Abstract
To optimize digital health interventions, intervention creators must determine what intervention dose will produce the most substantial health behavior change—the dose-response relationship—while minimizing harms or burden. In this manuscript we present important concepts, considerations, and challenges in studying dose-response relationships in digital health interventions. We propose that interventions make three types of prescriptions: (1) intervention action prescriptions, prescriptions to receive content from the intervention, such as to read text or listen to audio; (2) participant action prescriptions, prescriptions to produce and provide content to the intervention, such as to send text messages or post intervention-requested photos on social media; and (3) behavioral target action prescriptions, prescriptions to engage in behaviors outside the intervention, such as changing food intake or meditating. Each type of prescription has both an intended dose (i.e., what the intervention actually prescribes) and an enacted dose (i.e., what portion of the intended dose is actually completed by the participant). Dose parameters of duration, frequency, and amount can be applied to each prescription type. We consider adaptive interventions and interventions with ad libitum prescriptions as examples of tailored doses. Researchers can experimentally manipulate intended dose to determine the dose-response relationship. Enacted dose cannot generally be directly manipulated; however, we consider the applicability of “controlled concentration” research design to the study of enacted dose. We consider challenges in dose-response research in digital health interventions, including characterizing amount with self-paced activities and combining doses across modality. The presented concepts and considerations may help contribute to the optimization of digital health interventions.
MESH Keywords: Health Behavior, Treatment Adherence and Compliance, Treatment Outcome, telemedicine, behavioral research, research design
Designing digital interventions to improve health behavior requires making decisions about how much intervention to deliver and how to distribute that delivery over time. This is a matter of designing the intervention dose. Dose, like other elements of digital health interventions, should be selected based on empirical evidence of what will produce the most substantial behavioral change--the dose-response relationship--while minimizing harms or burden. Dose is a critical feature of an intervention. An intervention may be ineffective if the dose is too small or too large, even with otherwise perfectly selected and executed behavior change techniques. Thus, the study of dose in digital health interventions is essential for progress in developing maximally effective digital health interventions. Existing literature on the dose-response relationship has primarily focused on traditional intervention modalities such as in-person sessions or synchronous telephone calls (Lin et al., 2014; Martín Cantera et al., 2015; Powers, Vedel, & Emmelkamp, 2008). It is not clear if dose-response relationships observed in traditional intervention modalities apply to digital health interventions, which, more often than traditional interventions, are dynamic, delivered in individuals’ natural environments, and are delivered using frequent, brief contacts (e.g., daily text messages or social media postings).
The study of dose-response relationships in digital health interventions will be most productive if the concept of dose is clearly and consistently operationalized. Existing writings on operationalization of dose in behavioral interventions (Manojlovich & Sidani, 2008; Voils et al., 2012, 2014) do not address how dose concepts and parameters apply to digital health interventions. Given the differences between traditional and digital health interventions, there is a need to update the conceptualization of dose to better fit elements of digital health interventions. Thus, in the current manuscript we present a modified conceptualization of dose and provide suggestions for operationalizing and quantifying dose in digital health interventions. We purposefully design this conceptualization to apply across intervention modalities (including in-person modality) to facilitate discussion among researchers working in different modalities and to account for the many interventions that involve multiple modalities. After presenting a refined conceptualization of dose, we describe how this conceptualization might inform empirical research aiming to identify optimal doses for digital health interventions. Finally, we consider challenges that can arise when conducting dose-response research in digital health interventions and present strategies to overcome these challenges. The aim of our manuscript is to stimulate discussion, rather than to present a definitive or prescriptive description of these topics.
Conceptualizing Dose for Dose-Response Research in Digital Health Interventions
Dose, when applied to behavior change interventions, has been described as how much of an intervention has been delivered and received (Voils et al., 2012, 2014). The parameters of dose that have been suggested include duration, frequency, and amount (Manojlovich & Sidani, 2008; Voils et al., 2012). Duration is the total length of time over which an intervention is delivered; frequency is how often contact is made; and amount is the length of each contact. For example, an intervention can deliver content for a duration of six months at a frequency of twice per week, with each contact having an amount of one hour. Total number of contacts can be described by multiplying duration and frequency, while the total dose can be described by multiplying duration, frequency, and amount (Voils et al., 2012). Other ways of characterizing dose are tailored versus untailored and fixed versus variable (Voils et al., 2012). An intervention with a tailored dose provides different doses to different individuals based on individual characteristics, whereas an untailored dose provides the same dose across all participants. An intervention with a variable dose provides different doses across time during the intervention, whereas a fixed dose provides a consistent dose across time.
Determining What Counts Towards Dose in Digital Health Intervention.
A central challenge in applying these dose parameters to digital health interventions is determining to which components of the intervention dose should be applied. To explore this potential challenge, we consider the example of Track, a digital health intervention for weight loss among adults with obesity developed by Bennett and colleagues (2018). In Track, participants are assigned individualized diet and physical activity goals informed by their response to a series of questions. On a weekly basis, they receive a text message or an Interactive Voice Response (IVR) message (modality of message is based on participant preference) requesting that they report their progress on these goals over the prior week. They then have the opportunity to text or verbally respond to the IVR system to indicate extent of achievement of their goal. Based on their responses, the intervention sends a text or IVR message to the participant with feedback to encourage future goal attainment. The Track intervention also includes 30-minute counseling phone calls with a coach that occur every two weeks initially then every four weeks. To apply the dose parameters of frequency, amount, and duration to Track, one must first decide to which intervention elements these parameters apply. With Track, dose can apply to: texts/IVR calls sent by the intervention to the participant asking about goal attainment; texts/IVR calls sent by the participant to the intervention to report their goal attainment; texts/IVR calls sent by the intervention to the participant containing tailored feedback; counseling calls; and/or those activities that participants do in response to the intervention, such as walking more or increasing the frequency of grocery shopping. If dose can be applied to all of these components, then one must consider if they should be combined to provide one total dose of the intervention or if there are meaningful differences in these components that warrant presenting dose separately for different components.
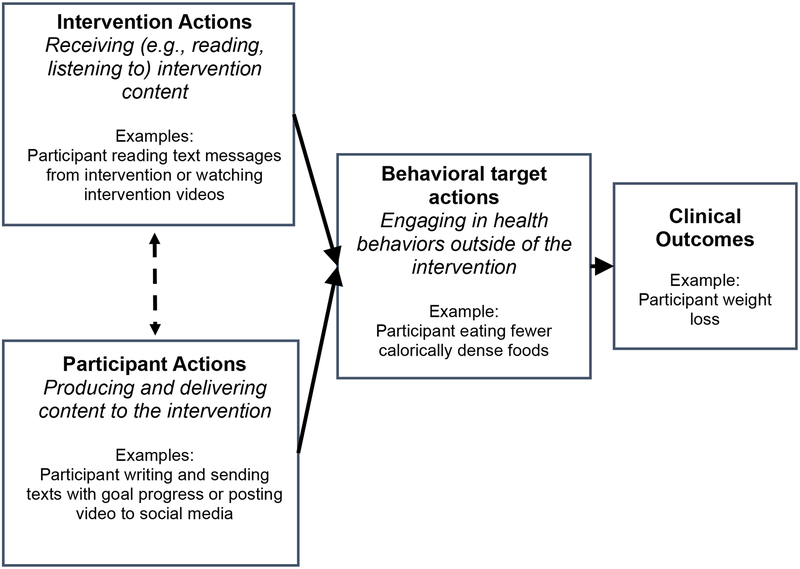
To determine what counts towards dose, it may be useful to focus on the behavioral prescription of the intervention—i.e., what the intervention asks the participants to do. Interventions’ behavioral prescriptions can be grouped into three meaningful categories: (1) prescriptions that participants receive content from the intervention, which can be called intervention action prescription; (2) prescriptions that participants produce and provide content to the intervention, which can be called participant action prescription; (3) prescriptions that participants engage in behaviors outside the intervention, which can be called behavioral target prescription (see Figure 1). There are commonalities of this categorization with existing conceptualizations of behavioral interventions. For example, the expert-developed ORBIT model of intervention development and testing distinguishes between the delivery of intervention and the behaviors in which participants engage (i.e., the behavioral targets; Czajkowski et al., 2015). Additionally, researchers in the area of intervention fidelity have distinguished between the delivery of the intervention and the receipt of the intervention (Bellg et al., 2004). In the next section, each of the three proposed prescriptions are considered in detail.
Figure 1.
Relationship between intervention actions, participant actions, behavioral target actions, and clinical outcomes.
Intervention action prescription.
Intervention action prescription refers to the intervention content participants are asked to receive. Depending on the modality, receiving can involve reading content (e.g., reading a mobile application notification), listening to content (e.g., listening to a message on a smart speaker), viewing content (e.g., watching a video in a social media feed), or some combination of these actions. Intervention action prescriptions are often stated explicitly by the intervention. For example, when individuals enroll in an intervention, they may be instructed to attend a one-hour group session each week for eight weeks, to enter a virtual reality world for 30 minutes once per week, or to log-in to their Twitter account daily to read messages from the study group. In some cases, the intervention action prescription is prescribed not only through explicit communication, but also is “pushed” by the intervention to participants (Klasnja & Pratt, 2012). Pushing content refers to a practice used by many digital health interventions in which content is delivered in a way that is intended to be integrated into their typical technology usage and thus is difficult to ignore. Examples include sending an email to a regularly used e-mail account, placing a post to appear in participants’ stream of social media, or sending a text message or an app notification to participants’ smart phones. The Track intervention, described previously, pushes text messages to participants’ smart phones; participants are thus prescribed to read these messages and respond accordingly.
Participant action prescription.
Participant action prescription refers to what the intervention asks the participant to produce and deliver in response to the intervention. Examples of participant action prescriptions include requesting a participant to send a text message indicating progress on a goal (as in the Track example); prompting participants to post a photo of themselves exercising on social media; encouraging participants to “talk” to a virtual person while in a virtual reality world; or asking participants to make an audio recording describing their mood.
Some interventions involve rapid exchange between interdependent intervention actions and participant actions. For example, a text messaging intervention can involve a constant exchange of texts with an interventionist (or bot). In the proposed framework, texts sent by the intervention comprise the intervention action prescription, and the request that the participant will respond is the participant action prescription. Describing the dose of these frequent, interdependent interactions poses a challenge, which is considered later.
Behavioral target prescription.
Behavioral target prescription refers to requests by the intervention to engage in the health behavioral targets of the intervention. This can include instructions to meditate twice a day; requests to consume more fruits and vegetables; or reminders to participants to take their medications. Behavioral target prescriptions may require intermediary behaviors that are implied rather than explicitly stated. For example, participants in the Track program may be assigned the goal of eating at least three fruits and vegetables per day; this also has the implicit prescription that the individual may need to go to the grocery store to buy these fruits and vegetables. On occasion, an intervention may influence individuals to engage in behaviors that are not directly prescribed by the intervention. For example, an intervention focused on stress reduction may inspire a participant to start using a meditation app, even if meditation was not prescribed. This “spontaneous” engagement in health-related activities (digitally mediated or otherwise) can be observed and described by the research team; however, because it does not involve prescribed activities, it would not count as part of dose in the present conceptualization.
The dose of the behavioral target prescriptions may have an important influence on outcomes, as it contributes to the total amount of time a participant devotes to the intervention. In the remainder of this manuscript, we focus on intervention and participant action prescriptions because they involve direct interaction between the participant and the intervention.
Intended and enacted dose for intervention and participant action prescriptions.
Previous conceptualizations of dose distinguished between how much intervention was intended by the intervention developers or implementers, called intended dose, and how much was actually received by the participants, called received dose (Saunders, Evans, & Joshi, 2005; Voils et al., 2012). These dose concepts can be applied to digital health interventions, and can be applied separately to the intervention action prescription and the participant action prescription (see Table 1). Enacted dose is used herein in place of received dose because it is inclusive of both intervention action and participant action prescriptions. These types of doses can be described using the dose parameters previously presented—frequency, amount, and duration.
Table 1.
Intended Dose and Enacted Dose for Intervention Action and Participant Actions.
| Intervention action | Participant action | |
|---|---|---|
| Intended Dose | Dose (duration, frequency, amount) of intervention content prescribed for the participant to see, hear, and/or read. | Dose (duration, frequency, amount) of prescribed actions (write, speak, photograph) for the participant to produce and provide to the intervention. |
| Enacted Dose | Portion of content that the participant sees, hears, and/or reads. | Portion of prescribed actions that the participant produces and provides to the intervention. |
Application of the dose parameter amount to digital health interventions is worth additional consideration. Describing the dose amount can be challenging in some digital health interventions, owing to the self-paced nature of many elements (e.g., reading of texts, navigating interactive web interventions) and individual differences (e.g., reading speed). For textual content, it may be sufficient to describe amount in terms of the number of words or characters. In other cases, it may be useful to convert written and other digital health content to a time unit, such as when comparing across different formats. Given that the average reader with an eighth grade level of education can read about 150 words per minute (Hasbrouck & Tindal, 2015.), one approach to translating textual data into time units is to describe content with 150 words or less as having an amount of one minute and content over 150 words a time value based on 150 words/minute. Alternatively, a time unit for an intervention can be estimated by having a small sample of participants complete an intervention task, perhaps during pilot testing, to identify an average or range of times.
Case studies.
We now present case studies to demonstrate the application of these dose parameters on digital health interventions. Consider again the Track intervention. One of the prescriptions is for participants to read weekly brief text messages (about 20–30 words) asking about goal progress. This is an intervention action prescription because the intervention is asking participants to receive this intervention content. The intended dose of this component can be characterized as duration of 12 months and frequency of once per week. The amount can be summarized as one minute based on the number of words presented to participants. The enacted intervention prescription dose for this intervention is how many of these IVR messages were listened to or text messages were read. The feasibility of determining the enacted dose varies depending on the technology used. In this example, the portion of IVR calls answered may be easy to obtain and provide a partial indicator of enacted dose for those participants who selected the IVR mode. With use of certain technology, it is also possible to determine if a text message was viewed.
Track participants are also asked to send a weekly text message indicating if they met their goals. This is a participant action prescription because they are asked to provide content to the intervention. The intended duration of this prescription is 12 months and frequency is once per week. There is no pre-specified number of words requested from participants, making the assignment of an intended amount unclear. However, given that the mode is texting and that participants are asked to answer a brief question, it can be assumed that the amount is one minute. Alternatively, the amount parameter may be deemed irrelevant to this particular intervention. The enacted dose of this participant action prescription can be determined by recording how many responses were sent to the intervention.
We next consider another digital health intervention, Tweet2Quit, a social media-based smoking cessation intervention that has been piloted (Pechmann, Delucchi, Lakon, & Prochaska, 2017) and is now being tested in a fully powered randomized controlled trial. Tweet2Quit intervention components include: a one-time automated email encouraging participants to start tweeting at least daily; a series of five automated emails with links to smokefree.gov modules (delivered at varying intervals); daily “auto-question” tweets to the group from the intervention; and daily “auto-feedback” text messages, sent to participants’ smart phones that encourage them to tweet. The intended intervention action prescription is as follows: (1) read automated emails and modules at smokefree.gov, which has a variable dose with a duration of 22 days and average frequency of every 5.5 days; (2) read Twitter auto-question messages, which have a duration of 100 days and frequency of daily; and (3) read auto-feedback text messages, which have a duration of 100 days and frequency of daily. The enacted intervention action of reading the smokefree.gov module can be partially estimated by determining how often the modules sent via email were opened. However, it may not be feasible to determine if the tweets were read. For Tweet2quit, the intended participant action prescription is to tweet daily over the course of the intervention, which has a duration of 100 days and frequency of once per day. The amount parameter for this prescription can be expressed in number of words prescribed to read or translated into a minute unit. The enacted participant action dose in this example is how much the participants tweet; the authors reported that an average of 72 tweets were written per participant, out of the prescribed 100 (Pechmann, Delucchi, Lakon, & Prochaska, 2017). Note that this value combines frequency (1/day) and duration (100 days) to provide total number of contacts.
Tailored and ad libitum interventions.
Digital health interventions have substantial promise for facilitating interventions that are precisely tailored to characteristics of the participants—that is, adaptive interventions. Examples of adaptive intervention approaches include just-in-time adaptive interventions (Nahum-Shani et al., 2016.) and control systems engineering-based approaches (Hekler et al., 2018). Adaptive interventions can tailor both the content and the intended dose. Consider an intervention that aims to improve adherence to oral medication for patients with diabetes by delivering a text message reminder only when the electronic pill container is not opened in a pre-specified time (Vervloet et al., 2012). The intended intervention action frequency can be described as 0–1 times/day. Describing the enacted dose of tailored interventions requires additional considerations. It may be most appropriate for enacted dose to be presented in relation to the intended dose for each particular participant based on the tailoring schema used. Alternatively, a range of enacted doses can be presented.
One important sub-type of tailored dose is ad libitum, in which no specific dosage of use is prescribed. Rather, with ad libitum prescriptions participants either are instructed to use the intervention according to their need or desire or are not given any explicit instructions on usage frequency, amount, or duration. All or just some components of an intervention may have an ad libitum dose. Consider as an example Healthy Mind, an app-based intervention that includes nine different stress management tools (Morrison et al. 2017). Healthy Mind participants are informed that they can use these tools “on demand” and are sent periodic push notification messages encouraging use of the tools. A dose can be described for both the push notifications and for the stress management tools. The dose of the push notifications may be described in terms of the standard parameters of frequency, duration, and amount. Describing the dose of the stress management tool component is less straightforward. The prescription for these tools may be best described as having a minimum dose corresponding to the dose of the notifications sent (since these notifications are functioning as “prescriptions”) as well as having an ad libitum dose beyond that minimum dose (since participants can use the tools beyond those times when they receive push notifications).
Passively acquired data.
Some interventions collect data on participant behavior that does not require participant input, such as step counts or Global Positioning System (GPS) location acquired from a smart phone. In many cases, these data are used to provide tailored intervention content. As an example, the HeartStep intervention uses automatically acquired geolocation information and other data to tailor the content, timing, and frequency of physical activity recommendations (Klasnja et al 2018). Thus, different doses of intervention are delivered across individuals and across time. Although passively acquired data can influence dose, the acquired data are not counted as part of the intervention dose per the currently presented conceptualization because there is no action prescribed to the participant.
Implications for the Empirical Study of Dose
Intended Dose
Researchers interested in optimizing intervention dose can manipulate the intended intervention action dose and/or the intended participant action dose and observe outcomes of interest (Voils et al., 2012). With regard to intervention action dose, researchers can systematically vary what is asked of participants to receive. For example, researchers can randomize individuals to be asked to complete daily or weekly virtual reality sessions or to receive a text message daily or weekly. Morrison et al. (2017) randomized participants to three different doses of app notifications in the previously described Healthy Minds intervention: daily, every 72 hours, or at intervals tailored based on participant data. While this is an example of testing frequency, other dose parameters can be varied. For example, a three-level factorial study can vary the prescription of duration (12 vs. 24 weeks), frequency (one per week vs. once per day), and amount (one vs. three minutes).
As with intervention action dose, the effects of participant action dose on outcomes can be studied by systematically varying what participants are prescribed to produce. In the Tweet2quit intervention, researchers can examine effects of requesting that participants write one tweet per week versus one tweet per day. Factorial design can be used to simultaneously manipulate both intervention and participant action dose. For example, a currently ongoing study by Bennett and colleagues (Bennett, 2018) is varying whether individuals are sent texts once per week or daily; this is a test of the intervention action prescription dose. In the same study, they are also randomizing individuals to different prescribed frequency of response; this is a test of participation action prescription dose. For interventions with tailored doses, researchers can utilize experimental studies to determine the most effective tailoring schema. For example, researchers can systematically vary different tailoring schemes and ranges of intended dose. In addition to these experimental methods, researchers can use non-randomized approaches to evaluate dose-response for intended intervention action and participant action prescriptions (see Voils et al., 2012, 2014).
Dose can also be categorized and studied based on the specific behavior change techniques used (Tate et al., 2016). In the Track intervention, for example, dose can be described separately for self-monitoring technique and feedback on self-monitoring. Many digital health intervention modalities lend themselves to this fine-grained analysis and thus provide an opportunity for further probing of mechanisms of change.
Enacted Dose
Unlike manipulating intended dose, researchers cannot directly manipulate enacted dose because the enacting is at the discretion of the participant. Thus, research on the relation between enacted dose and response is necessarily observational and subject to limitations in identifying causal relationships.
The difficulty of determining the relationship between enacted dose and outcomes has a parallel in the field of clinical pharmacology. In clinical pharmacology, investigators are interested in understanding how specific blood concentrations of a drug (vs. the amount of drug ingested or administered) influence clinical outcomes. This is challenging, however, because individual differences in metabolism of the same delivered drug dose can result in different blood concentrations, biasing estimates of the blood concentration-outcomes relationship. A parallel in digital health interventions is that the same intended dose can result in different enacted dose due to individual differences, biasing estimates of the enacted dose-outcome relationship. In clinical pharmacology, this challenge has been addressed via concentration-controlled trials (Sanathanan & Peck, 1991), in which participants are randomized to a specified blood concentration of a drug, their blood concentration levels are continuously monitored, and the delivered dose is adjusted to achieve the assigned blood concentration. A parallel design in digital health behavioral interventions might involve randomizing participants to a targeted enacted dose, continuously monitoring their achieved enacted dose, and delivering more dose as needed to obtain the assigned enacted dose. For example, consider a study aiming to compare daily versus weekly viewing (i.e., enacted dose) of smoking cessation motivational messages that are delivered by participants clicking on a web link embedded in text messages. A concentration-controlled design might involve monitoring participants’ opening of the web link and sending additional text messages in order to obtain the assigned amount of enacted dose. Such an approach is consistent with an adaptive design, in which participants may receive different intervention elements as a function of their initial response to an intervention. A potential challenge with this approach is that participants who are not initially engaging may not be responsive to efforts to increase engagement. Additionally, for this approach to work there must be an accurate indicator of enacted dose that can be continuously monitored.
Researchers can also study enacted dose by manipulating intervention features that are hypothesized to effect enacted dose. For example, researchers may hypothesize that participants will be more likely to read a text and respond to a text (i.e., have a greater enacted dose) if it is delivered at certain times of day. To test this, the researchers can randomize participants to be sent texts at different times of day and examine effects on enacted dose and clinical outcomes of interest.
One notable intervention characteristic that may affect enacted dose is the intended dose of the intervention (Voils et al., 2014). Intended dose may affect enacted dose by setting expectation of participants’ actions and by providing an upper limit on the dose (i.e., highest possible amount of content in which they can engage). Further, an intended dose that is very low may lead to a lack of engagement in the intervention, thus leading to less enacted dose. Alternatively, an intended dose may be high enough that it is viewed as burdensome or annoying, leading to withdrawal and a lower enacted dose, or lack of willingness to initiate the intervention in the first place (Voils et al., 2014; see further discussion below). Thus, researchers manipulating intended dose in an experimental design should examine the effect on enacted dose to help guide interpretations of outcomes and refine intervention dose for future studies.
The concepts of enacted dose and intervention engagement are closely related. While intervention engagement in digital health has been defined in many ways, a recent literature review developed an integrative definition of engagement that proposes two elements: extent of usage of an intervention, and subjective experience characterized by “attention, interest, and affect.” (Perski, Blandford, West, & Michie, 2017). Enacted dose as presented here has complete conceptual overlap with the usage component of engagement but does not overlap with the subjective experience portion. Researchers focusing on the usage components of engagement may consider applying the concepts outlined herein by distinguishing between engagement with the intervention action prescription and participant action prescription.
Intervention Burden and Adverse Events
Adverse events, unanticipated problems, and intervention burden are integral to consider when designing an intervention. An adverse event is an “untoward or unfavorable medical occurrence,” physical or psychological, attributed to an intervention (Unanticipated Problems Involving Risks & Adverse Events Guidance, 2007). Intervention burden is a subjective experience of the participant in which the intervention requirements are greater than the participants’ resources at a point in time (Nahum-Shani et al., 2016.). Burden and adverse events can be influenced by characteristics of the individual (e.g., monetary resources, motivation), the environment, and the intervention. Notably, intervention-related burden and risk of adverse events may differ for in-person compared to digital health interventions. For example, an in-person intervention may be perceived as burdensome if it requires a long drive to an intervention site. A digital health intervention may be perceived as burdensome if it interrupts participants’ daily activities with intrusive notifications (Dennison, Morrison, Conway, & Yardley, 2013). Intervention burden and adverse events are undesirable on their own accord and may make an intervention less effective, such as by leading to intervention disengagement. Potentially, burden and adverse events can be influenced by the intended dose of all three types of intervention prescriptions (intervention action, participant action, and behavioral target). Researchers can systematically measure how intended dose affects experience of burden and enacted dose, and if burden mediates relationships between intended dose and outcomes.
Overcoming Challenges in the Empirical Study of Dose-Response Relationships.
Combining Dose Prescriptions across Modalities.
Many digital health interventions use multiple modalities. For example, Track uses both text messages and counseling calls, while Tweet2quit sends text messages and tweets. When studying the dose of these interventions, should these modalities be combined to make a complete dose or should the dose of each be separately described? The answer should depend on the purpose of the research study. On some occasions, researchers may wish to study the dose of specific modalities separately. In other cases, the total dose of an intervention across modalities may be of interest, for example, if the goal is to compare an overall low dose and high dose.
Describing Dose When Intervention and Participant Action Prescriptions Occur in Interdependent Bursts.
As previously noted, some interventions include components for which the intervention actions and participant actions are interdependent and occur in rapid succession, such as with a series of text messages from the intervention and participant, or conversations in a virtual world (or real world) that typically involve inter-dependent exchanges of participant and intervention actions. When conducting experimental studies, it may not be possible or desired to control the total number of exchanges in these interactions. Instead, researchers might focus on manipulating the frequency with which new bursts of communication are started. For example, if researchers send text messages offering to engage in back-and-forth dialogue to help with problem solving, they may be interested in varying if that initial text is sent daily or three times a week (and ignoring the number of contacts within that burst of communication). If studying enacted dose in these types of exchanges, however, researchers may consider more fine-grained relationships.
Conclusions
Most research in dose-response relationship has focused on in-person modalities. As a result, little is known about the optimal doses for digital health interventions. Further, current treatment guidelines suggest doses based on research from in-person interventions, which may not be appropriate for digital health interventions. Further discussion about dose in digital health interventions and more dose-response research is warranted to optimize the impact of digital health interventions.
Acknowledgments
This work was supported by a career development award to Dr. McVay (K23 HL127334) from the National Heart, Lung, and Blood Institute, and a Research Career Scientist award to Dr. Voils (RCS 14–443) from Veteran’s Affairs Health Services Research & Development. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. Funding agencies did not review or approve of the manuscript.
Contributor Information
Megan A. McVay, University of Florida, Duke University School of Medicine
Gary G. Bennett, Duke University, Duke Global Health Institute
Dori Steinberg, Duke University School of Nursing, Duke Global Health Institute.
Corrine I. Voils, William S. Middleton Veterans Memorial Hospital, University of Wisconsin School of Medicine and Public Health
References
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, … Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. (2004). Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology, 23(5), 443–451. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- Bennett GG, (2018). Charge: A text messaging-based weight loss intervention clinical trial registry. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03254940.
- Bennett GG, Steinberg D, Askew S, Levine E, Foley P, Batch BC, … & DeVries A (2018). Effectiveness of an app and provider counseling for obesity treatment in primary care. American journal of preventive medicine, 55(6), 777–786. 10.1016/j.amepre.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, … Charlson ME (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology, 34(10), 971–982. 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison L, Morrison L, Conway G, & Yardley L (2013). Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. Journal of Medical Internet Research, 15(4), e86 10.2196/jmir.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbrouck J, & Tindal G (2005). Oral reading fluency: 90 years of measurement. Behavioral Research and Teaching Technical Report, 33. [Google Scholar]
- Hekler EB, Rivera DE, Martin CA, Phatak SS, Freigoun MT, Korinek E, … Buman MP (2018). Tutorial for using control systems engineering to optimize adaptive mobile health interventions. Journal of Medical Internet Research, 20(6), e214 10.2196/jmir.8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasnja P & Pratt W (2012). Healthcare in the pocket: Mapping the space of mobile-phone health interventions. Journal of Biomedical Informatics, 45(1), 184–198. 10.1016/j.jbi.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasnja P, Smith S, Seewald NJ, Lee A, Hall K, Luers B, … & Murphy SA (2018). Efficacy of contextually tailored suggestions for physical activity: a micro-randomized optimization trial of HeartSteps. Annals of Behavioral Medicine, 53(6), 573–582. 10.1093/abm/kay067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, O’Connor EA, Evans CV, Senger CA, Rowland MG, & Groom HC (2014). Behavioral Counseling to Promote a Healthy Lifestyle for Cardiovascular Disease Prevention in Persons With Cardiovascular Risk Factors: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US) Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK241537/ [PubMed] [Google Scholar]
- Manojlovich M & Sidani S (2008). Nurse dose: what’s in a concept? Research in Nursing & Health, 31(4), 310–319. 10.1002/nur.20265 [DOI] [PubMed] [Google Scholar]
- Martín Cantera C, Puigdomènech E, Ballvé JL, Arias OL, Clemente L, Casas R, … Granollers S (2015). Effectiveness of multicomponent interventions in primary healthcare settings to promote continuous smoking cessation in adults: a systematic review. BMJ Open, 5(10). 10.1136/bmjopen-2015-008807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LG, Hargood C, Pejovic V, Geraghty AW, Lloyd S, Goodman N, … & Yardley L (2017). The effect of timing and frequency of push notifications on usage of a smartphone-based stress management intervention: an exploratory trial. PloS one, 12(1), e0169162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Smith SN, Tewari A, Witkiewitz K, Collins LM, Spring B, & Murphy SA (2016). Just-in-Time Adaptive Interventions (JITAIs): An Organizing Framework for Ongoing Health Behavior Support. Methodology Center technical report, 2014, 14–126. [Google Scholar]
- Pechmann C, Delucchi K, Lakon CM, & Prochaska JJ (2017). Randomised controlled trial evaluation of Tweet2Quit: a social network quit-smoking intervention. Tobacco Control, 26(2), 188–194. 10.1136/tobaccocontrol-2015-052768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perski O, Blandford A, West R, & Michie S (2017). Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. Translational Behavioral Medicine, 7(2), 254–267. 10.1007/s13142-016-0453-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Vedel E, & Emmelkamp PMG (2008). Behavioral couples therapy (BCT) for alcohol and drug use disorders: A meta-analysis. Clinical Psychology Review, 28(6), 952–962. 10.1016/j.cpr.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Sanathanan LP & Peck CC (1991). The randomized concentration-controlled trial: an evaluation of its sample size efficiency. Controlled Clinical Trials, 12(6), 780–794. [DOI] [PubMed] [Google Scholar]
- Saunders RP, Evans MH & Joshi P (2005). Developing a process-evaluation plan for assessing health promotion program implementation: a how-to guide. Health Promotion Practice, 6(2), 134–147. 10.1177/1524839904273387 [DOI] [PubMed] [Google Scholar]
- Tate DF, Lytle LA, Sherwood NE, Haire-Joshu D, Matheson D, Moore SM, … Michie S (2016). Deconstructing interventions: approaches to studying behavior change techniques across obesity interventions. Translational Behavioral Medicine, 6(2), 236–243. 10.1007/s13142-015-0369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanticipated Problems Involving Risks & Adverse Events Guidance (2007). Retrieved from https://www.hhs.gov/ohrp/regulations-and-policy/guidance/reviewing-unanticipated-problems/index.html#Q2
- Vervloet M, van Dijk L, Santen-Reestman J, van Vlijmen B, van Wingerden P, Bouvy ML, & de Bakker DH (2012). SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. International Journal of Medical Informatics, 81(9), 594–604. 10.1016/j.ijmedinf.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Voils CI, Chang Y, Crandell J, Leeman J, Sandelowski M, & Maciejewski ML (2012). Informing the dosing of interventions in randomized trials. Contemporary Clinical Trials, 33(6), 1225–1230. 10.1016/j.cct.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voils CI, King HA, Maciejewski ML, Allen KD, Yancy WS, & Shaffer JA (2014). Approaches for informing optimal dose of behavioral interventions. Annals of Behavioral Medicine, 48(3), 392–401. 10.1007/s12160-014-9618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]