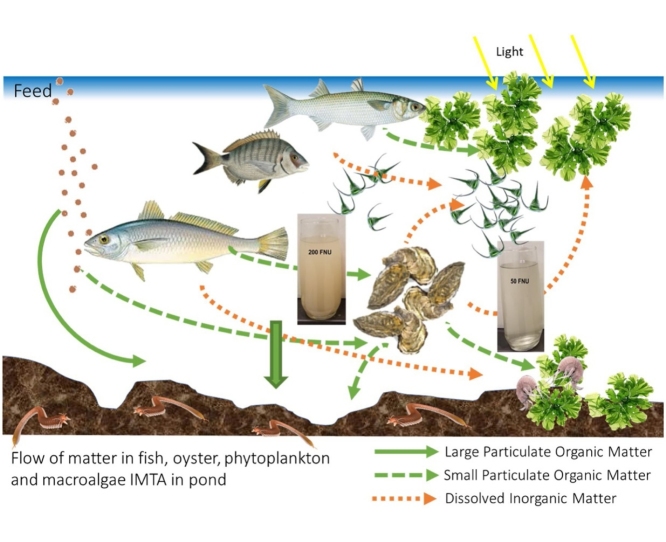
Graphical abstract
Method name: Pond IMTA
Keywords: Aquaculture, IMTA, Meagre, White seabream, Grey mullet, Japanese oyster, Phytoplankton, Ulva
Abstract
Production costs in extensive and semi-intensive fish culture in earthen ponds are often too high to offer sustainable economic activity due to the low productivity of these systems. The right combination of commercial finfish species with inorganic (primary producers) and organic extractive (bivalves) species in Integrated MultiTrophic Aquaculture (IMTA) create a balanced system with higher profitability and risk reduction. To achieve this, it is crucial to understand the role of each functional groups within the system what we did by comparing three different IMTA production three different IMTA production treatments with distinct combinations of trophic levels:
-
•
fish, filter feeders, phytoplankton and macroalgae,
-
•
fish, filter feeders and phytoplankton
-
•
fish, phytoplankton and macroalgae
Each treatment was carried out in two similar ponds under semi-intensive conditions and flow through system, in a total of 6 earthen ponds of 500 m2 surface and depth of 1.5 m.
Results showed that the presence of oysters in the ponds enhanced water quality by decreasing turbidity and by controlling phytoplankton which led to regulation of dissolved oxygen levels. The enhanced water quality in these systems lead to improved fish performance and higher biomass production contributing to greater profitability. The combination of fish, oyster, phytoplankton and macroalgae was particularly good providing much more fish supply compared with the other two treatments.
-
•
Oysters enhanced water quality in the ponds by decreasing turbidity and controlling phytoplankton which regulated the dissolved oxygen levels.
-
•
The enhanced water quality in systems with oysters improve fish performance resulting in higher biomass production and greater profitability.
-
•
The combination of fish, oyster, phytoplankton and macroalgae was particularly good providing much more fish supply compared with the other two treatments.
Specifications Table
| Subject Area: | Agricultural and Biological Sciences |
| More specific subject area: | Aquaculture |
| Method name: | Pond IMTA |
| Name and reference of original method: | NA. |
| Resource availability: | NA |
Method details
Context
In the face of global climate change, the world is required to change the ways of conducting economic activities and aquaculture is one of them. Fish, oyster and macroalgae integrated production in earthen ponds is an improved system compared to the regular semi-intensive fish production since marine bivalves and seaweeds are extractive species that can provide environmental benefits by removing waste materials, including waste from fed species, and lowering the nutrient load in the water. The water quality in these systems becomes of higher quality, leading to better fish performance and higher biomass production but also results in the reduction of energy needs, resulting in greater profitability.
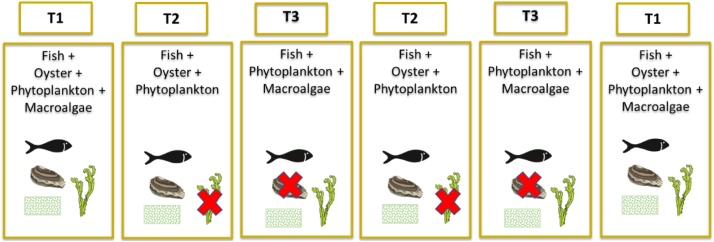
To evaluate the role of each of three fish species belonging to different functional groups (a carnivore, an omnivore and a deposit feeder), one filter feeder and two primary producers (phytoplankton and macroalgae), three treatments (Fig. 1) were compared from 15, April 2016 to 29, November 2016 at the Aquaculture Research Station (Estação Piloto de Piscicultura de Olhão, EPPO), of the Portuguese Institute of Sea and Atmosphere (Instituto Português do Mar e Atmosfera, IPMA), south Portugal (37° 02´ N; 07° 49´W). The combinations in each treatment were as follow: Treatment 1 (T1) fish + oysters + phytoplankton + macroalgae; Treatment 2 (T2) fish + oysters + phytoplankton; Treatment 3 (T3 fish + phytoplankton + macroalgae (comparable to the regular earthen pond production) (Fig. 1). The fish species were: a carnivore from trophic level 4.3 (meagre, Argyrosomus regius), an omnivore from trophic level 3.4 (white seabream, Diplodus sargus) and a deposit feeder from trophic level 2.5 (flathead mullet, Mugil cephalus) [1]; a filter feeder (oyster, Crassostrea gigas triploid) and unknown number of organic extractive primary producer species (phytoplankton and macroalgae, mainly Ulva spp.) that naturally developed in the ponds. Table 1 shows the number of fishes introduced in each of the ponds, considering the aim to obtain a final density of 1.5 kg.m−3 at the end of the experiment (8 months) and the number and mean weight of oyster seeded in each T1 and T2 pond. The results of these are presented in Cunha et al. [2].
Fig. 1.
IMTA treatments in the study of the role of fish, oysters, phytoplankton and macroalgae.
Table 1.
Initial zootechnical parameters of fish and oyster introduced in each earthen pond.
| Species | # /pond | Mean weight (g) | Mean total length (cm) | Condition Index |
|---|---|---|---|---|
| Argyrosomus regius | 1450 | 205 ± 63 | 27 ± 3 | 1.1 ± 0.1 |
| Diplodus sargus | 850 | 52 ± 19 | 14 ± 1 | 1.7 ± 0.5 |
| Mugil cephalus | 565 | 118 ± 96 | 19 ± 6 | 1.1 ± 0.2 |
| Crassostrea gigas | 18,000 | 0.5 ± 0.2 | – | – |
Pond management
Earthen ponds at EPPO were operated in a semi-intensive system with continuous water renewal. Water was pumped from a reservoir connected directly to Ria Formosa coastal lagoon into 6 ponds of 500 m2 and a mean depth of 1.5 m (Fig. 2). The water outlet, at the opposite side, was equipped with a plastic net to avoid fish escapees and a sluice gate that controlled water level and drained into a settling pond (Fig. 3). Water renewal was controlled according to water temperature and adjusted every two weeks according to water temperature and fish growth using Table 2 as a guide. The ponds were covered with anti-bird nets and equipped with automatic feeders (NR-A602 model, SINO-AQUA, Taiwan) and air injectors (model FORCE7, AQUA&Co, Italy). All ponds had oxygen and temperature probes (B&G SINERGIA, Italy) close to the water outlet for continuous monitoring. External aeration worked whenever DO levels drop below 50 % and stopped at 70 % saturation. Working periods of the air injector were automatically registered, together with DO and temperature measurements of the probes. Automatic probes (temperature and DO), pumps, aerators, dead fish, feeders and water renewal were checked daily, registered and rearranged when in need.
Fig. 2.
Aerial view of the Aquaculture Research Station (Estação Piloto de Piscicultura de Olhão, EPPO), south Portugal (37° 02´ N; 07° 49´W) and location of earthen ponds with IMTA treatments (T1, T2, and T3). Arrows indicate water inlet and outlet; R - water reservoir; S – settling pond.
Fig. 3.
Equipment location in earthen ponds.
Table 2.
Daily water renewal (% of pond volume) according to water temperature and daily feed quantity (EPPO, IPMA).
| Feed day−1 (kg) Temperature (°C) align="left" |
<10 | 10–20 | 20–30 | 30–40 | 40–50 | >50 |
|---|---|---|---|---|---|---|
| ≤12 | 10 | 15 | 20 | 25 | 30 | 35 |
| 15 | 15 | 20 | 30 | 40 | 50 | 60 |
| 18 | 20 | 30 | 40 | 50 | 75 | 90 |
| 21 | 30 | 40 | 50 | 75 | 100 | 120 |
| 24 | 40 | 60 | 75 | 90 | 120 | 140 |
| ≥25 | 50 | 80 | 100 | 120 | 140 | >160 |
Fish farming and monitoring
Except for grey mullet juveniles, that had wild origin, all the other fish were produced at the EPPO hatchery. Fish were introduced at the beginning of April (07/04/2016) and were regularly sampled for length-weight measurements as a basis for determining/estimating their biomass in the pond and therefore their daily feed rations. They were fed daily with a commercial diet (AQUASOJA, Portugal) and Table 3 shows the main feed composition and ingredients. Feed pellet size started at 5 mm in April 7 and was upgraded to 7 mm after July 11. Feed ration (daily feed quantity given to fish) was adjusted every two weeks according to fish mean weight and water temperature following Table 4. Fish were monitored closely and regularly to determine the general condition of the stock. Individuals were rod fished every week for observation of ectoparasites levels (prevalence and infestation). After being caught the fish parasite burden was assessed by microscopic observation of a wet mount of the first 2 branchial arches from the left operculum in a stereo microscope (UB200i SERIES, Proiser, Spain), after being euthanized by spinal cut close to the head [3]. Parasites were counted and identified to species level whenever possible. When A. ocellatum trophonts were detected, a treatment with copper sulphate at 250 ppm was used to avoid fish mortalities in the experiment [4]. Since oysters are highly susceptible to copper sulphate at this concentration, with mortality occurring after 96 h of exposure [5,6], they were placed in a quarantine pond with natural microalgae production until the end of copper sulphate treatment to avoid mortality. Fish growth was determined by sampling 50–100 individuals at the beginning of the experiment (end of March/beginning of April), July (meagre) and at the end of the experiment (all species). Fish was collected with a seine net, and 100 individuals were anesthetized in a 300−l tank with pond water at 100 mg L-1 of phenoxyethanol and all individuals were measured (total length in cm), weighed (total weight in g) and returned to the corresponding pond.
Table 3.
Composition of the commercial diet.
| Ingredients | Proximate and mineral elements |
|---|---|
| Fish meal | Crude protein, 42% |
| Transformed animal protein from farmed poultry | Crude fat, 17% |
| Rapeseed meal | Crude ash, 11% |
| Soy meal (peeled and toasted) | Cellulose, 2.0% |
| Feather meal | Calcium, 2.4% |
| Poultry fat | Phosphorus, 1.5% |
| Fish oil | Sodium, 0.3% |
| Pea starch | |
| Blood meal | |
| Carob germ | |
| Brewer’s yeast |
Table 4.
Feed ration table for meagre (EPPO, IPMA).
| Temperature (°C) | 12 | 15 | 18 | 21 | 24 | 26 | 28 |
|---|---|---|---|---|---|---|---|
| Fish size (g) | |||||||
| 50-100 | 0.4 | 1.5 | 2.0 | 3.0 | 3.4 | 3.8 | 4.0 |
| 100-150 | 0.4 | 1.2 | 1.6 | 2.4 | 2.8 | 3.0 | 3.2 |
| 150-200 | 0.4 | 1.1 | 1.5 | 2.0 | 2.6 | 2.8 | 3.0 |
| 200-250 | 0.4 | 1.0 | 1.4 | 1.6 | 2.0 | 2.6 | 2.8 |
| 250-300 | 0.3 | 0.8 | 1.1 | 1.6 | 1.8 | 2.0 | 2.4 |
| 300-350 | 0.3 | 0.8 | 1.1 | 1.6 | 1.8 | 2.0 | 2.2 |
| 350-400 | 0.3 | 0.8 | 1.1 | 1.6 | 1.8 | 2.0 | 2.2 |
| 400-450 | 0.2 | 0.8 | 1.1 | 1.6 | 1.8 | 2.0 | 2.2 |
| 450-500 | 0.2 | 0.8 | 1.0 | 1.6 | 1.8 | 2.0 | 2.0 |
Macroalgae farming
Macroalgae occur naturally in the ponds and the most abundant genus was Ulva. It is almost impossible to avoid their growth in the ponds and to control the proliferation in treatment T2 floating macroalgae were harvested manually with a fishing net every week. In the ponds with treatment T1 and T3 the harvesting occurred every other week. After harvesting, the macroalgae were washed with clean saltwater to remove most of the impurities and epibionts and weighed after being hand squeezed. Macroalgae growing at the bottom was not removed to avoid disturbances to the system (sediment resuspension and fish stress).
Oyster farming and monitoring
Oysters were seeded at the middle of May (18 May 2016) in 5 mesh bags in each pond. They were farmed in traditional oyster mesh bags, suspended close to the pond surface by polystyrene buoys (boards of 50 × 60 cm) attached to a longline (Fig. 3). Oyster mesh bags were air exposed every week for 24 h to avoid biofouling and to simulate tide effect by turning the buoys upside down (Fig. 4). This was done manually with human intervention inside the pond which allowed for oyster inspection. Oyster growth and mortality were monitored monthly by weighing 50 oysters from each of 5 bags pond−1 (n = 250 oyster pond−1). Dead individuals in each bag were removed and counted to estimate mortality for the whole stock as a cumulative % of dead oysters. Oysters were sorted and re-distributed to new bags (one bag divided in two) whenever the mesh bag attained 8 Kg, or oyster volume was 80 % of the mesh bag.
Fig. 4.
Oyster mesh bags exposed to the air.
Water quality monitoring
Physical parameters (temperature, dissolved oxygen - DO, pH, salinity, turbidity) were measured twice daily using a handheld multiprobe (model H9829, Hanna Instruments, EUA) between 8h30-9h00 and 16h30-17h00. Water transparency (Secchi disk) was determined weekly in all ponds around 12h00.
Sixty-liter integrated water samples composed of inlet, middle and outlet water (20 L each) were collected monthly for evaluation of nutrients (ammonia, nitrites, nitrates, phosphates and silica), chlorophyll a and phaeopigments. The samples were immediately refrigerated and taken to the laboratory where 250 mL of the water was filtered for chlorophyll a and phaeopigments through fiberglass filters (Whatman, 0,47 mm porosity) following Boyd [7]. Filters for pigments and nutrient samples were kept at -20 °C until analysis. Gross primary production was estimated using the light and dark bottle method for 2 h followed by oxygen Winkler titration. The conversion factor of released O2 during photosynthesis to Carbon was assumed to be 0.375 [8].
Chlorophyll_a was determined by spectrophotometry according to Lorenzen (1967) after 24 h extraction in acetone (90 %). Ammonium (NH4+), nitrate (NO3−), nitrite (NO2−), silicon (Si (OH)4) and phosphate (HPO42-) were analyzed using a “Skalar” autoanalyser according to the methodology described in Grasshoff et al. [9]. At the end of the trail microalgae were identified and counted. Samples were collected in each tank with a 10 L van Dorn bottle in 9 different points at 1 m depth and homogenized in a 250 L conic container. Duplicate samples were collected in 1 L flasks containing 5 mL of acidified Lugol’s iodine solution for sample preservation. Counts were done in three replicates of 12.5 mL sub-samples using the sedimentation procedure [10] in 50 mL settling tubes for 24 h. Cells were counted under an inverted microscope.
Acknowledgements
The experiment was performed within the framework of the ERA-Net COFASP project (IMTA-Effect project) with funding from FCT (COFASP/0003/2015). H. Quental-Ferreira, L. Ribeiro, and F. Soares were funded by Project DIVERSIAQUA (Mar2020 16-02-01-FMP-0066), S. Gamito by project UID/Multi/04326/2019 and I. Monteiro by project Algared+ (Poctep 0055_ALGARED_PLUS_5_E). M. Moreira has a PhD grant from FCT (SFRH/BD/118601/2016).
Acknowledgments
Declaration of Competing Interest
The authors confirm that there are no conflicts of interest.
References
- 1.FishBase, 2019. https://www.fishbase.de/.
- 2.Cunha M.E., Quental-Ferreira H., Parejo A., Gamito S., Ribeiro L., Moreira M., Monteiro I., Soares F., Pousão-Ferreira P. Understanding the individual role of fish, oyster, phytoplankton and macroalgae in the ecology of integrated production in earthen ponds. Aquaculture. 2019 doi: 10.1016/j.mex.2019.10.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreira M., Schrama D., Soares F., Wulff T., Pousão‐Ferreira P., Rodrigues P. Physiological responses of reared sea bream (Sparus aurata Linnaeus, 1758) to an Amyloodinium ocellatum outbreak. J. Fish Dis. 2017;40:1545–1560. doi: 10.1111/jfd.12623. [DOI] [PubMed] [Google Scholar]
- 4.Soares F., Quental-Ferreira H., Cunha E., Pousão-Ferreira P. Occurrence of Amyloodinium ocellatum in aquaculture fish production: a serious problem in semi-intensive earthen ponds. Aquac. Int. 2011;36(4):13–16. [Google Scholar]
- 5.Okazaki R.K. Copper toxicity in the pacific oyster Crassostrea gigas. Bull. Environ. Contam. Toxicol. 1976;16(6):658–664. doi: 10.1007/BF01685570. [DOI] [PubMed] [Google Scholar]
- 6.Pipe R.K., Coles J.A. Environmental contaminants influencing immune function in marine bivalve mollusks. Fish Shellfish Immunol. 1995;5(8):581–595. [Google Scholar]
- 7.Boyd C.E. Auburn University/Alabama Agricultural Experiment Station; Auburn, AL: 1990. Water Quality in Ponds for Aquaculture. [Google Scholar]
- 8.Cole G.A., Weihe P.E. fifth edition. Waveland Press Inc.; Long Grove, Ilinois: 2015. Textbook of Limnology. [Google Scholar]
- 9.Grasshoff K., Ehrhardt M., Kremling K. 2nd edition. Verlag Chemie Weinhein; New York: 1983. Methods of Seawater Analysis. [Google Scholar]
- 10.Utermöhl von H. Neue Wege in der quantitativen Erfassung des Planktons. (Mit besondereBeriicksichtigung des Ultraplanktons) Verh. Int. Verein. Theor. Angew. Limnol. 1931;5:567–595. [Google Scholar]