Abstract
Hurricane Harvey made landfall in Texas August 25, 2017, bringing massive rains and flooding that impacted soils in a residential neighborhood in East Houston. Trace elements, organochlorine pesticides, polycyclic aromatic hydrocarbons (PAHs), polybrominated diphenyl ether fire retardants (PBDEs) and polychlorinated biphenyls (PCBs) were determined in 24 soil samples. The highest concentrations found in soils were total PAHs, which ranged from 1,310 μg/kg to 85,700 μg/kg with a mean of 12,600 μg/kg. Analysis of specific PAH ratios indicate the source of the PAHs were dominated by pyrogenic rather than petrogenic sources. Chlordanes were detectable in the area where the likely local source is for ant control. The trace metal concentrations were below any environmental health concern concentrations but As, Cd, Hg, Pb, Se, Ag, Zn were enriched over the crustal abundance. While Hurricane Harvey was responsible for the redistribution of many contaminants, the large volume of rain and floodwater likely transported contaminants from the land areas and into the Houston Ship Channel and Galveston Bay. The findings from this study will serve as baseline data for determining the mobilization of contaminants caused by natural disasters.
Keywords: Environmental science, Environmental analysis, Environmental assessment, Environmental chemistry, Environmental hazard, Environmental health, Environmental impact assessment, Environmental pollution, Water quality, Contaminant transport, Environmental risk assessment, Baseline contaminants, Soil, Hurricane, Hydrocarbons, Chlorinated pesticides, Trace metals
1. Introduction
Understanding the potential mobilization of contaminants by natural disasters is an important element of hazard mitigation and response planning as mobilization can have both environmental and human health impacts (Knap and Rusyn, 2016; Plumlee et al., 2012). However, since there is wide variation across disasters, by disaster type and magnitude, over time, and in different geographic locations the impacts may vary (Peacock et al., 2001). In addition, there have been very few incidents in which environmental baselines have been established prior to an event. On August 25, 2017, Hurricane Harvey made landfall as a Category 4 storm (Saffir-Simpson scale) in Aransas County, Texas, approximately 200 miles southwest of Houston. Due to the atmospheric northeast and northwest steering currents, as well as the presence of two weak storm systems, Hurricane Harvey stalled near the Texas coast, resulting in unprecedented inland precipitation in Houston over the following six days.
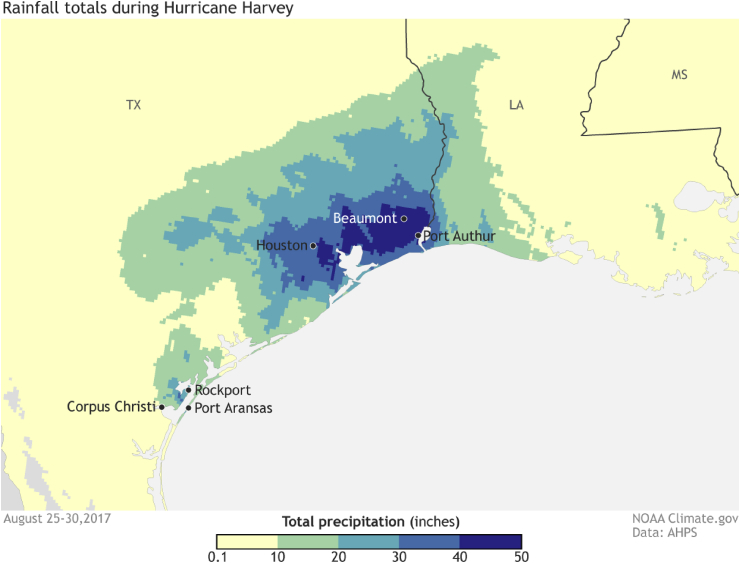
An estimated 44 trillion liters of rainwater fell over the Houston area during Hurricane Harvey flooding 70% of Harris County including the city of Houston, at a water level of at least 18 inches (Di Liberto, 2017). The intense rain led to widespread flooding (Fig. 1), which was so severe it reduced the salinity of Galveston Bay to zero, compared to the normal mean salinity of 15 Practical Salinity Units (PSU), for a period of two weeks (Steichen and Quigg, 2018).
Fig. 1.
Rainfall in inches over Houston area, (NOAA Climate.gov).
In Houston, a metropolitan area with 6.7 million residents and more than 25 Superfund sites (Texas Commission on Environmental Quality (TCEQ), Data USA, 2017; American Community Survey, 2016) flooding from Hurricane Harvey potentially exposed residents to a variety of mobilized contaminants. Houston is home to 40% of the US capacity for producing chemicals as well as more than 3,600 energy companies, including downstream components such as refining (City-Data, 2018). Its location on the US Gulf Coast makes it highly susceptible to extreme weather, including tropical storms and hurricanes, further exacerbating the risk of contaminant mobilization. The Galveston Bay and Houston Ship Channel (GB/HSC) area has a history of major hurricane landfalls, with any 50-mile segment of the Texas coast being impacted every 6 years (Roth, 2010).
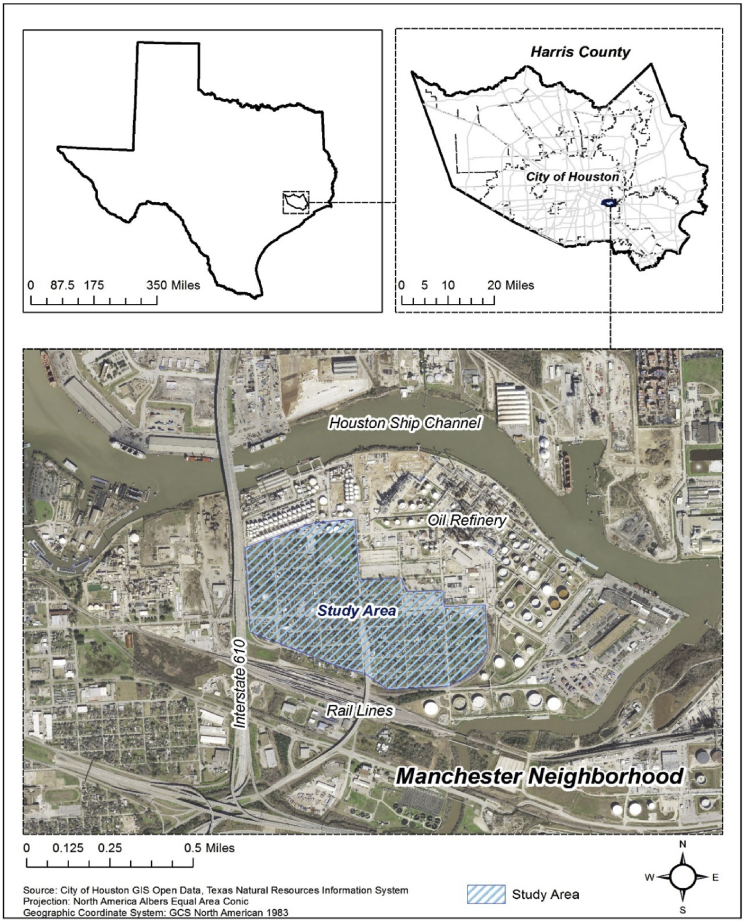
The present study focuses on contaminants of concern in Manchester, TX, a well-studied environmental justice neighborhood in Houston's East End (Chakraborty et al., 2014; Sansom et al., 2018; Horney et al., 2018). Environmental justice communities located along the HSC are at a particularly high risk for health impacts related to contamination mobilization due to their location at the nexus of exposure to hazardous substances and natural disasters (Fig. 2). Within 1 mile of the Manchester neighborhood, there are 21 facilities reporting to the EPA's Toxic Release Inventory: 11 large-quantity generators of hazardous waste; 4 facilities that treat, store, or dispose of hazardous wastes; 9 major dischargers of air pollution; and 8 major storm water discharging facilities (WEACT, 2013). The area is also highly vulnerable to the impacts of natural disasters, both socially and physically. The population of the Harrisburg/Manchester Super Neighborhood is 98% minority, with a median income that is one-third less that the City of Houston overall (City of Houston Planning and Development, 2014). The floodplains along Sims Bayou have also increased by 15% since 1980 due to an increase in development, additions of impervious covers like concrete and asphalt in the region, subsidence as well as rising sea-levels. It is estimated that another 35,000 residents in HSC and Sims Bayou neighborhoods could be exposed to regular flooding by 2050 (Muñoz Ordoñez, 2015). Thus, major goal of this study is to establish baseline for both organic and inorganic contaminants associated with soils in this area. The results from this study will help to investigate the redistribution/mobilization of contaminants caused by natural disasters and in turn, help to assess the potential threats from these contaminants.
Fig. 2.
Sample Map of Manchester, TX – shaded area is the site of 24 surface soil samples (after Horney et al., 2018).
2. Methods
2.1. Sampling
Hurricane Harvey associated flooding affected much of Houston's transportation infrastructure, making access to the neighborhood of Manchester difficult until one week after landfall. Soil samples were taken from 24 locations within the geographically compact neighborhood of Manchester, TX on September 1, 2017 (Fig. 2). Sampling sites included yards and ditches in the residential area of Manchester, with amounts consisting of approximately 150 g soil. Samples were collected by scooping soil from ponding areas into certified clean 300 ml glass containers. Samples were transported to the Geochemical and Environmental Research Group (GERG) at Texas A&M University and frozen until analysis.
2.2. Analysis
Prior to analyses, the samples were freeze-dried in a Model 75040 Labconco Freeze Drier 8. Sample dry weights were determined gravimetrically by weight until they were at a constant weight. All sample analyses are reported based on the sample dry weight. Quality Assurance procedures from the NOAA National Status and Trends Program, the US EPA Environmental Monitoring and Assessment Program-Near Coastal (EMAP-NC) and the U.S. Fish and Wildlife Service for trace contaminant analysis were followed. For every batch of 20 samples or less, a procedure blank, duplicate, matrix spike, lab control sample, and a standard reference material (NIST, 1944 for sediments) was run to evaluate overall accuracy of the procedures used for preparation and analysis. Duplicates were included to estimate sample homogeneity and analytical variability. To identify any digestion interferences and to evaluate the accuracy and performance of the analytical system, a laboratory control sample (LBS) and a matrix spiked sample (MS) are run with each batch.
2.3. Trace metal analysis
Soil samples for metal analysis (Table 1) were digested based on a total recoverable procedure. This procedure extracts the metals that would be removed by nitric acid treatment, but does not break-down the soil matrix. This approach is appropriate because leachable metals rather than the whole rock geochemistry are of interest. Anthropogenic trace element contaminants extracted using this method are more bio-available compared to elements tightly bound to the silicate matrix of the soil. Briefly, 1000 mg of dried sample was digested with 10 ml of a 1:1 HNO3/H2O by heating at 95 °C for 10 min in a hot block. After cooling the mixture to ambient temperature, 5 ml of concentrated HNO3 was added and heated at 95 °C for an additional 30 min. After cooling, 3 ml of 30% H2O2 was added and momentarily returned to the hot block. This was repeated in 1 mL increments until effervescence is completed; the sample is then heated until the volume is reduced to 10 ml. The cooled digestate is filtered and brought up to a final volume of 100 ml with milli-Q water. Instrumental analysis for the various metals was performed using a PerkinElmer NexION 300D inductively coupled plasma mass spectrometer (ICP-MS). The practical quantitation limits (PQLs) of the technique used for the analyses of trace metals were in the range of 0.02–0.05 μg/g.
Table 1.
Trace element concentrations of Manchester soil samples in mg/Kg. CA is the crustal abundance in the same units and Enrichment Factor (EF) provides the sample relationship to the CA in Bold.
| Trace Elements |
Incidence |
Median |
Mean ± 1 STD |
Range |
CA |
EF |
|---|---|---|---|---|---|---|
| (%) | (mg/Kg d.w.) | (mg/Kg d.w.) | (mg/Kg d.w.) | |||
| Aluminium(Al) | 100 | 16,200 | 15,800 ± 4,780 | 6,670 - 25,400 | 82,000 | 0.2 |
| Antimony (Sb) | 100 | 0.173 | 0.189 ± 0.0723 | 0.097 - 0.391 | 0.20 | 1.0 |
| Arsenic (As) | 100 | 3.84 | 5.11 ± 3.37 | 2.75 - 16.5 | 2.10 | 2.4 |
| Barium (Ba) | 100 | 115 | 121 ± 33.3 | 65.4 - 182 | 340 | 0.4 |
| Beryllium (Be) | 4.2 | 0.01 | 0.0208 ± 0.0996 | 0.01 - 0.498 | 1.90 | 0.0 |
| Cadmium (Cd) | 100 | 0.781 | 0.873 ± 0.327 | 0.395 - 1.62 | 0.15 | 5.8 |
| Chromium (Cr) | 100 | 10.5 | 11.2 ± 2.61 | 7.81 - 18.2 | 140 | 0.1 |
| Cobalt (Co) | 100 | 2.18 | 2.27 ± 0.444 | 1.75 - 3.58 | 30.00 | 0.1 |
| Copper (Cu) | 100 | 10.2 | 12.2 ± 8.04 | 3.66 - 42.4 | 68.0 | 0.2 |
| Iron(Fe) | 100 | 12,800 | 13,100 ± 3,340 | 6,640 - 19,700 | 63,000 | 0.2 |
| Lead (Pb) | 100 | 45.1 | 51.6 ± 22.2 | 17.2 - 116 | 10.00 | 5.1 |
| Lithium (Li) | 100 | 5.15 | 5.23 ± 1.16 | 3.52 - 9.07 | 17.0 | 0.3 |
| Manganese (Mn) | 100 | 114 | 143 ± 99.2 | 62.6 - 508 | 1100 | 0.1 |
| Nickel | 100 | 5.56 | 5.76 ± 1.46 | 3.45 - 9.69 | 90.00 | 0.1 |
| Selenium(Se) | 100 | 3.92 | 4.01 ± 0.656 | 2.91 - 5.46 | 0.05 | 82.0 |
| Silver (Ag) | 100 | 0.215 | 0.223 ± 0.0741 | 0.121 - 0.372 | 0.08 | 2.8 |
| Strontium (Sr) | 100 | 73.8 | 69.2 ± 23.4 | 25.6 - 114 | 360 | 0.2 |
| Zinc (Zn) | 100 | 487 | 493 ± 115 | 339 - 813 | 79.0 | 6.2 |
| Magnesium(Mg) | 100 | 2,600 | 2,650 ± 721 | 1630 - 4910 | 20,900 | 0.1 |
| Tin (Sn) | 100 | 0.403 | 0.587 ± 0.556 | 0.156 - 2.69 | 2.20 | 0.3 |
| Thallium (Tl) | 100 | 0.095 | 0.122 ± 0.131 | 0.032 - 0.696 | 0.53 | 0.2 |
| Vanadium (V) | 100 | 14.2 | 14.3 ± 1.78 | 11.2 - 19.5 | 190 | 0.1 |
| Mercury (Hg) | 100 | 0.091 | 0.125 ± 0.0891 | 0.054 - 0.407 | 0.070 | 1.8 |
The NexION 300D ICP Mass Spectrometer features Universal Cell Technology™ (UCT) with three modes of operation, standard, collision, and reaction mode to optimize collision/reaction cell (CRC) conditions for each trace element. A triple cone interface and quadrupole ion deflector maximizes ion definition and focus while removing most particulates, neutrals and photons. The ICP/MS resolution is less than or equal to 0.9 amu at 10% peak height from 6-253 amu and 1.0 amu at 5% peak height from 6-253 amu. An internal standard technique is utilized where internal standards and digestates are introduced and nebulized in the spray chamber where an argon carrier sweeps the sample aerosol through the quartz torch and into the RF plasma where the sample is de-solvated and decomposed. The ICP/MS is optimized and calibrated each day of operation using one blank and at least 4 standards using the average of three replicate integrations. The linearity of the initial calibration is deemed sufficiently linear if r2 ≥ 0.995. The ongoing validity of the calibration is determined by the subsequent calibration verifications performed every ten samples. Metal raw concentrations and metal crustal abundance (webelement.com) were normalized against iron to compensate the grain size variation. Enrichment factor (EF) values were calculated by dividing normalized measured metal concentration with normalized crustal abundance concentration.
Mercury (Hg) determinations are made using an acid-permanganate digestion of the dry powdered sampled followed by stannous chloride reduction to Hg metal and detection by cold vapor atomic absorption spectroscopy (CVAAS) using a PerkinElmer flow injection mercury system (Model FIMS-400). Briefly, 200 mg of dried sample was digested with 4 ml of a concentrated H2SO4/HNO3 (2.5:1.5 v/v) mixture by heating at 95 °C for 30 min in a hot block. After cooling the mixture to ambient temperature, 10 ml of distilled water, 10 ml of KMnO4 solution, and 5 ml of K2S2O8 solution was added and heated at 95 °C for 30 min. 5 ml of a sodium chloride/hydroxylamine sulfate solution was added, after cooling, to reduce excess permanganate and the sample diluted to 40 ml with milli-Q water. The CVAAS was calibrated by injections of standards at five different concentrations where the initial calibration is deemed sufficiently linear if r2 ≥ 0.995, calibration verification standards and blanks are analyzed every 10 samples or less.
2.4. Organic analysis
Once thawed and homogenized, the soil samples were split in two equal portions. One half was weighed and the placed in automated Accelerated Solvent Extractor (ASE) cells together with a drying agent (Hydromatrix) to remove any residual water and analyzed for organochlorine pesticides (OCs), PCB, PBDEs, PAHs and Aliphatic hydrocarbons following GERG's Standard Operating Procedures (SOPs). The other half of the samples were reserved for later analysis of more polar compounds. More details regarding these methods can be found elsewhere (e.g., Gardinali and Wade, 1998; Sbriz et al., 1998; Yogui and Sericano, 2009; Ruiz-Fernández et al., 2016).
Briefly, samples and associated QA/QC samples (i.e., blank, matrix spike, duplicate and Standard Reference material, if available) were spiked with the appropriate surrogate standards [d10-naphthalene,d10-acenaphthene,d10-phenanthrene, and d12-chrysene, for PAHs; d26-nC12, d42-nC20, d50-nC24, and d62-nC30, for aliphatic hydrocarbons; PCB congeners 103 and 198 and 4,4-dibromooctafluorobiphenyl (DBOFB), for PCBs, organochlorine pesticides, and PBDE's before extraction (El-Kady et al., 2017). Following the ASE extraction, the sample extracts were purified by partially deactivated silica/alumina column chromatography to eliminate interfering materials and treated with acid washed granulated copper to eliminate potential interference from sulfur compounds.
PAH, PCB, OC, and PBDE analyses were performed by gas chromatography/mass spectrometry (GC/MS, Agilent Technologies 6890N GC System/5975C inert MSD) in the selective ion mode (SIM) after the addition of the appropriate internal standards [i.e., d10-Fluorene, d12-Benzo(a)pyrene), for PAHs, and 2,4,5,6-tetrachloro-m-xylene (TCMX), for PCBs, OCs, and PBDEs] used to evaluate the efficiency of the analytical methods (El-Kady et al., 2017). The sample extracts were splitless-injected into a 30 m × 0.25 mm i.d. (0.25 μm film thickness) DB-5MS fused silica capillary column (J&W Scientific, Inc. or similar) at an initial temperature of 60 °C, held for 3 min, and temperature was then programmed at 12 °C min−1 to 300 °C with a hold of 6 min at the final temperature, for PAHs; for PCBs the initial temperature was set at 75 °C, held for 3 min, and then ramped to 150, 260, and 300 °C at 15, 2, and 20 °C min−1, respectively, with a final hold of for 3 min; for PCBs, and for PBDEs, the initial temperature was programed from 130 °C, after a hold of 1 min, to 154, 210, and 300 °C at 12, 2, and 3 °C min−1, respectively, with a final holding time of 5 min. Aliphatic hydrocarbons were analyzed by GC-FID, after the addition of d38-nC16 as internal standard, using a DB-5MS fused silica capillary column and an oven-temperature program starting at 40 °C, held for 2 min and ramped up to 320 °C at a rate of 6 °C per min and a final hold of 15 min. The GC/MS and GC-FID were calibrated by injections of standards at five different concentrations. Identification of target analytes was based on the retention time of their respective peaks (Aliphatic hydrocarbons) or the respective quantitation ions and a series of confirmation ions (PAHs, PCBs, OCs, and PBDE's).
2.5. Data visualization: Kriging
The statistical interpolation method, called Kriging (Matheron, 1963; Stein, 1999), was used to predict concentration levels over the entire area. That is, concentration levels measured at the 24 sites were modeled as a spatial stochastic process, accounting for the spatial dependence structure and the uncertainty of the concentration field. Specifically, if C(s) denotes the log-transformed concentration level of a pollutant at location s, the formula is:
| C(s) = b0 + b1 latitude(s) + b2 longitude(s) + e(s) --- (1) |
Here e is assumed to follow Gaussian distribution with mean zero, as commonly done in spatial statistics. We take log transformation of concentration to make the distribution of the error term in (1), e, more close to be Gaussian. The covariance structure of e is modeled with the Matérn covariance function (Stein, 1999), a widely used parametric covariance function class in spatial statistics. Regression coefficients, b0, b1, b2, along with the covariance parameters for the Matérn model are estimated using Maximum Likelihood Estimation method (Le Cam, 1990).
Then, at any given new location in the area, the Kriged (or predicted) value is obtained by a weighted average of the (log-transformed) observed concentration levels and the weights are determined by the estimated spatial dependence structure as well as estimated regression term as in (1). This method was used for the trace metal Zn and several individual PAHs. We then back-transform (that is, through exponential transformation) Kriged values to make the predicted values comparable to actual concentration levels.
3. Results and discussion
3.1. Concentrations of analytes
The concentrations of analytes in the soils are described in Tables 1, Table 2a, Table 2b, 3, 4, and 5.
Table 2a.
Individual PAH as analyzed and quantified by GC/MS. The EPA Priority 16 PAH are in Boldface and totals of both these 16 PAH and all those measured are provided at the bottom. Individual naphthalenes are listed out in 5 entries in the table but they are not part of the total as they are totaled as alkylated naphthalene under Naphthalene. Incidence and distributions of detected concentrations are shown. Diagnostic ratios of some of the individual PAH are shown and provide a determination if the PAH are pyrogenic or petrogenic sources. STD = standard deviation.
| Polycyclic aromatic hydrocarbons | Incidence |
Median |
Mean ± 1 STD |
Range |
Percent Distribution as ug/kg d.w. |
|||
|---|---|---|---|---|---|---|---|---|
| (%) | (ug/kg d.w.) | (ug/kg d.w.) | (ug/kg d.w.) | nd - <100 | 100 - <1, 000 | 1,000 - <10,000 | >100,000 | |
| Naphthalene | 100 | 787 | 869 ± 438 | 316 - 2,020 | 66.7 | 33.3 | ||
| C1-Naphthalenes | 100 | 669 | 748 ± 410 | 148 - 1,880 | 75.0 | 25.0 | ||
| C2-Naphthalenes | 100 | 304 | 353 ± 205 | 45.5–863 | 4.17 | 95.8 | ||
| C3-Naphthalenes | 100 | 88.7 | 97.2 ± 45.7 | 11.8–180 | 62.5 | 37.5 | ||
| C4-Naphthalenes | 100 | 24.5 | 24.3 ± 10.8 | 4.18–45.3 | 100 | |||
| Biphenyl | 100 | 235 | 258 ± 117 | 52.8–490 | 4.17 | 95.8 | ||
| Acenaphthylene | 100 | 58.3 | 90.4 ± 83.0 | 13.6–326 | 75.0 | 25.0 | ||
| Acenaphthene | 100 | 512 | 548 ± 254 | 98.7 - 1,330 | 4.17 | 95.8 | ||
| Fluorene | 100 | 180 | 230 ± 145 | 18.0–550 | 16.7 | 83.3 | ||
| C1-Fluorenes | 100 | 7.37 | 9.89 ± 8.30 | 1.32–35.7 | 100 | |||
| C2-Fluorenes | 100 | 6.69 | 8.80 ± 7.31 | 2.30–34.7 | 100 | |||
| C3-Fluorenes | 100 | 8.96 | 12.6 ± 13.4 | 3.40–70.8 | 100 | |||
| Phenanthrene | 100 | 121 | 453 ± 744 | 22.0–2,670 | 33.3 | 54.2 | 12.5 | |
| Anthracene | 100 | 39.5 | 152 ± 206 | 8.92–701 | 66.7 | 33.3 | ||
| C1-Phenanthrenes/Anthracenes | 100 | 51.5 | 144 ± 237 | 12.8–887 | 83.3 | 16.7 | ||
| C2-Phenanthrenes/Anthracenes | 100 | 41.4 | 104 ± 143 | 10.1–540 | 79.2 | 20.8 | ||
| C3-Phenanthrenes/Anthracenes | 100 | 30.1 | 69.2 ± 107 | 8.68–525 | 87.5 | 12.5 | ||
| C4-Phenanthrenes/Anthracenes | 100 | 17.2 | 34.3 ± 59.3 | 3.34–307 | 100 | |||
| Dibenzothiophene | 100 | 9.13 | 26.1 ± 39.2 | 2.07–151 | 95.8 | 4.17 | ||
| C1-Dibenzothiophenes | 100 | 9.90 | 18.4 ± 20.9 | 3.35–75.1 | 100 | |||
| C2-Dibenzothiophenes | 100 | 16.5 | 23.6 ± 24.2 | 4.33–127 | 100 | |||
| C3-Dibenzothiophenes | 100 | 16.1 | 22.7 ± 31.0 | 3.95–167 | 100 | |||
| Fluoranthene | 100 | 242 | 1,020 ± 1,960 | 43.8–8,150 | 12.5 | 70.8 | 16.7 | |
| Pyrene | 100 | 194 | 853 ± 1,640 | 37.0–6,910 | 20.8 | 66.7 | 12.5 | |
| C1-Fluoranthenes/Pyrenes | 100 | 91.5 | 242 ± 443 | 15.4 - 2,130 | 54.2 | 45.8 | ||
| C2-Fluoranthenes/Pyrenes | 100 | 50.1 | 168 ± 316 | 7.41 - 1,540 | 66.7 | 33.3 | ||
| C3-Fluoranthenes/Pyrenes | 100 | 33.0 | 104 ± 186 | 6.08–909 | 75.0 | 25.0 | ||
| Benzo(a)anthracene | 100 | 128 | 578 ± 1,130 | 22.8 - 4,780 | 41.7 | 45.8 | 12.5 | |
| Chrysene | 100 | 195 | 828 ± 1,610 | 42.9 - 7,480 | 25.0 | 58.3 | 16.7 | |
| C1-Chrysenes | 100 | 93.5 | 300 ± 514 | 28.0 - 2,240 | 50.0 | 45.8 | 4.17 | |
| C2-Chrysenes | 100 | 55.1 | 135 ± 203 | 11.7 - 941 | 75.0 | 25.0 | ||
| C3-Chrysenes | 100 | 26.6 | 59.0 ± 87.3 | 3.96 - 424 | 87.5 | 12.5 | ||
| C4-Chrysenes | 91.7 | 18.0 | 30.2 ± 34.6 | 0.6 - 157 | 100 | |||
| Benzo(b)fluoranthene | 100 | 219 | 1,120 ± 2,380 | 53.9 - 11,600 | 16.7 | 58.3 | 25.0 | |
| Benzo(k)fluoranthene | 100 | 35.8 | 257 ± 713 | 5.75 - 3,550 | 66.7 | 33.3 | ||
| Benzo(e)pyrene | 100 | 152 | 658 ± 1,410 | 38.6 - 6,930 | 33.3 | 50.0 | 16.7 | |
| Benzo(a)pyrene | 100 | 126 | 616 ± 1,310 | 26.1 - 6,220 | 37.5 | 50.0 | 12.5 | |
| Perylene | 100 | 42.6 | 164 ± 284 | 16.2 - 1,290 | 70.8 | 29.2 | ||
| Indeno(1,2,3-c,d)pyrene | 100 | 86.9 | 513 ± 1,290 | 13.7 - 6,350 | 54.2 | 37.5 | 8.33 | |
| Dibenzo(a,h)anthracene | 100 | 20.3 | 109 ± 255 | 3.93 - 1,250 | 83.3 | 16.7 | ||
| Benzo(g,h,i)perylene | 100 | 114 | 524 ± 1,230 | 26.1 - 6,120 | 37.5 | 54.2 | 8.33 | |
| 2-Methylnaphthalene | 100 | 284 | 319 ± 172 | 63.7 - 790 | 4.17 | 95.8 | ||
| 1-Methylnaphthalene | 100 | 386 | 429 ± 238 | 84.7 - 1,090 | 4.17 | 95.8 | ||
| 2,6-Dimethylnaphthalene | 100 | 96.3 | 104 ± 47.2 | 18.1 - 214 | 58.3 | 41.7 | ||
| 1,6,7-Trimethylnaphthalene | 100 | 8.33 | 9.09 ± 3.77 | 1.59 - 20.0 | 100 | |||
| 1-Methylphenanthrene | 100 | 11.0 | 36.7 ± 57.0 | 2.42 - 208 | 87.5 | 12.5 | ||
| Total PAHs | 6180 | 12,600 ± 17,800 | 1,310 - 85,700 | 70.8 | 29.2 | |||
| TOTAL Priority 16 PAH | 3,540 | 8,760 ± 13920 | 819 - 65,700 | 4.17 | 83.3 | 12.5 | ||
| Sum Alkylated PAHs | 2,180 | 2,710 ± 2,310 | 378 - 11,600 | |||||
| Sum of Parent PAHs | 4,060 | 9,870 ± 15,600 | 930 - 74,100 | |||||
| Ratio Alkylated/Parent PAHs | 0.44 | 0.426 ± 0.148 | 0.155 - 0.754 | |||||
Table 2b.
Diagnostic ratios of some of the individual PAH used to determine if observed PAHs are from pyrogenic or petrogenic sources.
| Diagnostic ratios | Median | Mean + 1STD | Range |
|---|---|---|---|
| Anthracene/(Anthracene + Phenanthrene) [ANT/(ANT + PHE)] | 0.225 | 0.272 ± 0.181 | 0.132 - 0.880 |
| Fluoranthene/(Fluoranthene + Pyrene) [FLA/(FLA + PYR)] | 0.544 | 0.534 ± 0.039 | 0.389 - 0.590 |
| Indeno(1,2,3-c,d)pyrene/Benzo(g,h,i)perylene (InP/BgP) |
0.825 | 0.786 ± 0.217 | 0.358 - 1.15 |
Table 3.
Data for Polybrominated Diethylethers in ug/Kg d.w.. The PBDE congeners listed are generally those which are the most common in environmental samples.
| Polybrominated Diphenyl Ethers | Incidence |
Median |
Mean ± 1 STD |
Range |
|---|---|---|---|---|
| (%) | (ug/kg d.w.) | (ug/kg d.w.) | (ug/kg d.w.) | |
| BDE 28 | 45.8 | 0.5 | 0.267 ± 0.480 | 0.5 - 2.08 |
| BDE 47 | 100.0 | 2.6 | 3.96 ± 5.61 | 0.530 - 29.6 |
| BDE 99 | 100.0 | 3.5 | 4.65 ± 3.99 | 0.8 - 18.8 |
| BDE 100 | 95.8 | 1.0 | 1.24 ± 1.13 | 0.8 - 5.28 |
| BDE 153 | 41.7 | 1.0 | 0.778 ± 1.88 | 1.0 - 9.43 |
| BDE 154 | 45.8 | 1.0 | 0.333 ± 0.437 | 1.0 - 1.37 |
| Total PBDEs | 8.7 | 12.5 ± 12.5 | 1.96 - 63.3 |
Table 4.
Individual PCB congeners – all 209 congeners are measured in ug/Kg d.w. The PCB congeners listed were detected in, at least, 50% of the samples and represented >60% of the total PCB loads. The degree of chlorination (homologs) information is shown at the bottom.
| Polychlorinated Biphenyls | Incidence |
Median |
Mean ± 1 STD |
Range |
|---|---|---|---|---|
| (%) | (ug/kg d.w.) | (ug/kg d.w.) | (ug/kg d.w.) | |
| PCB 5 | 62.5 | 0.260 | 0.489 ± 0.254 | 0.5 - 1.12 |
| PCB 8 | 87.5 | 0.505 | 0.581 ± 0.285 | 0.5 - 1.53 |
| PCB 18 | 87.5 | 0.460 | 0.522 ± 0.230 | 0.5 - 1.06 |
| PCB 28/31 | 100 | 0.450 | 0.559 ± 0.309 | 0.200 - 1.50 |
| PCB 43/52 | 95.8 | 0.410 | 0.471 ± 0.294 | 0.5 - 1.54 |
| PCB 49 | 66.7 | 0.225 | 0.419 ± 0.306 | 0.5 - 1.29 |
| PCB 88/95 | 79.2 | 0.395 | 0.514 ± 0.309 | 0.5 - 1.54 |
| PCB 99 | 66.7 | 0.315 | 0.547 ± 0.420 | 0.5 - 1.90 |
| PCB 101/113 | 91.7 | 0.500 | 0.699 ± 0.494 | 0.5 - 2.52 |
| PCB 105 | 79.2 | 0.425 | 0.647 ± 0.629 | 0.5 - 3.03 |
| PCB 110 | 95.8 | 0.690 | 1.07 ± 0.875 | 0.5 - 4.48 |
| PCB 118 | 83.3 | 0.630 | 1.06 ± 0.931 | 0.5 - 4.44 |
| PCB 128/162 | 58.3 | 0.400 | 0.717 ± 0.352 | 0.5 - 1.64 |
| PCB 132/153/168 | 100 | 1.39 | 1.76 ± 1.19 | 0.380 - 4.75 |
| PCB 134/143 | 70.8 | 0.555 | 0.804 ± 0.458 | 0.5 - 2.00 |
| PCB 138/158 | 100 | 1.78 | 2.25 ± 1.58 | 0.630 - 6.97 |
| PCB 160/163/164 | 70.8 | 0.555 | 0.937 ± 0.504 | 0.5 - 1.97 |
| PCB 170/190 | 91.7 | 0.880 | 1.07 ± 0.602 | 0.5 - 2.71 |
| PCB 174/181 | 79.2 | 0.515 | 0.721 ± 0.407 | 0.5 - 2.06 |
| PCB 177 | 50.0 | 0.200 | 0.593 ± 0.343 | 0.5 - 1.49 |
| PCB 180/193 | 100 | 1.35 | 1.67 ± 1.09 | 0.580 - 5.25 |
| PCB 182/187 | 100 | 0.665 | 0.805 ± 0.503 | 0.290 - 2.55 |
| PCB 183 | 62.5 | 0.255 | 0.451 ± 0.218 | 0.5 - 0.970 |
| PCB 206 | 58.3 | 0.185 | 0.386 ± 0.148 | 0.5 - 0.62 |
| PCB 209 | 62.5 | 0.330 | 0.552 ± 0.271 | 0.5 - 1.20 |
|
Total PCBs |
17.3 |
22.7 ± 16.2 |
5.91 - 77.5 |
|
|
PCB Homologs | ||||
| Monochlorobiphenyls | - | - | <0.5 | |
| Dichlorobiphenyls | 0.665 | 0.814 ± 0.560 | <0.5 - 2.02 | |
| Trichlorobiphenyls | 1.19 | 1.39 ± 0.955 | 0.310 - 4.39 | |
| Tetrachlorobiphenyls | 0.92 | 2.03 ± 2.57 | 0.190 - 11.3 | |
| Pentachlorobiphenyls | 3.77 | 4.54 ± 4.18 | 0.200 - 20.6 | |
| Hexachlorobiphenyls | 5.05 | 6.63 ± 4.60 | 1.40 - 19.9 | |
| Heptachlorobiphenyls | 4.36 | 4.95 ± 3.57 | 1.30 - 17.2 | |
| Octachlorobiphenyls | 1.86 | 3.65 ± 5.44 | <0.5 - 24.5 | |
| Nonachlorobiphenyls | 0.185 | 0.280 ± 0.299 | <0.5 - 0.960 | |
| Decachlorobiphenyl | 0.330 | 0.345 ± 0.342 | <0.5 - 1.20 | |
Table 5.
Individual organochlorine compounds in ug/Kg d.w. HCH isomers (alpha, beta, gamma, and delta), Heptachlor and its epoxide, Oxychlordane, Aldrin, Dieldrin, Endrin, Chlorpyrifos, Endosulfan I and II, 2,4′-DDE, 2,4′-DDD, 2,4′-DDT, and 4,4′-DDT were not detected.
| Chlorinated Pesticides | Incidence |
Median |
Mean ± 1 STD |
Range |
|---|---|---|---|---|
| (%) | (ug/kg d.w.) | (ug/kg d.w.) | (ug/kg d.w.) | |
| Tetrachlorobenzene 1,2,4,5 | 12.5 | - | 0.173 ± 0.0556 | 0.5 - 0.25 |
| Tetrachlorobenzene 1,2,3,4 | 20.8 | - | 0.196 ± 0.0625 | 0.5 - 0.28 |
| Pentachlorobenzene | 58.3 | 0.221 | 0.400 ± 0.201 | 0.5 - 0.99 |
| Hexachlorobenzene | 70.8 | 0.465 | 0.761 ± 0.525 | 0.5 - 2.00 |
| Alpha Chlordane | 91.7 | 3.94 | 15.1 ± 23.0 | 0.5 - 88.3 |
| Gamma Chlordane | 95.8 | 3.87 | 12.2 ± 17.7 | 0.5 - 65.9 |
| Cis-Nonachlor | 100 | 3.03 | 6.47 ± 7.75 | 0.66 - 30.9 |
| Trans-Nonachlor | 100 | 6.75 | 16.0 ± 20.8 | 1.17 - 84.8 |
| Total Chlordane | 17.60 | 48.0 ± 67.6 | 3.44 - 270 | |
| Pentachloroanisole | 16.7 | - | 0.580 ± 0.395 | 0.5 - 1.26 |
| Mirex | 4.17 | - | - | |
| 4,4′ DDE | 66.7 | 1.45 | 2.85 ± 2.43 | 0.5 - 9.68 |
| 4,4′ DDD | 12.5 | 0.5 | 1.98 ± 0.604 | 0.5 - 2.71 |
| Total DDTs | 1.56 | 2.14 ± 2.51 | 0.5 - 9.68 |
3.2. Trace metals
The trace metal results for all 24 soil samples are presented in Table 1 as mg/kg on a sediment dry weight basis normalized to Fe (Summers et al., 1996). The mean and relative percent standard deviation (RPSD) were low, with most samples below 40% RPSD, suggesting reasonably homogeneous samples. Only those elements that have very low concentrations, such as berylium and thallium are over 100 % RPSD. Generally, the concentrations of the metals are much lower than their Crustal Abundance (CA) by 6%–25% (Webelements.com, 2007). The only exception to the metals measured is Zn with a mean concentration of 493 ± 115 mg/kg and an Enrichment Factor (EF) of 6.2 times its CA. Other elements which are normally of concern to human health are arsenic (5.1 ± 3.4 mg/kg; EF 2.4), cadmium (0.9 ± 0.3 mg/kg; EF 5.8), lead (51.6 ± 22.1 mg/kg, EF 5.1), Methyl Hg in the samples was very low with a mean of 0.12 μg/kg and EF 1.8, Other metals such as Chromium (11.2 ± 2.6 mg/kg), copper (12.2 ± 8.0 mg/kg), nickel (5.7 ± 1.5 mg/kg) were well below their Crustal Abundance (Table 1). Selenium (4 ± 0.6 mg/kg) was 82 times its crustal abundance.
3.3. Kriging
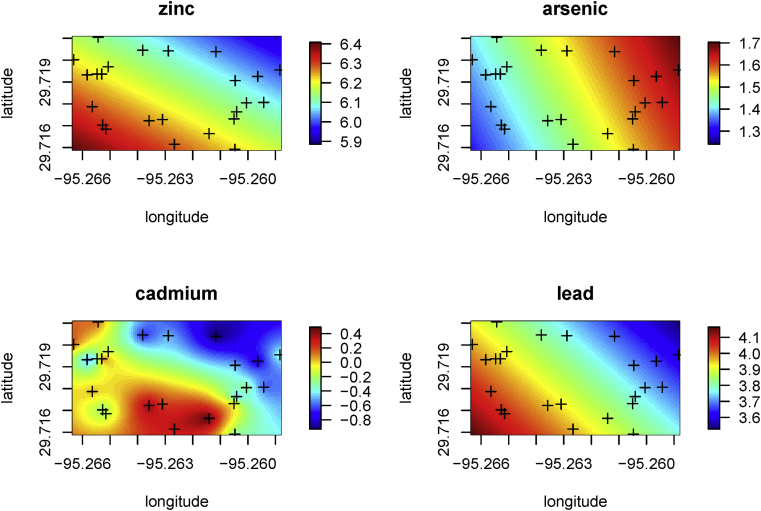
Kriging was applied for every element measured but there were only a few definitive relationships with concentration versus location. Kriging the elevated Zn concentrations did point to higher concentrations of Zn in the Southwest of our study site (Fig. 3), which is proximal to a 24-line Union Pacific railyard. It is possible that this was the source of the high Zn concentrations. For organics, only PAHs data are shown because there were no hotspots for other organic contaminants.
Fig. 3.
Kriged results (log scale) of Zn, Cd, As, Pb in soils from 24 samples in Manchester, Texas.
3.4. Polycyclic aromatic hydrocarbons
The U.S. Environmental Protection Administration (EPA) classified 16 PAHs as priority pollutants in 1976 (ATSDR, 2005). Table 2a provides the data from the 24 sampling sites in Manchester, Texas, this community is surrounded by refineries and highways, known PAH sources. The total PAH measured is provided in the table as well as the concentrations of the 16 priority pollutant PAHs in Table 2a. The mean of all 45 PAH measured is 12,600 μg/kg dry weight (range 1,310 - 85,700 μg/kg) and the mean of the 16 Priority Pollutant PAH are 8,760 μg/kg with a range of 819–65,700 μg/kg (Table 2a).
Baseline PAH soil concentrations are considered to range between 10 and 10,000 μg/kg for total PAH, while urban background range between 1,000 to 100,000 μg/kg (Bakker et al., 2000; IARC, 1983; MDEP, 1996). Among the more volatile carcinogenic PAHs, Menzie et al. (1992) noted a range of 10 to 1,300 μg/kg in forest and rural soil. Based on the 15 samples described in Teaf et al. (2008) for Florida, urban soils ranged from 60 to 5,800 μg/kg (median of 1,100 μg/kg); these values are lower than the mean and median of 12,293 μg/kg reported for this study.
Determination of petrogenic and pyrogenic sources are possible through analyzing branched versus the parent compounds rather than just the EPA priority pollutants. These sources can be determined from key ratios of these pyrogenic versus petrogenic compounds (Table 2b).
PAH's molecular masses (e.g. 178, 202, and 228) have been used to distinguish between combustion and petroleum sources (Gschwend and Hites, 1981; Socolo et al., 2000; Yunker et al., 2002). An anthracene to (anthracene + phenanthrene) ratio is used where a ratio less than 0.1 indicates a predominantly petrogenic source while over 0.1 is pyrogenic (Budzinski et al., 1997). The mean of the Manchester samples was 0.272 + 0.181 indicating pyrogenic. A fluoranthene to (fluoranthene + pyrene) ratio greater than 0.50 is considered pyrogenic, while a ratio 0.4 to 0.5 is considered petrogenic. The mean of the samples reported here are 0.534 + 0.039. A ratio of indeno(2,2,3,-c,d)pyrene to (indeno(2,2,3,-c,d)pyrene + benzo(g,h,i)perylene, Yunker et al., 2002) where a ratio of less than 0.2 indicates a petrogenic source and over 0.2, pyrogenic. The results on Table 2b suggest a mean of 0.786 +/- 0.217. The 24 Manchester samples are overwhelmingly a petrogenic source assumed to come from the major highways from the area as well as from the many refinery complexes nearby.
3.5. Polybrominated diphenyl ethers (PBDE)
In this study 40 PBDE congeners, which are usually found in the environment, were analyzed and the results of the 6 most prevelant PBDE's are presented in Table 3. There were many non-detected PBDEs of those normally reported. Only BDE 28 (2,4,4′-TriBDE), BDE 47 (2,2′,4,4′-TetraBDE), BDE 99 (2,2′,4,4′,5-PentaBDE) and BDE 100 (2,2′,4,4′,6-PentaBDE) were detected in nearly all samples with total concentrations ranging from 2.0 μg/kg soil to 63.3 μg/kg soil. Generally, the penta BDE (e.g. PBDE 100) are of the most concern as they accumulate in humans (Lorber, 2017). McDonald (2005) cited Geyer et al. (2004) to assign half-lives within the body of 3.0 years for BDE 47, 5.4 years for BDE 99, and 2.9 years for BDE 100. Offenberg et al. (2006) studied 33 surface soil concentrations from 15 US states and found that BDE 47 was present in 31 out of the 33 samples at an average of 1.9 μg/kg compared to 3.9 μg/kg in this study. BDE 99 was found in 30 of the 33 soil samples at 3.6 μg/kg (Offenberg et al., 2006) where these 24 Manchester sample mean was very similar at 4.9 μg/kg.
3.6. Polychlorinated biphenyls
Aroclor was produced from approximately 1930 to 1979. It is one of the most commonly known trade names for PCB mixtures. There are many types of Aroclors and each has a distinguishing suffix number that indicates the degree of chlorination. The numbering standard for the different Aroclors are the following: the first two digits usually refer to the number of carbon atoms in the phenyl rings (for PCBs this is 12) and the second two numbers indicate the percentage of chlorine by mass in the mixture. For example, the name Aroclor 1254 means that the mixture contains approximately 54% chlorine by weight.
The results from our analysis of PCBs in the 24 soil samples from Manchester, Texas are presented in Table 4. Many of the isomers that were not detected PCB congeners are not listed, although we did analyze for all 209 PCBs, either as a single molecule or co-eluting congeners. The mean of all samples 27.7 +/- 16.2 μg/kg and the range was 5.9–88.5 μg/kg.
3.7. Organochlorine pesticides
Table 5 provides data on the analysis of organochlorines in the 24 soil samples from Manchester. Pentachlorbenzene and hexachlorobenzene were the most prevalent, although concentrations were very low, as expected since samples were from neighborhood sites as opposed to industrial sites. Surprisingly, there were no hexachlorocyclohexanes present and no heptachlor, heptachlor epoxide or oxychlordane. However, alpha, gamma, cis and trans nonachlor were present in significant concentrations with the range from the 24 sites to be 3.5 μg/kg to 270 μg/kg with a mean of 48.0 μg/kg. A previous study of soil concentrations of 30 homes in the US ranged from 22.4 to 2,540 μg/kg (ATSDR, 1994). Chlordanes are from the cyclodiene family and were sold in the US from 1948 to 1988 due to their effective pest control for fire ants. Because of growing concern for adverse health, primarily liver cancer, and environmental effects, the US EPA banned all uses of chlordane in 1983 except for termite control in wooden structures (e.g. houses). Chlordane use was permanently banned in 1988; although it is still manufactured in the US, it must be sold outside of the US. Since chlordanes take 25–35 years to breakdown, they are considered as persistent compounds. However, to effectively evaluate our chlordane values, it was difficult to locate comparable modern literature concentration values of chlordane in soils. Pentachloroanisole was found in 4 samples but at very low concentrations and Mirex was found in one sample (4) at a concentration of 10.9 μg/kg. Mirex was banned in 1978 and like chlordane was very effective against fire ants.
The only other major compound found in 16 of the 24 sites in this study was 4,4′ DDE. This para, para DDE is the main breakdown product of the banned pesticide DDT and is present at range of nd 9.7 μg/kg. DDT (dichloro-diphenyl-trichloroethane) was developed as the first of the modern synthetic insecticides in the 1940s. It was initially used with great effect to combat malaria, typhus, and the other insect-borne human diseases among both military and civilian populations. It also was effective for insect control in crop and livestock production, institutions, homes, and gardens. DDT's quick success as a pesticide and broad use in the United States and other countries led to the development of resistance by many insect pest species. In 1972, EPA issued a cancellation order for DDT based on its adverse environmental effects, such as wildlife toxicity, as well as its potential human health risks (Bitman et al., 1970). Thus 4,4′ DDE is ubiquitous in the environment well after the parent compound (DDT) has gone.
3.8. Distribution of contaminants
The fact the Galveston estuary is regularly impacted by tropical storms and hurricanes makes it an interesting site for the study of the redistribution of contaminated sediments in a vulnerable coastal area (Roth, 2010). According to Freedman (2012), the Houston-Galveston area is the 5th most vulnerable city in the US to the impacts of hurricanes. In 2008, Hurricane Ike, a Category 2 storm, made landfall on the east end of Galveston Island, traveling north up Galveston Bay along Houston's east side (NOAA, 2009). Hurricane Ike caused an estimated $37.6 billion in damages and killed 37 people in Texas. Hurricane Ike generated a storm surge of up to 20 feet across much of eastern Texas and also produced up to 20 inches of rain along Spring Creek near Houston, which exacerbated inland flooding (NOAA, 2009). In the immediate aftermath of Ike, large piles of debris were transported far inland and reports of sludge by many residents in the area. Chemical substances were also found to have spilled during the storm (TCEQ, 2008).
The Houston area is highly vulnerable to flooding from the combined threat of storm surge and inland precipitation, which is exacerbated by subsidence and sea level rise. Extensive subsidence, caused mainly by extracting of ground-water for community needs as well as oil and gas extractions (Holzer and Galloway, 2005) has increased the frequency of flooding, as well as the likelihood that flooding will cause damage to industrial and transportation infrastructure and lead to substantial loss of wetland habitat. Levees, reservoirs, and surface-water distribution facilities have been developed across the region for mitigation of flooding. The main increase in “nuisance flooding” is on the US east coast and along the Gulf coast. In fact, the average sea-level rise globally is 3.1 mm/yr (Church and White, 2011); however, Sabine Pass and Galveston, Texas have recorded increases of 5.49 and 6.1 mm/yr (Sweet et al., 2014).
Although there have been many studies on the mobilization of contaminants by disasters (Plumlee et al., 2012) there have only been a few on tropical cyclones (Knap and Rusyn, 2016). This is primarily due to the relative infrequency of major land-falling hurricanes in the US that would mobilize contaminated soils and sediments. Each storm is different; however, what made Hurricane Harvey unique was that residents experienced the full complement of hurricane hazards (Shultz and Galea, 2017; Shultz et al., 2005). Hurricane Harvey made landfall with Category 4 winds (up to 130 mph), which impacted some Texas coastal towns initially and subsequently produced up to 60 inches of rainfall over Houston, with total rainfall amounts estimated to be 11 trillion gallons, 44 trillion liters (National Weather Service, 2017). Storm surge was less than the predicted 13 feet, measuring only about 6.7 feet at Port Lavaca, TX, south of Houston, and 9.3 feet at the HSC in Manchester (NOAA, 2017). Although the neighborhood of Manchester was heavily impacted by rain, we did not access the area until the flooding had subsided on September 1, 2017. It was expected that many of the contaminants would have been washed out into the nearby HSC or Sims Bayou, on to Galveston Bay, and into the ocean. Sediment cores taken after Hurricane Harvey showed a mixed layer of sediment of up to 12 cm which had been scoured from the land over the whole area (Dellepena, personal communication). The results of this present study did show variable but measurable values of soil contaminants as one would expect while contaminants soluble in water (e.g. trace metals), showed the least variability.
The growth of disaster frequencies and human populations living near industrialized areas has brought greater attention to the potential health effects of environmental contamination associated with joint natural and technological (na-tech) disasters (IFRC, 2016; UNISDR, 2016; Young et al., 2004; Noji, 1997; WHO, 2018). Na-tech events occur as a result of disaster-associated technological malfunction or failure, leading to the unintentional release of hazardous materials (Young et al., 2004). For example, after Hurricane Floyd, more than 50 large-scale hog waste lagoons in North Carolina were inundated or breached, contaminating nearby waterways with animal waste (Schmidt, 2000). Following the Indian Ocean Tsunami of 2004, sewage overflow, chemical releases from farms and factories, and hazardous medical and household chemical debris threatened the quality of ground and surface water (Srinivas and Nakagawa, 2008). In the aftermath of Hurricane Harvey, potential environmental hazards resulting from chemical plant explosions (Newkirk, 2017), flooding of a designated Superfund site (Chapin, 2017), and toxic emissions from a refinery located in the Manchester neighborhood (Evans, 2017) were documented by the media.
Perhaps the most impressive, but unpublished, collection of baseline data available for the Houston area was collected by the Texas Council on Environmental Quality (TCEQ) following Hurricane Ike in 2008. Sampled areas included approximately 380 square miles of Brazoria, Chambers Fort Bend, Galveston, Hardin, Jefferson, Liberty, Montgomery and Orange Counties, with up to 3 samples taken per one square mile grid (unpublished data report). Of 85 residue samples, only two showed elevated levels of contaminants and soils were removed as remediation. Only a few of the analytes measured by the present study were comparable to 2008 TCEQ samples. For inorganic compounds, Arsenic, Barium, Chromium and Lead were reported for 76 samples. With a mean of 7.3 mg/kg As, Ba was 171.6 mg/kg, Cr was 37.1 mg/kg, Pb was 23.8 mg/kg and Hg was 0.06 mg/kg. Selenium was also measured in some samples and was close to the detection limit for only a few samples and not reported in Tables. These levels of mainly rural residues compare to those of the Manchester area of 5.1 mg/kg As, Ba was 122.1 mg/kg, Cr was 11.1 mg/kg. Pb was slightly elevated at Manchester at 51.6 mg/kg and Hg was double at 0.12 mg/kg. Organic analyses, when reported, were reported for much fewer samples than the inorganic samples and only some, not all, of the EPA Priority pollutants were reported as there was no opportunity to determine sources of PAHs (pyrogenic versus petrogenic). This makes comparisons very difficult to our Manchester study. We set the non-reported data to zero as the TCEQ report only highlighted certain PAH found in some samples. If we take the mean of all of the Priority Pollutant samples we get an under-reported mean of 4,311 μg/kg compared to our mean of Priority Pollutants (PP) of 7,796 μg/kg. The two contaminated sites, which were remediated by TCEQ, had a reported mean of PP of 46,491 and 59,245 μg/kg. These fit within the range of 819–65,700 μg/kg PP PAH for Manchester with the range of the individual PAH, Benzo (a) pyrene was 26.1 to 6,220 μg/kg. When the range (819–65,700 μg/kg) of PAHs for Manchester soils were compared with PAHs ranges (for 16 priority PAHs) found in soils from highly urbanized cities around the world (Table 6), PAHs ranges in Manchester are on the higher side. It was also interesting to see that Manchester, Texas soil has highest amount of PCBs in soil (5.91–77.5 μg/kg) compared to other cities in the World. For example, Cachada et al. (2009) found 0.62–73 μg/kg PCBs in soils of 5 European cities or Creaser et al. (2007a) found 0.98–39.34 μg/kg of PCBs in UK (Table 6)).
Table 6.
PAHs and PCBs concentrations in Manchester, Texas soils and compared with the soils from around the world.
| PAHs | Mean (μg/kg) | Range(μg/kg) | ||
|---|---|---|---|---|
| Manchester, Houston, Texas | 16 Priority PAHs | 8760 ± 13920 | 819 - 65700 | This study |
| Taragona County, Spain | 16 Priority PAHs | 97.2 ± 104.3 | Nadal et al. (2009) | |
| Glasgow, UK | 16 Priority PAHs | 16190 ± 12030 | 15300 - 88070 | Kim et al. (2017) |
| Shanghai, China | 16 Priority PAHs | 1970 ± 1450 | 80 - 7220 | Wang et al. (2013) |
| London, UK | 16 Priority PAHs | 18000 ± 14000 | 4000 - 67000 | Vane et al. (2014) |
| South China | 16 Priority PAHs | 4940 | 1170 - 10600 | Wang et al. (2012) |
| Beijing, China |
16 Priority PAHs |
1228.1 ± 180 |
93.3 - 13141.5 |
Peng et al. (2011) |
|
PCBs | ||||
| Manchester, Houston, Texas | 25 PCBs | 22.7 ± 16.2 | 5.91 - 77.5 | This study |
| UK | 26 PCBs | 3.04 ± 1.86 | 0.98 - 39.34 | Creaser et al. (2007a) |
| 5 Europian Cities | 19 PCBs | 7.9 | 0.62 - 73 | Cachada et al. (2009) |
| Madrid | 20 PCBs | 32.0 | 9.0 - 66 | Garcia-Alonso and Perez-Pastor (2003) |
| Moscow | 17 PCBs | 13.9 ± 9.9 | 3.1 - 42 | Wilcke et al. (2006) |
| Harfleur, France | 22 PCBs | 40.1 | Motelay-Massei et al. (2004) | |
| Taragona County, Spain | 7 PCBs | 12.0 ± 11.6 | Schuhmacher et al. (2004) | |
Post-Harvey findings can also be compared to data on contaminants collected following Hurricane Katrina. Hurricane Katrina made landfall in Plaquemines Parish, Louisiana in August 2005 as a strong Category 3 storm. Similar to the City of Houston, the Louisiana coast had been subsiding, while wetlands were concurrently being damaged by oil and gas development. The City of New Orleans is below sea level, but was thought to be protected by pumps and an extensive levee system. Due to pump failures associated with Katrina, levees were breached, leading to severe flooding similar to flooding associated with Hurricane Harvey. However, Hurricane Katrina brought only 4 inches of rain in the New Orleans area, compared with up to 60 inches associated with Hurricane Harvey in Houston; the difference was the pump and levee failures. Roper et al., (2006) report data from three of the highest contaminant values in samples of sediment deposited on land from Katrina in 2005. Benzo (a) pyrene was 0.77 mg/kg in their study where our concentrations ranged from 0.06 mg/kg to a high of 6.22 mg/kg with a mean from all our 24 samples at 0.62 mg/kg, they did not report the range. Following Hurricane Katrina in September, 2005, Presley et al. (2006) reported PAH concentrations measured in soil from New Orleans found the highest concentrations of B[a]A, B[b]F, and B[a]P to be 1.64, 1.88, and 1.26 mg kg−1 soil, respectively. Our concentration after Harvey were B(a)A 4.8, B(b)F 11.5 and B(a) P 6.3 mg/kg of soil, significantly higher but highly influenced by site 7 and 22. Their 3 highest arsenic numbers were 14.4–17.6 mg/kg where our highest three were 10.6–16.5 mg/kg – both values for each area are very similar. However, Rotkin-Ellman et al. (2010) compared pre-Katrina samples to be between 2.7 to 4.9 mg/kg but saw an increase in 20.3–25.8 mg/kg. Over an 18-month time period, these observed levels decreased significantly to a mean of 3.6 mg/kg. With background levels of arsenic in the Mississippi Delta region reported to be 10 mg/kg (Gustavsson et al., 2002), the observed levels post-Katrina saw a dramatic flux in arsenic levels.
The EPA tested sediments for Pb after Katrina, since lead has been a long standing concern for the inner city of New Orleans. About 40 percent of nearly 5,000 soil samples showed Pb levels in excess of 400 mg/kg (Pelley, 2006). Presley et al. (2006) found lead in excess of 400 mg/kg (405 and 642 mg/kg) in two of 12 sediment samples, consistent with the pre-Katrina observations of Mielke et al., 2005. The highest Pb level in this study was 91 mg/kg with the mean of 52 mg/kg Bera et al. (2018) investigated pollutant distribution caused by Katrina in St. Louis Bay, Mississippi (maximum inundation zone during Katrina). They found that sediments enriched with anthropogenic stable cesium (EF value up to 13) were redistributed from a nearby DuPont factory and deposited in the surrounding marsh. However, Reibl et al. (2008) concluded that with so little baseline data, it was impossible to determine statistically the effects Katrina had on the mobilization of contaminants in New Orleans after Katrina. Bera et al. (2018) paper describes the impact of Hurricane Katrina on pollution distribution in St. Louis Bay. It shows that during Katrina pollutants from a nearby DuPont factory deposited in marsh sediment.
Contaminant mobilization was demonstrated in New York and New Jersey after Superstorm Sandy made landfall there as a Category 2 storm in October, 2012. While Mandigo et al. (2016) reported contaminated soil concentrations for pre- and post-Sandy for Arsenic, Lead, PCBs and PAH, this present study had no pre-Harvey soil samples. Mandigo et al. (2016) reported the lead levels from Flushing pre–Sandy were 375 mg/kg and for another area in Rockaway point were 88.4 mg/kg, Arsenic was 20 mg/kg from Flushing. After Sandy, there was a reduction in both lead and arsenic concentrations to 289 mg/kg and 9 mg/kg respectively; the authors also suggested the presence of high intra-sample variability. In contrast, our mean concentration for Pb was 52 mg/kg and As mean concentration was 5 mg/kg; both were much lower than the concentrations reported post-Sandy. The post-Sandy samples had total PAH concentrations between 6.8 and 9.9 mg/kg for 2 areas with PCB's at 0.4–0.7 mg/kg. Our PCB concentrations were over an order of magnitude lower with a mean of 0.027 mg/kg, while our PAH concentrations were at a much higher range between 1.3 and 85.7 mg/kg. Since the Manchester community is surrounded by a number of refineries, our PAH ratio analysis indicates the main contributing PAH source results from combustion; two likely point sources include the surrounding refineries as well as the major highways in the Houston area.
We have used Kriging to look at the distribution of zinc, cadmium, lead, the only metals above their CA (Fig. 3). The data show increased zinc in the southwest of the study area. The most probable source is from railyard activities and Highway 610 (Fig. 2). Arsenic levels were elevated in the Northwest of the site but we have no view why the pattern looked like this.
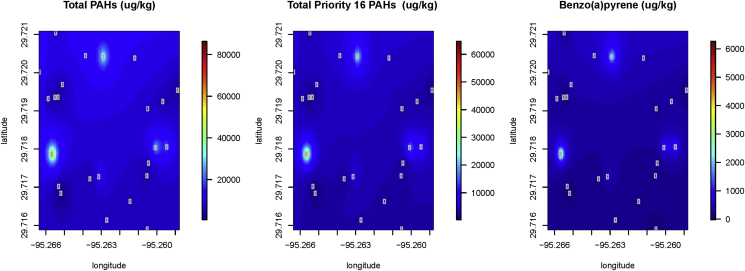
Fig. 4 provides a visual description of the comparison of sites through Kriging. The only values that show a recognizable pattern are the high PAH, EPA Priority 16 PAH and Benzo(a)pyrene concentrations compared to other sites. The western part of the sampling area, which are adjacent the elevated Interstate Highway 610, a major road artery for the City of Houston, had higher concentrations of these pyrogenic-sourced compounds. We propose that these elevated concentrations came from the washout of PAH from car exhaust rather from the Valero refinery to the north of the study area (Fig. 1).
Fig. 4.
Kriged results of Total PAH, 16 Priority PAH and Benzo(a)pyrene in soils from 24 samples in Manchester, Texas.
4. Conclusion
The long-term environmental effects of natural disasters are little considered, except by those residents living in the affected area. This may be changing as the focus on long-term recovery grows through the support for multiyear studies that attempt to quantify the ongoing human and environmental impacts (Cutter et al., 2006; Manuel, 2013; Lichtveld et al., 2016). For example, studies conducted after Hurricane Katrina and the Deep Water Horizon oil spill in Louisiana are coalescing around the importance of assessing immediate human and economic damages as well as the long-term impacts on housing, business, and physical and mental health (Knap and Rusyn, 2016).
In this small case study, we were able to collect 24 surface soil samples in the Manchester neighborhood of Houston one week after Hurricane Harvey made landfall. Sampling locations were established as part of an ongoing pilot cohort study that began in December of 2015 (Horney et al., 2018). The analysis of contaminants was extensive (e.g., trace metals, PAHs, PBDE (fire retardants), organochlorine pesticides and pesticides, and will provide critically needed baseline data for a neighborhood which is subject to frequent flooding. There were two sites where concentrations were notably elevated. This finding was somewhat surprising given the volume of rain associated with Hurricane Harvey and the expected dilution. After flood waters recede, the land dries and contaminants may be left behind. In this case, PAH contaminants were left behind in measurable quantities. No analyses have reported measurements in relation to other disasters across as wide a range of contaminants as this study, so a comprehensive comparison to other areas and storms was not possible for many of the analytes. Some studies exist on marine sediment concentrations for these areas, but very few examined the potential for storm-mobilized contaminants. Nonetheless, given the likelihood of future storms in the Houston area, these reported concentrations can serve as a useful baseline for future investigations.
Declarations
Author contribution statement
Gopal Bera: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Krisa Camargo, Stephen T Sweet: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jose L Sericano, Yina Liu, Mikyoung Jun, Weihsueh A Chiu: Performed the experiments; Analyzed and interpreted the data.
Jennifer Horney, Ivan Rusyn, Terry L Wade: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Anthony H Knap: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award P42 ES027704.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank two anonymous reviewers for their comments. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- American Community Survey . 2016. Houston-the Woodlands – Sugar Land TX Metro Area.https://censusreporter.org/profiles/31000US26420-houstonthe-woodlandssugar-land-tx-metro-area/ Available at. [Google Scholar]
- ATSDR . Public Health Service, U.S. Department of Health and Human Services; Atlanta, GA: 1994. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Chlordane (Update) [Google Scholar]
- Bakker M.I., Casado B., Koerselman J.W., Tolls J., Kolloffel C. Polycyclic aromatic hydrocarbons in soil and plant samples from the vicinity of an oil refinery. Sci. Total Environ. 2000;263:91–100. doi: 10.1016/s0048-9697(00)00669-0. [DOI] [PubMed] [Google Scholar]
- Bera G., Yeager K.M., Shiller A.M. Hurricane Katrina impact on trace metal and dioxin deposition in fringing marshes of St. Louis Bay, MS. Sci. Total Environ. 2018;624(2018):517–529. doi: 10.1016/j.scitotenv.2017.12.156. [DOI] [PubMed] [Google Scholar]
- Bitman J., Cecil H.C., Fries G.F. DDT-induced inhibition of avian shell gland carbonic anhydrase: a mechanism for thin eggshells. Science. 1970;168(3931):594–596. doi: 10.1126/science.168.3931.594. [DOI] [PubMed] [Google Scholar]
- Budzinski H., Jones I., Bellocq J., PieÅLrard C., Garrigues P. Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Mar. Chem. 1997;58:85–97. [Google Scholar]
- Cachada A., Lopes L.V., Hursthouse A.S., Biasioli M., Grcman H., Otabbong E., Davidson C.M., Duarte A.C. The variability of polychlorinated biphenyls levels in urban soils from five European cities. Environ. Pollut. 2009;157:511–518. doi: 10.1016/j.envpol.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Chakraborty J., Collins T., Grineski S., Montgomery M., Hernandez M. Comparing disproportionate exposure to acute and chronic pollution risks: a case study in Houston, Texas. Risk Anal. 2014;34(11):2005–2020. doi: 10.1111/risa.12224. PMID: 24913274. [DOI] [PubMed] [Google Scholar]
- Chapin J. 2017. EPA Approves Plan to Clean up San Jacinto Waste Pits. KHOU [newspaper on the Internet]. Oct 11 [cited 2017 Oct 23]http://www.khou.com/news/local/epa-approves-plan-to-clean-up-san-jacinto-waste-pits/482678370 Available from. [Google Scholar]
- Church J., White N. Sea-level rise from the late 19th to the early 21st century. Surv. Geophys. 2011;32(4):585–602. [Google Scholar]
- City-Data . 2018. Houston Economy.http://www.city-data.com/us-cities/The-South/Houston-Economy.html Available at. [Google Scholar]
- City of Houston Planning and Development . 2014. Super Neighborhood Resource Assessment.http://www.houstontx.gov/planning/Demographics/docs_pdfs/SN/65_Harrisburg_Manchester.pdf [cited 2015 November 7]. Available from: [Google Scholar]
- Creaser C.S., Wood M.D., Alcock R., Copplestone D., Crook P.J. Environment Agency; Bristol, UK: 2007. UK Soil and Herbage Pollutant Survey: UKSHS Report No. 8, Environmental Concentrations of Polychlorinated Biphenyls (PCBs) in UK Soil and Herbage. [Google Scholar]
- Cutter S.L., Emrich C.T., Mitchell J.T., Boruff B.J., Gall M., Schmidtlein M.C., Melton G. The long road home: race, class, and recovery from Hurricane Katrina. Environment. 2006;48(2):8–20. [Google Scholar]
- Data, USA. 2017. https://datausa.io/profile/geo/houston-sugar-land-baytown-tx-metro-area/ [Google Scholar]
- Di Liberto R. National Oceanographic and Atmospheric Administration; Silver Spring, MD: 2017. Reviewing Hurricane Harvey's Catastrophic Rain and Flooding.https://www.climate.gov/news-features/event-tracker/reviewing-hurricane-harveys-catastrophic-rain-and-flooding Available: [Google Scholar]
- El-kady A.A., Wade T.L., Sweet S.T., Sericano J.L. Distribution and residue profile of organochlorine pesticides and poly chlorinated biphenyls in sediment and fish of lake Manzala, Egypt. Environ. Sci. Pollut. Control Ser. 2017;24:10301–10312. doi: 10.1007/s11356-017-8714-1. [DOI] [PubMed] [Google Scholar]
- Evans M. After oil refinery is damaged by Harvey, benzene is detected in Houston area. Wall Str. J. 2017 https://www.wsj.com/articles/after-oil-refinery-is-damaged-by-harvey-benzene-is-detected-in-the-air-in-houston-area-1504638772 [newspaper on the Internet]. Sept 5 [cited 2017 Oct 23]. Available from. [Google Scholar]
- Freedman A. 2012. Top 5 Most Vulnerable U.S. Cities to Hurricanes.http://www.climatecentral.org/news/top-5-most-vulnerable-us-cities-to-hurricanes Available at: [Google Scholar]
- Garcia-Alonso S., Perez-Pastor R.M. Occurrence of PCBs in ambient air and surface soil in an urban site of Madrid. Water Air Soil Pollut. 2003;146:283–295. [Google Scholar]
- Gardinali P.R., Wade T.L. Contribution of PAHs, PCBs and PCDD/PCDF to the 2,3,7,8-TCDD induction equivalents (IEQs) in environmental samples. Mar. Pollut. Bull. 1998;37:27–31. [Google Scholar]
- Geyer H.J., Schramm K.W., Darnerud P.O., Aune M., Anton F.E., Fried K.W. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 2004;2004(66):3820–3825. [Google Scholar]
- Gschwend P.M., Hites R.A. Fluxes of polycyclic aromatic hydrocarbons to marine and lacustrine sediments in the northeastern United States. Geochem. Cosmochim. Acta. 1981;45:2359–2367. [Google Scholar]
- Gustavsson N., Blviken B., Smith D., Severson R. U.S. Geological Survey; Denver, Colo.: 2002. Geochemical Landscapes of the Conterminous United States—New Map Presentations for 22 Elements. U.S. Geological Survey Professional Paper 1648.http://pubs.usgs.gov/pp/p1648/p1648.pdf Available online at. [Google Scholar]
- Holzer T.L., Galloway D.L. Impacts of land subsidence caused by withdrawal of underground fluids in the United States. In: Ehlen J., Haneberg W.C., Larson R.A., editors. Humans as Geologic Agents: Reviews in Engineering Geology. Vol. 16. 2005. pp. 87–99. Available online at http://reg.gsapubs.org/content/16/87.abstract. [Google Scholar]
- Horney J.A., Casillas G.A., Baker E., Stone K.W., Kirsch K.R., Camargo K., Wade T.L., McDonald T.J. Comparing residential contamination in a Houston environmental justice neighborhood before and after Hurricane Harvey. PLoS One. 2018;13(2):e0192660. doi: 10.1371/journal.pone.0192660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) vol. 32. World Health Organization; Lyons, France: 1983. (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Polynuclear Aromatic Compounds: Part I. Chemical, Environmental and Experimental Data). [PubMed] [Google Scholar]
- International Federation of the Red Cross and Red Crescent Societies (IFRC) The Federation; Geneva: 2016. World Disasters Report.http://media.ifrc.org/ifrc/publications/world-disasters-report-2016/ Available from: [Google Scholar]
- Kim A., Vane C., Moss-Hayes V., Beriro D., Nathanail C., Fordyce F., Everett P. Polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in urban soils of Glasgow, UK. Earth Environ. Sci. Trans. Roy. Soc. Edinb. 2019;108(2-3):231–247. [Google Scholar]
- Knap A.H., Rusyn I. Environmental exposures due to natural disasters. Rev. Environ. Health. 2016;31:89–92. doi: 10.1515/reveh-2016-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cam Lucien. Maximum likelihood – an introduction. ISI Review. 1990:153–171. [Google Scholar]
- Lichtveld M., Sherchan S., Gam K.B., Kwok R.K., Mundorf C., Shankar A., Soares L. The Deepwater Horizon oil spill through the lens of human health and the ecosystem. Curr. Environ. Health Rep. 2016;3(4):370–378. doi: 10.1007/s40572-016-0119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated dipheny ethers. J. Expo. Sci. Environ. Epidemiol. 2017;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- Mandigo A.C., DiScenza D.J., Keimowitz A.R., Fitzgerald N. Chemical contamination of soils in the New York city area following hurricane Sandy. Environ. Geochem. Health. 2016;38:1115–1124. doi: 10.1007/s10653-015-9776-y. [DOI] [PubMed] [Google Scholar]
- Manuel J. The long road to recovery: environmental health impacts of Hurricane Sandy. Environ. Health Perspect. 2013;121(5):a152. doi: 10.1289/ehp.121-a152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheron G. Principles of geostatistics. Econ. Geol. 1963:1246–1266. [Google Scholar]
- McDonald T.A. Polybrominated diphenyl ether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Int. Env. Ass. Mgmt. 2005;1:343–354. [PubMed] [Google Scholar]
- MDEP . 1996. Background Levels of Polycyclic Aromatic Hydrocarbons and Metals in Soil.https://semspub.epa.gov/work/05/424346.pdf [Google Scholar]
- Menzie C.A., Potocki B.B., Santodonato J. Ambient concentrations and exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 1992;26(7):1278–1284. [Google Scholar]
- Mielke H.W., Gonzales C., Powell E., Mielke P.W., Jr. Changes of multiple metal accumulation (MMA) in New Orleans soil: preliminary evaluation of differences between Survey I (1992) and Survey II (2000) Int. J. Environ. Res. Public Health. 2005;2(2):84–90. doi: 10.3390/ijerph2005020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motelay-Massei A., Ollivon D., Garban B., Teil M.J., Blanchard M., Chevreuil M. Distribution and spatial trends of PAHs and PCBs in soils in the Seine River basin, France. Chemosphere. 2004;55:555–565. doi: 10.1016/j.chemosphere.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Muñoz-Ordoñez L.A. Texas A&M University; College Station (TX): 2015. The Effect of Urbanization on the Streamflows of the Sims Bayou Watershed. [Google Scholar]
- Nadal M., Mari M., Schuhmacher M., Domingo J.L. Multi-compartmental environmental surveillance of a petrochemical area: levels of micropollutants. Environ. Int. 2009;35:227–235. doi: 10.1016/j.envint.2008.06.001. [DOI] [PubMed] [Google Scholar]
- National Weather Service . 2017. Hurricane Harvey Info.https://www.weather.gov/hgx/hurricaneharvey Available at: [Google Scholar]
- Newkirk V.R. 2017. The looming superfund nightmare. The Atlantic [newspaper on the Internet]. 2017 Sept 12 [cited 2017 Oct 23]https://www.theatlantic.com/health/archive/2017/09/the-looming-superfund-nightmare/539316/ 2nd. Available from: [Google Scholar]
- Noji E. The nature of disaster: general characteristics and public health effects. In: Noji E.R., editor. The Public Health Consequences of Disasters. Oxford; New York: 1997. pp. 3–20. [Google Scholar]
- NOAA . 2009. Tropical Cyclone Report: Hurricane Ike. National Hurricane Center Rep. 55 pp. [Available online at http://www.nhc.noaa.gov/2008atlan.shtml. [Google Scholar]
- NOAA . 2017. Highest Water Levels during Hurricane Harvey as of 8.28.17.https://twitter.com/noaaocean/status/902604823429820418/photo/1?ref_src=twsrc%5Etfw&ref_url=https%3A%2F%2Fweather.com%2Fstorms%2Fhurricane%2Fnews%2Ftropical-storm-harvey-forecast-texas-louisiana-arkansas Available at: [Google Scholar]
- Offenberg J.H., Stapleton H.M., Stryner M.J., Lindstrom A.B. 2006. Polybrominated Diphenyl Ethers in US soilsPresented at Dioxin 2006, August 21–25, Oslo, Norway.http://www.dioxin2006.org Abstract available at. [Google Scholar]
- Peacock W.G., Tripoli G., Wood S.L. Hazard Reduction and Recovery Center; College Station, TX: 2001. Creating a More Disaster Resilient America (CAMRA) [Google Scholar]
- Pelley J. Lead: a hazard in post-Katrina sludge. Environ. Sci. Technol. 2006;40(2):414–415. Available online at: http://pubs.acs.org/subscribe/journals/esthag-a/40/i02/html/011506news3.html. [PubMed] [Google Scholar]
- Peng C., Chen W., Liao X., Wang M., Ouyang Z., Jiao W., Bai Y. Polycyclic aromatic hydrocarbons in urban soils of Beijing, polycyclic aromatic hydrocarbons in urban soils of Beijing: status, sources, distribution and potential risk. Environ. Pollut. 2011;159(3):802–808. doi: 10.1016/j.envpol.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Plumlee G.S., Morman S.A., Meeker G.P., Hoefen T.M., Hageman P.L. The environmental and medical geochemistry of potentially.hazardous materials produced by disasters. Lollar B.S.L., editor. Treatise Geochem. 2012;11:257–304. http://www.sciencedirect.com/science/article/pii/B9780080959757009074 Available at: (invited chapter) [Google Scholar]
- Presley S.M., Rainwater T.R., Austin G.P., Platt S.G., Zak J.C., Cobb G.P., Marsland E.J., Tian K., Zhang B., Anderson T.A., Cox S.B., Abel M.T., Leftwich B.D., Huddleston J.R., Jeter R.M., Kendall R.J. Assessment of pathogens and toxicants in New Orleans, LA following hurricane Katrina. Environ. Sci. Technol. 2006;40(2):468–474. doi: 10.1021/es052219p. [DOI] [PubMed] [Google Scholar]
- Reibl D.D., Haas C.N., Pardue J.H., Walsh W.J. Toxic and contaminant concerns generated by Hurricans Katrina. Aftermath Katrina. 2008;36(1) https://www.nae.edu/19582/Bridge/TheAftermathofKatrina/ToxicandContaminantConcernsGeneratedbyHurricaneKatrina.aspx [Google Scholar]
- Roper W.E., Weiss K.J., Wheeler F. 2006. Water Quality Assessment and Monitoring in New Orleans Following Huricane Katrina EPA Archived Document.https://archive.epa.gov/emergencies/content/fss/web/pdf/roper_3.pdf [Google Scholar]
- Roth D. National Weather Service; Camp Springs, MD: 2010. Texas hurricane history. [Google Scholar]
- Rotkin-Ellman M., Soloman G., Gonzales C., Agwaramgbo L., Mielke H.W. Arsenic contamination in New Orleans soil: temporal changes associated with flooding. Environ. Res. 2010;110:19–25. doi: 10.1016/j.envres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Ruiz-Fernández A.C., Betancourt Portela J.M., Sericano J.L., Sanchez-Cabeza J.-A., Espinosa L.F., Cardoso-Mohedano J.G., Pérez-Bernal L.H., Garay Tinoco J.A. Coexisting sea-based and land-based sources of contamination by PAHs in the continental shelf sediments of Coatzacoalcos River discharge area (Gulf of Mexico) Chemosphere. 2016;144:591–598. doi: 10.1016/j.chemosphere.2015.08.081. [DOI] [PubMed] [Google Scholar]
- Sansom G.T., Kirsch K.R., Stone K.W., McDonald T.J., Horney J.A. Domestic exposures to polycyclic aromatic hydrocarbons in a Houston, Texas, environmental justice neighborhood. Environ. Justice. 2018;11(5):183–191. doi: 10.1089/env.2018.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbriz L., Aquino M.R., Alberto de Rodriguez N.M., Fowler S.W., Sericano J.L. Levels of chlorinated hydrocarbons and trace metals in bivalves and nearshore sediments from the Dominican Republic. Mar. Pollut. Bull. 1998;36:971–979. [Google Scholar]
- Schmidt C.W. Lessons from the flood: will Floyd change livestock farming? Environ Health Perspect. 2000;108(2):A74–A77. doi: 10.1289/ehp.108-a74. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher M., Nadal M., Domingo J.L. Levels of PCDDFs PCBs and PCNs in soils and vegetation in an area with chemical and petrochemical industries. Environ. Sci. Technol. 2004;38:1960–1969. doi: 10.1021/es034787f. [DOI] [PubMed] [Google Scholar]
- Shultz J.M., Galea S. Mitigating the mental and physical health consequences of Hurricane Harvey. Jama. 2017;318(15):1437–1438. doi: 10.1001/jama.2017.14618. [DOI] [PubMed] [Google Scholar]
- Shultz J., Russell J., Espinel Z. Epidemiology of tropical cyclones: the dynamics of disaster, disease, and development. Epidemiol. Rev. 2005;27(1):21–35. doi: 10.1093/epirev/mxi011. [DOI] [PubMed] [Google Scholar]
- Socolo H.H., Garrigues P., Ewald M. Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine(France) areas. Mar. Pollut. Bull. 2000;40:387–396. [Google Scholar]
- Srinivas H., Nakagawa Y. Environmental implications for disaster preparedness: lessons learnt from the Indian Ocean Tsunami. J Environ Manage. 2008;89(1):4–13. doi: 10.1016/j.jenvman.2007.01.054. Oct. [DOI] [PubMed] [Google Scholar]
- Steichen J.L., Quigg A. Fish species as indicators of freshwater in flow within a subtropical estuary in the Gulf of Mexico. Ecol. Indicat. 2018;85:180–189. [Google Scholar]
- Stein M.L. Springer; New York: 1999. Statistical Interpolation of Spatial Data: Some Theory for Kriging. [Google Scholar]
- Summers J.K., Wade T.L., Engle V.D., Malaeb Z.A. Normalization of elemental concentrations of contaminants in estuarine sediments of the Gulf of Mexico, concentrations of contaminants in estuarine sediments of the Gulf of Mexico. Estuaries. 1996;19:581–584. [Google Scholar]
- Sweet W., Park J., Marra J., Zervas C., Gill S. US Department of Commerce; 2014. Sea Level Rise and a Nuisance Flood Frequency Changes Around the Unites States. NOAA Technical Report NOS CO-OPS 073. 58pp. [Google Scholar]
- Texas Commission on Environmental Quality (TCEQ) 2008. Post-Hurricane Ike Sediment Residue Screening Sampling of the Upper Gulf Coast of Texas. November, 2008. [Google Scholar]
- Teaf C.M., Covert D.J., Kothur S.R. Poylcyclic aromatic hydrocarbons (PAHs) in urban soil: a Florida risk assessment perspective. Int. J. soil Sediment Water. 2008;1(2):1–14. [Google Scholar]
- Texas Commission on Environmental Quality . 2017. Superfund Sites in Harris County.https://www.tceq.texas.gov/remediation/superfund/sites/county/harris.html Available at: [Google Scholar]
- United Nations Office for Disaster Risk Reduction (UNISDR) 2016. 2015 Disasters in Numbers.https://www.unisdr.org/we/inform/publications/47804 Available from: [Google Scholar]
- Vane C.H., Kim A.W., Beriro D.J., Cave M.R., Knights K., Moss-Hayes V., Nathanail P.C. Polycyclic aromatic hydrocarbons (PAH) and polychlorinated biphenyls (PCB) in urban soils of Greater London, UK. Appl. Geochem. 2014;51:303–314. [Google Scholar]
- Wang Y., Tian Z., Zhu H., Cheng Z., Kang M., Luo C., Li J., Zhang G. Polycyclic aromatic hydrocarbons (PAHs) in soils and vegetation near an e-waste recycling site in South China: concentration, distribution, source, and risk assessment. Sci. Total Environ. 2012;15:187–193. doi: 10.1016/j.scitotenv.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Wang X.-T., Miao Y., Zhang Y., Li Y.C., Wu M.H., Yu G. Polycyclic aromatic hydrocarbons (PAHs) in urban soils of the megacity Shanghai: occurrence, source apportionment and potential human health risk. Sci. Total Environ. 2013;447:80–89. doi: 10.1016/j.scitotenv.2012.12.086. [DOI] [PubMed] [Google Scholar]
- WEACT for Environmental Justice . 2013. Assisting Congress to Better Understand Environmental justice.http://mpaenvironment.ei.columbia.edu/files/2014/06/WEACT-for-Environmental-Justice.pdf [cited 2016 March 15]. Available from: [Google Scholar]
- Abundance in Earths Crust. 2007. Webelements.comhttp://www.webelements.com/webelements.com/webelements/properties/text/image-flash/abund-crustcrust.html 9 March w007. [Google Scholar]
- WHO . World Health Organization; Geneva: 2018. Chemical Releases Caused by Natural hazard Events and Disasters – Information for Public Health Authorities. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Wilcke W., Krauss M., Safronov G., Fokin A.D., Kaupenjohann M. Polychlorinated biphenyls (PCBs) in soils of the Moscow region: concentrations and small-scale distribution along an urban-rural transect. Environ. Pollut. 2006;141:327–335. doi: 10.1016/j.envpol.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Yogui G.T., Sericano J.L. Levels and pattern of polybrominated diphenyl ethers in eggs of Antarctic seabirds: endemic versus migratory species. Environ. Pollut. 2009;157:975–980. doi: 10.1016/j.envpol.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Young S., Balluz L., Malilay J. Natural and technologic hazardous material releases during and after natural disasters: a review. Sci. Total Environ. 2004;322(1-3):3–20. doi: 10.1016/S0048-9697(03)00446-7. [DOI] [PubMed] [Google Scholar]
- Yunker M.B., Macdonald R.W., Vingarzan R., Mitchell R.H., Goyette D., Sylvestre S. PAH’s in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002;33:489–515. [Google Scholar]