Abstract
Background:
Cardiac troponin T, measured using a high-sensitive assay (hs-cTnT), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are associated with increased stroke risk and perhaps with cognitive decline. However, there are few well-designed, prospective studies with extended follow-up.
Design:
Prospective cohort study.
Setting:
Four U.S. communities.
Participants:
A total of 9,114 and 9,108 participants from the Atherosclerosis Risk in Communities study for analyses of hs-cTnT and NT-proBNP, respectively.
Measurements:
We examined association of hs-cTnT and NT-proBNP with 15-year change (1996–1998 to 2011–2013) in three cognitive tests of executive function (Digit-Symbol Substitution Test), verbal learning memory (Delayed Word Recall Test), and semantic fluency (Word Fluency Test), and an overall score combining the three tests using multivariable linear mixed effect models. We conducted several sensitivity analyses, including multiple imputations to address bias due to missing data and attrition, and compared associations within groups combining hs-cTnT and NT-proBNP into a 3-level categorical variable.
Results:
At baseline (1996–1998), mean (SD) age was 63.4 (5.7) years; 56.4% were women and 17.5% were black. Hs-cTnT at baseline was not associated with cognitive change in any measure. There was some evidence of accelerated decline in verbal learning and memory when comparing those in the highest to lowest NT-proBNP quintiles; however this association was not replicated when considering clinically relevant cut-offs or declies of exposure in survivors. Sensitivity analyses were consistent with our primary analyses. There was little evidence to support effect modification by any considered factors. People with highest levels of both biomarkers had excessive decline in global z-scores than people with lowest levels (−0.342, 95% CI: −0.638, −0.045).
Conclusions:
Markers of myocardial injury and stretch were not associated with cognitive decline following 15 years among survivors, but when combined together, were suggestive in post-hoc analysis. Whether this represents targets of intervention should be examined in the future.
Keywords: Cognitive Change, Dementia, High-sensitivity troponin, N-Terminal pro-B-type Natriuretic Peptide
INTRODUCTION
Mounting evidence suggests cardiovascular risk factors and cardiovascular health may affect cognitive decline and dementia years to decades later.1–5 Understanding whether cardiac biomarker levels are associated with accelerated cognitive decline over the following years to decades may offer insights into mechanisms by which cardiovascular health impacts cognitive health, and into the potential effectiveness of targeted strategies in slowing cognitive decline or preventing dementia.6
Cardiac troponin T, measured using a highly sensitive assay (hs-cTnT), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are sensitive biomarkers of myocardial injury7 and stretch,8 respectively. Both are associated with increased risk for stroke9,10 and subclinical cerebrovascular brain injury such as silent brain infarcts and white matter hyperintensities.11–13 Existing literature report an association between higher hs-cTnT 14–18 as well as NT-proBNP15,17,19–23 and lower cognitive function or increased risk of dementia. However, limitations of existing studies include case-control or cross sectional designs, small sample size and short follow-up. Thus, the goal of this study was to estimate the association of hs-cTnT and NT-proBNP with 15-year cognitive change in the Atherosclerosis Risk In Communities (ARIC) study.
METHODS
Study population
The ARIC study is a prospective cohort study of 15,792 individuals aged 45–64 years at ARIC Visit 1 (1987–1989) enrolled from four U.S. communities: Washington County, Maryland; Jackson, Mississippi; Forsyth County, North Carolina; and suburbs of Minneapolis, Minnesota.24 The ARIC investigators subsequently assessed participants at three follow-up visits at approximately 3-year intervals (Visits 2 to 4), and after an extended interval at Visit 5 between 2011–2013. The baseline for the current analyses was ARIC Visit 4 (1996–1998) as hs-cTnT and NT-proBNP were quantified in stored blood samples obtained at this study visit. For both primary and sensitivity analyses, we excluded participants who died before Visit 4 (n=976), individuals who did not consent to provide genetic data (n=45), non-black and non-white participants and non-white participants from Minnesota or Maryland sites (n=105). However, for the primary analyses we also required participants to have attended Visit 4 and have complete covariate, exposure, and Visit 4 cognitive data with a sample of 9,114 for hs-TnT and 9,108 for NT-proBNP (Supplemental Figure s). The institutional review boards at each site approved study protocols and all study participants provided informed consents.
Exposure Assessment
We measured hs-cTnT on stored blood samples collected during Visit 4 (1996–1998) with a high-sensitivity assay, Elecsys Troponin T (Roche Diagnostics®), on an automated Cobas e411 analyzer.25 We used hs-cTnT cutoffs based on prior studies, with the limit of detection of 5 ng/L.25,42, Similarly, we also measured NT-proBNP on the automated Cobas e411 analyzer (Roche Diagnostics) using an electrochemiluminescent immunoassay with a measurement range of 5–35,000 pg/mL and a limit of quantification of 35 pg/mL.26
Outcome Assessment
Trained examiners collected neurocognitive data at ARIC Visits 2, 4 and 5 following standard protocols; this study considers associations between hs-TnT and NT-proBNP and change in cognitive performance from Visit 4 (baseline) to Visit 5. The Delayed Word Recall Test (DWRT) is a test of verbal learning and recent memory,27 the Digit-Symbol Substitution Test (DSST) is a test of executive function,28 and Word Fluency Test (WFT) is a test of semantic fluency.29 There was little evidence of floor or ceiling effects, as each score was approximately normally distributed. We calculated normalized z-scores for each test using the mean and standard deviation of scores from ARIC participants at Visit 2, and created global z-scores by normalizing the sum of the mean of the three individual test z-scores in a similar manner.1 Higher scores reflect better cognitive function.
Covariates
We considered multiple confounders: age (linear, squared), sex (male/female), race-center (Mississippi-black/North Carolina-black/ North Carolina-white/Minnesota-white/Maryland-white), education (<high school, high school or equivalent, >high school) and health insurance status (yes/no) data from Visit 1, dietary patterns and physical activity data from Visit 3, and all other covariates from Visit 4 (study baseline). A participant was considered to be physically inactive with a sport activity score of <2 from the Baecke Questionnare.30,31 We defined dietary pattern based on degree of adherence to either a “prudent” or “Western” dietary pattern.32 We defined diabetes as fasting blood glucose level ≥126 mg/dL, non-fasting blood glucose level ≥200 mg/dL, or self-reported physician diagnosis of or treatment for diabetes. We defined hypertension as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg measured with random-zero mercury manometers or use of antihypertensive medications. We measured fasting plasma total cholesterol and high-density lipoprotein cholesterol using enzymatic measures (98% fasting >8 hours). We determined medication use through visual inspection of medications at the study visit and linkage to Medi-Span Therapeutic Classification codes. Other pertinent variables were self-reported alcohol use and smoking status (current, former or never), apolipoprotein ε4 allele status (APOE ε4 alleles: 0, 1, or 2), and adjudicated stroke at any point before Visit 5 (yes/no).
Statistical Methods
We examined the association of hs-cTnT and NT-proBNP and 15-year change (from Visit 4 to 5) in global and individual cognitive test z-scores using linear mixed models. We parameterized hs-cTnT based on previously established cutoffs (<5, ≥5 to <14 and ≥14 ng/L),25,33 and parameterized NT-proBNP based on quintiles as well as clinically relevant cutoffs (<120, 120 to <300 or ≥300 pg/mL).34 We used time on study as the time scale and an independent covariance matrix for the two random effects -- a random intercept and a random slope. We adjusted all models for the aforementioned potential confounders determined a priori; models were adjusted for the main effect and the interaction of each covariate with time. We used three-way multiplicative interaction terms (effect modifier*cardiac biomarker*time) to determine whether the association of cardiac biomarkers on cognitive change was modified by race, sex, APOE ε4 status, systolic blood pressure ≥ 140 mmHg, hypertension, lipid-lowering medication use, and statin use at Visit 4.
To understand the influence of missing exposure, covariate, and cognitive data, we performed several sensitivity analyses using an adaptation of multiple imputation methods that was developed for this purpose.35 Specifically, we examined associations after (1) imputing exposure and covariate data only, (2) additionally imputing cognitive data during Visits 4 and 5 for those known to be alive at the time of each study visit but who did not complete cognitive testing, and (3) additionally imputing cognitive data from 6 months prior to death for those who died between Visits 4 and 5. For all estimates from sensitivity analyses implementing multiple imputation, we used a burn-in of 25 iterations, and report results based on combined estimates from 5 imputations. For these analyses, we considered hs-cTnT categories of <5, ≥5 to <14 and ≥14 ng/L, and NT-proBNP as quintile categories.
In additional sensitivity analyses, we excluded (i) persons with clinically diagnosed coronary heart disease or heart failure at Visit 4 (n=867), and (ii) persons with stroke prior to Visit 5 (n=317) as stroke can affect cognitive function. In post hoc analyses, we explored the association with polynomials and deciles of exposure to understand dose-response and non-linear relationship. As the models using polynomials were extremely unstable, we report only the associations with deciles. We considered using other continuous parameterizations (e.g. linear, log-transformed), but felt that these were not justifiable given the extreme skew of the distribution of hs-cTnT and the combination of skewed distribution and relatively large proportion of non-detectable levels of NT-proBNP. In additional post hoc analyses, we considered combining hs-cTnT and NT-proBNP into a single 3-level categorical variable allowing comparison of those with uniformly low hs-cnT and pro-BNP (hs-cTnT <5 ng/L and pro-BNP <120 ng/L, reference) to those with uniformly high levels on both (hs-cTnT ≥14 ng/L and NT-proBNP ≥300 ng/L), and those who did not show uniformly high or low hs-cTnT and NT-proBNP levels.
We report 95% confidence intervals and assume 2-tailed P<0.05 to be statistically significant. We performed analysis using SAS version 9.4 (Cary, NC) and STATA version 13 (College Station, TX).
RESULTS
Baseline data
A total of 9,114 and 9,108 individuals were included in our primary analyses of hs-cTnT and NT-proBNP, respectively (Supplemental Figure S1). The mean (SD) age was 63.4 (5.7) years at Visit 4 (baseline) and 76.2 (5.2) years at Visit 5. At baseline (Visit 4), 43.6% of our participants were men, 17.5% were black, and 7% of both black and white participants were likely cognitively impaired, indicated by global cognitive z-scores 1.5 SD or more below the mean in race-specific analyses. The distributions of hs-cTnT and NT-proBNP were highly right skewed; the median (25th percentile, 75th percentile) of hs-cTnT was 5 ng/L (1.5, 3.8) while the median (25th percentile, 75th percentile) of NT-proBNP was 69.5 pg/mL (34.5, 134.4). Of those included in our sample, all of whom contributed to Visit 4 cognitive data, 28% were alive but with missing cognitive data at Visit 5 and 22% were deceased by the time of Visit 5. Among those who completed all three cognitive tests at Visit 5, average follow-up time was 14.8 years (range: 12.6 to 17.4 years). Mean (SD) change in cognitive test z-scores from Visit 4 to Visit 5 was −0.79 (0.72) for the global z-scores, −0.62 (0.55) for DSST, −1.06 (1.23) for DWRT, and −0.19 (0.68) for WFT. Baseline data by categories of cardiac biomarkers are shown in Supplemental Table S1.
Primary analysis
There were no significant associations between higher baseline hs-cTnT and cognitive change (Table 1). Compared to persons in the lowest NT-proBNP quintile, those in the second NT-proBNP quintile exhibited greater cognitive decline in global z-scores (−0.069, 95% CI: −0.123, −0.014), which was largely driven by accelerated decline in DWRT scores (−0.129, 95% CI: −0.219, −0.039, Table 1). There was similar association for change in global z-score for the highest vs. lowest NT-proBNP quintile (−0.129, 95% CI: −0.252, −0.006), which was also largely driven by the association between NT-proBNP and accelerated decline in DWRT scores (−0.129, 95% CI: −0.327, 0.069). There was no evidence of an association when we considered clinically relevant cut-offs of NT-proBNP, in which the reference group (<120 pg/mL) contains most people in the first three quintiles of NT-proBNP (Table 1).
Table 1.
Average Adjusted Difference in 15-year Cognitive Change (1996–1998 to 2011–2013) by Cardiac Biomarker Levels at Visit 4 (1996–1998) in Eligible ARIC Participants
| Cardiac biomarkers | Adjusted difference in 15-year cognitive change1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Global z-score | DWRT z-score | DSST z-score | WFT z-score | |||||
| Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | |
| hs-cTnT (ng/L), n=9,114 | ||||||||
| <5 | Ref | Ref | Ref | Ref | ||||
| ≥5 to <14 | −0.006 −0.051, 0.039 |
0.79 | 0.008 −0.067, 0.082 |
0.84 | 0.017 −0.016, 0.05 |
0.31 | −0.026 −0.068, 0.016 |
0.22 |
| ≥14 | −0.039 −0.144, 0.067 |
0.47 | −0.001 −0.17, 0.168 |
0.99 | 0.013 −0.063, 0.09 |
0.73 | −0.075 −0.172, 0.021 |
0.13 |
| NT-proBNP (pg/mL), n=9,108 | ||||||||
| <34 | Ref | Ref | Ref | Ref | ||||
| ≥34 to <69 |
−0.069 −0.123, −0.014 |
0.01 |
−0.129 −0.219, −0.039 |
<0.01 | −0.023 −0.063, 0.016 |
0.25 | −0.02 −0.071, 0.031 |
0.45 |
| ≥69 to <134 | 0.022 −0.035, 0.08 |
0.45 | 0.001 −0.094, 0.097 |
0.98 | 0.025 −0.017, 0.066 |
0.25 | −0.002 −0.056, 0.052 |
0.94 |
| ≥134 to <300 | 0.009 −0.059, 0.076 |
0.80 | 0.02 −0.092, 0.132 |
0.73 | −0.002 −0.051, 0.047 |
0.94 | −0.024 −0.087, 0.039 |
0.45 |
| ≥300 |
−0.129 −0.252, −0.006 |
0.04 | −0.129 −0.327, 0.069 |
0.20 | −0.069 −0.157, 0.019 |
0.12 | −0.053 −0.167, 0.061 |
0.36 |
| NT-proBNP (pg/mL), n=9108 | ||||||||
| <120 | Ref | Ref | Ref | Ref | ||||
| ≥120 to <300 | 0.034 −0.018, 0.087 |
0.20 | 0.053 −0.035, 0.141 |
0.24 | 0.019 −0.02, 0.057 |
0.34 | 0 −0.049, 0.049 |
0.99 |
| ≥300 | −0.110 −0.228, 0.007 |
0.06 | −0.086 −0.275, 0.104 |
0.38 | −0.065 −0.15, 0.019 |
0.13 | −0.043 −0.152, 0.067 |
0.45 |
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
Statistically significant findings are shown in bold
Effect modification
There was little evidence for effect modification (all interaction P>0.05), except by sex on the association between hs-cTnT and global z-score (interaction P=0.049) and by sex on the association between hs-cTnT and WFT z-score (interaction P=0.016). In stratified analyses, men appear to have excess cognitive decline associated with higher hs-cTnT; however, the sex-specific estimated association of hs-cTnT on rate of cognitive change were not individually statistically significant (data not shown).
Sensitivity analyses
Associations of hs-cTnT on cognitive change were similar in most sensitivity analyses addressing missing data and informative attrition, except we observed greater decline in WFT z-score for the highest vs. the lowest hs-cTnT categories (−0.089, 95% CI: −0.176, −0.002) when missing exposure, covariates and cognitive scores at Visit 5 were imputed (Supplementary Table S2–S4). Results of sensitivity analyses of NT-proBNP were also similar to that of primary analyses (Supplementary Tables S2–S4), with a few exceptions. We observed a significant association for decline in WFT z-score with the highest vs. the lowest NT-proBNP quintile (−0.108, 95% CI: −0.209, −0.007) when imputing missing exposure, covariates, and cognitive data at the time of Visit 5 for those who were alive but did not attend the visit (Supplementary Table S3); but the association was no longer statistically significant when we additionally imputed scores at 6 months prior to death for those who died between Visits 4 and 5 (Supplementary Table S4). In analyses restricting to persons without (i) coronary heart disease or heart failure at Visit 4 or (ii) stroke by the time of Visit 5, the associations of hs-cTnT as well as NT-proBNP with cognitive change were similar to that of the primary analyses (Tables 2 and Supplementary Table S5), although the association between the highest versus lowest quintile of NT-proBNP was no longer statistically significant.
Table 2.
Average Adjusted Difference in 15-year Cognitive Change (1996–1998 to 2011–2013) by Cardiac Biomarker Levels at Visit 4 (1996–1998) After Excluding Persons with Prevalent CHD or Heart Failure at Visit 4 (1996–1998) in Eligible ARIC Participants
| Cardiac biomarkers | Adjusted difference in 15-year cognitive change1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global z-score | DWRT z-score | DSST z-score | WFT z-score | ||||||||||
| Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | ||||||
| hs-cTnT (ng/L), n=8,247 | |||||||||||||
| <5 | Ref | Ref | Ref | Ref | |||||||||
| ≥5 to <14 | 0.006 −0.041, 0.052 |
0.81 | 0.018 −0.059, 0.095 |
0.64 | 0.02 −0.014, 0.054 |
0.25 | −0.016 −0.06, 0.027 |
0.47 | |||||
| ≥14 | −0.031 −0.144, 0.083 |
0.60 | −0.017 −0.197, 0.163 |
0.85 | 0.023 −0.059, 0.105 |
0.58 | −0.072 −0.175, 0.031 |
0.17 | |||||
| NT-proBNP, (pg/mL), n=8,241 | |||||||||||||
| <34 | Ref | Ref | Ref | Ref | |||||||||
| ≥34 to <69 |
−0.068
−0.124, −0.013 |
0.02 |
−0.132
−0.224, −0.04 |
0.005 | −0.017 −0.057, 0.024 |
0.42 | −0.022 −0.074, 0.03 |
0.41 | |||||
| ≥69 to <134 | 0.018 −0.041, 0.077 |
0.56 | 0.004 −0.094, 0.102 |
0.94 | 0.027 −0.016, 0.07 |
0.22 | −0.008 −0.063, 0.048 |
0.78 | |||||
| ≥134 to <300 | −0.011 −0.082, 0.059 |
0.75 | −0.023 −0.14, 0.094 |
0.70 | −0.001 −0.053, 0.051 |
0.97 | −0.026 −0.092, 0.041 |
0.45 | |||||
| ≥300 | −0.131 −0.272, 0.01 |
0.07 | −0.157 −0.384, 0.071 |
0.18 | −0.049 −0.15, 0.052 |
0.34 | −0.054 −0.185, 0.077 |
0.42 | |||||
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
Statistically significant findings are shown in bold
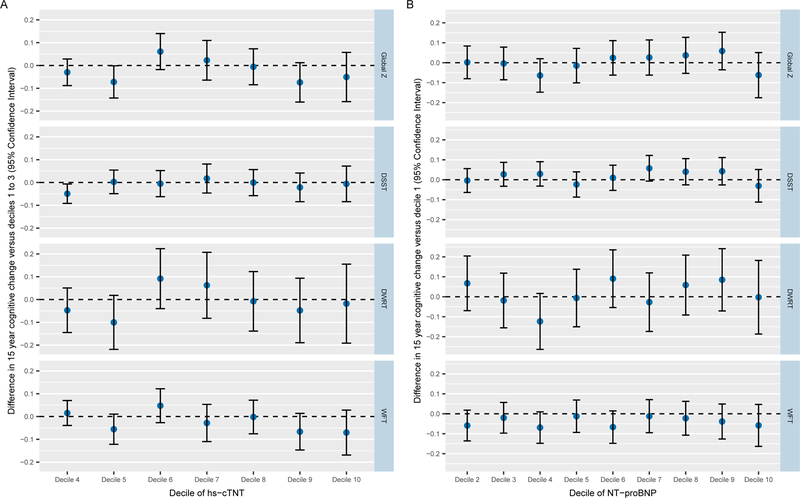
The results of the post hoc analyses with each exposure variable treated as deciles are shown in Figure Panels A and B. Note that we used the lowest three hs-cTnT deciles as the reference due to ties related to non-detectable levels of exposure. Decile analyses did not provide any evidence of a non-linear dose-response between either of the cardiac biomarkers and cognitive decline.
Figure. Deciles of cardiac biomarkers and cognitive change.
Panel A. hs-cTnT deciles and cognitive change
The lowest three hs-cTnT deciles were treated as the reference because of ties related to non-detectable levels.
Abbreviation: hs-cTnT = cardiac troponin T measured using high-sensitivity assay
Panel B. NT-proBNP deciles and cognitive change
Abbreviation: NT-proBNP = N-Terminal pro- B-type Natriuretic Peptide
The results of additional post hoc analyses combining the two biomarkers are shown in Table 3. As compared with uniformly low biomarker levels, there were no signification associations of either uniformly high biomarker levels or neither uniformly high nor uniformly low levels for change in individual cognitive z-scores. However, people with uniformly high biomarker levels had greater cognitive decline in global z-scores (−0.342, 95% CI: −0.638, −0.045) when compared with uniformly low biomarker levels.
Table 3.
Average Adjusted Difference in 15-year Cognitive Change (1996–1998 to 2011–2013) by Combined Cardiac Biomarker Levels at Visit 4 (1996–1998) in Eligible ARIC Participants
| Cardiac biomarkers | Adjusted difference in 15-year cognitive change1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Global z-score | DWRT z-score | DSST z-score | WFT z-score | |||||
| Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | |
| n=9108 | ||||||||
| Uniformly Low | Ref | Ref | Ref | Ref | ||||
| Neither Uniformly High nor Uniformly Low |
−0.003 −0.045, 0.04 |
0.91 | 0.02 −0.05, 0.091 |
0.58 | 0.007 −0.024, 0.037 |
0.67 | −0.029 −0.069, 0.01 |
0.14 |
| Uniformly High |
−0.342 −0.638, −0.045 |
0.02 | −0.238 −0.704, 0.228 |
0.32 | −0.195 −0.407, 0.016 |
0.07 | −0.215 −0.488, 0.059 |
0.12 |
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
Statistically significant findings are shown in bold
DISCUSSION
Myocardial injury as indexed by hs-cTnT level and myocardial stretch as reflected by NT-proBNP level were not associated with accelerated cognitive decline over 15-years follow up among survivors in a large, biracial, community-based prospective cohort study. There was no evidence to support effect modification by important covariates. Results were similar in sensitivity analyses where we addressed bias due to informative non-death attrition and the impact of preexisting coronary heart disease and intervening stroke. Nevertheless, in post-hoc analysis, people with highest levels of both biomarkers had excessive decline in global z-scores than people with lowest levels of both biomarkers. It is possible that these cardiac biomarkers may be truly associated with cognitive deterioration but we did not observe such association given intervening mortality and the relative infrequency of cognitive follow-up.
It is known that hs-cTnT and NT-proBNP are sensitive biomarkers of myocardial injury7 and stretch,8 respectively. Both predict not only congestive heart failure25,36,37 and coronary heart disease,10,25,38 but also stroke9,10,36,38 and subclinical brain injury.11,12 Findings of our primary analyses are at odds with prior studies. Prior studies of high sensitive troponins14–18 and NT-proBNP,15,17,19–23 have shown significant associations with cognitive function, decline or dementia. However, these studies were limited by smaller sample size, 15,19,20,22,23 either cross-sectional or case case-control designs,15,18–20,23 or in the case of cohort studies, short follow up,16,22 typically involved a late-life study population,14–16,22,23,39 or considered associations with level of cognitive function (and not cognitive change)15,19,20,23 or ICD diagnostic codes used for dementia diagnosis.17,18,21 Thus, the discrepancy in findings may be related to methodological differences between the current and previous studies. First, cross-sectional studies estimate associations between prevalent disease and prevalent cognition, and such associations are not always replicated in prospective studies for several reasons, including issues of reverse causation. In fact, in the current analysis we see evidence of a cross-sectional association between levels of the cardiac biomarkers and cognition, even though there is little support for a longitudinal association with cognitive change. Second, there were only a few prospective studies examining cognitive decline,16, 21, 42 and the cognitive tests used in our study were different than those used in these prior studies.16, 21 Indeed, a prior prospective study that used change in WFT as one of the outcomes showed null association, similar to our current analysis.42 Our tests may not have sufficiently captured decline in the relevant cognitive domain. However, it is notable that others have reported associations between other cardiovascular risk factors and cognitive change in the ARIC study, indicating that the tests we used in our analyses are sensitive to late-life cognitive decline.1,2 Third, many prior studies used dementia as the outcome.14,15,17,20,42,43 Dementia is the advanced form of accelerated cognitive decline, and is diagnosed when cognitive impairment is sufficient to interfere with functional ability. Associations between risk factors and the related outcomes of cognitive decline and dementia do not always converge in other settings;1,40 this may be related to issues of confounding by pre-morbid cognition.41 Furthermore, several studies diagnosed dementia using ICD codes with possibility of misclassification error. This methodological choice could also contribute to the differing findings between our study of cognitive decline and other studies of dementia diagnosed using ICD codes. 17,18,21 Fourth, selective attrition from loss to follow is important in study of cognitive change because people with poor cognitive function are less likely to continue participantion.35 Most prior prospective studies did not account for selective attrition from loss to follow up or death.16,21,22 In a prior study, association of NT-proBNP for dementia attenuated but still remained significant and for Alzheimer’s disease reversed after using competing risk analysis accounting for death as a competing risk for dementia.39 Our results were similar after implementation of a multiple imputation approach, which minimizes the likelihood that bias due to non-death attrition can explain the lack of strong evidence of association between the cardiac biomarkers and cognitive change. However, as these biomarkers are strongly associated with death25,33 clinical and subclinical brain disease,11,12 as well as dementia related hospitalization, 23 it is possible that earlier mortality in persons with myocardial injury and stretch may have prevented observation of otherwise truly present association.25,33 However, our current results suggest no association between the biomarkers when assessed individually and cognitive change in the survivors, and sensitivity analyses imputing cognitive status prior to death in those who die did not materially change our findings. However, in post-hoc analysis, we found that those with highest levels of both biomarkers were more likely to have excessive cognitive decline than those with lowest levels. Given that there is considerable evidence that cardiac disease and risk factors may be associated with cognitive decline,1–5 future studies may consider testing whether biomarkers of myocardial injury and stretch could be an intervention target.
Fifth, it is possible that the actual degree of cognitive decline associated with cardiac biomarkers examined here may be so small that our study was underpowered to detect this difference. However, other studies in ARIC have reported small, yet meaningful associations between vascular risk factors and cognitive decline1–3 and smaller associations that could be detected in a larger sample are unlikely to be clinically relevant.
While there was some suggestion that the effect of hs-cTnT on cognitive decline was modified by sex, and we observed a higher prevalence of elevated hs-cTnT in men (hs-cTnT ≥14: 16% of men versus 3% of women), estimates of the sex-specific associations were not statistically significant. Although myocardial stretch as reflected by NT-proBNP level was associated with greater cognitive decline when comparing the highest NT-proBNP quintile with the lowest quintile, we did not see similar association when comparing the highest to lowest clinically relevant categories. While it remains possible that there is a true association whereby very high NT-proBNP levels confers excess risk of cognitive decline relative to the lowest levels, we also cannot exclude the possibility of chance findings.
Our study has several limitations. Although the three cognitive tests studied are usually affected in dementia, they do not cover the complete range of cognitive domains. The mean age of our study population was 63 years. As midlife cardiovascular risk factors are often more relevant to cognitive decline and dementia than late life measures, it is possible that future studies in a younger population may find different result. The interaction by sex with hs-cTnT could be a chance finding and should be further investigated. Despite multiple imputations, residual bias from selective attrition is possible as missing cognitive assessments is likely not-at-random,42 and as such it can be difficult to make firm conclusions. However, this is going to be almost unavoidable issue in most studies of aging. Not all key baseline covariates were collected at the same visit. Physical activity, diet, and health insurance status were assessed several years prior to baseline could have changed over time. Additional factors affecting association of these biomarkers with cognitive change include analytical and biological variations in biomarkers’ sampling and various biomarker trajectories over time, which was not captured in this study. Although we believe that we have adequately addressed confounding, we cannot exclude residual confounding or the role of chance.
CONCLUSION
Among survivors, myocardial injury as indexed by hs-cTnT was not associated with cognitive decline. Although there was evidence of an association between NT-proBNP and accelerated decline in verbal learning and memory when comparing those in the highest to lowest quintile, we did not see similar evidence to support increased risk with high NT-proBNP when we considered clinically relevant categories or using deciles. However, when considering both biomarkers together in a post-hoc analysis, people with highest levels of both biomarkers were more likely to have excessive cognitive decline than those with lowest levels. Whether cardiac biomarkers can be an intervention target should be examined further.
Supplementary Material
Supplemental Figure S1. Flowchart describing derivation for primary analytical sample used to assess association between cardiac biomarkers and 15-year cognitive decline.
Abbreviations: hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide
Supplementary Table S1. Summary of Observed Demographic and Health Characteristics of ARIC Participants Eligible for Inclusion in Primary Analyses at Study Baseline (Visit 4, 1996–1998)
Abbreviations: ARIC = Atherosclerosis Risk in Communities, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide
1Higher values indicate greater adherence to the dietary pattern
Categorical variables presented as percentage and continuous variables as mean (standard deviation)
Supplementary Table S2. Average adjusted difference in 15-year cognitive change (1996–1998 to 2011–2013) by cardiac biomarker levels at Visit 4 (1996–1998) after imputing missing exposure and covariate data in eligible ARIC participants
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test,,hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
1Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
2Categories determined using quintile cut-offs.
Statistically significant findings are shown in bold.
Supplementary Table S3. Average adjusted difference in 15-year cognitive change (1996–1998 to 2011–2013) by cardiac biomarker levels at Visit 4 (1996–1998) after imputing missing exposure, covariates and cognitive scores among alive persons at visit 5 in eligible ARIC participants
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
1Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
2Categories determined using quintile cut-offs.
Statistically significant findings are shown in bold.
Supplementary Table S4. Average adjusted difference in 15-year cognitive change (1996–1998 to 2011–2013) by cardiac biomarker levels at Visit 4 (1996–1998) after imputing missing exposure, covariates and cognitive scores 6 months prior to death for those who died between visits 4 and 5, in eligible ARIC participants
1Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
2Categories determined using quintile cut-offs.
Statistically significant findings are shown in bold.
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, Hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
Supplementary Table S5. Average Adjusted Difference in 15-year Cognitive Change (1996–1998 to 2011–2013) by Cardiac Biomarker Levels at Visit 4 (1996–1998) After Excluding Persons with Prevalent Stroke at Visit 5 (2011–2013) in Eligible ARIC Participants
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
1Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
Statistically significant findings are shown in bold
ACKNOWLEDGEMENT:
Conflict of Interest:
Dr. Knopman serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network study; is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California; and receives research support from the National Institutes of Health.
Dr Nambi has received honorarium for event adjudication for a study sponsored by Siemens Lab. A provisional patent (patent no. 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” was filed by Roche and Baylor College of Medicine on behalf of Dr. Nambi.
Dr. Ballantyne has received research grant from Roche (Significant); and served as consultants to Denka Seiken. A provisional patent (patent no. 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” was filed by Roche and Baylor College of Medicine on behalf of Dr. Ballantyne.
Dr. Hoogeveen has received research grant from Roche and Denka Seiken (Significant); and served as consultants to Denka Seiken. A provisional patent (patent no. 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” was filed by Roche and Baylor College of Medicine on behalf of Dr. Hoogeveen.
Dr. Selvin- advisory board, Roche.
Others: None
Reagents for the high sensitivity troponin and NTproBNP assays were donated by the Roche Diagnostics Corporation.
Funding Sources:
This work was supported in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I and HHSN268201700005I). Neurocognitive data are collected by the support of the National Heart, Lung, and Blood Institute U01 HL096812, HL096814, HL096899, HL096902, and HL096917, with previous brain magnetic resonance imaging examinations funded by R01‐HL70825. This research was also supported by National Institutes of Health/ National Heart, Lung, and Blood Institute grant R01 HL134320 to Drs. Ballantyne and Selvin.
Dr. Pokharel was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837 and the American Heart Association SWA Summer 2014 Postdoctoral Fellowship Award (15POST23080014).
Dr. Schneider was supported by the NIH/NINDS through an administrative supplement to award R25NS065729.
Dr. Selvin was supported by NIH/NIDDK grants K24DK106414 and R01DK089174.
Dr. Power was supported by the National Institute of Aging R03AG055485 during the period of this work.
The authors thank the staff and participants of the ARIC study for their important contributions. The authors also thank Dr. Pamela Lutsey for providing the dietary pattern scores.
Abbreviations
- APOE ε4
apolipoprotein ε4
- ARIC
Atherosclerosis Risk In Communities
- DSST
Digit-Symbol Substitution Test
- DWRT
Delayed Word Recall Test
- hs-cTnT
cardiac troponin T measured using high-sensitivity assay
- NT-proBNP
N-Terminal pro-B-type Natriuretic Peptide
- WFT
Word Fluency Test
Footnotes
Sponsors’ Role:
The sponsors have no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
REFERENCES:
- 1.Power MC, Rawlings A, Sharrett AR, et al. Association of midlife lipids with 20-year cognitive change: A cohort study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2017. [DOI] [PMC free article] [PubMed]
- 2.Rawlings AM, Sharrett AR, Schneider AL, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Annals of internal medicine 2014;161(11):785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA neurology 2014;71(10):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolters FJ, Segufa RA, Darweesh SKL, et al. Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2018. [DOI] [PubMed]
- 6.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361(9):858–867. [DOI] [PubMed] [Google Scholar]
- 8.Hall C Essential biochemistry and physiology of (NT-pro)BNP. European journal of heart failure 2004;6(3):257–260. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Nambi V, Bell EJ, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke; a journal of cerebral circulation 2013;44(4):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oluleye OW, Folsom AR, Nambi V, Lutsey PL, Ballantyne CM. Troponin T, B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Annals of epidemiology 2013;23(2):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pikula A, Beiser AS, DeCarli C, et al. Multiple biomarkers and risk of clinical and subclinical vascular brain injury: the Framingham Offspring Study. Circulation 2012;125(17):2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dadu RT, Fornage M, Virani SS, et al. Cardiovascular biomarkers and subclinical brain disease in the atherosclerosis risk in communities study. Stroke; a journal of cerebral circulation 2013;44(7):1803–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zonneveld HI, Ikram MA, Hofman A, et al. N-Terminal Pro-B-Type Natriuretic Peptide and Subclinical Brain Damage in the General Population. Radiology 2017;283(1):205–214. [DOI] [PubMed] [Google Scholar]
- 14.Sabayan B, van Buchem M, de Craen T, et al. High-sensitivity serum troponin t and future risk of dementia: The ages-reykjavik study. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 11(7):P512. [Google Scholar]
- 15.Hilal S, Chai YL, Ikram MK, et al. Markers of cardiac dysfunction in cognitive impairment and dementia. Medicine 2015;94(1):e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijsman LW, de Craen AJ, Trompet S, et al. High-sensitivity cardiac troponin T is associated with cognitive decline in older adults at high cardiovascular risk. European journal of preventive cardiology 2016;23(13):1383–1392. [DOI] [PubMed] [Google Scholar]
- 17.Tynkkynen J, Hernesniemi JA, Laatikainen T, et al. High-sensitivity cardiac troponin I and NT-proBNP as predictors of incident dementia and Alzheimer’s disease: the FINRISK Study. Journal of neurology 2017;264(3):503–511. [DOI] [PubMed] [Google Scholar]
- 18.Schneider AL, Rawlings AM, Sharrett AR, et al. High-sensitivity cardiac troponin T and cognitive function and dementia risk: the atherosclerosis risk in communities study. Eur Heart J 2014;35(27):1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kara K, Mahabadi AA, Weimar C, et al. N-Terminal Pro-B Type Natriuretic Peptide is Associated with Mild Cognitive Impairment in the General Population. Journal of Alzheimer’s disease : JAD 2017;55(1):359–369. [DOI] [PubMed] [Google Scholar]
- 20.Feinkohl I, Sattar N, Welsh P, et al. Association of N-terminal pro-brain natriuretic peptide with cognitive function and depression in elderly people with type 2 diabetes. PloS one 2012;7(9):e44569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tynkkynen J, Laatikainen T, Salomaa V, et al. NT-proBNP and the risk of dementia: a prospective cohort study with 14 years of follow-up. Journal of Alzheimer’s disease : JAD 2015;44(3):1007–1013. [DOI] [PubMed] [Google Scholar]
- 22.van Vliet P, Sabayan B, Wijsman LW, et al. NT-proBNP, blood pressure, and cognitive decline in the oldest old: The Leiden 85-plus Study. Neurology 2014;83(13):1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels LB, Laughlin GA, Kritz-Silverstein D, et al. Elevated natriuretic peptide levels and cognitive function in community-dwelling older adults. Am J Med 2011;124(7):670.e671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology 1989;129(4):687–702. [PubMed] [Google Scholar]
- 25.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123(13):1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nambi V, Liu X, Chambless LE, et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk--the atherosclerosis risk in communities study. Clinical chemistry 2013;59(12):1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol 1989;46(2):141–145. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler D Wechsler Adult Intelligence Scale-Revised (WAIS-R) San Antonio, Texas: The Psychological Corporation; 1981. [Google Scholar]
- 29.Lezak M Neuropsychological Assessment New York: Oxford University Press; 1995:335–384. [Google Scholar]
- 30.Demerath EW, Lutsey PL, Monda KL, et al. Interaction of FTO and physical activity level on adiposity in African-American and European-American adults: the ARIC study. Obesity (Silver Spring) 2011;19(9):1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer AM, Evenson KR, Couper DJ, Stevens J, Pereria MA, Heiss G. Television, physical activity, diet, and body weight status: the ARIC cohort. The international journal of behavioral nutrition and physical activity 2008;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 2008;117(6):754–761. [DOI] [PubMed] [Google Scholar]
- 33.Pokharel Y, Sun W, de Lemos JA, et al. High-sensitivity troponin T and cardiovascular events in systolic blood pressure categories: atherosclerosis risk in communities study. Hypertension 2015;65(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HN, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011;123(18):2015–2019. [DOI] [PubMed] [Google Scholar]
- 35.Rawlings AM, Sang Y, Sharrett AR, et al. Multiple imputation of cognitive performance as a repeatedly measured outcome. European journal of epidemiology 2017;32(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350(7):655–663. [DOI] [PubMed] [Google Scholar]
- 37.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. Jama 2010;304(22):2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation 2009;120(22):2177–2187. [DOI] [PubMed] [Google Scholar]
- 39.Mirza SS, de Bruijn RF, Koudstaal PJ, et al. The N-terminal pro B-type natriuretic peptide, and risk of dementia and cognitive decline: a 10-year follow-up study in the general population. Journal of neurology, neurosurgery, and psychiatry 2016;87(4):356–362. [DOI] [PubMed] [Google Scholar]
- 40.Gottesman RF, Albert MS, Alonso A, et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA neurology 2017;74(10):1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor DH Jr., Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. Journal of Alzheimer’s disease : JAD 2009;17(4):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Bari M, Pahor M, Franse LV et al. Dementia and disability outcomes in large hypertension trials: lessons learned from the systolic hypertension in the elderly program (SHEP) trial. Am J Epidemiol 2001. January 1; 153(1):72–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Flowchart describing derivation for primary analytical sample used to assess association between cardiac biomarkers and 15-year cognitive decline.
Abbreviations: hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide
Supplementary Table S1. Summary of Observed Demographic and Health Characteristics of ARIC Participants Eligible for Inclusion in Primary Analyses at Study Baseline (Visit 4, 1996–1998)
Abbreviations: ARIC = Atherosclerosis Risk in Communities, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide
1Higher values indicate greater adherence to the dietary pattern
Categorical variables presented as percentage and continuous variables as mean (standard deviation)
Supplementary Table S2. Average adjusted difference in 15-year cognitive change (1996–1998 to 2011–2013) by cardiac biomarker levels at Visit 4 (1996–1998) after imputing missing exposure and covariate data in eligible ARIC participants
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test,,hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
1Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
2Categories determined using quintile cut-offs.
Statistically significant findings are shown in bold.
Supplementary Table S3. Average adjusted difference in 15-year cognitive change (1996–1998 to 2011–2013) by cardiac biomarker levels at Visit 4 (1996–1998) after imputing missing exposure, covariates and cognitive scores among alive persons at visit 5 in eligible ARIC participants
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
1Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
2Categories determined using quintile cut-offs.
Statistically significant findings are shown in bold.
Supplementary Table S4. Average adjusted difference in 15-year cognitive change (1996–1998 to 2011–2013) by cardiac biomarker levels at Visit 4 (1996–1998) after imputing missing exposure, covariates and cognitive scores 6 months prior to death for those who died between visits 4 and 5, in eligible ARIC participants
1Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
2Categories determined using quintile cut-offs.
Statistically significant findings are shown in bold.
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, Hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
Supplementary Table S5. Average Adjusted Difference in 15-year Cognitive Change (1996–1998 to 2011–2013) by Cardiac Biomarker Levels at Visit 4 (1996–1998) After Excluding Persons with Prevalent Stroke at Visit 5 (2011–2013) in Eligible ARIC Participants
Abbreviations: ARIC = Atherosclerosis Risk in Communities, DSST = digit-symbol substitution test, DWRT = delayed word recall test, hs-cTnT = cardiac troponin T measured using high-sensitivity assay, NT-proBNP = N-Terminal pro-B-type Natriuretic Peptide, WFT = word fluency test
1Adjusted for age, age-squared, education, sex, race-center, body mass index, smoking status, alcohol use, diabetes, hypertension, physical activity, adherence to a prudent dietary pattern, adherence to a Western dietary pattern, use of lipid-lowering medications, health insurance status, Apolipoprotein ε4 allelle status, and total cholesterol/high-density lipoprotein cholesterol ratio, including main effects and interactions with time.
Statistically significant findings are shown in bold