Abstract
Background/Objective:
Regular physical activity (PA) has been associated with improved cognitive function2,3 but its effect on postoperative delirium (POD) has not been established. Our objectives were to determine the effect of baseline PA on the incidence of POD in older patients undergoing elective orthopedic surgery and to determine whether these effects were independent of cognitive reserve. We hypothesize that PA protects against POD by bolstering physiologic reserve needed to withstand the stressors of surgery.
Design:
Secondary analysis of a prospective, single-center cohort study.
Setting:
Urban academic hospital
Participants:
132 non-demented, English-speaking adults over 60 years undergoing elective orthopedic surgery
Measurements:
Subjects were screened for POD and delirium severity using the Confusion Assessment Method and the Memorial Delirium Assessment of Severity scale. Baseline cognitive and physical activities (PA) were assessed with a validated Leisure Activity Scale (LAS).4–6 Regular PA was categorized as 6–7 days per week. Association of regular PA with incidence of POD was assessed using multivariable logistic regression adjusting for age, sex, Charlson Comorbidity Index, cognitive reserve and cognitive function. Linear regression was used to assess the association of delirium severity with regular PA.
Results:
41 of 132 (31.1%) patients developed POD. Regular PA was associated with a 74% lower odds of developing POD (OR 0.26 95% CI 0.08–0.82). There was no significant interaction between PA and cognitive reserve (p=0.70). 25 of 85 women (29.4%) and 16 of 47 men (34.0%) men developed POD. In stratified analysis, women who engaged in regular PA had dramatically lower odds of POD, OR 0.08 (95% CI 0.01–0.63) compared with men OR 0.93 (95% CI 0.18–4.97).
Conclusions:
Regular PA is associated with decreased incidence of POD, especially among women. Future studies should address the basis of sex differences in PA benefits on delirium.
Keywords: Postoperative delirium, Physical Activity, Cognitive reserve
INTRODUCTION
Post-operative delirium (POD) affects between 9.9–67%7 of older adults undergoing non-cardiac surgery and 5.1–61% of older adults undergoing orthopedic surgery.8 POD can have devastating long-term sequelae such as cognitive dysfunction, decline in functional status, increased morbidity and mortality9,10. Given that there are an estimated 13.6 million surgeries performed annually on patients age 65 and over in the U.S. alone, and around 5 million knee and hip replacements, the magnitude of this problem is staggering11. Several intra-operative strategies to reduce delirium such as opiate sparing analgesia, avoidance of general anesthesia using regional and neuraxial techniques, better hemodynamic control, and pharmacologic interventions have had limited benefit12–14. This suggests that the best strategies for prevention of POD may rest in the pre-operative setting.
Physical activity (PA) is universally recognized to have positive health benefits and has been associated with improved cognitive function and brain health.15 Several studies have shown PA to be associated with a reduced risk of developing dementia.16,17 In contrast, a longitudinal study of older, community dwelling adults by Verghese et. al. showed that physical activity was not associated with a decrease in risk for development of dementia.4 The difference in these two findings may in large part be due to the fact that Verghese et. al. adjusted for leisure activities as a proxy for cognitive reserve, which seems to play a much stronger influential role than physical activity alone. For this reason, we thought it was important to study the effect of PA on delirium in the context of cognitive reserve.
The benefits of PA have also been demonstrated in regards to delirium. Among hospitalized patients, Yang et. al. demonstrated that regular exercise was associated with a 28% decrease in risk for delirium.1 Among surgical patients, Marcantonio et. al. found that patients with very poor tolerance of physical activity (less than 2 metabolic equivalents) had a 3-fold increased risk for POD.18 Although these are persuasive findings, the role of physical activity and the risk of POD is critically understudied, especially in the surgical population who are undergoing much higher burdens of physical stress and in whom the risk for POD is the highest.
Interestingly, women have routinely shown more of a benefit in response to physical activity compared to men. Samitz et. al recently published a meta-analysis that showed that women consistently demonstrated a greater reduction in risk of mortality per each 1000 kcal increment per week.19 And Kramer et. al. observe that studies with more women show a greater cognitive benefit in response to exercise, theorizing this is related to increased levels of brain-derived neurotrophic factor observed with both exercise and estrogen replacement.15
Our objectives were to measure the association of baseline physical activity with the incidence and severity of POD in older patients undergoing elective orthopedic surgery, to determine whether this association is independent of cognitive reserve, and to examine gender as a biologic variable. We hypothesize that physical activity protects against POD by bolstering the physiologic and cognitive reserve needed to withstand the stressors of surgery.
METHODS
We performed a secondary analysis of a prospective, single-center cohort study. Patients were recruited between October 2011- August 2014 from the Montefiore Medical Center Joint Replacement Center in the Bronx, NY. English-speaking adults over 60 years undergoing elective orthopedic surgery were included in the study. Patients were excluded for baseline delirium, physician-diagnosed dementia, Mini Mental Status Examination (MMSE) score below 24, or severe auditory or visual deficits. Both the initial study design and the secondary analysis of this dataset were approved by our institutional review board.
Primary outcome: Incidence of POD.
Patients were screened for POD using the Confusion Assessment Method (CAM)20 on postoperative days 1 and 2. The CAM is the most widely used screening instrument for delirium. Compared to assessment by a clinical psychiatrist, the CAM has a sensitivity of 94–100% and a specificity of 91–94%. It bases the diagnosis of delirium on presence of both 1) acute change of mental status and fluctuating course and 2) inattention and at least one or both 3) disorganized thinking and 4) altered level of consciousness. Delirium screening was conducted by a single investigator trained in the CAM who was blinded to the baseline cognitive and physical activity assessments. Cognitive testing was performed assessing patient orientation, registration and delayed recall of 3 words, forward and backward digit span, days of the week backward and months of the year backward. Each assessment took a median time of 8 minutes (IQR 6–10). In patients who did not meet delirium criteria during in-person assessments, physician and nursing documentation was reviewed for description of confusion, agitation or altered mental status. As reported in our previous publication, there was excellent agreement with delirium rating conducted by a senior neurologist.21
Secondary outcome: Severity of POD.
Severity of delirium was assessed using the Memorial Delirium Assessment Scale (MDAS)22, a scale with 10 items and possible scoring range of 0–30 points. This instrument was developed to be used repeatedly among medically ill patients with high inter-rater reliability.
Primary predictors:
A previously validated Leisure Activity Scale (LAS)4–6 was administered prior to day of surgery to measure cognitive and physical components. The LAS has been previously described, but briefly, participants were asked “In the last month, how many days in a week did you participate in exercise or sport?” Additionally, the type(s) of activity in which they participated was collected. Physical activity was analyzed both as a continuous variable (number of days of PA per week) as well as a dichotomous variable. Regular physical activity (PA) was categorized using two different cut points to explore a threshold effect: at 5 or more days per week and 6 or more days per week. Cognitive activities were measured and used as a proxy for late-life cognitive reserve. Weekly frequency of participation in 12 different cognitive activities was recorded which included reading the newspaper, reading books, knitting, playing cards, playing board games, playing computer games, electronic mail, singing, writing, doing crossword puzzles, playing bingo, or participating in group meetings. Our previous report in this cohort demonstrated an association between higher levels of participation in cognitive activities measured by the LAS with lower risk for delirium.21 Similar to the variable for PA, a threshold effect for regular cognitive activity was explored and defined as 21 or more cognitive activities per week.
Gender:
Men and women have demonstrated varied responses to PA. As such, gender was examined as a biological variable.
Covariates:
At the time of preoperative patient interview, demographics as well as baseline covariates that have been established in the literature as risk factors for POD were assessed. Cognitive status was measured using the MMSE, a continuous scale from 0–30 with a score equal to or over 24 being indicative of normal cognition23. Comorbidity was assessed using the Charlson Comorbidity Index (CCI)24. The Short Form of the Geriatric Depression Scale (GDS) was administered to assess mood. This is a validated instrument with a 92% sensitivity and 89% specificity25. A score from 5–9 on this scale is indicative of increased risk for depression and a score 10 or over is indicative of depression. In addition, information on age, years of education, smoking status, alcohol use, medication list and functional status using the Instrument for Activities of Daily Living (ADL) were collected.
Statistical methods:
Analysis was performed using Stata version 15.1 software (StataCorp College Station, TX). Differences in demographics, Charlson Comorbidity Index, ADL scale, GDS scale, MMSE, cognitive activity scale, physical activity, and MDAS between those developing POD and those who did not were compared using either t-tests or Mann Whitney U tests for continuous variables and chi-squared or Fisher’s exact tests for categorical variables.
Multivariable logistic regression was performed to assess the role of regular physical activity on risk of developing POD adjusting for age, sex, Charlson Comorbidity Index, cognitive reserve based on the LAS (number of cognitively stimulating activities performed per week) and cognitive function (MMSE). Physical activity and cognitive activities were analyzed both as continuous variables (activities per week) as well as dichotomized variables, described earlier. GDS scale was included in the model initially but lost significance when controlling for other factors and was removed from the final model. An interaction between cognitive reserve and regular physical activity was included in the model to examine whether effects of regular physical activity on risk of developing POD were modified by cognitive reserve. As men and women have demonstrated varied responses to physical activity, the final logistic regression model was stratified by gender. Analysis was performed to examine potential differences in comorbidities, age, depression or education between gender groups. Multiple linear regression was used to test if physical activity significantly predicted delirium severity (highest MDAS score recorded over two days), controlling for age, sex, cognitive status, cognitive reserve and comorbidity. An analysis of delirium severity stratified by gender was also performed.
RESULTS
Detailed information regarding results of patient recruitment, screening, and reasons for exclusion have been previously reported.21 155 subjects were enrolled. 8 subjects withdrew prior to their surgery, 2 had their surgery cancelled, and 3 were lost to follow up. Of the remaining 142 subjects, 6 subjects were excluded for missing data on physical activity and 4 subjects were excluded due to missing information on comorbidity index or baseline cognitive status. Hence, 132 subjects were eligible for this analysis.
Of the 132 subjects, 41 developed POD, making the incidence 31.1%. The majority of subjects in our sample were women (64%). 16 of 47 (34%) men developed POD compared to 25 of 85 (29.4%) women (p=0.36). The median age was 70.5 years (IQR 64.5–76.3). By in large, this was a healthy cohort, with 71.1% having a CCI score of 0 or 1 and 56.1% having an American Board of Anesthesiologist (ASA) score of 1 or 2. The median number of years of education was 12.5 (IQR 11.5–16). Age, gender, BMI, comorbidity index or ADL score were similar in those who developed delirium and those who did not, as shown in Table 1. Subjects who did not develop POD had higher median MMSE scores and increased cognitive reserve reflected by cognitive activities per week. Subjects with Geriatric Depression Scale (GDS) under 5 were less likely to develop POD. There was no significant difference in rates of POD depending on the mode of anesthesia delivered, either general, neuraxial, or with a peripheral nerve block or catheter.
Table 1.
Baseline Characteristics of Study Population (N=132)
| Risk Factors | Total (N=132) | Subjects who did not develop POD (N=91) | Subjects who developed POD (N=41) | p value |
|---|---|---|---|---|
| Age, yearsa | 70.5 (64.5–76.3) | 69.8 (64.4–75.8) | 72.7 (65–76.6) | 0.46 |
| Sex, N (%) | ||||
| Male | 47 (35.6) | 31 (34.0) | 16 (39.0) | 0.36 |
| Female | 85 (64.4) | 60 (65.9) | 25 (61.0) | |
| Years of Educationb | 13.2 ± 0.3 | 13.5 ± 0.3 | 12.5 ± 0.7 | 0.15 |
| Body mass index kg/m2 b | 32.6 ± 0.6 | 32.9 ± 0.7 | 32.0 ± 0.9 | 0.47 |
| Charlson Comorbidity Index, N (%) | ||||
| 0 | 54 (40.9) | 38 (41.8) | 16 (39.0) | 0.38 |
| 1 | 40 (30.3) | 25 (27.5) | 15 (36.6) | |
| 2 | 22 (16.7) | 14 (15.4) | 8 (19.5) | |
| 3 | 11 (8.3) | 9 (9.9) | 2 (4.9) | |
| 4 | 5 (3.8 ) | 5 (5.5) | 0 (0) | |
| ADL scale (1–14)a | 1 (0, 1) | 1 (0,1) | 1 (0,1) | 0.53 |
| Geriatric Depression Scale | <0.01 | |||
| >5 | 12 (9.2) | 11 (12.1) | 1 (2.5) | |
| 5–9 (at risk for depression) | 115 (87.8) | 80 (87.9) | 35 (87.5) | |
| 10–12 (indicative of depression) | 4 (3.1) | 0 (0) | 4 (10.0) | |
| MMSE (0–30)a | 29 (27– 30) | 29 (27.5–30) | 27 (25–29) | <0.01 |
| Cognitive Activity | ||||
| Continous scale (0–41)a | 13.5 (7–19) | 14.5 (8–22) | 7.5 (2–15) | <0.01 |
| Regular Cognitive Activity, N (%) | 29 (22.0) | 27 (93.1) | 2 (4.9) | <0.01 |
| Physical Activity | ||||
| Continous scale, days per week a | 1 (0–6) | 1 (0–7) | 1 (0–4) | 0.45 |
| Regular PA (6–7 days/wk), N (%) | 35 (26.5) | 28 (80) | 7 (20) | 0.10 |
| Regular PA (5–7 days/wk), N (%) | 41 (31.1) | 32 (78.1) | 9 (22) | 0.13) |
| Highest recorded MDAS a,c | 5 (3–7) | 4 (2.5–5) | 8 (7–9) | <0.01 |
| ASA score,e N (%) | ||||
| 1 | 1 (0.8) | 1 (1.1) | 0 (0.0) | 0.90 |
| 2 | 73 (55.3) | 51 (56.0) | 22 (53.6) | |
| 3 | 58 (43.9) | 39 (42.9) | 19 (46.3) | |
| Type of surgery, N (%) | ||||
| Hip replacement | 39 (29.6) | 30 (32.9) | 9 (22.0) | 0.39 |
| Knee replacement | 91 (68.9) | 60 (65.9) | 31 (75.1) | |
| Spinal surgery | 2 (1.5) | 1 (1.1) | 1 (2.4) | |
| Type of anesthesia, N (%) | ||||
| General | 48 (36.4) | 32 (66.7) | 16 (33.3) | 0.67 |
| Spinal | 79 (59.9) | 57 (72.2) | 22 (27.9) | 0.33 |
| Epidural | 41 (31.1) | 26 (63.4) | 15 (36.6) | 0.36 |
| Peripheral nerve block | 53 (40.2) | 39 (73.6) | 14 (26.4) | 0.35 |
| Peripheral nerve catheter | 38 (29.7) | 23 (60.5) | 15 (39.5) | 0.19 |
reported as Median (IQR)
reported as Mean ± SD
Memorial Delirium Assessment Scale
Defined as 21–41 cognitive activities per week
American Society of Anesthesiologist’s score
Reported physical activities listed in Table 2 included walking, taking part in physical therapy, lifting weights, cycling, stretching, engaging in competitive sports, and dancing. The most commonly reported activity was walking. While the median number of days per week of PA was only 1 (IQR 0–6), 25.6% of participants were able to engage in PA 6–7 days per week and 31.1% were able to engage in PA 5–7 days per week. Using bivariate analysis, there was no statistically significant difference in PA habits between those who developed POD and those who did not.
Table 2.
Types of Physical Activity Reported, N (%)
| Walking | 21 (15.9) |
| Physical therapy | 3 (2.3) |
| Weights | 4 (3.0) |
| Biking | 3 (2.3) |
| Stretching | 6 (4.6) |
| Heavy housework | 2 (1.5) |
| Dancing | 3 (2.3) |
In fully adjusted models, participation in regular PA (6–7 days per week) was associated with a 74% lower odds of POD (OR 0.26; 95%C.I. 0.08 – 0.82). See Table 3, Model 2. Using a lower threshold to define regular PA (5–7 days per week), the association was borderline significant (OR 0.35; 95% C.I. 0.12–0.99). The role of cognitive reserve, as previously reported,21 remains one of the strongest factors. Each unit of cognitive activity per week is associated with a 8% lower odds of POD (0.92; 95% C.I. 0.87–0.98), as demonstrated in Table 3, Model 1, 22% of participants who partook in regular cognitive activities, defined as 21 activities or more per week. Regular cognitive activities was associated with 81% lower odds of POD; OR 0.19 (95% CI 0.04–0.89).
Table 3.
Logistic regression model of delirium risk (N=132)
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| PA (days per week) | 0.88 (0.75–1.02) | 0.11 | * | * | * | * | * | * |
| Cognitive activities per weekb | 0.92 (0.87–0.98) | 0.01 | * | * | * | * | * | * |
| Regular PA a | * | * | 0.26 (0.08–0.82) | 0.02 | 0.35 (0.12–0.99) | 0.05 | 0.33 (0.12–0.92) | 0.03 |
| Regular cognitive activitiesc | * | * | 0.19 (0.04–0.89) | 0.04 | 0.19 (0.04–0.88) | 0.03 | * | * |
| Age | 0.97 (0.91–1.03) | 0.37 | 0.98 (0.92–1.04) | 0.48 | 0.97 (0.91–1.04) | 0.38 | 0.97 (0.92–1.03) | 0.39 |
| Female sex | 0.78 (0.32–1.88) | 0.58 | 0.67 (0.28–1.64) | 0.39 | 0.68 (0.28–1.66) | 0.40 | 0.67 (0.28–1.59) | 0.37 |
| MMSEd | 0.68 (0.54–0.86) | <0.01 | 0.64 (0.51–0.81) | <0.01 | 0.66 (0.52–0.84) | <0.01 | 0.62 (0.49–0.78) | <0.01 |
| CCI e | 0.79 (0.53–1.19) | 0.26 | 0.80 (0.53–1.20) | 0.29 | 0.77 (0.51–1.16) | 0.21 | 0.75 (0.50–1.12) | 0.16 |
Model 1: Days per week of physical activity and number of cognitive activities per week as continuous variables
Model 2: Regular physical activity and cognitive activities are dichotomized: Regular PA defined as 6–7 days activity per week; Regular cognitive activities defined as 21 or more activities per week
Model 3: Regular PA defined as 5–7 days of PA per week
Model 4: Cognitive activities are removed from the model with minimal change in OR for regular PA
Regular Physical Activity defined as 6–7 days per week in Model 2 and defined as 5–7 days in Model 3
On a scale of 0–41 unit activities per week
Regular Cognitive Activity defined as 21 or more activities per week
Mini Mental Status Examination score, range from 0–30
Charlson Comorbidity Index
omitted from model
There was no significant interaction between cognitive reserve and regular physical activity (p=0.70), indicating that cognitive reserve and regular physical activity are independently associated with risk of POD. Removing cognitive reserve from the model (Table 3, Model 4) had negligible effect on the OR for regular physical activity.
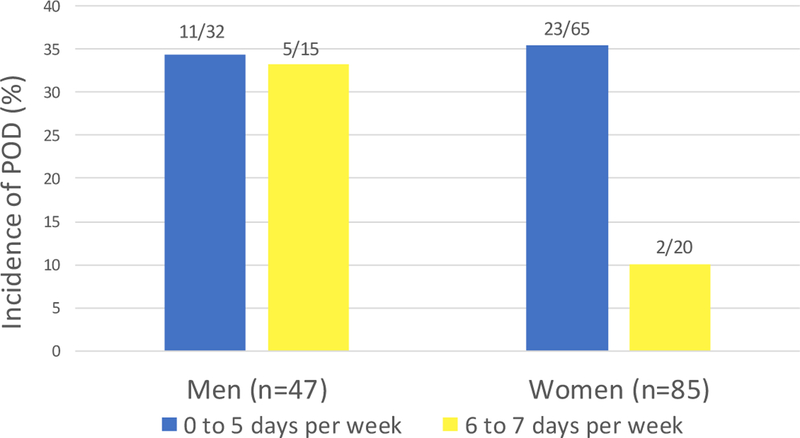
Among men, the unadjusted relative risk of POD was 0.97, with similar incidence among those who partook in regular PA (n=5/16, 33.3%) and those who did not (n=11/16, 34.4%), Among women, however, the unadjusted relative risk was 0.14, with an incidence of 10% (n=2/20) among those who partook in regular PA compared to 35.4% (n=23/65) among those who did not. In the fully adjusted model, the interaction term between PA and gender was not significant (p=0.21). However, stratified logistic regression showed a marked difference in the association between regular PA and POD among men and women. While women who partook in regular physical activity had a 93% lower odds for POD, OR 0.07 (95% CI 0.01–0.62), men showed no difference, OR 0.99 (95% CI 0.19–5.16). Comparing men and women, there was no significant difference in age, CCI score, MMSE score, number of cognitive activities performed per week or median number of days of physical activity per week.
The median delirium severity score (highest MDAS score recorded) was 4 (IQR 2.5–5) in the non-delirious group compared to 8 (IQR 7–9) among those developing POD (p<0.01). The median MDAS score was 4 (IQR 2–5) among those who partook in regular PA compared with 5 (IQR 3–7.5) among those who didn’t, but this was not statistically significant (p=0.07) Using multiple linear regression to adjust for age, sex, cognitive status, cognitive reserve and comorbidity, we found there was a trend towards a reduction in MDAS among those who exercised 6–7 days per week (ß= −0.99, p=0.06). In analysis stratified by gender, baseline regular PA was not associated with decreased delirium risk among males; B=−0.43 (95% CI −2.04 to 1.18), p=0.59. Among females, there appeared to be a trend towards a reduction in delirium severity, but this also was not statistically significant; B= −1.21 (95% CI −2.59 to 0.17), p=0.09.
DISCUSSION
This study investigated the effect of regular physical activity relative to cognitive reserve on the risk of POD among older adults undergoing elective orthopedic surgery. We found that older adults who participated in regular PA of 6–7 days per week had a 73% lower odds for developing POD. This finding confirms earlier reports examining the role of PA on delirium among hospitalized patients1, and shows that the relationship is similar, if not more marked among elective surgery patients. A fairly robust amount of physical activity was needed before a statistically significant difference in POD risk was observed.
We found that the benefit of regular PA was independent of cognitive reserve and cognitive status in this population of non-demented subjects. Though our study is observational and does not establish causation, clinicians may take away a couple of points. First, regular PA is associated with decreased risk of POD regardless of cognitive reserve or cognitive status within a non-demented population. Persons with mild cognitive impairment or relatively low level of participation in stimulating cognitive activities will likely still benefit from exercise if tolerated. On the other hand, adherence to exercise programs may be difficult for many. Patients with joint pain or injuries may find it difficult to exercise and exercise could entail risk in patients with clinical entities such as coronary artery disease or impaired balance. In such cases, participating in stimulating cognitive activities such as reading or doing puzzles could be more beneficial and is certainly very low risk.
Our results showed that regular PA was associated with a trend towards lower delirium severity, but this was not statistically significant. Given that we have yet to discover a single pharmacologic remedy to POD prevention and most interventions are multifactorial, the study of delirium severity is crucial in that it gives us potential insight into the dose response to treatment. Although our results did not establish an association between regular PA and delirium, the study was underpowered. Our sample size of 132 gave us only a 35.7% power to detect a 15% effect size. Further analysis of a larger dataset is warranted.
Though these results are preliminary and based on a small sample size, the difference in association between PA and POD among women compared to men is quite striking. While regular PA among women was associated with a 92% lower odds for POD, there was no such association observed among men. This may be due to a few different reasons. Women and men may report PA differently (misclassification bias). Alternatively, men may have taken part in physical activity with higher risk of injury. Thirdly, we had many more women in this sample compared to men, and the difference may have been due to chance or unmeasured confounders. However, given that there are several studies consistently showing women to have greater reduction in mortality and morbidity associated with PA, however, further investigation of this gender difference is worthwhile.
Interestingly, Erikson et. al. have proposed that both exercise and estrogen upregulate production of brain-derived neurotrophic factor (BDNF) which is involved in neurogenesis and neuroprotection.15 The same investigator also reported that hormone replacement therapy and exercise was associated with increased prefrontal cortex volumes on magnetic resonance imaging and superior scores on tests of executive functioning. In this older population where hormone replacement therapy was not prescribed, it is unclear if post-menopausal levels of estrogen among women could account for the difference in response to PA or if other biological, psychosocial or genetic differences may be at play. Further exploration of possible mechanisms accounting for such differences may be warranted.
One challenge in studying the effects of PA is the inherent difficulty in measuring PA in clinical and research populations, where direct measurements such as pedometers is not necessarily accurate in geriatric populations and direct observation is not necessarily feasible26. For these reasons, a majority of studies on PA rely on self-reporting, which has the potential for recall and misclassification bias. While some PA assessment instruments may seek to provide more granular information such as intensity, duration, frequency and habituation which would allow for quantification of MET/hours/week, the value of this additional detail has to be weighed against the additional burden on the research subjects who are already volunteering for to participate during a stressful surgical period. The optimal assessment of PA is especially tricky among orthopedic surgery patients, as the ability to exercise is a key moderator of PA and vigorous activity is likely not possible in this group. These patients are likely to have painful symptoms which result in a change in their exercise habits leading up to the time of surgery and it is unclear how a lifetime of exercise habits may relate to delirium risk compared to the most recent exercise habits.
Our PA instrument (LAS) asked the participants to characterize the type, frequency and habituation of the PA, but much of the data regarding the historical habits was incomplete. We chose to dichotomize the PA variable for two important reasons. We observed a biphasic distribution in the days per week, with clustering around 0–1 days and 6–7 days. We also felt that attempting to capture a more precise measure of physical activity beyond “regular” and “not regular” had the potential to be misleading. With larger sample sizes and/or more detailed quantification of “doses” of PA, one might be able to give a more detailed picture of how much each day of physical activity reduces POD risk and delirium severity.
As our study is observational, we cannot establish causation. In the future, if the results if this study are confirmed in larger datasets, randomized controlled trials to test the efficacy of regular physical activity as well as cognitive exercises in reducing the risk for POD should be conducted. It remains unclear if pre-conditioning with exercise and cognitive training would prospectively decrease POD risk, As most successful interventions in the post-operative period to decrease incidence and severity have been multi-pronged approaches, it is likely that pre-operative optimization may also necessitate multimodal targets.
We demonstrated that older adults undergoing elective orthopedic surgery who engage in regular physical activity had a reduced risk of incident POD but not severity. Though women benefit more from physical activity in terms of reduced risk of POD, there are several other health benefits enjoyed by men engaging in PA. While our population was restricted to orthopedic surgery patients, we feel this finding would likely be generalizable to other types of surgical procedures but should be tested in larger, more diverse surgical populations. PA appears to decrease risk for POD regardless of cognitive status or reserve and suggests that practitioners may recommend low-risk lifestyle modifications to decrease risk of POD where appropriate.
Figure 1. Incidence of postoperative delirium and physical activity.
Men who engaged in regular physical activity had similar rates of POD as those who did not, with an unadjusted relative risk for POD of 0.97. Among women, however, a marked difference was observed. The unadjusted relative risk was 0.14, with an incidence of 10% among those who partook in regular PA compared to 35.4% among those who did not.
Table 4.
Logistic regression of delirium risk, stratified by gender
| Men (N=47) | Women (N=85) | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Regular PAa | 0.93 (0.18–4.97) | 0.94 | 0.08 (0.01–0.63) | 0.02 |
| Regular Cognitive activitiesb | 0.44 (0.04–5.02) | 0.51 | 0.12 (0.01–1.04) | 0.05 |
| Age | 1.01 (0.92–1.11) | 0.83 | 0.96 (0.89–1.05) | 0.37 |
| MMSEc | 0.53 (0.34–0.82) | <0.01 | 0.66 (0.49–0.89) | <0.01 |
| CCId | 0.77 (0.38–1.55) | 0.46 | 0.84 (0.49–1.43)) | 0.52 |
6–7 days of physical activity per week
21 or more cognitive activities per week
Mini Mental Status Examination score, range 0–30
Charlson Comorbidity Index
ACKNOWLEDGMENTS
Funding: Dr. Lee was supported by the NIH/National Center for Advancing Translational Science through Einstein-Montefiore Institute for Clinical and Translational Science Award, Grant Number UL1TR001073. Joe Verghese receives funding from National Institute of Aging grants 1RO1 AGO44829–01A1, R01 AG050448–01, and 1UG3NS105565–01 and is a member of the editorial board at Journal of the American Geriatrics Society. The funding sources had no role in the design, conduct or reporting of this study.
Footnotes
Presented at the 2019 Annual Meeting of American Delirium Society, Boston, MA
Conflict of Interest: The authors have no conflicts to report.
Special thanks to Amanda Tow for sharing her data for this analysis, the CRTP program for support and insight, and to Drs. Joe Verghese and Ellise Delphin for their mentorship.
REFERENCES
- 1.Yang FM, Inouye SK, Fearing MA, Kiely DK, Marcantonio ER, Jones RN. Participation in activity and risk for incident delirium. J Am Geriatr Soc 2008;56(8):1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman SB, Aslan S, Spence JS, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci 2013;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: recent findings and future directions. J Mol Neurosci 2004;24(1):9–14. [DOI] [PubMed] [Google Scholar]
- 4.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003;348(25):2508–2516. [DOI] [PubMed] [Google Scholar]
- 5.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006;66(6):821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verghese J, Cuiling W, Katz MJ, Sanders A, Lipton RB. Leisure activities and risk of vascular cognitive impairment in older adults. J Geriatr Psychiatry Neurol 2009;22(2):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avidan MS, Fritz BA, Maybrier HR, et al. The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study: protocol for an international multicentre randomised controlled trial. BMJ Open 2014;4(9):e005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neitzel J, Sendelbach S, Larson LR. Delirium in the orthopaedic patient. Orthop Nurs 2007;26(6):354–363; quiz 364–355. [DOI] [PubMed] [Google Scholar]
- 9.Aranake-Chrisinger A, Avidan MS. Postoperative delirium portends descent to dementia. Br J Anaesth 2017;119(2):285–288. [DOI] [PubMed] [Google Scholar]
- 10.Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg 2015;150(12):1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Classification of Diseases. Ninth Revision(Clinical Modification ICD 9-CM) [Google Scholar]
- 12.Leung JM, Sands LP, Chen N, et al. Perioperative Gabapentin Does Not Reduce Postoperative Delirium in Older Surgical Patients: A Randomized Clinical Trial. Anesthesiology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. The Lancet 2017;390(10091):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neerland BE, Krogseth M, Juliebo V, et al. Perioperative hemodynamics and risk for delirium and new onset dementia in hip fracture patients; A prospective follow-up study. PLoS One 2017;12(7):e0180641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 2007;11(8):342–348. [DOI] [PubMed] [Google Scholar]
- 16.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144(2):73–81. [DOI] [PubMed] [Google Scholar]
- 17.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA 2004;292(12):1447–1453. [DOI] [PubMed] [Google Scholar]
- 18.Marcantonio Edward R., Goldman Lee, Orav E. John, Cook E. Francis, Lee Thomas H.. The Association of Intraoperative Factors with the Development of Postoperative Delirium. Am J Med July 9, 1998;105:380–384. [DOI] [PubMed] [Google Scholar]
- 19.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol 2011;40(5):1382–1400. [DOI] [PubMed] [Google Scholar]
- 20.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 21.Tow A, Holtzer R, Wang C, et al. Cognitive Reserve and Postoperative Delirium in Older Adults. J Am Geriatr Soc 2016;64(6):1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997;13(3):128–137. [DOI] [PubMed] [Google Scholar]
- 23.Horton AM Jr., Alana S. Validation of the Mini-Mental State Examination. Int J Neurosci 1990;53(2–4):209–212. [DOI] [PubMed] [Google Scholar]
- 24.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- 25.Yesavage JA. Geriatric depression scale: consistency of depressive symptoms over time. Percept Mot Skills 1991;73(3 Pt 1):1032. [DOI] [PubMed] [Google Scholar]
- 26.Sylvia LG, Bernstein EE, Hubbard JL, Keating L, Anderson EJ. Practical guide to measuring physical activity. J Acad Nutr Diet 2014;114(2):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]