Highlights
-
•
Subarachnoid hemorrhage patients show a reduced sensitivity to negative feedback, depicted by diminished amplitude of the feedback-related negativity (FRN).
-
•
A delayed increase of theta oscillatory activity (4–8 Hz) was found for the patient group in presence of monetary losses compared to the healthy control group.
-
•
No significant differences between groups were found at positive feedback event-related (ERP) components, such as the feedback P300 (FB-P3), neither on the time-frequency domain (beta-gamma band −25–35).
-
•
Damage to medial prefrontal cortex (mPFC) regions might be altering the performance monitoring mechanisms associated to feedback processing and belief updating, resulting in altered day-to-day decision-making functioning.
Keywords: Subarachnoid hemorrhage, Medial prefrontal cortex, Performance monitoring, Feedback-related negativity, Theta activity, Event-related brain potentials, Oscillations
Abbreviations: ACC, anterior cingulate cortex; AComA, anterior communicating artery; CAT, computerized axial tomography; EEG, electroencephalography; ERN, error-related negativity; ERPs, event-related potentials; fMRI, functional magnetic resonance imaging; FB-P3, feedback P300; FRN, feedback-related negativity; GCS, Glasgow coma scale; IGT, Iowa gambling task; mPFC, medial prefrontal cortex; MFN, medial frontal negativity; MRI, magnetic resonance imaging; QI, quotient of intelligence; RL, reinforcement learning; SAH, Subarachnoid hemorrhage; WAIS-III, Wechsler Adult Intelligence Scale -III; WM, working memory
Abstract
Patients with subarachnoid hemorrhage (SAH) secondary to anterior communicating artery (AComA) aneurysm rupture often experience deficits in executive functioning and decision-making. Effective decision-making is based on the subjects’ ability to adjust their performance based on feedback processing, ascribing either positive or negative value to the actions performed reinforcing the most adaptive behavior in an appropriate temporal framework. A crucial brain structure associated to feedback processing is the medial prefrontal cortex (mPFC), a brain region frequently damaged after AComA aneurysm rupture. In the present study, we recorded electrophysiological responses (event-related potentials (ERPs') and oscillatory activity (time frequency analysis) during a gambling task in a series of 15 SAH patients. Previous studies have identified a feedback related negativity (FRN) component associated with an increase on frontal medial theta power in response to negative feedback or monetary losses, which is thought to reflect the degree of negative prediction error. Our findings show a decreased FRN component in response to negative feedback and a delayed increase of theta oscillatory activity in the SAH patient group when compared to the healthy controls, indicating a reduced sensitivity to negative feedback processing and an effortful signaling of cognitive control and monitoring processes lengthened in time, respectively. These results provide us with novel neurophysiological markers regarding feedback processing and performance monitoring patterns in SAH patients, illustrating a dysfunctional reinforcement learning system probably contributing to the maladaptive day-to-day functioning in these patients.
1. Introduction
Subarachnoid hemorrhage (SAH) is a subset of stroke characterized by the accumulation of blood in the subarachnoid space. Although it only accounts for 5% of all strokes (Donnan et al., 2008), the consequences of this pathology are severe. Only 50% of those who experience SAH survive (van Gijn et al., 2007), and among these, 50% show cognitive impairments (Rinkel and Algra, 2011), mainly in the domains of memory, language and executive functions (Al-Khindi et al., 2010; Hütter and Gilsbach, 1993).
One of the main causes of SAH is the sudden rupture of aneurysms located in the anterior communicating artery (AComA), a small anastomotic artery at the anterior aspect of the Circle of Willis connecting both anterior cerebral arteries. Aneurysm rupture provokes two main bleeding patterns: (a) SAH, defined as the presence of blood in the subarachnoid space, which causes lesions secondary to the ischemic effect of vasospasm and (b) intraparenchymal bleeding, frequently associated to SAH, which occurs when the aneurysm rupture site is in direct contact with the brain parenchyma. The brain areas most likely to be damaged in these patients are the medial prefrontal cortex (mPFC), especially in its orbital and ventral parts, and the adjacent anterior cingulate cortex (ACC), in its ventral, dorsal and subgenual portions (von Cramon and Müller, 1998), brain regions implicated in evaluative decision-making processes as well as conflict detection (Bush et al., 2002; Knutson et al., 2003).
An essential aspect of executive functioning and decision-making for resuming daily activities is feedback processing. In everyday life, we choose to approach some things and to avoid others based on our previous experiences. Our actions are reinforced with either positive or negative feedback, characterizing our actions as rewarding or non-rewarding, embodying the process of reinforcement learning (RL) (Holroyd and Coles, 2002). Several neuroimaging studies have shown that activity in the ventral striatum/nucleus accumbens, a target area of dopaminergic neurons, correlates with reward prediction errors, positive or negative depending on whether the outcome is better or worse than expected, correspondingly (Camara et al., 2010; Knutson et al., 2000; McClure et al., 2003, 2004; O'Doherty et al., 2003; Pagnoni et al., 2002). Relatedly, activity in the mPFC has been associated with the expected subjective value of reward outcome, serving to strengthen the incentive motivation to maintain behavioral responses (Grabenhorst and Rolls, 2011; Knutson et al., 2003; Levy and Glimcher, 2012; Rangel et al., 2008). Representations in the mPFC are continuously updated by means of frontostriatal connections, providing a linkage between prediction errors (ventral striatum) and mPFC representations necessary to regulate and adapt our behavior (Camara et al., 2009; Münte et al., 2008).
Several studies have explored reward-based decision-making in SAH survivors. Initial research conducted by Damasio, Bechara, and colleagues using the Iowa Gambling Task (IGT) (Bechara et al., 1994, 1996; Bechara et al., 2000; Alexander and Brown, 2011; Damasio, 1996) highlighted a pattern of sub-optimal decision-making in patients with prefrontal lesions (some of them SAH patients secondary to AComA rupture). In this task, patients had to select from four decks of cards (two of them associated with high monetary rewards and penalties and the other two with small rewards and penalties). Patients with prefrontal damage took greater risks in their decision-making (drew more frequently from decks with high rewards and penalties) compared to normal controls. More recent studies using the IGT have also reported this pattern of increased risk-taking behavior and poor social judgment in SAH patients (Al-Khindi et al., 2014; Escartin et al., 2012). The present study seeks to clarify whether these dysfunctional decision-making patterns in SAH patients at the behavioral level might be related to alterations in the underlying neurophysiological processing related to feedback processing by means of studying event-related brain potentials (ERP's).
Many studies using ERP's have been carried out during the last decade on decision-making and reward processing. An interesting line of research has been devoted to the investigation of ERP neural signatures associated to feedback processing (Cui et al., 2013) which is indeed the focus of the present study. As we have already mentioned in previous paragraphs, feedback processing plays a key role in decision-making as it enables us to evaluate our actions based on previous experience ascribing a negative or a positive valence to our actual state and allowing us to anticipate and give an efficient response selection. In one of the earliest studies by Gehring and Willoughby (2002), these authors described a mid-frontal negativity component (called Medial frontal negativity or feedback-related negativity, FRN) that develops at about 260–300 ms following a negative feedback (e.g., monetary loss compared to monetary win) (Gehring and Willoughby, 2002; Hajcak et al., 2006; Holroyd and Coles, 2002; Holroyd et al., 2006; Toyomaki and Murohashi, 2005; Wu and Zhou, 2009; Yeung and Sanfey, 2004).
It has been suggested that the FRN might reflect the early judgment of feedback as a binary categorization of good vs. bad (i.e. gain vs. loss). Accordingly, the FRN is thought to reflect the degree of negative prediction error due to decreased mesencephalic dopaminergic activity that is transmitted throughout the ACC to the mPFC from the basal ganglia (Holroyd and Coles, 2002; Nieuwenhuis et al., 2004). Thus, these signals conveyed in the mPFC might help the organism to detect potential cognitive conflicts arising from previous expectations and unexpected outcomes, enhancing action monitoring and control processes and allowing belief updating (Botvinick et al., 2004; Holroyd and Coles, 2002; Padrão et al., 2013; Ridderinkhof et al., 2004). However, this idea based on RL principles has been challenged in several studies. For example, in a study in which participants were asked to evaluate their own performance as either correct or incorrect, and then gave them real feedback, the FRN was observed for both unexpected “correct” and “incorrect” feedbacks, regardless of the motivational value (Oliveira et al., al., 2007). Furthermore, Pfabigan and colleagues (2011) found that the FRN did not only reflect a negative reward prediction error, but to a minor extent also a positive reward prediction error, hypothesizing that the FRN might be signaling the mismatch between internal and external representations by detecting the motivational salience of outcomes (Pfabigan et al., 2011).
Source localization analyses have frequently identified the ACC as the most likely generator of the FRN (Bellebaum and Daum, 2008; Gehring and Willoughby, 2002; Luu et al., 2003; Mathewson et al., 2008; Miltner et al., 1997; Müller et al., 2005; Ruchsow et al., 2002). However, there's still no consensus on the literature regarding the neural source of this component (for a review see Walsh and Anderson, 2012). These findings have also been supported by functional magnetic resonance imaging (fMRI) studies (Bush et al., 2002; Holroyd et al., 2004; Monchi et al., 2001; Ullsperger and von Cramon, 2003), showing significant clusters of activation in the ACC related to feedback processing. Importantly, recent studies using combined EEG-fMRI approaches have been performed tackling the possible neural sources associated to these components. For example, Hauser et al. (2014) encountered a single cluster located in the dorsal anterior cingulate cortex (dACC) signaling reward prediction error signals, as well as additional regions related to the salience network (anterior insula, dorsolateral prefrontal cortex and posterior parietal cortex). These results led them to conclude that the FRN might be signaling surprise and originating at the dACC. Similarly, in another study by Becker et al. (2014), the authors found feedback-related BOLD-responses in the ventral striatum, middle cingulate cortex (MCC), and middle frontal cortices (MFC) associated to positive but not to negative feedback in the time range of the FRN (Becker et al., 2014), supporting the hypothesis that the FRN might result from the superposition of a feedback-related positivity (FRP) upon the N2 component (driven by variance from rewarding feedback) (Holroyd et al., 2008; Carlson et al., 2011; Foti et al., 2015) but nonetheless corroborating the role of the ACC/MCC in the elicitation of the FRN component. Finally, in a magnetoencephalography (MEG) study using source localization with low-resolution electromagnetic tomography (Doñamayor et al., 2012), the authors found a generator in the posterior cingulate cortex (PCC) with subsequent activity in the anterior cingulate cortex (ACC) as the magnetic correlate of the FRN.
Another feedback-related component is the feedback P300 (FB-P3), a positive deflection found at centro-parietal locations at 300–600 ms after feedback presentation. It has been related to attention-driven categorization of salient outcome-related information, such as context updating, as well as in signaling of the motivational salience of rewarding feedback (Donchin, 1981; Polich, 2007; Sutton et al., 1965; see Glazer et al., 2018 for a review). The FB-P3 has been found to be sensitive to reward probability (higher amplitude for low probable rewards) and magnitude (larger for maximum conditions), while the evidence regarding valence sensitivity is quite inconsistent. Some studies have reported an enhanced response following positive outcomes (Wu and Zhou, 2009; Yeung et al., 2005), while others have reported larger amplitudes following negative outcomes (De Pascalis et al., 2010), and others still report no difference (Yeung and Sanfey, 2004).
An important factor in measuring both FRN and FB-P3 is that these two ERP components partially overlap in time, complicating standard time domain methods of response quantification (Glazer et al., 2018). To better isolate these feedback-locked components, time-frequency analysis can be applied in order to separate ERP components that overlap in time but presenting different spectral oscillatory characteristics. Previous studies have revealed that the FRN and FB-P3 operate at different frequencies. Specifically, the FRN (like the ERN) is characterized by medial-frontal theta oscillatory activity (4 –8 Hz), showing larger theta power increases after an erroneous response or negative performance feedback (Cavanagh and Frank, 2014; Cavanagh et al., 2010; Cohen, 2011; Cohen et al., 2007; Cunillera et al., 2012; Luu and Tucker, 2001; Luu et al., 2004; Marco-Pallarés et al., 2008, 2009; Padrão et al., 2013; Trujillo and Allen, 2007). It has been proposed that increases of the medial-frontal theta component may reflect a general top-down mechanism operating over expectation violation and behavioral adaptation in order to improve performance and learning (Cavanagh et al., 2010; Cunillera et al., 2012; Tzur and Berger, 2009; van de Vijver et al., 2011; Womelsdorf et al., 2010). On the other hand, the FB-P3 is composed mainly by activity in the delta range (1–3 Hz), specially following gains (Bernat et al., 2015, 2011; Delorme et al., 2007; Luu et al., 2004), leading some to suggest that FB-delta may be a reward-specific index of feedback processing signaling feedback expectancy (Watts et al., 2017). Additionally, consummatory responses to positive outcomes (i.e. monetary gains) have been associated to beta-gamma oscillatory activity (20–35 Hz) (Cohen et al., 2007; Cunillera et al., 2012; Marco-Pallarés et al., 2008, 2009; for a review see Marco-Pallarés et al., 2015). Previous evidence using electroencephalographic (EEG) recordings have reported increased EEG beta-gamma band power during reward processing, particularly after positive feedback (Cohen et al., 2007; Hallschmid et al., 2002; Marco-Pallarés et al., 2008, 2009; Mas-Herrero et al., 2015), suggesting that beta-gamma activity may reflect a brain signature of reward processing.
Considering that the anterior ACC and mPFC are supplied by the AComA and its perforators, and based on previous reports showing behavioral differences during the IGT in SAH patients (Al-Khindi et al., 2014; Bechara et al., 1994, 1996; Alexander and Brown, 2011; Damasio, 1996; Escartin et al., 2012), we wanted to investigate the presence of dysfunctional feedback-related neurophysiological components in SAH patients secondary to AComA aneurysms rupture. With this aim, SAH patients treated with endovascular coiling performed a well characterized gambling task (Camara et al., 2010; Marco-Pallarés et al., 2008; Padrão et al., 2013) while undergoing EEG recording. Based on previous findings in patients with prefrontal lesions in which differences were observed regarding reward-based decision-making (Al-Khindi et al., 2014; Bechara et al., 1994, 1996, 2000; Damasio, 1996; Escartin et al., 2012), we predicted that differences between groups would be observed in the processing of positive (monetary gains) and negative (monetary losses) feedback EEG components (FB-P3 and FRN respectively) and oscillatory responses (the beta-gamma, medial-frontal theta and delta components), supporting the proposal of dysfunctional feedback-based decision making processing in SAH patients.
2. Materials and methods
2.1. Participants
A series of 15 patients who had suffered from aneurysmal subarachnoid hemorrhage (SAH) due to anterior communicating artery (AComA) aneurysm rupture treated with endovascular simple coiling were recruited (10:5 males; mean age = 50.40 ± 7.56; mean education years = 10.53 ± 3.83). Neuroradiologic CAT and MRI evaluation was blinded in respect to study results evaluation. Demographic and clinical characteristics of the sample, including Glasgow Coma Scale (GCS) (Teasdale and Jennett, 1974) and Fisher grade (Fisher et al., 1980) scores, are summarized in Table 1A and B. Patients over 65 years, with multiple aneurysms or with previous history of neurological or psychiatric disorders or drug abuse were excluded. Twelve control participants were also recruited and matched by age, gender and years of education (5:7 males; mean age = 45.00 ± 8.25; mean education years = 11.08 ± 2.64). All patients signed an informed consent for participation in this study and the protocol was approved by the Ethical Committee of the Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona (Spain).
Table 1.
(A) Demographic characteristics. (B) Clinical characteristics. Fisher grade: 1: No SAH detected; 2: less than 1 mm thick SAH; 3: SAH thicker than 1 mm; 4: intracerebral hemorrhage or intraventricular hemorrhage with or without diffuse SAH. GCS: ≤ 8: severe head injury; 9–12: moderate head injury; 13–15: mild head injury. Complications column refers to: 0: none; 1: hydrocephalus; 2: vasospasm; 3: infarction; 4: other. C).Neuropsychological assessment. Neuropsychological results are shown in raw scores and its corresponding scaled scores (≤6 considered as impairment).
GCS: Glasgow Coma Scale; Complic: Complications; IQ: Intelligence Quotient.
| A. Demographic characteristics | B. Clinical characteristics | C. Neuropsychological assessment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age | Gender | Education years | Fisher grade | GCS | Complic. | Digit span forward | Digit span backward | Digits | Letters and numbers | Arithmetic | Working memory IQ | Semantic fluency | Phonetic fluency |
| 1 | 41 | M | 10 | 2 | 15 | 0 | 6 | 4 | 14 (10) | 8 (9) | 13 (11) | 98 | 17 (6) | – |
| 2 | 51 | M | 9 | 1 | 15 | 0 | – | – | – | – | – | – | – | – |
| 3 | 51 | M | 8 | 2 | 15 | 0 | 4 | 4 | 12 (8) | 11 (12) | 12 (10) | 98 | 13 (5) | 9 (7) |
| 4 | 53 | F | 8 | 2 | 14 | 2 | 5 | 4 | 13 (9) | 6 (7) | 8 (7) | 83 | 10 (2) | 8 (5) |
| 5 | 55 | M | 12 | 3 | 15 | 0 | 4 | 2 | 7 (5) | 10 (13) | 10 (11) | 96 | 18 (9) | 10 (7) |
| 6 | 48 | M | 6 | 1 | 14 | 3 | – | – | – | – | – | – | – | – |
| 7 | 64 | M | 12 | 3 | 13 | 4 | 4 | 3 | 10 (9) | 7 (11) | 10 (11) | 100 | 19 (10) | 14 (10) |
| 8 | 46 | M | 12 | 3 | 14 | 1 | 5 | 3 | 11 (7) | 11 (12) | 14 (12) | 100 | 32 (15) | – |
| 9 | 39 | M | 22 | 2 | 15 | 0 | – | – | – | – | – | – | – | – |
| 10 | 65 | F | 6 | 4 | 15 | 4 | 5 | 4 | 11 (10) | 6 (10) | 9 (10) | 98 | 19 (11) | 12 (10) |
| 11 | 50 | F | 12 | 3 | 13 | 1 | 4 | 3 | 9 (6) | 7 (8) | 11 (10) | 86 | 22 (11) | 9 (7) |
| 12 | 43 | F | 11 | 2 | 14 | 2 | 4 | 4 | 11 (7) | 6 (7) | 10 (9) | 83 | 19 (7) | 14 (9) |
| 13 | 47 | M | 12 | 4 | 3 | 2 | 6 | 3 | 14 (10) | 5 (6) | 8 (7) | 83 | 24 (10) | 15 (9) |
| 14 | 57 | F | 10 | 3 | 15 | 3 | 6 | 5 | 14 (12) | 8 (12) | 8 (9) | 104 | 11 (4) | 11 (8) |
| 15 | 46 | M | 8 | 4 | 14 | 2 | 5 | 5 | 15 (11) | 11 (12) | 9 (8) | 100 | 21 (10) | 8 (5) |
| T score Asymp. p-value | −2.755 | −1.936 | −2.539 | −1.543 | – | – | −1.339 | −3.615 | ||||||
| 0.012 | 0.066 | 0.019 | 0.137 | – | – | 0.194 | 0.002 | |||||||
2.2. Event-related brain potentials (ERP's)
2.2.1. Experimental task
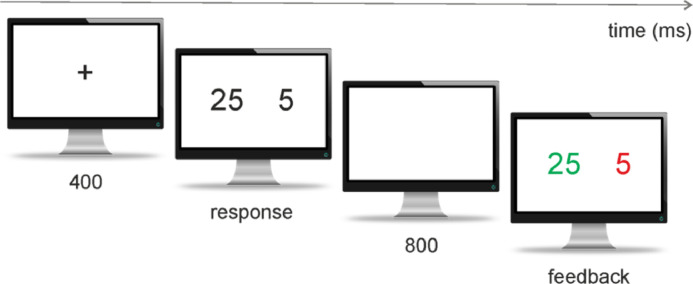
The gambling task consisted of selecting one of two numbers presented in white on a black background in the middle of a screen (Camara et al., 2010; Marco-Pallarés et al., 2008; Padrão et al., 2013). Two possible displays were shown, either [25 5] or [5 25] (for a trial example, see Fig. 1). Participants had to make a mandatory button press response with their left or right index finger, indicating their selection. For example, in the [25 5] display, a left button press would indicate selection of number 25, and a right button press selection of number 5. After the response choice (with a fixed interval of 800 ms), one of the numbers would turn red while the other one turned green. If the number selected by the participant turned red, this signaled a loss of the corresponding amount in Euro cents; a green number indicated a gain of this amount in Euro cents. The duration of the feedback stimulus was 1000 ms. The next trial began with the presentation of a warning signal (“+,” with a 400 ms duration), followed by a new trial.
Fig. 1.
Sequence of stimulus and response events presented in one single trial in the gambling paradigm used in the present study (Camara et al., 2010; Marco-Pallarés et al., 2008; Padrão et al., 2013). After a warning signal (400 ms), a set of two numbers ([5 25] or [25 5]) was shown. Participants were instructed to select one of the two alternatives by pressing the corresponding button on the left- or right-hand side (response). One second after the response choice (800 ms), one of the numbers turned red and the other green (feedback) indicating a gain (green) or loss (red) of the corresponding amount of money in Euro cents. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The experiment consisted of 17 blocks of 40 trials. In each block, the four different feedback types were presented in random order: [25 5], [25 5], [5 25], and [5 25] (note: non-bold font stands for red [a loss], while bold font for green [a win]). Participants were prompted to gain as much as possible. When combined with the two response options (Left or Right), this generated eight different types of stimulus–response combinations. For example, if the volunteer had chosen the left number in a [25 5] condition, this was scored as a “maximum gain” trial. If the participant had gone for the right number, the trial was scored as a minimum loss. For the analysis presented here, left and right choices were combined into four different averages: maximum gain (+25), minimum gain (+5), minimum loss (−5), and maximum loss (−25). Relevantly, the mean expected value of the monetary outcome was zero on each block, in order to avoid potential confounding influences of a differential probability of gains or losses. The participants were informed about their accumulated amount of money (10 s duration) after blocks of ten trials.
2.2.2. Electrophysiological recording
The EEG data were recorded using tin scalp electrodes mounted in an elastic cap and located at 29 standard positions (Fp1/2, Fz, F7/8, F3/ 4, Fc1/2, Fc5/6, Cz, C3/4, T7/8, Cp1/2, Cp5/6, Pz, P3/4, P7/P8, Po1/2, and O1/2). Biosignals were referenced online to the right outer canthus eye electrode and re-referenced offline to the mean of the activity at the two mastoid electrodes. Vertical eye movements were monitored with an electrode at the infraorbital ridge of the right eye. Electrode impedances were kept below 5 kΩ. The electrophysiological signals were filtered with a bandpass of 0.01–70 Hz (half-amplitude cutoffs) and digitized at a rate of 250 Hz. We used ERPLAB (Lopez-Calderon and Luck, 2014) for processing and filtering the data. Artifact rejection was performed offline and tailored to each participant.
2.3. Neuropsychological assessment
The neuropsychological examination was carried out at least 6 months after the SAH at the Neuropsychology Unit of the Hospital Universitari de Bellvitge. Three patients failed to participate in the neuropsychological assessment.
A specific neuropsychological protocol addressing attention, working memory and executive functioning was designed. It included the “Digits” (both direct and Inverse), “Letters and Numbers” and “Arithmetic” subtests from the Wechsler Adult Intelligence Scale-III (WAIS-III) (Spanish Edition; Wechsler, 2001). Verbal fluency was also assessed with semantic and phonological verbal fluency tests (Goodglass and Kaplan, 1983). Additionally, a more general cognitive examination was also carried out although results are not reported in this work.
Neuropsychological examination scores were interpreted by using Spanish normative data from the WAIS-III Spanish Edition (Wechsler, 2001). Additionally, Working Memory (WM) intelligence quotient (IQ) was also calculated. Verbal fluencies were corrected using Neuronorma Spanish normative data, and corrected by age and years of education (Peña-Casanova et al., 2009). In both cases, impairment was defined as a scaled score ≤6.
2.4. Data analysis
2.4.1. Electrophysiological data
Event-related potentials were time-locked to the appearance of the feedback (color change of the number-displays) and averaged for epochs of 1000 ms, starting 100 ms prior to the stimulus (baseline). The possible differences were tested using repeated measures analyses of variance (ANOVAs) with the factors Valence (gain and loss), Magnitude (maximum and minimum) and Midline Electrode Locations (frontal, Fz; central, Cz; and parietal, Pz) as within subjects’ factors and Group (Patients and Controls) as the between-subjects factor.
Source localization analyses of the major generators of the FRN on the grand average for the difference waveform (Maximum loss minus Maximum gain) was performed for each group, using Brain Electrical Source Analysis software (BESA Research 7.0). Cortical source estimation using the minimum-norm estimation (MNE) technique (Hämäläinen and Ilmoniemi, 1994) was also performed. This approach involves the computation of the source activities of a large number of regional sources evenly distributed over 1420 standard locations of the smoothed surface of a standard brain. A unique current distribution explaining the surface measurements is obtained by applying the minimum norm constraint, which selects the solution with minimum overall intensity. In this case, the Tikhonov–Philips approach was applied for spatial regularization (λ = 0.01) to suppress uncorrelated noise. Finally, a CLARA distributed method (Classic LORETA Recursively Applied; Hoechstetter et al., 2010) and dipole source model (Scherg and von Cramon, 1985) were computed in a 4-shell ellipsoidal head model.
To study the time frequency behavior of the electrical activity related to the feedback, we generated 4000 ms epochs (2000 ms before the stimulus appearance and 2000 ms after). Data from each single trial were convoluted with a variable cycle complex Morlet wavelet (between 4 and 9 cycles). Changes in time varying power (square of the convolution between the signal and the wavelet) in the frequency range from 1 to 40 Hz (linear increase) with respect to the baseline were computed for each trial and averaged for each participant before performing the grand average for the whole group. Repeated measures ANOVA with the factors Valence (gain, loss), Magnitude (maximum, minimum) and Electrode Location (Fz, Cz, Pz) was performed to test for differences in mean power changes between groups.
Statistical analyses were run with SPSS 21. In repeated-measures ANOVA, the Greenhouse-Geisser correction was applied whenever the sphericity assumption was not met. In case a theoretically relevant interaction was found to be significant, we disentangled the effect by means of post-hoc t tests.
2.4.2. Behavioral analysis of risk
In order to explore the risk-seeking behavior in both groups, we analyzed the evolution of the risk choice (choosing 25 instead of 5) across the whole gambling task. In order to do so, we clustered all trials in 17 bins of 40 trials each. For exploring this behavior, we performed an ANOVA on the proportions of risk-choices (selecting 25 instead of 5) for the 17 bins of the task on both groups (controls and SAH patients).
Because previous evidence shows that risk decisions patterns are influenced by the outcome on previous trials (Gehring and Willoughby, 2002; Padrão et al., 2013), we compared the risk choices following losses (both maximum and minimum) to those that followed gains (maximum and minimum). To do so, we first computed the probability of risk-choice depending on the previous trial by calculating the number of times that participants selected the risky option (25) by the number of times participants selected either 25 or 5 with respect to the previous trial. For analyzing the effect of the previous trial on decision-making, we used repeated measures ANOVA with Risk (after maximum gain, minimum gain, minimum loss and maximum loss) and Group (controls and SAH patients) as factors.
3. Results
3.1. Event-related brain potentials (ERP's)
3.1.1. Feedback related negativity (FRN)
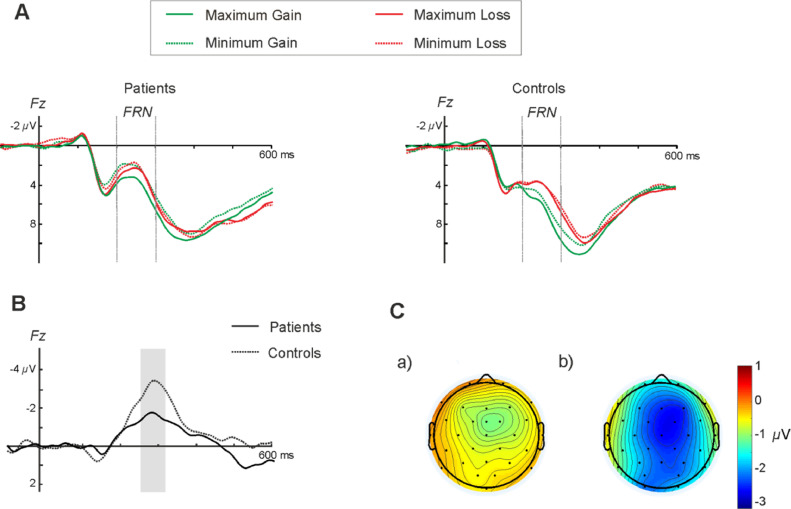
As explained on previous introductory paragraphs, when an incorrect response or negative feedback is produced, the FRN component is usually elicited reflecting the degree of negative prediction error. An analysis of variance (ANOVA) on the mean amplitude in the time window 200–300 ms was performed with Valence (gain and loss), Magnitude (maximum and minimum), Midline Electrode Locations (frontal, Fz; central, Cz; and parietal, Pz) and Group (Patients and Controls) as factors (Fig. 2). As expected, differences in amplitude of the FRN were found depending on Valence (F(1, 25) = 13.22, p = .001, ηp2 = 0.34) (Fig. 2A). Additionally, a significant main effect of Magnitude was observed [F(1, 25) = 6.78, p = .015, ηp2 = 0.18] (see Fig. 2A). Post-hoc tests revealed that the FRN elicited more negative amplitudes for Losses than for Gains (Losses: 3.12 ± 0.53 μV, Gains: 4.05 ± 0.55 μV, pBF = 0.001) as well as larger mean amplitudes for the Maximum conditions compared to the Minimum (Maximum: 3.90 ± 0.57 μV, Minimum: 3.28 ± 0.52 μV, pBF = 0.025), as reported on previous studies (Hajcak et al., 2006; Holroyd et al., 2004; Marco-Pallarés et al., 2008). A significant Valence x Magnitude interaction was found [F(1, 25) = 15.72, p < .001, ηp2 = 0.39], evidencing larger differences between gains and losses at the maximum conditions. Importantly, a significant interaction between Valence x Group [F(1, 25) = 5.06, p = .033, ηp2 = 0.15 ] was also found, observing greater differences in amplitude between gains and losses for the control group compared to the patient group (Fig. 2A).
Fig. 2.
(A) Grand average waveforms at Fz for both groups. (B) Difference waveforms (Maximum Loss minus Maximum Gain trials) at frontal location Fz. (C) Scalp distribution of the MFN component derived from the difference waveforms for the Patients (a) and Controls (b) at the peak time window (250–350 ms).
To examine the mean averages of the FRN for both groups, we estimated the difference waveform (Maximum loss minus Maximum gain) at Fz (see Fig. 2B). Significant differences were observed on the mean amplitude [t(25) = 2.85, p = .032] and the peak amplitude [t(25) = 2.592, p = .021] between the control and patient groups at Fz for the time window 250–350 ms, showing a significant reduction of the MFN/FRN in the patient group, clearly illustrated on the scalp distributions figure (Fig. 2C).
3.1.2. Feedback P300 (FB-P3)
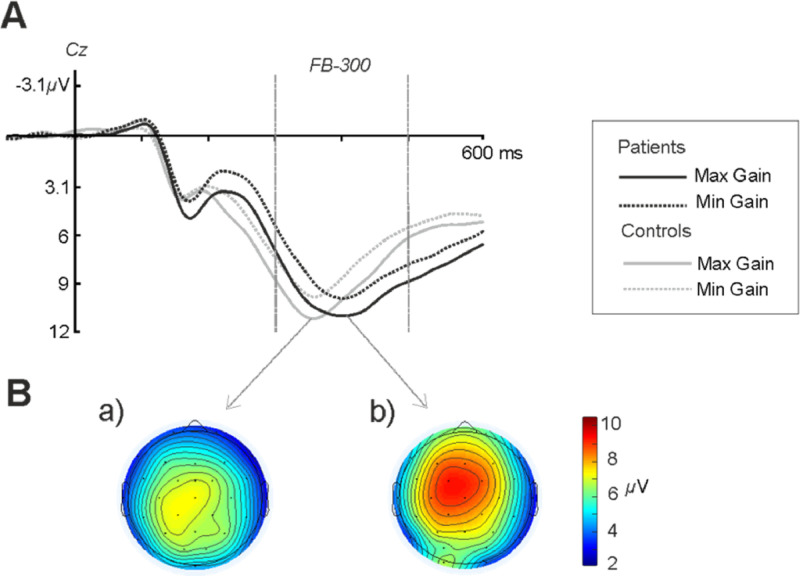
In order to examine possible differences in the elicitation of the FB-P3 component, an ANOVA was carried out following the same factorial design as in the previous paragraph but for the time window 300–500 ms after feedback. A significant main effect of Magnitude was found (F(1, 25) = 11.35, p = .002, ηp2 = 0.31), showing a larger amplitude for the Maximum condition (Maximum: 8.44 ± 0.76 μV, Minimum: 7.95 ± 0.78 μV, pBF = 0.002) (see Fig. 3). Also, a significant main effect for Electrode location was observed (F(1, 25) = 5.19, p = .010, ηp2 = 0.17), with Cz displaying larger amplitudes at this time window (Fz: 7.63 ± 0.76 μV, Cz: 8.93 ± 0.90 μV, Pz: 8.03 ± 0.75 μV) than Fz (pBF = 0.008) and Pz (pBF = 0.038). A significant interaction Valence x Magnitude was also obtained (F(1, 25) = 6.29, p = .0169, ηp2 = 0.20), confirming previous evidence on the sensitivity of the FB-P3 to reward Magnitude (larger for maximum conditions) as well as Valence (larger after gains) (Wu and Zhou, 2009; Yeung et al., 2005). Group differences were not found to be statistically significant at this level of analysis.
Fig. 3.
A) Grand average waveforms at Cz for the gain conditions (maximum and minimum) for both groups depicting the FB-P3 component at the time window between 300 and 500 ms after feedback. B) Scalp distributions for the FB-P3 component for the patient (a) and healthy control group (b) at the peak time window 250–350 ms.
3.2. Source localization analysis
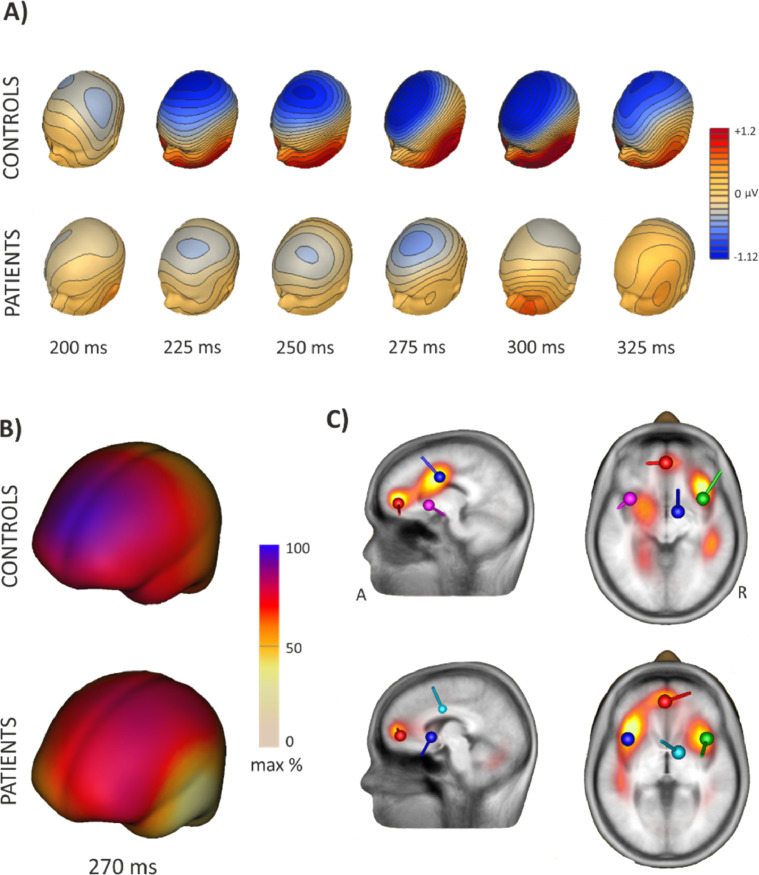
Firstly, we computed the surface voltage maps for each group corresponding to the time window 200–325 ms after the feedback onset. Electrophysiological responses to the Loss conditions depicted a larger difference waveform at the ERP's in the time window 200–300 ms for the healthy control group than for the SAH patient group. This distinction can be clearly observed when looking at the surface voltage maps (Fig. 4A), showing increased negative voltage at frontocentral locations for both groups, but significantly reduced in the case of the SAH patients. Similar results were obtained when estimating the cortical sources using the minimum-norm estimation (MNE) technique (Hämäläinen & Ilmoniemi, 1984) (Fig. 4B). In this case, the minimum norm image at the peak latency 270 ms after negative feedback revealed a frontal midline location for the FRN component, depicting higher activation for the control group than for the SAH patient group (see Fig. 4B). The CLARA distributed model (Classic LORETA Recursively Applied; Hoechstetter et al., 2010) showed activations in the ACC and bilateral insular regions for both the control and patient groups (Fig. 4C). Interestingly, the control group showed additional activity at the MCC (Fig. 4C). Finally, we applied a 4-dipole model at the time window 240–280 ms after negative feedback based on the grand average ERP's of the difference waveform. The dipole locations were fixed to the main sources, namely the ACC, MCC and symmetric bilateral insulae, orientation was free. For the control group, this model resulted in a residual variance of 2.8%. In the SAH patient group, a residual variance of 10.87% was still present using these dipole localizations (see Fig. 4C).
Fig. 4.
A) Three-dimensional maps illustrating the scalp distribution of the ERP difference waveforms voltage at their maximal peaks for the Control (A) and SAH patients (B) groups at the time window 200–325 ms. B) Cortical surface estimates based on Minimum norm at the 270 ms peak of the averaged FRN component are shown for both controls (above) and patients (below). C) Source locations by distributed CLARA and discrete dipole solutions for the FRN at the peak time window 240–280 based on the grand average ERP's for the Controls (superior) and SAH patients (inferior) groups. The location of the main sources for both groups are shown (Anterior cingulate gyrus –ACC- (Red), Middle cingulate gyrus –MCC- (Blue) and bilateral insula (Green and Pink)). A: anterior; R: right. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Time frequency analysis
3.3.1. Delta activity (1–3 Hz)
As exposed on the introductory paragraphs, parietal delta oscillatory activity following feedback has been shown to be sensitive to performance evaluation, reward evaluations and magnitude (Bernat et al., 2015, 2011; Delorme et al., 2007; Luu et al., 2004; Vilà-Balló et al., 2014). In order to inspect this delta activity related to reward-outcome (i.e. monetary gains and losses), we performed an ANOVA of the mean power change for the delta frequency band (1–3 Hz) with Valence (gain, loss), Magnitude (maximum, minimum) and Midline Electrode Location (Fz, Cz, Pz) as within subjects factors and Group (Patients and Controls) as between subjects factor at the time window 300–500 ms. A significant main effect of Valence was found (F(1, 25) = 10.54, p = .003, ηp2 = 0.30), showing increased delta-activity for the positive feedback condition (Gain) (Gain: 0.32 ± 0.07 μV, Loss: 0.23 ± 0.06 μV, pBF = 0.003) (see Fig. 5B) . Also, a main effect of Electrode was obtained (F(1, 50) = 20.83, p < .0001, ηp2 = 0.45), evidencing larger power at Pz location (Fz: 0.16 ± 0.07 μV, Cz: 0.29 ± 0.07 μV, Pz: 0.37 ± 0.06 μV) than in Fz (pBF < 0.001) and Cz (pBF = 0.018). Significant interactions between factors were also found, between Valence x Magnitude (F(1, 25) = 4.23, p = .05, ηp2 = 0.15), depicting larger power increases for the Maximum gain condition and Valence x Electrode (F(1, 50) = 6.29, p = .012, ηp2 = 0.20), showing greater delta power for Gain at Pz location (Fig. 5B). However, non-significant differences between groups were found.
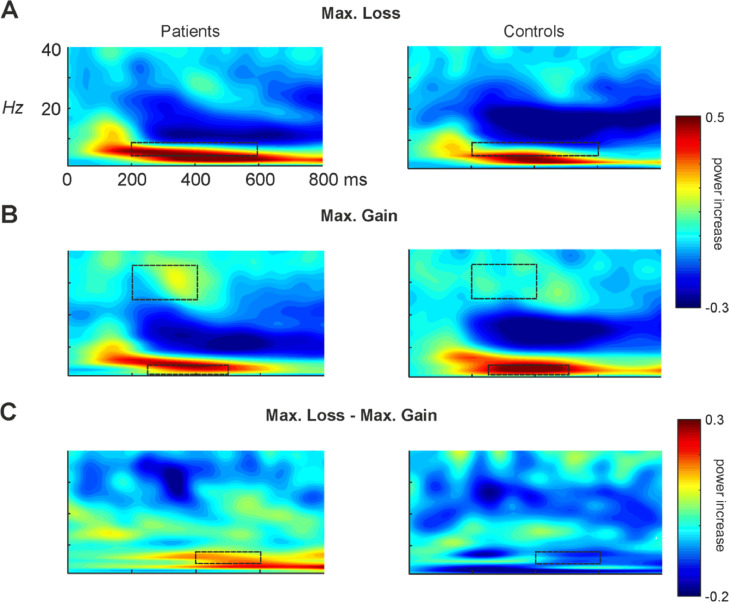
Fig. 5.
Changes in power with respect to baseline (100 ms) of A) Maximum loss condition, B) Maximum gain condition and C) Difference between Maximum loss minus Maximum gain for both the patient and the control group. Dotted contours show the areas where ANOVA's were computed.
3.3.2. Theta activity (4–8 Hz)
Considering medial-frontal theta activity in response to different monetary losses, repeated measures ANOVA was performed in the same manner as described above at the time window 200–600 ms. A significant increase of the theta frequency was encountered at Fz for losses as compared to gains [interaction between Valence x Electrode F(2, 50) = 10.58, p < .001, ηp2 = 0.28] (see Fig. 5A). No significant effects of the Group factor were found.
To further explore group differences at theta activity, the difference Maximum loss minus Maximum gain was computed and two time windows (200–400 ms and 400–600 ms) were defined based on visual inspection and previous studies using the same task (Camara et al., 2010; Marco-Pallarés et al., 2008; Padrão et al., 2013). Noteworthy, significant group differences at theta power were found for the period 400–600 ms at Fz electrode location [t(25) = 2.47, p = .021] (Fig. 5C), depicting larger negative values (power of loss > power of gain) for the patient group in comparison to the control group.
3.3.3. Beta-gamma activity (25–35 Hz)
The same analysis was performed for the beta-gamma band (25–35 Hz) at the time window 200–400 ms after feedback, in order to inspect consummatory responses to positive outcomes (i.e. monetary gains). As expected considering previous literature (Cohen et al., 2007; Cunillera et al., 2012; Marco-Pallarés et al., 2008, 2009, 2015), the ANOVA results showed a Valence effect (F(1, 25) = 12.09, p = .033, ηp2 = 0.33), evidencing larger beta oscillatory activity for gains as compared to losses (pBF = 0.002), as well as a significant Magnitude effect (F(1, 25) = 8.26, p = .008, ηp2 =0.25) showing an increase of beta activity for the maximum condition (pBF = 0.008). A significant Valence x Magnitude interaction [F(1, 25) = 6.75, p = .016, ηp2 = 0.21] was found, evidencing larger beta oscillatory activity at the maximum gain condition. No significant differences between groups were observed (see Fig. 5B).
3.4. Behavioral analysis of risk
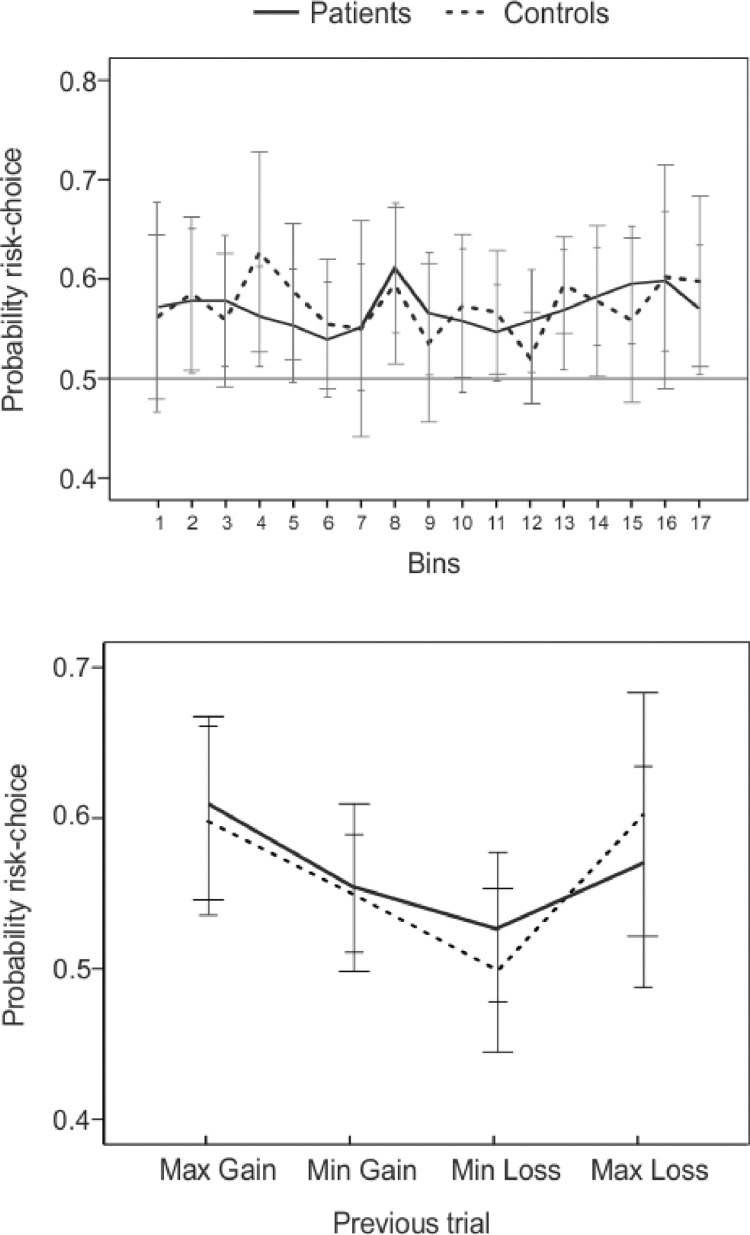
Regarding the total amount of money collected by each group at the end of the gambling task, no significant differences were observed [SAH patients ~ €4.00; Controls ~ €4.37; t(25) < 1]. For exploring the evolution of the risk choices across the task, an ANOVA directly comparing both groups in regard of the time bin showed neither significant main effects nor interactions, indicating that the proportions of risk choices across the task did not vary between groups (see Fig. 6, top).
Fig. 6.
(A) Evolution of risk choices (choosing 25 instead of 5) across the whole task (17 bins). Each bin is comprised of 40 trials, reporting the mean proportion of choosing 25 in that particular bin. The horizontal dotted line reflects chance level (p = .5). (B) Probabilities of risk choices on the current trial (n) when the previous trial (n-1) had been a maximum gain, minimum gain, minimum loss and maximum loss. Error bars represent SEMs.
To investigate how the risk-seeking behavior was influenced by the previous trial, repeated measures ANOVAs with Risk (after maximum gain, minimum gain, minimum loss and maximum loss) and Group (SAH patients vs. controls) factors were performed. As shown in Figure 6 , a significant main effect of risk was found [F(3, 78) = 4.02, p = .017] highlighting that the tendency to make risky decisions increased after gaining the maximum amount of money for both groups, as previously seen by Padrão et al. (2013). In line with previous reports (Gehring and Willoughby, 2002), the control group showed a notable increase in their tendency to undertake risks after losing the maximum amount of money, in contrast to the pattern observed for patient group, although this difference was not statistically significant [t(25) < 1] (Fig. 6).
3.5. Neuropsychological assessment
Table 1C shows raw and scaled scores of each neuropsychological test. Attention deficits (Digits) were observed in 2 out of 15 patients (cases 5 and 11), whereas working memory deficits were observed in 1 patient (case 13) in Letters and numbers subtest but none in the Arithmetic subtest. The WM IQ was normal for all patients, even though cases 4, 12 and 13 performed in the low average range.
Regarding verbal fluency, semantic fluency impairment was observed in 4 out of 15 patients (cases 1, 3, 4 and 14) and phonetic fluency difficulties were observed in 2 patients (cases 4 and 15).
In order to do between-groups comparisons, T tests for independent samples were performed. Significant differences on digit span forward [Patients, 4.83 ± 0.83; Controls, 6.00 ± 1.21; t(22) = −2.755, p = .012], “Digits” [Patients, 11.75 ± 2.38; Controls, 15.00 ± 3.742; t(22) = −2.539, p = .019] and phonetic fluency test [Patients, 11.00 ± 2.62; Controls, 17.08 ± 4.74; t(20) = −3.615, p = .002) were found, showing a lower performance of the patient group when compared to the control group. The arithmetic subtest and working memory IQ was not compared since the control group did not have such punctuations.
4. Discussion
We are constantly deciding among different options and possibilities and we have to do so in an appropriate temporal framework in connection with a given situation. Deciding well is selecting a response that will be ultimately advantageous to the individual in terms of its survival, and of the quality of that survival (Damasio, 1994, page 169). Bearing in mind that subarachnoid hemorrhage (SAH) occurs at a relatively young age, many patients have important responsibilities with respect to work and family. Therefore, deficits in decision-making might have a major impact on their lives, leading to personal, social, economic or health problems. In the present study our main aim was to characterize the neurophysiological correlates related to feedback processing in a group of SAH patients using a gambling paradigm (Camara et al., 2010; Marco-Pallarés et al., 2008; Padrão et al., 2013). Several studies have been performed assessing decision-making in SAH patients from a behavioral perspective suggesting altered neural response patterns to rewards and punishments and significant differences in the processing of monetary gains and losses (Al-Khindi et al., 2014; Bechara et al., 1994, 1996, 2000; Damasio, 1996; Escartin et al., 2012). In the present work, a different approach was employed using event-related brain potentials (ERPs) and trial-by-trial wavelet-based time-frequency analysis of the EEG signal to investigate the effects of positive and negative feedback associated with monetary gains and losses in this group of patients. To our knowledge, no previous neurophysiological report assessing ERP's and time frequency analysis have been performed addressing this question in SAH patients.
Previous evidence using ERPs have highlighted the presence of a medial frontal negative deflection known as feedback-related negativity (FRN) 200–300 ms after feedback onset (Gehring and Willoughby, 2002; Holroyd et al., 2006; Nieuwenhuis et al., 2004; Toyomaki and Murohashi, 2005; Wu and Zhou, 2009; Yeung and Sanfey, 2004), thought to reflect the activation of a reinforcement learning system (RL) that assesses the outcomes of our decisions determining whether feedback is better or worse than expected, encoding this difference between the expected vs. actual state as a reward prediction error (Holroyd and Coles, 2002; Nieuwenhuis et al., 2004). This idea based on RL principles has been challenged in several studies, pointing out to the possibility that the FRN might be driven by the surprisingness (Courville et al., 2006; Hauser et al., 2014; Hayden et al., 2011) or saliency (Alexander and Brown, 2011; Bromberg-Martin et al., 2010; Oliveira et al., 2007; Pfabigan et al., 2011; Talmi et al., 2013) of an event. As previously exposed on previous paragraphs, a key region for feedback and reward processing and value representations is the mPFC (Grabenhorst and Rolls, 2011; Knutson et al., 2003; Levy and Glimcher, 2012), a brain region frequently damaged in SAH patients caused by AComA aneurysm rupture. As in previous reports, larger negativities for losses (significantly larger for the maximum condition) than for gains in the healthy controls were found (Gehring and Willoughby, 2002; Hajcak et al., 2006; Holroyd et al., 2006; Toyomaki and Murohashi, 2005; Wu and Zhou, 2009; Yeung and Sanfey, 2004). Noteworthy, this difference was diminished for the patient group (see Fig. 2A), depicting altered signal processing in the presence of negative monetary feedback. Moreover, the FRN component signaling negative prediction error showed significant amplitude differences between groups, illustrated as a weaker difference waveform (Maximum loss minus Maximum gain) for SAH patients when compared to healthy controls (Fig. 2B), pointing out to a possible alteration in the feedback processing mechanisms. Source localization analysis using CLARA distributed model (Classic LORETA Recursively Applied; Hoechstetter et al., 2010) showed bilateral insular activity as well as the MCC in the case of the control group, confirming the role of the cingulate cortex in the generation of the FRN (see Fig. 4) (Becker et al., 2014; Bellebaum and Daum, 2008; Doñamayor et al., 2012; Gehring and Willoughby, 2002; Hauser et al., 2014; Luu et al., 2003; Mathewson et al., 2008; Miltner et al., 1997; Müller et al., 2005; Ruchsow et al., 2002).
Previous evidence from other studies addressing other pathological conditions has also shown differences at the FRN component. In one study with schizophrenic patients, diminished differentiation between correct and incorrect feedback was found during a probabilistic learning task that manipulated the feedback validity (Morris et al., 2008). A reduced FRN amplitude has also been reported in adults and children with depressive symptoms (Bress et al., 2012; Foti and Hajcak, 2009; Liu et al., 2014). One possible explanation of the present results regarding the FRN component in this clinical population is that lesions occurring at the reward-based decision-making circuitry, especially at the mPFC, might be altering the processing of negative reward prediction errors failing to inform the system about potential conflicts and need for re-adjustment, supporting the hypothesis of the RL framework (Holroyd and Coles, 2002). As a result of dysfunctional RL mechanisms, reward prediction errors cannot be used optimally by the mPFC to monitor and control the patient's performance resulting in a maladaptive goal directed behavior in everyday functioning.
Given that previous studies have described an association between the FRN and medial-frontal theta oscillatory activity (4–8 Hz) (Cavanagh et al., 2010; Cohen et al., 2007; Marco-Pallarés et al., 2008; Padrão et al., 2013; Trujillo and Allen, 2007) we performed wavelet-based time frequency analysis of the EEG data to study to what extent the theta oscillations were involved in negative feedback processing. It has been proposed that theta power enhancement over the mPFC cortex might be reflecting a general top-down mechanism mirroring the degree of reward prediction error in the service of behavioral adjustment (Cavanagh and Frank, 2014; Cavanagh et al., 2010; Tzur and Berger, 2009; van de Vijver et al., 2011; Womelsdorf et al., 2010). Previous studies have provided evidence that the mPFC theta power increases more after errors or negative performance feedback than after successful trials or positive feedback (Cavanagh and Frank, 2014; Cavanagh et al., 2010; Cohen, 2011; Cohen et al., 2007; Cunillera et al., 2012; Luu et al., 2003, 2004; Marco-Pallarés et al., 2008, 2009; Padrão et al., 2013; Trujillo and Allen, 2007). Accordingly, when feedback denotes that an unfavorable outcome has occurred and that an adjustment in behavior is needed, theta-band oscillations in the frontal network seem to be responsible of transmitting this signal and implementing performance and behavioral adjustments of the system (Cavanagh et al., 2009). Our results clearly illustrate an increase in theta power in presence of negative feedback (monetary losses) independently of the magnitude (maximum or minimum) significantly larger at Fz electrode location. This pattern was observed for both healthy controls and SAH patients groups, indicating no alterations at this level of analysis. Nevertheless, when computing the difference Maximum loss minus Maximum gain, significant differences between groups appeared showing increased theta oscillatory activity in the SAH patient group at the 400–600 ms time window of analysis. This finding might be pointing out to an increase in the cognitive effort needed to signal the need for control and action monitoring, resulting in an augmented theta oscillatory activity in SAH patients. In principle, these results in theta oscillatory power might seem contradictory with the results observed for the FRN (circumscribed in an earlier time window) as the FRN component is associated to this frequency band (Cavanagh and Frank, 2014). One possibility is that in the patient group, an amplitude reduction in the FRN component could reflect a prolongation of the signal due to slow information processing or less availability of cognitive resources needed for performing the task and therefore recruitment of additional cognitive control mechanisms are indispensable. Increased load and scarce cognitive resources could be translated into larger variations of the latency of the component from trial to trial (referred as latency jitter effect), with the consequence of a reduction of the FRN amplitude after averaging multiple trials and increasing the error in the amplitude estimation. As oscillatory wavelet analysis applied on single-trial basis is less affected by the latency jitter effect, mitigating this problem. The present results might reflect the recruitment of further cognitive control resources as well as compensatory mechanisms in a more effortful context.
Differences in a later ERP component, the FB-P3, a positive deflection found at centro-parietal locations at 300–600 ms after feedback presentation related to reward outcome processing (Donchin, 1981; Polich, 2007; Sutton et al., 1965; see Glazer et al., 2018 for a review) was also examined (Fig. 3). In this case, no statistically significant differences between groups were found. Nevertheless, significant results regarding the FB-P3 sensitivity to reward magnitude (depicting larger amplitudes for the maximum conditions) as well as Valence (larger for gains than for losses) were found, confirming previous results in the literature (Wu and Zhou, 2009; Yeung et al., 2005). These findings showing no significant differences in the FB-P3 suggest a preservation of this positive feedback-related component. Relatedly, we examined the FB-P3 related parietal delta power (1–3 Hz), thought to reflect feedback expectancy and reward evaluation during reward outcome. Confirming previous findings, increased delta oscillatory activity for gains compared to losses, especially for the maximum condition, at parietal locations were found, confirming its sensitivity to reward evaluation and magnitude. Moreover, previous studies have reported the association between monetary gains and positive feedback with the appearance of beta-gamma oscillations (25–35 Hz) (Cohen et al., 2007; Cunillera et al., 2012; Marco-Pallarés et al., 2008, 2009, 2015), supporting the idea that beta activity orchestrates reward processing by signaling the appearance of salient positive events on the environment. Our results clearly support this hypothesis, showing an enhancement of beta power significantly larger for gains than for losses in both groups. Furthermore, this beta-power oscillatory activity was boosted depending on the magnitude of the positive feedback as previously reported (Marco-Pallarés et al., 2008), confirming the role of the beta-oscillatory component not only in positive feedback processing but also in the magnitude evaluation of reward processing. Correspondingly as to what we found for the FB-P3 component and the FB-P3 related delta activity, no significant differences between groups were found at the beta-gamma range, probably indicating a more specific alteration of the RL system circumscribed to the processing or monitoring in presence of negative feedback or in the cognitive control exerted by the mPFC necessary for adjusting an individual's performance.
At the behavioral level, no significant differences on task performance, neither on the total amount of money collected nor on their risk choice patterns throughout the task were observed between groups (see Fig. 6, top). When looking at how the risk-seeking behavior was influenced by the previous trial, the tendency to make risky decisions increased after gaining the maximum amount of money for both groups (Fig. 6, bottom) as previously seen by Padrão et al. (2013). Also, the control group showed a noteworthy increase in their predisposition to undertake risks after losing the maximum amount of money, as described in previous reports (Gehring and Willoughby, 2002). Importantly, a reduction in this risk tendency after losing the maximum amount of money was observed for the patient group (Fig. 6, bottom), probably indicating a reduced sensitivity to losses compared to the healthy controls, in line with our findings in the ERP results regarding differences in the FRN component, although this difference did not reach statistical significance.
Regarding the neuropsychological results, most patients performed normally in standard tests of attention, working memory and executive function. Different studies have demonstrated the dissociation between the execution on standard neuropsychological frontal tests and the results obtained on gambling tasks (such as the Iowa Gambling task) in patients with mPFC lesions (Bechara, 2004; Bechara et al., 2000). Given that SAH secondary to AComA aneurysms might affect the integrity of the mPFC subserving feedback processing and the RL system, it would be important to assess systematically decision-making in this group of patients using more specific and directed tasks.
Concluding remarks
Overall, our results provide new evidence on how the reward-based decision-making circuit is altered in SAH patients from a neurophysiological perspective, revealing altered electrophysiological signatures related to feedback processing. At frontal locations, a diminished FRN component was observed for the patient group, probably reflecting larger variability on the processing of the negative feedback in single-trials. On the other hand, time frequency analysis showed a delayed increase in the theta oscillatory activity in the SAH group, indicating an effortful signaling of cognitive control and monitoring processes lengthened in time when compared to healthy control subjects. Taken together, our study points out to a dysfunctional RL pattern characterized by an altered processing of negative reward prediction errors, affecting performance adjustment and belief updating in SAH patients, probably contributing to their day-to-day cognitive complaints. More studies using ERP's as well as time frequency analysis should be performed in this group of patients to try clarify the nature of their cognitive complaints, allowing us to address specific rehabilitation therapies in order to improve these patients’ day-to-day functioning.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Spanish Government with a MINECO Grant (PSI2015-69178-P) awarded to A.R.F. We would like to specially thank our patients for their help during this project, support and willingness to collaborate in all cases.
References
- Alexander W.H., Brown J.W. Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 2011;14(10):1338. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khindi T., Macdonald R.L., Schweizer T.A. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–e536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- Al-Khindi T., Macdonald R.L., Schweizer T.A. Decision-making deficits persist after aneurysmal subarachnoid hemorrhage. Neuropsychology. 2014;28(1):68–74. doi: 10.1037/neu0000003. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cognit. 2004;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. Vol. 10. 2000. Emotion, decision making and the orbitofrontal cortex; pp. 295–307. (Cereb. Cortex). [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H., Damasio A.R. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb. Cortex. 1996;6(2):215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Becker M.P., Nitsch A.M., Miltner W.H., Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J Neurosci. 2014;34(8):3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C., Daum I. Learning‐related changes in reward expectancy are reflected in the feedback‐related negativity. Eur. J. Neurosci. 2008;27(7):1823–1835. doi: 10.1111/j.1460-9568.2008.06138.x. [DOI] [PubMed] [Google Scholar]
- Bernat E.M., Nelson L.D., Baskin‐Sommers A.R. Time‐frequency theta and delta measures index separable components of feedback processing in a gambling task. Psychophysiology. 2015;52(5):626–637. doi: 10.1111/psyp.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat E.M., Nelson L.D., Steele V.R., Gehring W.J., Patrick C.J. Externalizing psychopathology and gain–loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. J. Abnorm. Psychol. 2011;120(2):352. doi: 10.1037/a0022124. http://dx.doi.org/10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cognit. Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Smith E., Foti D., Klein D.N., Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol. Psychol. 2012;89(1):156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin E.S., Matsumoto M., Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Vogt B.A., Holmes J., Dale A.M., Greve D., Jenike M.A., Rosen B.R. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara E., Rodríguez-Fornells A., Münte T.F. Functional connectivity of reward processing in the brain. Front. Hum. Neurosci. 2009;2:19. doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara E., Rodríguez-Fornells A., Münte T.F. Microstructural brain differences predict functional hemodynamic responses in a reward processing task. J. Neurosci. 2010;30(34):11398–11402. doi: 10.1523/JNEUROSCI.0111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J.M., Foti D., Mujica-Parodi L.R., Harmon-Jones E., Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Cavanagh J.F., Cohen M.X., Allen J.J. Prelude to and resolution of an error: eeg phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 2009;29(1):98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. Frontal theta as a mechanism for cognitive control. Trends Cognit. Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J., Klein T.J., Allen J.J. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49(4):3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. Neuroimage. 2011;55(3):1373–1383. doi: 10.1016/j.neuroimage.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Cohen M.X., Elger C.E., Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courville A.C., Daw N.D., Touretzky D.S. Bayesian theories of conditioning in a changing world. Trends Cognit. Sci. 2006;10(7):294–300. doi: 10.1016/j.tics.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Cui J.F., Chen Y.H., Wang Y., Shum D.H., Chan R.C. Neural correlates of uncertain decision making: ERP evidence from the Iowa gambling task. Front. Hum. Neurosci. 2013;7:776. doi: 10.3389/fnhum.2013.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunillera T., Fuentemilla L., Periañez J., Marco-Pallarès J., Krämer U.M., Càmara E., ..., Rodríguez-Fornells A. Brain oscillatory activity associated with task switching and feedback processing. Cognit. Affect. Behav. Neurosci. 2012;12(1):16–33. doi: 10.3758/s13415-011-0075-5. [DOI] [PubMed] [Google Scholar]
- Damasio A.R. New York: Putnam; 1994. Descartes’ Error: Emotion, Rationality and the Human Brain. [Google Scholar]
- Damasio A.R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B. 1996;351(1346):1413. doi: 10.1098/rstb.1996.0125. 1320. [DOI] [PubMed] [Google Scholar]
- Delorme A., Sejnowski T., Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascalis V., Varriale V., D'Antuono L. Event-related components of the punishment and reward sensitivity. Clin. Neurophysiol. 2010;121(1):60–76. doi: 10.1016/j.clinph.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Doñamayor N., Schoenfeld M.A., Münte T.F. Magneto-and electroencephalographic manifestations of reward anticipation and delivery. Neuroimage. 2012;62(1):17–29. doi: 10.1016/j.neuroimage.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise!… surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Escartin G., Junqué C., Juncadella M., Gabarrós A., de Miquel M.A., Rubio F. Decision-making impairment on the Iowa gambling task after endovascular coiling or neurosurgical clipping for ruptured anterior communicating artery aneurysm. Neuropsychology. 2012;26(2):172. doi: 10.1037/a0024336. [DOI] [PubMed] [Google Scholar]
- Fisher C.M., Kistler J.P., Davis J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Foti D., Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: evidence from event-related potentials. Biol. Psychol. 2009;81(1):1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D., Weinberg A., Bernat E.M., Proudfit G.H. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clin. Neurophysiol. 2015;126(7):1338–1347. doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Willoughby A.R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Glazer J.E., Kelley N.J., Pornpattananangkul N., Mittal V.A., Nusslock R. Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. Int. J. Psychophysiol. 2018;132:184–202. doi: 10.1016/j.ijpsycho.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Goodglass H., Kaplan E. Philadelphia: Lea & Febiger; 1983. The Assessment of Aphasia and Related Disorders. [Google Scholar]
- Grabenhorst F., Rolls E.T. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cognit. Sci. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Holroyd C.B., Simons R.F. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol. Psychol. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hallschmid M., Mölle M., Fischer S., Born J. EEG synchronization upon reward in man. Clin. Neurophysiol. 2002;113(7):1059–1065. doi: 10.1016/s1388-2457(02)00142-6. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M.S., Ilmoniemi R.J. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput. 1994;32(1):35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hauser T.U., Iannaccone R., Stämpfli P., Drechsler R., Brandeis D., Walitza S., Brem S. The feedback-related negativity (FRN) revisited: new insights into the localization, meaning and network organization. Neuroimage. 2014;84:159–168. doi: 10.1016/j.neuroimage.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Hayden B.Y., Heilbronner S.R., Pearson J.M., Platt M.L. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J. Neurosci. 2011;31(11):4178–4187. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K., Berg P., Scherg M. BESA research tutorial 4: distributed source imaging. BESA Res. Tutor. 2010;4:1–29. [Google Scholar]
- Holroyd C.B., Coles M.G. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109(4):679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Hajcak G., Larsen J.T. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Res. 2006;1105(1):93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Nieuwenhuis S., Yeung N., Nystrom L., Mars R.B., Coles M.G., Cohen J.D. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat. Neurosci. 2004;7(5):497. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Pakzad‐Vaezi K.L., Krigolson O.E. The feedback correct‐related positivity: Sensitivity of the event‐related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Hütter B.O., Gilsbach J.M. Which neuropsychological deficits are hidden behind a good outcome (Glasgow= I) after aneurysmal subarachnoid hemorrhage? Neurosurgery. 1993;33(6):999–1006. doi: 10.1227/00006123-199312000-00007. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Bennett S.M., Adams C.M., Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Levy D.J., Glimcher P.W. The root of all value: a neural common currency for choice. Curr. Opin. Neurobiol. 2012;22(6):1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.H., Wang L.Z., Shang H.R., Shen Y., Li Z., Cheung E.F., Chan R.C. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J., Luck S.J. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P., Tucker D.M. Regulating action: alternating activation of midline frontal and motor cortical networks. Clin. Neurophysiol. 2001;112(7):1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Luu P., Tucker D.M., Derryberry D., Reed M., Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol. Sci. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Luu P., Tucker D.M., Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin. Neurophysiol. 2004;115(8):1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Marco-Pallarés J., Cucurell D., Cunillera T., García R., Andrés-Pueyo A., Münte T.F., Rodríguez-Fornells A. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46(1):241–248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Marco-Pallarés J., Cucurell D., Cunillera T., Krämer U.M., Càmara E., Nager W., ..., Rodríguez-Fornells A. Genetic variability in the dopamine system (dopamine receptor D4, catechol-O-methyltransferase) modulates neurophysiological responses to gains and losses. Biol. Psychiatry. 2009;66(2):154–161. doi: 10.1016/j.biopsych.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Marco-Pallarés J., Münte T.F., Rodríguez-Fornells A. The role of high-frequency oscillatory activity in reward processing and learning. Neurosci. Biobehav. Rev. 2015;49:1–7. doi: 10.1016/j.neubiorev.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Mas-Herrero E., Ripollés P., HajiHosseini A., Rodríguez-Fornells A., Marco-Pallarés J. Beta oscillations and reward processing: coupling oscillatory activity and hemodynamic responses. Neuroimage. 2015;119:13–19. doi: 10.1016/j.neuroimage.2015.05.095. [DOI] [PubMed] [Google Scholar]
- Mathewson K.J., Dywan J., Snyder P.J., Tays W.J., Segalowitz S.J. Aging and electrocortical response to error feedback during a spatial learning task. Psychophysiology. 2008;45(6):936–948. doi: 10.1111/j.1469-8986.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- McClure S.M., Berns G.S., Montague P.R. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Miltner W.H., Braun C.H., Coles M.G. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. J. Cognit. Neurosci. 1997;9(6):788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Monchi O., Petrides M., Petre V., Worsley K., Dagher A. Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J. Neurosci. 2001;21(19):7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.E., Heerey E.A., Gold J.M., Holroyd C.B. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr. Res. 2008;99(1-3):274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S.V., Möller J., Rodríguez-Fornells A., Münte T.F. Brain potentials related to self-generated and external information used for performance monitoring. Clin. Neurophysiol. 2005;116(1):63–74. doi: 10.1016/j.clinph.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Münte T.F., Heldmann M., Hinrichs H., Marco-Pallarés J., Krämer U.M., Sturm V., Heinze H.J. Contribution of subcortical structures to cognition assessed with invasive electrophysiology in humans. Front. Neurosci. 2008;2:6. doi: 10.3389/neuro.01.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S., Holroyd C.B., Mol N., Coles M.G. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neurosci. Biobehav. Rev. 2004;28(4):441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- O'Doherty J.P., Dayan P., Friston K., Critchley H., Dolan R.J. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Oliveira F.T., McDonald J.J., Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. J. Cognit. Neurosci. 2007;19(12):1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- Padrão G., Mallorquí A., Cucurell D., Marco-Pallarés J., Rodríguez-Fornells A. Neurophysiological differences in reward processing in anhedonics. Cognit. Affect. Behav. Neurosci. 2013;13(1):102–115. doi: 10.3758/s13415-012-0119-5. [DOI] [PubMed] [Google Scholar]
- Pagnoni G., Zink C.F., Montague P.R., Berns G.S. Activity in human ventral striatum locked to errors of reward prediction. Nat. Neurosci. 2002;5(2):97. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Peña-Casanova J., Quiñones-Ubeda S., Gramunt-Fombuena N., Quintana-Aparicio M., Aguilar M., Badenes D., ..., Blesa R. Spanish multicenter normative studies (NEURONORMA project): norms for verbal fluency tests. Arch. Clin. Neuropsychol. 2009;24(4):395–411. doi: 10.1093/arclin/acp042. [DOI] [PubMed] [Google Scholar]
- Pfabigan D.M., Alexopoulos J., Bauer H., Sailer U.T.A. Manipulation of feedback expectancy and valence induces negative and positive reward prediction error signals manifest in event‐related brain potentials. Psychophysiology. 2011;48(5):656–664. doi: 10.1111/j.1469-8986.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A., Camerer C., Montague P.R. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 2008;9(7):545. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rinkel G.J., Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10(4):349–356. doi: 10.1016/S1474-4422(11)70017-5. [DOI] [PubMed] [Google Scholar]
- Ruchsow M., Grothe J., Spitzer M., Kiefer M. Human anterior cingulate cortex is activated by negative feedback: evidence from event-related potentials in a guessing task. Neurosci. Lett. 2002;325(3):203–206. doi: 10.1016/s0304-3940(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Scherg M., Von Cramon D. Two bilateral sources of the late AEP as identified by a spatio-temporal dipole model. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Section. 1985;62(1):32–44. doi: 10.1016/0168-5597(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Sutton S., Braren M., Zubin J., John E.R. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150(3700):1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Talmi D., Atkinson R., El-Deredy W. The feedback-related negativity signals salience prediction errors, not reward prediction errors. J. Neurosci. 2013;33(19):8264–8269. doi: 10.1523/JNEUROSCI.5695-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet N. Am. Ed. 1974;304(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Toyomaki A., Murohashi H. Discrepancy between feedback negativity and subjective evaluation in gambling. Neuroreport. 2005;16(16):1865–1868. doi: 10.1097/01.wnr.0000185962.96217.36. [DOI] [PubMed] [Google Scholar]
- Trujillo L.T., Allen J.J. Theta eeg dynamics of the error-related negativity. Clin. Neurophysiol. 2007;118(3):645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Tzur G., Berger A. Fast and slow brain rhythms in rule/expectation violation tasks: Focusing on evaluation processes by excluding motor action. Behav. Brain Res. 2009;198(2):420–428. doi: 10.1016/j.bbr.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., Von Cramon D.Y. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J. Neurosci. 2003;23(10):4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Vijver I., Ridderinkhof K.R., Cohen M.X. Frontal oscillatory dynamics predict feedback learning and action adjustment. J. Cognit. Neurosci. 2011;23(12):4106–4121. doi: 10.1162/jocn_a_00110. [DOI] [PubMed] [Google Scholar]
- Van Gijn J., Kerr R.S., Rinkel G.J.E. Subarachnoid haemorrhage. Lancet N. Am. Ed. 2007;369(9558):306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- Vilà-Balló A., Hdez-Lafuente P., Rostan C., Cunillera T., Rodríguez-Fornells A. Neurophysiological correlates of error monitoring and inhibitory processing in juvenile violent offenders. Biol. Psychol. 2014;102:141–152. doi: 10.1016/j.biopsycho.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Von Cramon D.Y., Müller U. The septal region and memory. In: Cohadon F., editor. Advances and Technical Standards in Neurosurgery. Springer; Vienna: 1998. pp. 3–40. (Eds.) pp. [DOI] [PubMed] [Google Scholar]
- Walsh M.M., Anderson J.R. Learning from experience: event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neurosci. Biobehav. Rev. 2012;36(8):1870–1884. doi: 10.1016/j.neubiorev.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A.T., Bachman M.D., Bernat E.M. Expectancy effects in feedback processing are explained primarily by time-frequency delta not theta. Biol. Psychol. 2017;129:242–252. doi: 10.1016/j.biopsycho.2017.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual de Aplicación y Corrección; Madrid: TEA: 2001. Escala De Inteligencia De Wechsler Para Adultos-III (WAIS-III) [Google Scholar]
- Womelsdorf T., Johnston K., Vinck M., Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc. Natl. Acad. Sci. 2010;107(11):5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou X. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Res. 2009;1286:114–122. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yeung N., Holroyd C.B., Cohen J.D. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb. Cortex. 2005;15(5):535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yeung N., Sanfey A.G. Independent coding of reward magnitude and valence in the human brain. J. Neurosci. 2004;24(28):6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]