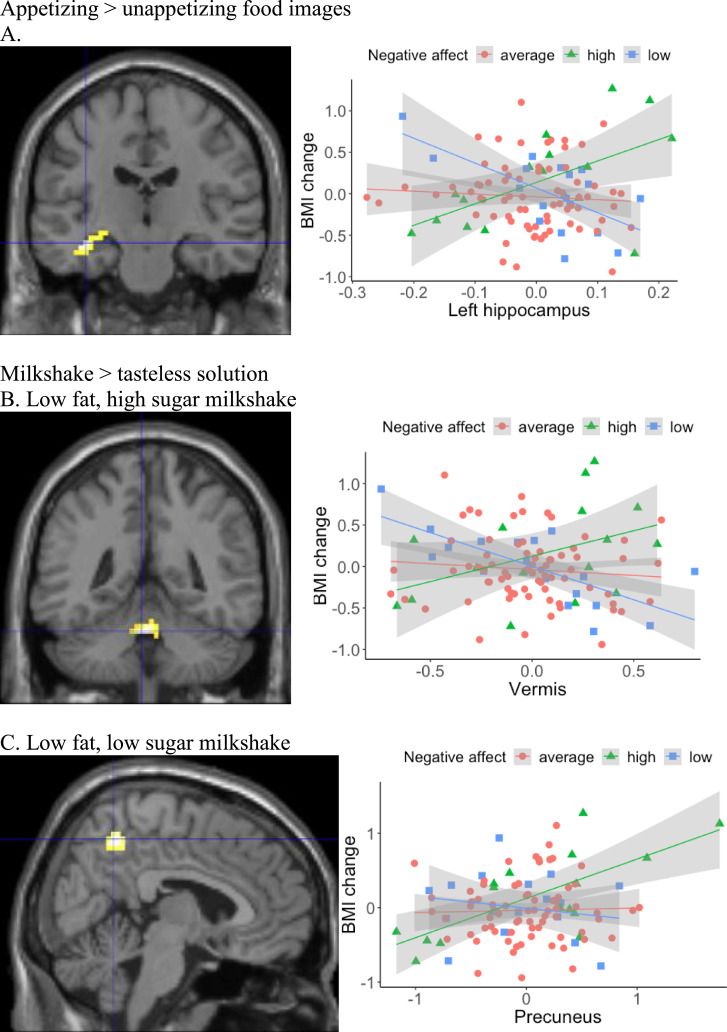
Appetizing > unappetizing food images. Baseline negative affect positively moderated the relation between BOLD response to appetizing food images in the left hippocampus and future BMI gain in the unimputed data (Table 1; Fig. 1A; the same region was then detected 4/10 times in the multiply imputed data sets). Simple slope analyses with the raw eigenvalues extracted from SPM for the neural region were conducted and the graph of BOLD response by BMI change for participants with low (1 SD below the mean; n = 18), average (n = 73), and high negative affect (1 SD above the mean; n = 17) indicated that when participants with high negative affect were instructed to imagine eating the appetizing food, higher BOLD response in left hippocampus was linked with higher future weight gain. The direction of association was reversed in participants with low negative affect: lower BOLD response in left hippocampus during the presentation of appetizing food images was associated with higher future weight gain. No relation between hippocampal BOLD response and BMI gain was detected in participants with average negative affect.
Table 1.
Baseline negative affect and stressful events as moderators of the relationship between food reward-related BOLD responses and BMI changes over three years.
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Moderator/Condition | Regions | x | Y | z | k | t |
| Negative affect | df = 102 | |||||
| Appetizing > unappetizing food images | left-hippocampus (BA20) | −36 | −18 | −15 | 44 | 5.03 |
| LFHS milkshake > tasteless solution | vermis | −3 | −45 | −27 | 94 | 5.58 |
| LFLS milkshake > tasteless solution | bilateral-precuneus | 6 | −54 | 51 | 43 | 4.42 |
| Stressful events | df = 100 | |||||
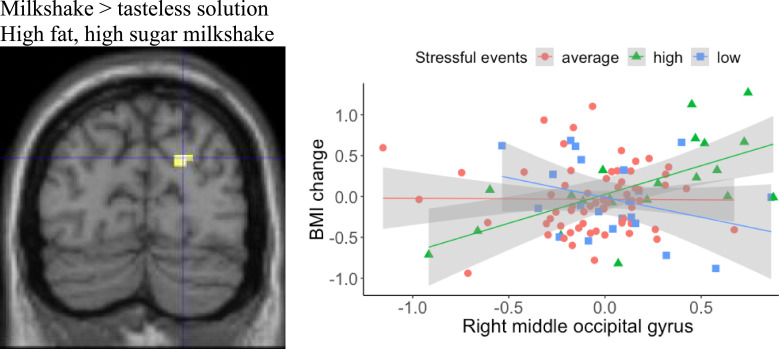
| HFHS milkshake > tasteless solution | right-middle occipital gyrus (BA19) | 21 | −78 | 30 | 38 | 4.10 |
Note. k means the cluster size for contiguous voxels. LFHS means low fat high sugar; LFLS means low fat low sugar; HFHS means high fat high sugar. To correct for multiple comparisons across the brain and keep the overall significance level < 0.05, peaks within all the regions (k ≥ 32) reached p < .001 uncorrected.
Fig. 1.
Negative affect moderated the association between food-related BOLD response and future BMI gain. fMRI images are presented for the significant neural regions: (A) left hippocampus (MNI coordinates: −36, −18, −15) in the food images condition, (B) vermis (MNI coordinates: 3, −45, −27) and (C) bilateral-precuneus (MNI coordinates: 6, −54, 51) in the milkshake conditions. Scatterplots present the data points of the average BOLD response in respective region (x-axis) and BMI change (y-axis). A linear regression line with 95% confidence interval is presented for average, high (1 SD above the mean) and low (1 SD below the mean) levels of negative affect.
Milkshake > tasteless solution. Baseline negative affect positively moderated the relation between BOLD response to LFHS and LFLS milkshakes and future BMI gain. Simple slope analyses with the raw eigenvalues extracted from SPM for the neural region were conducted and graphs of BOLD response by BMI change indicated that among participants with high negative affect, vermis activation to LFHS milkshake positively correlated with BMI gain in the unimputed data (Table 1; Fig. 1B; the same region was then detected 10/10 times in the imputed data sets). In the LFLS milkshake contrast, participants with high negative affect and high precuneus activation to the milkshake tended to have greater increases in BMI in the unimputed data (Table 1; Fig. 1C; the same region was detected 6/10 times). In comparison, this relation was reversed in participants with low negative affect, and no relation between BOLD response and BMI gain was present in participants with average negative affect.
3.2.2. Baseline stressful events as a moderator of reward-related brain function and future weight gain
Appetizing > unappetizing food images. No significant regional activation was moderated by stressful events in the food image paradigm.
Milkshake > tasteless solution. Stressful events positively moderated the association between BOLD response to HFHS milkshake solution in right middle occipital gyrus and BMI gain. Simple slope analyses with the raw eigenvalues extracted from SPM for the neural region were conducted, and graphs of BOLD response by BMI change for participants with low (n = 21), average (n = 64), and high (n = 21) stressful events indicated that participants with high stressful events showed a positive relation between right middle occipital gyrus activation and BMI gain in the unimputed data (Table 1; Fig. 2; the same region was then detected 4/10 times in the imputed datasets). The direction was reversed in participants with fewer stressful events: lower BOLD response in right middle occipital gyrus during the milkshake receipt was associated with higher weight gain. No relation between BOLD response during milkshake receipt and BMI gain was detected in participants with average stressful events.
Fig. 2.
Stressful events moderated the association between food-related BOLD response and future BMI gain. An fMRI image is presented for the right middle occipital gyrus (MNI coordinates: 21, −78, 30) in the milkshake condition. A scatterplot presents the data points of the average BOLD response in the right middle occipital (x-axis) and BMI change (y-axis). A linear regression line with 95% confidence interval is presented for average, high (1 SD above the mean) and low (1 SD below the mean) levels of stressful events.
3.3. Secondary exploratory analyses
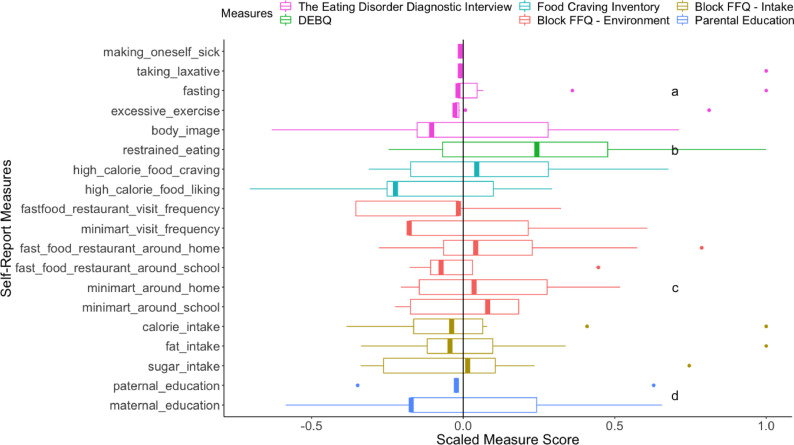
The profile of the participants with high baseline negative affect and BMI gain. Key self-report measures were compared across four groups: high negative affect (1 SD above the mean) and stable BMI (BMI gain < 0; n = 9; nmale = 3), high negative affect (1 SD above the mean) and BMI gain (BMI gain > 0; n = 10; nmale = 2), low to average negative affect and stable BMI (n = 51; nmale = 25), and low to average negative affect and BMI gain (n = 38; nmale = 22); Fig. 3. Notable distinct features of the high negative affect and BMI gain group included: (a) more eating-disorder related behaviors (taking laxative, fasting and excessive exercise) and relatively high importance attached to body weight for self-evaluation, (b) higher self-reported endorsement of eating restraint, (c) in general more access to unhealthy food, and (d) low maternal education level. Fig. 2 in Supplementary Materials presents the breakdown of measure means for all groups.
Fig. 3.
Boxplots of self-report measures from the participants of high baseline negative affect and BMI gain. The scores of each measure are standardized across the whole sample and divided by the largest absolute value to limit the range to −1 and 1. A vertical black line at the grand mean (z = 0) is provided for comparison. The median of each measure from the participants of high baseline negative affect and BMI gain is highlighted with a thick band. A median to the right of the black line suggests higher value among the participants of high baseline negative affect and BMI gain for a measure, and vice versa. The lower and upper hinges show the first and third quartiles. The upper/lower whisker extends from the hinge to the largest/smallest value within 1.5 x inter-quartile range of the hinge. Data points outside the range are outliers. The Eating Disorder Diagnostic Interview included items measuring different self-reported maladaptive weight management behaviors: making oneself sick, taking laxative, fasting, and excessive exercise. Body image is a question on how weight influences one's self-evaluation, ranging from no importance (a raw score of 0) to supreme importance (a raw score of 6). The number of binge eating episodes was not reported due to extremely low occurrence in this sample. Restrained_eating refers to the restrained eating subscale from DEBQ. Food Craving Inventory included two subscales: craving and liking for high calorie food. Block FFQ – Environment included frequency of visiting fast food restaurants and minimarts, numbers of fast food restaurants and minimarts around the home and the school. Block FFQ – Intake refers to the self-reported weekly calorie, fat and sugar intake. Paternal_education and maternal_education refer to paternal and maternal education levels. Notation “a” highlights the outlier data points detected for eating-disorder related behaviors (taking laxative, fasting and excessive exercise) and relatively high importance attached to body weight for self-evaluation. Notation “b” highlights higher self-reported endorsement of eating restraint. Notation “c” highlights in general more access to unhealthy food. Notation “d” highlights low maternal education levels.
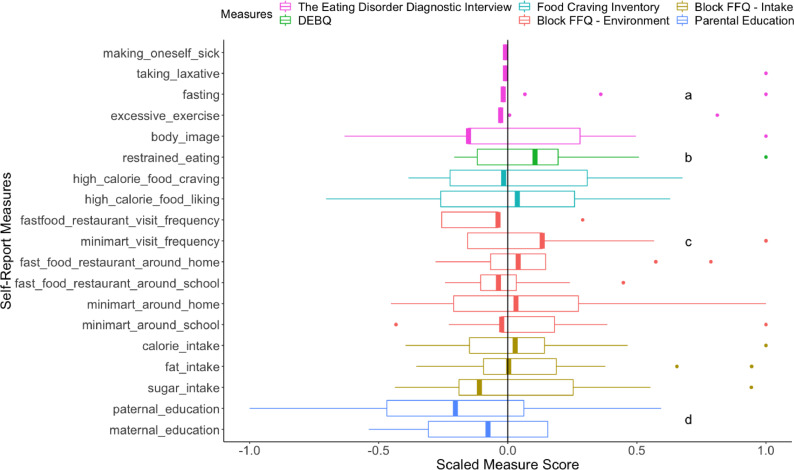
The profile of the participants with a high number of baseline stressful events and BMI gain. Key behavioral correlates were computed for four groups: high stressful event scores (1 SD above the mean) and stable BMI (BMI gain < 0; n = 8; nmale = 5), high stressful event scores (1 SD above the mean) and BMI gain (BMI gain > 0; n = 13; nmale = 5), low to average scores of stressful events and stable BMI (n = 51; nmale = 23), and low to average scores of stressful events and BMI gain (n = 34; nmale = 18); Fig. 4. Characteristics among the high stressful event and BMI gain group highly resembled those in the high negative affect and BMI gain group: (a) more eating-disorder related behaviors (taking laxative, fasting and excessive exercise) and relatively high importance attached to body weight for self-evaluation, (b) higher self-reported endorsement of eating restraint, (c) in general more access to unhealthy food, and (d) low parental education levels. Fig. 3 in Supplementary Materials presents the breakdown of measure means for all groups.
Fig. 4.
Boxplots of self-report measures from the participants of high baseline stressful events and BMI gain. The scores of each measure are standardized across the whole sample and divided by the largest absolute value to limit the range to −1 and 1. A vertical black line at the grand mean (z = 0) is provided for comparison. Median of each measure from the participants of high baseline stressful events and BMI gain is highlighted with a thick band. A median to the right of the black line suggests higher value among the participants of high baseline stressful events and BMI gain for a measure and vice versa. The lower and upper hinges show the first and third quartiles. The upper/lower whisker extends from the hinge to the largest/smallest value within 1.5 x inter-quartile range of the hinge. Data points outside the range are outliers. The Eating Disorder Diagnostic Interview included items measuring different self-reported maladaptive weight management behaviors: making oneself sick, taking laxative, fasting, and excessive exercise. Body_image is a question on how weight influences one's self-evaluation, ranging from no importance (a raw score of 0) to supreme importance (a raw score of 6). The number of binge eating episodes was not reported due to extremely low occurrence in this sample. Restrained_eating refers to the restrained eating subscale from DEBQ. Food Craving Inventory included two subscales: craving and liking for high calorie food. Block FFQ – Environment included frequency of visiting fast food restaurants and minimarts, numbers of fast food restaurants and minimarts around the home and the school. Block FFQ – Intake refers to the self-reported weekly calorie, fat and sugar intake. Paternal_education and maternal_education refer to paternal and maternal education levels. Notation “a” highlights the outlier data points detected for eating-disorder related behaviors (taking laxative, fasting and excessive exercise) and relatively high importance attached to body weight for self-evaluation. Notation “b” highlights higher self-reported endorsement of eating restraint. Notation “c” highlights in general more access to unhealthy food. Notation “d” highlights low parental education levels.
4. Discussion
This study examined the effects of baseline negative affect and stressful events on the relation between adolescents’ baseline neural activation towards food images and tastants and future weight gain over 3-year follow-up. We did not find strong evidence to support the original hypothesis – higher negative affect and more stressful events at the initial assessment did not enhance the association between food-related response in neural regions that are typically implicated in reward processing and weight gain. However, in participants who later gained weight, high baseline negative affect or more stressful events were associated with elevated activation in four neural regions: hippocampus, precuneus, middle occipital gyrus, and vermis. The hippocampus (Knierim, 2015; Maguire et al., 2016; Stoeckel et al., 2008) and precuneus (Cavanna and Trimble, 2006) are both involved in episodic memory formation, and the hippocampus, precuneus, and middle occipital gyrus are implicated in mental imagery (Addis et al., 2007). The vermis is involved in coordination of motor behavior, including eating behavior (Amianto et al., 2013). The results of this study suggest that neural response to appetitive foods varies as a function of negative affect or stressful events in regions that are associated with memory, mental imagery, and eating behavior.
4.1. Learned associations between negative affect or stress and food
One interpretation of these results is that negative affect and stressful events may serve as food cues to consume preferred food in participants who later gained weight. Participants with high negative affect or stressful events who gained weight may have stronger mental visual imagery in response to food cues (Tiggemann and Kemps, 2005) at baseline. The construction of these mental images may be represented by elevated activation in the hippocampus, precuneus (Ganis et al., 2004; Zeidman et al., 2015), occipital gyrus (Giuliani et al., 2014; Zeidman et al., 2015), and cerebellar vermis (Ganis et al., 2004). Elevated activation in the vermis during appetitive tastes may also correspond to eating behavior-related motor preparedness (Yakusheva et al., 2007). The association between negative affect and neural response in regions that are involved in food-related memory, imagery, and motor preparedness may be particularly likely in adolescents who have paired negative affect with consuming high-calorie foods.
4.2. Food craving and maladaptive eating beliefs and behaviors
Elevated hippocampal response to palatable food images and elevated precuneus, middle occipital gyrus, and vermis response to palatable food tastes in individuals with high negative affect or stressful events who later gained weight may also reflect strong baseline food craving in those participants. Hippocampus activation is implicated in animal (Crombag et al., 2008) and human (Tang et al., 2012; Wang et al., 2007) drug craving, and craving for liked and familiar food (e.g., chocolate) may be similar to drug-related cravings (Tuomisto et al., 1999). Hippocampus activation is also consistently found in human imaging studies that contrast states of hunger with satiation (LaBar et al., 2001; Paolini et al., 2014; Pelchat et al., 2004; Stevenson and Francis, 2017). The left lateralization of hippocampus activation could be because the left hippocampus is more active given its higher involvement with the speech function (left lateralization in 97% of the right-handers and 75% of the left-handers; Goldstein, 2010) and the default mode network (Ushakov et al., 2016). There is also evidence implicating precuneus responsivity in cue-reactivity (Kroemer et al., 2013) and craving (Li et al., 2018), and the vermis belongs to a bottom-up appetitive network which drives motivation in feeding behavior for appetitive food (Brooks et al., 2012; Zhu and Wang, 2008).
Food craving alone may not lead to subsequent weight gain, as the profile analyses failed to show large food craving differences between the individuals who gained weight relative to those who did not. We speculate that it is the combination of food craving and maladaptive food-related beliefs (e.g., restrained eating) and behaviors (e.g., compensatory exercises, fasting, taking laxatives) that may eventually lead to weight gain. The profile analyses revealed that, in contrast to adolescents with stable weight, at baseline the participants with high negative affect or stressful events and later weight gain reported more restrained eating, more disordered eating behaviors including fasting and taking laxatives to control weight, and comparable frequency of visits to fast food restaurants and mini-marts despite in general easier access to unhealthy food. Access to healthy and unhealthy food may have a profound impact on the human appetitive system (Hoyt et al., 2014) whereby immediately available high calorie foods are particularly rewarding (Blechert et al., 2016). In the context of comparable frequency of visits to fast food restaurants and minimarts despite high availability, more restrained eating may reflect more strenuous effort to avoid high fat/sugar foods in spite of high food craving. Consistent with this interpretation, in lean undergraduates, restrained eating is a risk factor for binge-eating (Coffino et al., 2016), and restrained eating is often associated with weight gain over time (Schur et al., 2010). Weight gain in restrained eaters may be due to mood fluctuations disrupting dieting behaviors (Neimeijer et al., 2017; Polivy et al., 1994) and higher neurophysiological sensitivity to the reward value of highly palatable food (Burger and Stice, 2011; Gibson, 2006). In individuals with limited material and cognitive resources to cope with stress, experiencing higher negative affect or more stressful events may make energy-dense comforting food more desirable as an emotion-regulation tool. This interpretation is also supported by the observation in human research that highly palatable food may provide stress relief (Gibson, 2006) and in animal studies that restrained rats eat more calorically dense lard and sucrose (Pecoraro et al., 2004) and experience consequent reductions in stress reactivity (Foster et al., 2009).
4.3. Strengths and limitations
The focus on adolescents in this study aligns with the idea that adolescence is a convergent period of reward-related neural development, higher negative affect and stress, and elevated risk for obesity and eating disorders. Additional strengths of this study include the relatively large sample size, three-years of follow-ups, the use of standard fMRI experimental paradigms, and a new reporting metric for multiply imputed datasets in fMRI analyses. Although the missing data from the delayed collection of negative affect and stressful events measures is a weakness, the application of multiple imputation to mitigate this weakness is a strength. The metric approach provides insight into the reliability and statistical power of regional neural activation findings. Given 10 batches of imputed data, the ratio of detecting a significant hyperactivation varied across different brain regions (between 4/10 and 10/10). With higher number of detections, the effect size may be larger, and the finding may be more replicable. One advantage in this method compared to split-half analyses is the retainment of the large sample size, thus higher power for detecting existing moderation effects.
There are also limitations to the study and caveats to our interpretation of these findings. One challenge to our interpretations is that neural hyperresponsivity to food tastants was inconsistent across milkshake conditions (with a slight preference for more added sugar). This inconsistency weakens the arguments for functional associations among the regions involved in food-related memory, mental imagery, or food craving. The inconsistent interactions of negative affect or stress with BOLD response during various milkshake conditions may reflect different sensitivity to foods with varied fat and sugar combinations in healthy-weight participants with high stress or high negative affect. Among lean adolescents, fat and sugar have demonstrated differential effects on reward and gustatory regions, with sugar eliciting stronger activations (Stice et al., 2013). We speculate that sensitivity to fat and sugar in the context of stressors or negative affect may differ in individuals with healthy weight compared to individuals with overweight or obesity. The inclusion of adolescent participants who were initially of healthy weight, as well as the relatively small weight fluctuation over the course of the study, limit our ability to understand how neural activation and cognitive/behavioral patterns among healthy-weight participants may generalize to overweight and obese samples or samples with more pronounced weight gain. Relatedly, the samples from previous studies were cross-sectional (Bohon et al., 2009; Jastreboff et al., 2013) and composed of overweight individuals and individuals with obesity (Jastreboff et al., 2013) or emotional eating (Bohon et al., 2009). Negative affect and/or stressful life events may be lower in the present study of initially healthy weight participants compared to other samples. The small effect size may underlie the reasons that, despite support from animal models that chronic stress enhances the reward value of highly palatable food (Adam and Epel, 2007), we did not uncover significant activation in other conventional food-related neural regions [e.g., corticolimbicstriatal circuity (Small et al., 2003)]. Further evaluation of the current relations in a non-adolescent sample and/or a sample with larger weight gain during follow-up is needed.
In addition, despite our effort to characterize participants who experienced increased food-related responsivity and weight gain with high baseline negative affect or stressful events, self-report questionnaires may be susceptible to reporting bias. It is also noted that negative affect was evaluated based on the past week, whereas stressful life events were reported considering the past year. The negative affect scale has high temporal stability across measurement time frames ranging from present-moment up to past-year (Watson et al., 1988). Therefore, negative affect measured over the past week should correlate highly with negative affect measured over longer time intervals. However, it is possible that negative affect measured over the past year may reflect accumulation of stress over time (Watson et al., 1988), and hence may be a stronger moderator of food reward-related brain function and future weight gain than negative affect measured over the past week.
5. Conclusion
The findings from this study indicate that high negative affect or more stressful events may amplify the relations of heightened neural response during food anticipation and receipt to future weight gain in healthy-weight adolescents. Specifically, hyperactivation in hippocampus, precuneus, middle occipital gyrus and vermis may represent learned associations between negative affect and appetitive food, and stronger mental imagery and/or food craving in the context of negative affect or stressful events. The association between food-related neural response and BMI gain in adolescents with high negative affect or more stressful events was related to reported behavior patterns of restrained eating and engaging in maladaptive weight management. The results enrich our understanding of the brain-body basis of eating behaviors in the context of negative affect or stress.
Negative affect and stressful events as risk factors in healthy-weight adolescents provide additional clinical insights. The associations between negative affect or stressful events and visual food stimuli and tastants identified in this study suggest that negative affect and stressful events may be related to restrained eating, disordered eating, and paradoxical weight gain in adolescents with high food craving and easy access to high calorie foods. Future research could shed light on the directionality of the effects and evaluate the temporal consistency of the neural findings in this study by assessing longitudinal associations between negative affect and stressful events, neural response to food reward-related stimuli, and weight gain using cross-lagged models. If these results are supported in future research, clinical interventions for obesity may be improved by targeting youth with high negative affect and more stressful events to provide motivational or cognitive-behavioral interventions that address learned associations between stress, food stimuli, and maladaptive food-related beliefs and behaviors (e.g., eating restraint, disordered eating) among adolescents.
Declaration of Competing Interest
None.
Acknowledgments
Support for this work was provided by National Institutes of Health (R01-DK092468 to E.S. and S.Y.). Salary support for Dr. Casement was provided by the National Institute of Mental Health (K01-MH103511).
Footnotes
Modifications to the original 14-item scale were made to decrease redundancy, increase age-appropriateness and gender neutrality. Five items from the original scale were omitted: “Did you experience academic failure”, “Did you have an abortion, miscarriage, or stillbirth”, “Was your home damaged by fire, flood, or other disaster”, “Did you move or change residence”, and “Were you a victim of crime, violence, or assault”. One item from the original scale was changed from “Did you lose a close friend” into “Did one or more of your friends end a close relationship”. Two items were added to the original scale: “Did a serious dating partner break-up with you”, and “Were you rejected from a group that you wanted to join”.
For completeness, this outlier's data was included in sensitivity analyses using unimputed data. The results from this analysis were broadly consistent with the primary hypothesis tests.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102067.
Contributor Information
X. Yang, Email: xiy@uoregon.edu.
E. Stice, Email: Estice@stanford.edu.
Appendix. Supplementary materials
References
- Adam T.C., Epel E.S. Stress, eating and the reward system. Physiol. Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Addis D.R., Wong A.T., Schacter D.L. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amianto F., D’Agata F., Lavagnino L., Caroppo P., Abbate-Daga G., Righi D., Fassino S. Intrinsic connectivity networks within cerebellum and beyond in eating disorders. Cerebellum. 2013;12(5):623–631. doi: 10.1007/s12311-013-0471-1. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Araiza A.M., Lobel M. Stress and eating: definitions, findings, explanations, and implications. Soc. Personal. Psychol. Compass. 2018 No Pagination Specified-No Pagination Specified. [Google Scholar]
- Berridge K.C., Ho C.-Y., Richard J.M., DiFeliceantonio A.G. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J., Klackl J., Miedl S.F., Wilhelm F.H. To eat or not to eat: effects of food availability on reward system activity during food picture viewing. Appetite. 2016;99:254–261. doi: 10.1016/j.appet.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Block G., Hartman A.M., Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- Block J.P., He Y., Zaslavsky A.M., Ding L., Ayanian J.Z. Psychosocial stress and change in weight among US adults. Am. J. Epidemiol. 2009;170(2):181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C., Stice E., Spoor S. Female emotional eaters show abnormalities in consummatory and anticipatory food reward: a functional magnetic resonance imaging study. Int. J. Eating Disorders. 2009;42(3):210–221. doi: 10.1002/eat.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.J., O’Daly O., Uher R., Friederich H.-C., Giampietro V., Brammer M., Campbell I.C. Thinking about eating food activates visual cortex with reduced bilateral cerebellar activation in females with anorexia nervosa: an fMRI study. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0034000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger K.S., Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 2011;55(1):233–239. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casement M.D., Guyer A.E., Hipwell A.E., McAloon R.L., Hoffmann A.M., Keenan K.E., Forbes E.E. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Dev. Cogn. Neurosci. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casement M.D., Shaw D.S., Sitnick S.L., Musselman S.C., Forbes E.E. Life stress in adolescence predicts early adult reward-related brain function and alcohol dependence. Soc. Cogn. Affect. Neurosci. 2015;10(3):416–423. doi: 10.1093/scan/nsu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain J. Neurol. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chao A.M., Jastreboff A.M., White M.A., Grilo C.M., Sinha R. Stress, cortisol, and other appetite-related hormones: prospective prediction of 6-month changes in food cravings and weight. Obesity. 2017;25(4):713–720. doi: 10.1002/oby.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino J.A., Orloff N.C., Hormes J.M. Dietary restraint partially mediates the relationship between impulsivity and binge eating only in lean individuals: the importance of accounting for body mass in studies of restraint. Front. Psychol. 2016;7 doi: 10.3389/fpsyg.2016.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H.S., Bossert J.M., Koya E., Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G., Yücel M., Allen N.B. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci. Biobehav. Rev. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Demos K.E., Heatherton T.F., Kelley W.M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012;32(16):5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E., Lapidus R., McEwen B., Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Foster M.T., Warne J.P., Ginsberg A.B., Horneman H.F., Pecoraro N.C., Akana S.F., Dallman M.F. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150(5):2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganis G., Thompson W.L., Kosslyn S.M. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Cognit. Brain Res. 2004;20(2):226–241. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Ge X., Natsuaki M.N., Conger R.D. Trajectories of depressive symptoms and stressful life events among male and female adolescents in divorced and nondivorced families. Dev. Psychopathol. 2006;18(1):253–273. doi: 10.1017/S0954579406060147. [DOI] [PubMed] [Google Scholar]
- Gibson E.L. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol. Behav. 2006;89(1):53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Giuliani N.R., Mann T., Tomiyama A.J., Berkman E.T. Neural systems underlying the reappraisal of personally craved foods. J. Cogn. Neurosci. 2014;26(7):1390–1402. doi: 10.1162/jocn_a_00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E.B. Encyclopedia of Perception. SAGE; 2010. [Google Scholar]
- Hilbert A., Vögele C., Tuschen-Caffier B., Hartmann A.S. Psychophysiological responses to idiosyncratic stress in bulimia nervosa and binge eating disorder. Physiol. Behav. 2011;104(5):770–777. doi: 10.1016/j.physbeh.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Hoyt L.T., Kushi L.H., Leung C.W., Nickleach D.C., Adler N., Laraia B.A., …, Yen I.H. Neighborhood influences on girls’ obesity risk across the transition to adolescence. Pediatrics. 2014;134(5):942–949. doi: 10.1542/peds.2014-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff A.M., Sinha R., Lacadie C., Small D.M., Sherwin R.S., Potenza M.N. Neural correlates of stress- and food cue–induced food craving in obesity. Diabetes Care. 2013;36(2):394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery R.W., Drewnowski A., Epstein L.H., Stunkard A.J., Wilson G.T., Wing R.R., Hill D.R. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1S):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- Klein A., Andersson J., Ardekani B.A., Ashburner J., Avants B., Chiang M.-C., Parsey R.V. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim J.J. The hippocampus. Curr. Biol. 2015;25(23):R1116–R1121. doi: 10.1016/j.cub.2015.10.049. [DOI] [PubMed] [Google Scholar]
- Kroemer N.B., Krebs L., Kobiella A., Grimm O., Vollstädt-Klein S., Wolfensteller U., Smolka M.N. (Still) longing for food: insulin reactivity modulates response to food pictures: insulin reactivity modulates response to food pictures. Hum. Brain Mapp. 2013;34(10):2367–2380. doi: 10.1002/hbm.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K.S., Gitelman D.R., Parrish T.B., Kim Y.-H., Nobre A.C., Mesulam M.-M. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav. Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Larson R., Ham M. Stress and “storm and stress” in early adolescence: the relationship of negative events with dysphoric affect. Dev. Psychol. 1993;29(1):130–140. [Google Scholar]
- Lewinsohn P.M., Roberts R.E., Seeley J.R., Rohde P., Gotlib I.H., Hops H. Adolescent psychopathology: II. Psychosocial risk factors for depression. J. Abnorm. Psychol. 1994;103(2):302–315. doi: 10.1037//0021-843x.103.2.302. [DOI] [PubMed] [Google Scholar]
- Li G., Ji G., Hu Y., Xu M., Jin Q., Liu L., Wang G.-J. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum. Brain Mapp. 2018;39(12):4755–4765. doi: 10.1002/hbm.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R., Drinkwater M., Yin Y., Anderson S.J., Schmidt L.C., Crawford A. Stability of family interaction from ages 6 to 18. J. Abnorm. Child Psychol. 2000;28(4):353–369. doi: 10.1023/a:1005169026208. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Intraub H., Mullally S.L. Scenes, spaces, and memory traces: what does the hippocampus do? Neuroscientist. 2016;22(5):432–439. doi: 10.1177/1073858415600389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.E., Holsen L.M., Chambers R.J., Bruce A.S., Brooks W.M., Zarcone J.R., Savage C.R. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18(2):254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- Masih T., Dimmock J.A., Epel E.S., Guelfi K.J. Stress-induced eating and the relaxation response as a potential antidote: a review and hypothesis. Appetite. 2017;118(Supplement C):136–143. doi: 10.1016/j.appet.2017.08.005. [DOI] [PubMed] [Google Scholar]
- McGue M., Elkins I., Walden B., Iacono W.G. Perceptions of the parent-adolescent relationship: a longitudinal investigation. Dev. Psychol. 2005;41(6):971–984. doi: 10.1037/0012-1649.41.6.971. [DOI] [PubMed] [Google Scholar]
- Murdaugh D.L., Cox J.E., Cook E.W., Weller R.E. FMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59(3):2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimeijer R.A.M., Roefs A., Ostafin B.D., de Jong P.J. Automatic approach tendencies toward high and low caloric food in restrained eaters: influence of task-relevance and mood. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Paolini B.M., Laurienti P.J., Norris J., Rejeski W.J. Meal replacement: calming the hot-state brain network of appetite. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N., Reyes F., Gomez F., Bhargava A., Dallman M.F. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pelchat M.L., Johnson A., Chan R., Valdez J., Ragland J.D. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Soc. Cogn. Affect. Neurosci. 2007;2(1):67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Mumford J.A., Nichols T.E. Cambridge University Press; 2011. Handbook of Functional MRI Data Analysis. [Google Scholar]
- Polivy J., Herman C.P., McFarlane T. Effects of anxiety on eating: does palatability moderate distress-induced overeating in dieters? J. Abnorm. Psychol. 1994;103(3):505–510. doi: 10.1037//0021-843x.103.3.505. [DOI] [PubMed] [Google Scholar]
- Rabasa C., Dickson S.L. Impact of stress on metabolism and energy balance. Curr. Opin. Behav. Sci. 2016;9:71–77. [Google Scholar]
- Romain A.J. An ounce of prevention outweighs kilograms of weight loss. Psychiatry Res. 2018;262:341–342. doi: 10.1016/j.psychres.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Rubin D. John Wiley and Sons; New York: 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- Ruttle P.L., Javaras K.N., Klein M.H., Armstrong J.M., Burk L.R., Essex M.J. Concurrent and longitudinal associations between diurnal cortisol and body mass index across adolescence. J. Adoles. Health. 2013;52(6):731–737. doi: 10.1016/j.jadohealth.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur E.A., Heckbert S.R., Goldberg J.H. The association of restrained eating with weight change over time in a community-based sample of twins. Obesity (Silver Spring) 2010;18(6):1146–1152. doi: 10.1038/oby.2009.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearrer G.E., Stice E., Burger K.S. Adolescents at high risk of obesity show greater striatal response to increased sugar content in milkshakes. Am. J. Clin. Nutr. 2018;107(6):859–866. doi: 10.1093/ajcn/nqy050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Role of addiction and stress neurobiology on food intake and obesity. Biol. Psychol. 2018;131:5–13. doi: 10.1016/j.biopsycho.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinharay S., Stern H.S., Russell D. The use of multiple imputation for the analysis of missing data. Psychol. Methods. 2001;6(4):317–329. [PubMed] [Google Scholar]
- Small D.M., Jones-Gotman M., Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Smeets P.A.M., Dagher A., Hare T.A., Kullmann S., van der Laan L.N., Poldrack R.A., Veldhuizen M.G. Good practice in food-related neuroimaging. Am. J. Clin. Nutr. 2019;109(3):491–503. doi: 10.1093/ajcn/nqy344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sominsky L., Spencer S.J. Eating behavior and stress: a pathway to obesity. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. Adolescent neurodevelopment. J. Adoles. Health. 2013;52(2 0 2):S7–13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A.C., White I.R., Carlin J.B., Spratt M., Royston P., Kenward M., Carpenter J.R. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ Br. Med. J. 2009;339(7713):157–160. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R.J., Francis H.M. The hippocampus and the regulation of human food intake. Psychol. Bull. 2017;143(10):1011–1032. doi: 10.1037/bul0000109. [DOI] [PubMed] [Google Scholar]
- Stice E. Interactive and mediational etiologic models of eating disorder onset: evidence from prospective studies. Annu. Rev. Clin. Psychol. 2016;12(1):359–381. doi: 10.1146/annurev-clinpsy-021815-093317. [DOI] [PubMed] [Google Scholar]
- Stice E., Burger K.S., Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA Allele. J. Neurosci. 2015;35(28):10316–10324. doi: 10.1523/JNEUROSCI.3607-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Burger K.S., Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage. 2013;67:322–330. doi: 10.1016/j.neuroimage.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Marti C.N., Spoor S., Presnell K., Shaw H. Dissonance and healthy weight eating disorder prevention programs: long-term effects from a randomized efficacy trial. J. Consult. Clin. Psychol. 2008;76(2):329–340. doi: 10.1037/0022-006X.76.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Shaw H., Marti C.N. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol. Bull. 2006;132(5):667–691. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Spoor S., Ng J., Zald D.H. Relation of obesity to consummatory and anticipatory food reward. Physiol. Behav. 2009;97(5):551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S. Relation of neural response to palatable food tastes and images to future weight gain: using bootstrap sampling to examine replicability of neuroimaging findings. Neuroimage. 2018;183:522–531. doi: 10.1016/j.neuroimage.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel L.E., Weller R.E., Cook E.W., Twieg D.B., Knowlton R.C., Cox J.E. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Sulkowski M.L., Dempsey J., Dempsey A.G. Effects of stress and coping on binge eating in female college students. Eat. Behav. 2011;12(3):188–191. doi: 10.1016/j.eatbeh.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Tang D.W., Fellows L.K., Small D.M., Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol. Behav. 2012;106(3):317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Thesen S., Heid O., Mueller E., Schad L.R. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn. Reson. Med. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tiggemann M., Kemps E. The phenomenology of food cravings: the role of mental imagery. Appetite. 2005;45(3):305–313. doi: 10.1016/j.appet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Tuomisto T., Hetherington M.M., Morris M.F., Tuomisto M.T., Turjanmaa V., Lappalainen R. Psychological and physiological characteristics of sweet food “addiction.”. Int. J. Eat. Disord. 1999;25(2):169–175. doi: 10.1002/(sici)1098-108x(199903)25:2<169::aid-eat6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ushakov V., Sharaev M.G., Kartashov S.I., Zavyalova V.V., Verkhlyutov V.M., Velichkovsky B.M. Dynamic Causal Modeling of Hippocampal Links within the Human Default Mode Network: Lateralization and Computational Stability of Effective Connections. Frontiers in Human Neuroscience. 2016;10 doi: 10.3389/fnhum.2016.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S., Boshuizen H.C., Knook D.L. Multiple imputation of missing blood pressure covariates in survival analysis. Stat. Med. 1999;18(6):681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]; doi:10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R.
- van Buuren S., Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. J. Stat. Softw. 2011;45(3) [Google Scholar]
- van Strien T., Frijters J.E.R., Bergers G.P.A., Defares P.B. The Dutch eating behavior questionnaire (DEBQ) for assessment of restrained emotional, and external eating behavior. Int. J. Eat. Disord. 1986;5(2):295–315. [Google Scholar]
- Voelker D.K., Reel J.J., Greenleaf C. Weight status and body image perceptions in adolescents: current perspectives. Adolesc. Health Med. Ther. 2015;6:149–158. doi: 10.2147/AHMT.S68344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Faith M., Patterson F., Tang K., Kerrin K., Wileyto E.P., …, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J. Neurosci. 2007;27(51):14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J., Chida Y., Gibson E.L., Whitaker K.L., Steptoe A. Stress and adiposity: a meta-analysis of longitudinal studies. Obesity (Silver Spring) 2011;19(4):771–778. doi: 10.1038/oby.2010.241. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- White M.A., Whisenhunt B.L., Williamson D.A., Greenway F.L., Netemeyer R.G. Development and validation of the food-craving inventory. Obes. Res. 2002;10(2):107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York: 2016. [Google Scholar]
- Yakusheva T.A., Shaikh A.G., Green A.M., Blazquez P.M., Dickman J.D., Angelaki D.E. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007;54(6):973–985. doi: 10.1016/j.neuron.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Yau Y.H.C., Potenza M.N. Stress and eating behaviors. Minerva Endocrinol. 2013;38(3):255–267. [PMC free article] [PubMed] [Google Scholar]
- Yokum S., Gearhardt A.N., Harris J.L., Brownell K.D., Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring) 2014;22(12):2544–2551. doi: 10.1002/oby.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S., Ng J., Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 2011;19(9):1775–1783. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P., Mullally S.L., Maguire E.A. Constructing, perceiving, and maintaining scenes: hippocampal activity and connectivity. Cerebral Cortex. 2015;25(10):3836–3855. doi: 10.1093/cercor/bhu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.-N., Wang J.-J. The cerebellum in feeding control: possible function and mechanism. Cell. Mol. Neurobiol. 2008;28(4):469–478. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.