Abstract
Objective.
Most attempts at smoking cessation are unsuccessful, and stress is frequently characterized both as a momentary precipitant of smoking lapse and a predictor of subsequent changes in other key precipitants of lapse. The current study examined longitudinal associations among stress, multiple precipitants of lapse, and lapse among smokers attempting to quit.
Methods.
Ecological momentary assessments (EMAs) were gathered from a multi-ethnic, gender-balanced sample of 370 adults enrolled in a smoking cessation program. EMAs (N=32,563) assessed smoking lapse and precipitants of lapse including stress, negative affect, smoking urge, abstinence self-efficacy, motivation to quit, difficulty concentrating, coping outcome expectancies, and smoking outcome expectancies. A multilevel structural equation model simultaneously estimated within person paths from stress to multiple precipitants and subsequent smoking lapse. Indirect effects of stress to smoking lapse through precipitants were computed.
Results.
Results indicated that increased stress was significantly associated with all precipitants of lapse consistent with a greater risk for lapse (i.e., increased negative affect, smoking urge, difficulty concentrating, and smoking outcome expectancies, and reduced abstinence self-efficacy, motivation to quit, and coping outcome expectancies). All precipitants were significantly associated with subsequent lapse. Indirect effects indicated that stress was uniquely connected to lapse through negative affect, smoking urge, abstinence self-efficacy, coping outcome expectancies, and smoking outcome expectancies.
Conclusions.
Results of this study highlight the broad importance of stress for smoking lapse during a quit attempt. Smoking cessation programs should pay close attention to the role of stress in exacerbating key precipitants of lapse to improve cessation success rates.
Keywords: stress, smoking lapse, multilevel modeling, ecological momentary assessment
Successful smoking cessation provides substantial health benefits (USDHHS, 2014), but the vast majority of smokers who try to quit each year fail to achieve long-term abstinence (Wu, Wilson, Dimoulas, & Mills, 2006). Further examination of the factors associated with failure to quit can provide important information for improving smoking cessation programs (Vangeli, Stapleton, Smit, Borland, & West, 2011). Cessation researchers differentiate a single instance of smoking lapse during a quit attempt from smoking relapse, or a failed quit attempt characterized by a return to regular smoking (Shiffman, 2006). Smokers who lapse earlier and more often during a quit attempt are at a higher risk for full relapse, but this process often entails multiple instances of lapse across several days or weeks (Shiffman, 2006).
Smokers regularly report that smoking offers a potential reprieve from the sequelae of stressful experiences (Kassel, Stroud, & Paronis, 2003). Empirical studies have supported these reports by highlighting stress as a key precipitant of smoking lapse during a cessation attempt (Kassel et al., 2003; McKee et al., 2011; Oberleitner, Moore, Verplaetse, Roberts, & McKee, 2018; Richards et al., 2011; Shiffman et al., 1997; Shiffman & Waters, 2004). Importantly, many of the sequelae of stressful experiences have been independently identified as precipitants of smoking lapse during a cessation attempt. Prominent theoretical models of addiction and relapse prevention have hypothesized that stress operates as a central factor in smoking lapse and relapse (Marlatt, 1985; Sinha, 2007, 2008; Witkiewitz & Marlatt, 2004). The current study explicitly draws from Witkiewitz and Marlatt’s dynamic model of relapse prevention which highlights stress as a “high risk” momentary factor that increases the likelihood of lapse by exacerbating other precipitants (Witkiewitz & Marlatt, 2004, p. 229).
Empirical studies have demonstrated that stressful experiences are associated with increased negative affect (McKee et al., 2011), stronger smoking urges (McKee et al., 2011; Volz et al., 2014), reduced abstinence self-efficacy (i.e., belief in one’s ability to quit) (Maciejewski, Prigerson, & Mazure, 2000), reduced motivation to quit (Sinha, 2008), difficulty concentrating (LeBlanc, 2009), reduced coping outcome expectancies (i.e., belief in one’s ability to cope with stress without smoking) (Kassel et al., 2003; Shadel & Mermelstein, 1993), and increased smoking outcome expectancies (i.e., belief that smoking would improve one’s mood) (Kassel et al., 2003; Shadel & Mermelstein, 1993). These factors are closely linked to Witkiewitz and Marlatt’s model and have an established association with increased likelihood of smoking lapse (Bolman et al., 2018; Gwaltney, Metrik, Kahler, & Shiffman, 2009; Gwaltney, Shiffman, Balabanis, & Paty, 2005; McKee et al., 2011; Richards et al., 2011; Shadel & Mermelstein, 1993; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996; Shiffman & Waters, 2004; Sinha, 2008; Zhou et al., 2009). Additionally, biobehavioral models suggest that stress triggers neurophysiological changes which enhance smoking urges (McKee et al., 2011; Richards et al., 2011), increase nicotine-seeking behavior (Mantsch, Baker, Funk, Lê, & Shaham, 2016), reduce motivation to quit, and increase risk of lapse (Sinha, 2008). As such, stress is likely to be an important factor influencing multiple cognitive and physiological precipitants of smoking lapse (Richards et al., 2011; Shiffman, 2006; Sinha, 2008; Witkiewitz & Marlatt, 2004).
Few empirical studies, however, have sought to explicitly test associations among stress, precipitants of lapse, and smoking lapse during a quit attempt. One reason for this dearth of research may be the inherent challenge in measuring near real-time responses to experiences of stress outside of the laboratory (Richards et al., 2011; Shiffman & Waters, 2004). Health behavior researchers have established that individual perceptions of a stressor are a key factor in determining the extent to which stress impacts health (Cohen, Kamarck, & Mermelstein, 1983). During smoking cessation, it is perceptions of stress that likely degrade coping resources and exacerbate cognitive and physiological precipitants to increase risk for lapse (Cohen et al., 1983; Ng & Jeffery, 2003; Witkiewitz & Marlatt, 2004). Furthermore, retrospective reports of perceived stress, affective or cognitive states, and smoking behavior are likely subject to substantial recall biases and errors creating further assessment challenges (Shiffman, 2009). Experience sampling methods such as ecological momentary assessment (EMA) offer one method of gathering near real-time data about experiences, affective and cognitive states, and behaviors on multiple occasions throughout each day and across multiple weeks. EMA produces highly detailed longitudinal datasets, minimizes retrospective recall biases and errors, and is particularly well-suited to examining dynamic relations among stressful experiences, precipitants of lapse, and smoking lapse as they occur in real-time (Shiffman, 2009).
EMA-based research by Shiffman and colleagues (1996) reported that intrapersonal stressors were a trigger for smoking lapse during a quit attempt. Additionally, negative affect resulting from episodic intrapersonal stressors was more likely to trigger a lapse when compared to chronic stress or negative affect emanating from other sources. Shiffman et al. (1997) noted that 48% of 105 smokers trying to quit cited stress as a cause of smoking lapse. Approximately 60% of those reporting stress as a cause of lapse also reported higher negative affect scores at the time of lapse. Shiffman and Waters (2004), however, found no association between daily stress and smoking lapse the following day, but did find that lapses were preceded by within day increases in momentary negative affect resulting from stress. Thus, the momentary result of stress (i.e., increasing negative affect) was more strongly associated with lapse than were day-today changes in stress. Minami and colleagues (2011) reported that a single episode of stress was not related to lapse during the following 48 hours, but the accumulation of stressful experiences during a 48-hour period was positively associated with lapse concurrently during that 48-hour period. Businelle et al. (2014) found that lower stress during the days leading up to a quit attempt predicted a greater likelihood of maintaining quit day abstinence among smokers experiencing homelessness. A second study from Businelle and colleagues (2016b) identified momentary stress as a precipitant of first lapse during a quit attempt in a sample of low socioeconomic status smokers and noted that 62% of lapses were preceded by three or more precipitants occurring simultaneously. Stress and other precipitants were measured in the 4 hours prior to lapse, but the authors did not report how often stress co-occurred with other momentary precipitants of lapse in this time frame. Taken together, results from studies assessing the stress-lapse relationship with EMA data support theoretical models suggesting that momentary stress is a key precipitant of lapse and associated with increased negative affect. No study to date, however, has examined how momentary stress might be linked to changes in other precipitants in ways that increase the likelihood of smoking lapse. A real-time examination of the associations between stress, multiple precipitants of smoking lapse, and lapse can help refine models of tobacco cessation, and provide detailed information to improve smoking cessation programs.
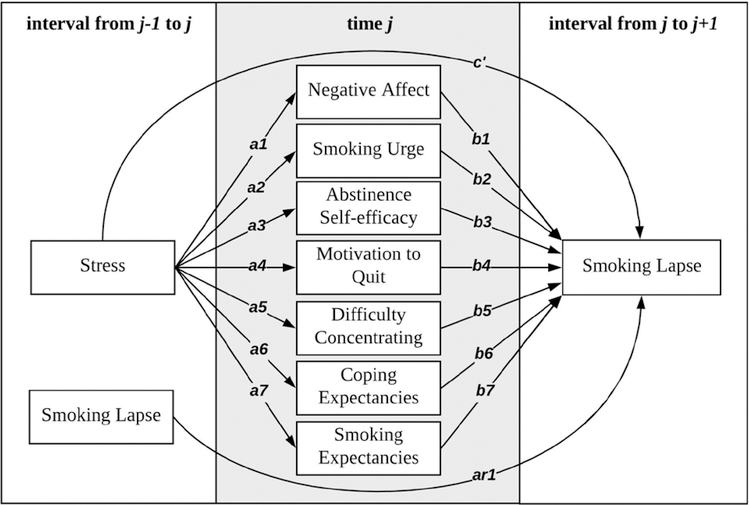
The current study utilized EMA data to examine associations among momentary measures of current and ongoing stressful experiences, multiple precipitants theoretically related to both stress and smoking lapse, and smoking lapse during a quit attempt. Drawing from Witkiewitz and Marlatt’s dynamic model of relapse prevention, we examined momentary associations from stress to multiple precipitants for lapse, and, ultimately, to smoking lapse. First, momentary increases in stress were hypothesized to be associated with an increased likelihood of subsequent smoking lapse. Second, momentary increases in stress were hypothesized to be associated with subsequent changes in precipitants that increased risk for lapse. These changes included increased negative affect, smoking urge, difficulty concentrating, and smoking outcome expectancies, and reduced abstinence self-efficacy, motivation to quit, and coping outcome expectancies. Finally, stress was hypothesized to be indirectly associated to subsequent lapse through these multiple precipitants of lapse. Figure 1 depicts the hypothesized model that was tested.
Figure 1.
Model for within person associations among stress, negative affect, smoking urge, abstinence self-efficacy, motivation to quit, difficulty concentrating, coping outcome expectancies, and smoking outcome expectancies, and subsequent smoking lapse. Paths from smoking lapse during interval j-1 to j to precipitants at time j and residual covariances among all precipitants at time j were estimated but are not shown for clarity.
Method
Participants
Data for the current study were drawn from a longitudinal cohort study designed to examine the potential role of race/ethnicity and other social determinants in smoking cessation. Participants from multiple racial/ethnic groups were recruited in the Houston, TX area via media advertisements to enroll in a smoking cessation study conducted from May 2005 to June 2007. Participants were required to be at least 21 years of age, have smoked an average of at least five cigarettes per day for the past year, have a home address and telephone number, demonstrate proficiency in English at a 6th grade level or higher, and be motivated to quit smoking in the next 30 days. Exclusion criteria included contraindication for nicotine patch use, use of tobacco products other than cigarettes, an active substance use disorder, use of nicotine replacement products other than the patch, participation in a cessation program in the past 90 days, or a household member enrolled currently in the study. The study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center. Potential participants were screened over the phone to determine eligibility (N=944). Those who met eligibility criteria (n=837) were invited to in-person screening and orientation sessions where informed consent and baseline measures were collected. Among those who met the eligibility criteria, 424 participants attended orientation sessions and were enrolled in the study. Additional description of sample recruitment, retention, and participant characteristics has been published elsewhere (Kendzor et al., 2008).
Smoking cessation treatment was administered based on guidelines provided by the Treating Tobacco Use and Dependence Clinical Practice Guideline (Fiore, Jaen, & Baker, 2008) and all participants received identical treatment consisting of nicotine patch therapy for six weeks and six brief smoking cessation counseling sessions (1 week prior to quit day, on quit day, and at 1, 2, 3, and 4 weeks post-quit). Participants completed six in-person assessments at 1 week prior to quit day, on quit day, and at 1, 2, 4, and 26 weeks post-quit. For each completed in-person assessment, participants received a $30 gift card.
Measures
EMA data were collected via palmtop personal computer from one week prior to quit day to 28 days after quit day. Among the 424 participants who enrolled in the study, 391 completed EMAs during the study period. Across 35 days, these participants successfully completed 40,198 out of 53,047 randomly-initiated EMAs (75.8%) and completed an additional 30,093 non-random, self-initiated EMAs. In the post-quit period, 376 participants completed random EMAs and only those with at least 3 completed random EMAs were included in the current analyses (n=370). Over the 28 days of post-quit monitoring, participants completed 32,563 random EMAs for an average of 88 EMAs per person and approximately 3 completed random EMAs per person per day. An additional 25,065 non-random, event-based EMAs were completed in the post-quit period. Four random EMAs were scheduled to be audibly and visually cued each day by the palmtop computer during normal waking hours. Additionally, participants were instructed to self-initiate a non-random EMA when they were experiencing a strong urge to smoke or in process of a smoking lapse. Data from non-random EMAs were only used to indicate smoking lapses that occurred between two random EMAs. Measures of stress and precipitants of smoking lapse were drawn from random EMAs only to capture the most ecologically valid reports and protect against capturing aspects of stress and precipitants directly connected to heightened withdrawal symptoms. The average length of interval between two EMAs was approximately 6 hours.
Smoking lapse was measured by both random and non-random EMAs. For random EMAs, participants responded to a single item asking if they had smoked any cigarettes that they had not already recorded in the computer. Those indicating lapse responded to an additional item “How long ago did you smoke the most recent cigarette that you did not record?” For non-random EMAs, participants responded to two items, “How many cigarettes did you smoke during this slip?” and “How long ago did you smoke your last cigarette?” Both random and non-random smoking lapse items indicating time of smoking offered seven response options ranging from “0–15 minutes” to “8 hours or more.” Time of lapse was measured by subtracting the midpoint of the response option interval (e.g. 0–15 minutes = 7.5 minutes) from the timestamp of the EMA. Responses of lapse 8 hours or more were set to missing given that the specific time of lapse could not be identified. Any indication of 1 or more smoking lapses in the interval between two random EMAs was coded as 1 with no indication of smoking lapse coded as 0.
Stress was measured at each random EMA by two items. One item asked if participants had experienced or thought about an ongoing stressor since their last completed assessment, and the second item asked if participants had experienced or thought about a new stressor since their last completed assessment. Response options of “Definitely No,” “Mostly No,” “Mostly Yes,” or “Definitely Yes” were coded as 1 through 4 respectively. Combined current stress was measured as the mean of responses to ongoing and new stressor items (Correlation = .83) and higher scores indicated more stress.
All precipitants were measured at random EMAs, used an identical 5-point Likert for response options (“Strongly Disagree,” “Disagree”, “Neutral,” “Agree” or “Strongly Agree”), and were coded 1 through 5 respectively. Precipitants were coded such that higher scores indicated more of each variable name. Negative affect was measured by the mean of three items asking if the respondent felt sad, angry, or anxious (Cronbach’s alpha = .77). Smoking urge was measured by the mean of three items “I have an urge to smoke”, “I really want to smoke”, and “I need a cigarette” (Cronbach’s alpha = .92). Abstinence self-efficacy was measured by one item “I am confident in my ability not to smoke in this situation.” Motivation to quit was measured by the mean of two items “I am very motivated not to smoke” and “Not smoking is very important to me” (Correlation = .64). Difficulty concentrating was measured by a single item “I am having a hard time concentrating.” Coping outcome expectancies were measured by a single item “I am confident I could do something other than smoke to improve my mood.” Smoking outcome expectancies were measured by a single item “I am confident that smoking would improve my mood.”
Data analytic strategy
Psychological researchers and tobacco scholars alike have noted the importance of separating within person, momentary estimates from between person, average estimates (Curran & Bauer, 2011; Shiffman & Waters, 2004). Analyses seeking to understand the momentary precipitants of smoking lapse should focus on the within person, momentary estimates as opposed to between person, average estimates. For example, while persons with higher average levels of stress across the study period may be more likely to lapse during a quit attempt when compared to other persons (as assessed at the between person level), the within person process of lapse is observed when an individual experiences a momentary increase in stress above their average experience of stress across the study period, and then subsequently lapses (Bolger & Laurenceau, 2013; Preacher, Zyphur, & Zhang, 2010). This person-mean centering approach is commonly used in EMA studies and was adopted for stress and all precipitants used in this study (Hamaker & Grasman, 2015). Multilevel structural equation models (MSEMs) offer researchers one method to examine within person, momentary changes in stress and precipitants of lapse. The general advantages of MSEMs and those specific to analyses of indirect effects relative to other approaches have been described in detail by Preacher and colleagues (2011; 2010) and MacKinnon and Pirlott (2015). In particular to the current study, the MSEM permitted all precipitants to be modeled concurrently in the moment. This allowed for an interpretation of indirect effects as unique, co-occurring paths through which stress was associated with lapse(MacKinnon & Pirlott, 2015). The shared momentary variance among multiple precipitants such as negative affect, smoking urge, and difficulty concentrating likely captures instances of heightened withdrawal.
MSEM analyses were conducted with Mplus 8.1 (Muthen & Muthen, 2017) using the Bayes estimator and probit link to estimate the effects of predictors on the continuous latent response underlying a binary measure of smoking lapse (Muthén & Asparouhov, 2012). MSEMs employing the Bayes estimator with non-informative priors often provide similar estimates to a maximum likelihood estimator, but offer two important advantages. The Bayesian approach 1) produces asymmetric credibility intervals (CIs) for all effects, and 2) reduces computational complexity of MSEMs that require many dimensions of numerical integration. The latter reduces convergence issues and substantially hastens model estimation. The current models were estimated with a minimum of 20,000 iterations. To determine significance, the 95% highest probability density interval (HPD) was calculated from the posterior distribution of the model and CIs based on the HPD that did not include zero were considered statistically different from zero (Muthén & Asparouhov, 2012). The Bayes estimator in Mplus is a full information estimator for handling missing data (Muthen & Muthen, 2017).
To allow for heterogeneity in the process of smoking lapse across participants, all within level paths were estimated with random intercepts and slopes such that each random slope is decomposed into an average slope and variance component representing the distribution of slopes across participants (Bolger & Laurenceau, 2013). MSEMs allowed for simultaneous estimation of paths from stress to multiple precipitants (a paths) and each precipitant to subsequent smoking lapse (b paths). The Model Constraint command was used to calculate indirect effects as the product of a and b paths plus the covariance of a and b paths at the between person level (Bolger & Laurenceau, 2013; Preacher et al., 2010). Substantively, the covariance of a and b paths represents the degree to which the association of stress and a precipitant covaries with the association of that precipitant and smoking lapse across persons (e.g., the covariance of the path from stress to negative affect [a1] and the path from negative affect to lapse [b1]). While a multiple precipitant model tested without the covariance of a and b added produced largely similar results, best practices for intensive longitudinal data recommend adding this covariance to protect against potential omitted variable bias (Vuorre & Bolger, 2017).
Preliminary analyses first demonstrated that ongoing stressors, new stressors, and combined current stress were each significantly associated with increased likelihood of smoking lapse in the subsequent interval after controlling for prior smoking lapse. Second, single precipitant models were estimated to determine if stress was indirectly associated with subsequent smoking lapse through each precipitant. Each single precipitant model indicated a significant indirect effect of stress on lapse through the precipitant. Single and multiple precipitant models using ongoing stressors alone, new stressors alone, and combined current stress produced substantively similar results. Therefore, only the results of the multiple precipitant model for combined current stress are presented to avoid redundancy. The final multiple precipitant MSEM controlled for the association between previous lapse and precipitants, the passage of time, and the length intervals, and estimated residual covariances among all precipitants.
Multiple sensitivity tests were conducted to examine the robustness of results to different subsets of the sample and to the inclusion of lagged measures of each precipitant. MSEMs employing participants with any post-quit EMA data, and participants with 3, 10, and 20 or more EMAs respectively were estimated and produced substantively identical results. Additionally, a MSEM including measures of each precipitant at time j regressed on measures of each precipitant at time j-1 was estimated to test the robustness of associations between stress and subsequent precipitants after accounting for prior levels of each precipitant. For example, we would expect that participants reporting higher than average smoking urge due to heightened withdrawal symptoms at time j-1 would be more likely to report higher than average smoking urge at time j. Therefore, it is important to examine if stress during interval j-1→j predicts smoking urge at time j while controlling for smoking urge at time j-1. The results of this sensitivity test mirrored those reported in Table 3 and confirmed that stress during interval j-1→j was uniquely associated with precipitants at time j (the a paths in Figure 1/Table 3) above and beyond accounting for measures of precipitants at time j-1.
Table 3.
Estimates, posterior standard deviations (SDs), and 95% credibility intervals (CI) from multilevel structural equation model of stress, mutliple precipitants, and smoking lapse
| Variable | Est. | SDa | p | [95% CI]b | |
|---|---|---|---|---|---|
| Stressi(j-1→j) → Smoking Lapsei(j→j+1) | (c’ ) | −.043 | .029 | .076 | [−.104, .012] |
| Stressi(j-1→j) → Precipitants | (a paths) | ||||
| Negative Affectij | (a1 ) | .199 | .015 | .000 | [.170, .229] * |
| Smoking Urgeij | (a2 ) | .160 | .017 | .000 | [.132, .198] * |
| Abstinence Self-efficacyij | (a3 ) | −.056 | .017 | .000 | [−.089, −.025] * |
| Motivation to Quitij | (a4 ) | −.061 | .013 | .000 | [−.089, −.037] * |
| Difficulty Concentratingij | (a5 ) | .170 | .018 | .000 | [.134, .202] * |
| Coping Expectanciesij | (a6 ) | −.076 | .018 | .000 | [−.109, −.042] * |
| Smoking Expectanciesij | (a7 ) | .108 | .018 | .000 | [.070, .139] * |
| Precipitants → Smoking Lapsei(j→j+1) | (b paths) | ||||
| Negative Affectij | (b1 ) | .248 | .036 | .000 | [.175, .319] * |
| Smoking Urgeij | (b2 ) | .430 | .028 | .000 | [.376, .485] * |
| Abstinence Self-efficacyij | (b3 ) | −.188 | .026 | .000 | [−.239, −.134] * |
| Motivation to Quitij | (b4 ) | −.173 | .036 | .000 | [−.247, −.109] * |
| Difficulty Concentratingij | (b5 ) | .086 | .022 | .000 | [.042, .131] * |
| Coping Expectanciesij | (b6 ) | −.099 | .023 | .000 | [−.143, −.052] * |
| Smoking Expectanciesij | (b7 ) | .114 | .021 | .000 | [.073, .158] * |
| Smoking Lapsei(j-1→j) → Smoking Lapsei(j→j+1) | (ar1 ) | .255 | .015 | .000 | [.228, .284] * |
| Indirect Estimatesc | |||||
| Negative Affectij | (a1 × b1 ) | .047 | .010 | .000 | [.026, .067] * |
| Smoking Urgeij | (a2 × b2 ) | .070 | .011 | .000 | [.050, .094] * |
| Abstinence Self-efficacyij | (a3 × b3 ) | .018 | .007 | .008 | [.003, .032] * |
| Motivation to Quitij | (a4 × b4 ) | .004 | .007 | .280 | [−.011, .018] |
| Difficulty Concentratingij | (a5 × b5 ) | .010 | .008 | .091 | [−.003, .026] |
| Coping Expectanciesij | (a6 × b6 ) | .020 | .006 | .000 | [.008, .033] * |
| Smoking Expectanciesij | (a7 × b7 ) | .016 | .007 | .010 | [.001, .029] * |
Notes. Est. = undstandardized estimate (probit); p = Bayesian one-tailed p -value; i indexes participants (N=370) and j indexes EMAs (N=32,561); paths control for the association between previous lapse and precipitants, the passage of time, and the length of time between intervals
= for positive values, the one-tailed p -value is the the proportion of posterior distribution above 0
= significance
is determined by 95% asymmetric posterior distributions that do not include 0.
= cov(a, b ) added to each indirect estimate.
Results
Table 1 displays participant characteristics. Participants were 42 years old on average were and 56% were female (N=203). For race/ethnicity, 33% identified as White, non-Hispanic, 33% identified as African American/Black, non-Hispanic, 33% identified as Hispanic/Latino, and 2% identified as another race/ethnicity. Participants smoked an average of 21 cigarettes per day prior to the quit attempt and had smoked for an average of 22 years. Table 2 presents descriptive statistics and correlations for stress, smoking lapse, and precipitants.
Table 1.
Participant characteristics
| Variable | Mean (SD) / % |
|---|---|
| Demographics | |
| Age (years) | 41.61 (11.09) |
| Female | 55% |
| Married/Living with Partner | 37% |
| Race/Ethnicity | |
| White, non-Hispanic | 33% |
| Black, non-Hispanic | 33% |
| Hispanic / Latino | 33% |
| Other Racea | 2% |
| Socioeconomics | |
| Education (years) | 12.97 (1.96) |
| Annual Income (< $20,000) | 42% |
| Employed | 58% |
| Health Insurance | 41% |
| Smoking Characteristics (pre-quit) | |
| Cigarettes per day | 20.79 (9.64) |
| Years smoked | 21.58 (11.13) |
| Smoke within 5 minutes of waking | 49% |
Note. N=370
= other race includes participants identifying as Asian American, Native American, Pacific Islander, or Mixed Race.
Table 2.
Descriptive statistics and correlations for time-varying variables
| Variable | M (%) | SD | Min | Max | Correlations |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||||
| Smoking Lapsei(j→j+1) | 7.71% | - | 0 | 1 | 1 | - | .28 | .22 | .30 | −.33 | −.33 | .21 | −.28 | .23 |
| Stressij | 1.70 | .99 | 1 | 4 | 2 | .05 | - | .54 | .21 | −.20 | −.18 | .39 | −.17 | .19 |
| Negative Affectij | 2.10 | .81 | 1 | 5 | 3 | .05 | .24 | - | .46 | −.50 | −.48 | .70 | −.43 | .40 |
| Smoking Urgeij | 2.29 | 1.06 | 1 | 5 | 4 | .15 | .13 | .24 | - | −.45 | −.52 | .69 | −.41 | .58 |
| Abstinence Self-efficacyij | 4.09 | .86 | 1 | 5 | 5 | −.07 | −.08 | −.15 | -.13 | - | .83 | −.46 | .75 | −.46 |
| Motivation to Quitij | 4.29 | .70 | 1 | 5 | 6 | −.09 | −.07 | −.15 | -.21 | .38 | - | −.49 | .79 | −.44 |
| Difficulty Concentratingij | 2.22 | 1.03 | 1 | 5 | 7 | .06 | .14 | .30 | .46 | −.11 | −.15 | - | −.40 | .50 |
| Coping Expectanciesij | 3.92 | .95 | 1 | 5 | 8 | −.08 | −.07 | −.14 | −.14 | .36 | .30 | −.10 | - | −.40 |
| Smoking Expectanciesij | 2.35 | 1.15 | 1 | 5 | 9 | .07 | .07 | .13 | .26 | −.06 | −.11 | .17 | −.06 | - |
Notes. Ni = 370, Nj = 32,563, i indexes study participants, j indexes EMAs; within person values shown below the diagonal represent correlations among momentary deviations from between person averages on each variable; between person values above the diagonal represent correlations among between person means on each variable; all correlations significant at p < .01.
Table 3 provides direct and indirect estimates from the fully-controlled, multiple precipitant model. Estimates for the a paths from stress precipitants and b paths from precipitants to lapse as shown in Table 3 should be interpreted with respect to person-mean centering (Hamaker & Grasman, 2015). Results are, therefore, interpreted such that momentary changes in stress or precipitants are relative to each participant’s average score on that measure throughout the 28-day study period. Results of the multiple precipitant model indicated that combined current stress during interval j-1→j was significantly associated with all precipitants at time j (a paths). Increased stress during interval j-1→j was associated with increased negative affect, smoking urge, difficulty concentrating, and smoking outcome expectancies at time j, and decreased abstinence self-efficacy and coping outcome expectancies at time j. Results of the multiple precipitant model indicated that all precipitants at time j (b paths) were significantly associated with smoking lapse during interval j→j+1. Increased negative affect, smoking urge, difficulty concentrating, and smoking outcome expectancies and decreased abstinence self-efficacy and coping outcome expectancies at time j were associated with increased likelihood of lapse during interval j→j+1. The association between increased stress during interval j-1→j and smoking lapse during interval j→j+1 identified in the initial model did not persist in the fully-controlled, multiple precipitant model. The autoregressive path from smoking lapse during interval j-1→j to smoking lapse during interval j→j+1 remained significant in the fully-controlled, multiple precipitant model. The fully-controlled model also accounted for the association between smoking lapse during interval j-1→j and all precipitants at time j. As expected, smoking lapse during interval j-1→j was significantly associated with all precipitants as time j (these paths are not shown on Figure 1 or Table 3). For example, lapse during j-1→j was associated with increased negative affect, smoking urge, difficulty concentrating, and smoking outcome expectancies, and reduced abstinence self-efficacy, motivation to quit, and coping outcome expectancies at time j. The fully-controlled, multiple precipitant model accounted for 41% of the variance in smoking lapse during interval j→j+1. A preliminary model including only combined stress and smoking lapse during interval j-1→j as a predictor of smoking lapse during interval j→j+1 accounted for 7% of the variance in smoking lapse during interval j→j+1.
Discussion
The current study is among the first to examine the momentary effects of stress on multiple precipitants of smoking lapse in near real-time. Most importantly, the results demonstrated that: 1) stress was broadly associated with a range of known precipitants of smoking lapse including negative affect, smoking urge, abstinence self-efficacy, motivation to quit, difficulty concentrating, coping outcome expectancies, and smoking outcome expectancies; 2) each association was in the theoretically expected direction such that increased stress was related to subsequent measures of each precipitant that increased risk for smoking lapse; 3) increased negative affect, smoking urge, difficulty concentrating, and smoking outcome expectancies, and decreased abstinence self-efficacy, motivation to quit, and coping outcome expectancies were uniquely associated with a higher likelihood of subsequent lapse after accounting for prior lapse; 4) negative affect, smoking urge, abstinence self-efficacy, coping outcome expectancies, and smoking outcome expectancies operated as unique mechanisms linking momentary stress to subsequent smoking lapse. The findings of this study provide support for Witkiewitz and Marlatt’s dynamic model of relapse prevention which hypothesizes a central role for stress in the process of smoking lapse (2004). Additionally, results highlight the broad effect of stress on multiple precipitants of lapse and the unique impact of multiple, theoretically connected precipitants on smoking lapse (Richards et al., 2011; Sinha, 2007). Employing near real-time, EMA measures of stress, precipitants, and lapse in a diverse sample of smokers, this study suggests that there are multiple co-occurring pathways through which stress can increase risk of lapse during a quit attempt.
These findings have important implications for intervention-focused research. First, consistent with current treatment approaches which often recommend cultivating stress management skills, research has shown that cessation attempts are aided by improving distress tolerance and stress management skills prior to and during a quit attempt (Farris, Aston, Leyro, Brown, & Zvolensky, 2018; Volz et al., 2014). As such, cessation programs regularly recommend some form of coping skills training for dealing with stressful situations (Fiore et al., 2008). Recently, therapeutic approaches based on mindfulness have been shown to not only reduce stress and negative affect, but also may weaken the link between stress and lapse (Heppner et al., 2016; Spears et al., 2017). The current findings reinforce the need for these types of approaches by demonstrating the broad impact of stress on precipitants of lapse and the multiple, co-occurring pathways through with stress can influence lapse. Specifically, research testing strategies to improve distress tolerance or stress management skills to enhance smoking cessation programs should investigate the capacity for these strategies to not only manage stress, but also to impact the range of downstream precipitants indirectly connecting stress to lapse. Second, these results may help inform the development and refinement of just-in-time-adaptive-interventions (JITAIs) by identifying real time factors that increase risk for lapse. JITAIs delivered by a smartphone have the potential to provide tailored therapeutic approaches during a stressful experience to mitigate lapse risk (Businelle et al., 2016a; Hébert et al., 2018). Finally, recent human and animal studies suggest that pharmacological interventions may be able to disrupt stress-induced nicotine seeking during a quit attempt (Mantsch et al., 2016). Future research should examine real-time reports or markers of stress in response to various behavioral and pharmacological interventions.
Given the central role of stress in smoking lapse processes identified here, it is important that future studies improve upon identification of the onset, duration, and severity of stressful experiences. The current study employed self-reported measures on the presence of new or ongoing stress captured by randomly initiated EMAs. While stress research has long recognized the meaningful impact of self-reports of stress on health behaviors (Cohen et al., 1983), physiological and behavioral measures of stress can add additional dimensions. Study designs using on-body sensing technology that utilize multiple indicators of stress (e.g., self-report, physiological, and behavioral) are likely to improve data collection precision and foster important future directions for stress-based health behavior research (Ertin et al., 2011; Richards et al., 2011). Additionally, the measures employed by the current study did not assess the source of stress and it is possible that different stressors may differentially influence precipitants or lapse risk (e.g., financial, interpersonal, work, health, withdrawal-related, etc.). Examinations of stress specifically emanating from heightened withdrawal symptoms may be of particular interest for researchers developing stress reduction strategies to promote cessation (Lawless, Harrison, Grandits, Eberly, & Allen, 2015). Overall, detailed and precise measures of stress obtained from both self-reports and on-body sensing in conjunction with information on the sources of stress have the potential to vastly improve health behavior research.
The current study provides evidence that increased stress exacerbates precipitants on the path the lapse. But it is highly likely that additional dynamics among stress and precipitants are operating prior to and during lapse events. Some researchers have suggested that precipitants may operate sequentially to influence smoking lapse. For example, Baker and colleagues (2004) proposed a model of addiction whereby negative affect initiates changes in other precipitants of lapse. From this perspective, changes in negative affect may precede changes in other precipitants temporally on the path to smoking lapse, a hypothesis which has been supported with respect to smoking urge by recent EMA studies (Serre, Fatseas, Denis, Swendsen, & Auriacombe, 2018). The current findings are not inconsistent with the possibility of a sequential chain of events (e.g., stress triggering changes in negative affect which, in turn, triggers changes in smoking urge or other precipitants of lapse), but did not seek to tease out such effects. Additionally, it is possible that multiple precipitants, operating in unison, increase risk for lapse over and above momentary changes in any single precipitant. Research has shown that the additive effect of co-occurring risk factors for lapse increased the likelihood of lapse during a quit attempt beyond the presence of individual risk factors alone (Lam et al., 2014). Further studies should expand upon the stress-lapse model presented here to examine both sequential and additive risk models for smoking lapse.
Motivation to quit and difficulty concentrating were associated with both prior stress and subsequent lapse, but our model did not show either precipitant operating as a unique factor linking stress to lapse. While the model showed significant a and b paths for motivation to quit (i.e., paths a4 and b4 on Figure 1/Table 3) and difficulty concentrating (i.e., paths a5 and b5 on Figure 1/Table 3), the failure to show a unique indirect effect from stress to lapse through these precipitants was due to adding the covariance of a and b paths. The addition of this covariance to the indirect effect represents a conservative approach intended to protect against omitted variables that may influence both a and b paths (Bolger & Laurenceau, 2013; Tofighi, West, & MacKinnon, 2013). Additionally, given that many of the precipitants considered here are correlated and may converge during experiences of heightened withdrawal (Castro et al., 2014; Shiffman et al., 1997; Xian et al., 2005), it is not surprising that unique paths were not identified for each precipitant when accounting for potentially omitted variables. Importantly, all precipitants examined in this model showed links to both stress (a paths) and lapse (b paths) after accounting for prior smoking lapse.
These findings should be considered in light of some important limitations. First, the nature of the EMA assessments yielded detection of a stress and smoking lapse within a time interval between two random assessments as opposed to at an exact moment. Detecting the precise timing of the onset and duration of a stressor in conjunction with the momentary indications of lapse could shed additional light on more fine-grained dynamic processes at work. Second, self-report EMAs of stress and smoking behavior likely fail to capture all instances of increased stress or smoking lapse. Third, given that the measure of stress employed here did not capture the source of stress, it is not possible to rule the influence of heightened withdrawal symptoms on self-reports of new or ongoing stressors. Improved detection of stress and smoking drawn from additional sources including on-body sensing of physiology and behavior can alleviate many of these concerns and provide important data for future research (Ertin et al., 2011). Finally, these data were collected from a sample of relatively heavy smokers (i.e., 21 cigarettes per day on average prior to the quit attempt) and were collected from 2005 to 2007 prior to recent drops in smoking prevalence rates. Caution should be taken in generalizing these findings to light or intermittent smokers.
The current study provides important and novel information concerning the broad impact of stress on precipitants of smoking lapse as well as the near real-time processes linking stress, precipitants of smoking lapse, and lapse. Importantly, this study employed a within person design connecting stress to increased risk of lapse that provides insight on momentary processes as they unfold in real life (Curran & Bauer, 2011). Additionally, the analytic approach employed here allowed within person paths to vary across participants. Assuming fixed slopes for indirect estimates may overestimate the size of effects when testing within person hypotheses using intensive longitudinal data (Bolger & Laurenceau, 2013; Vuorre & Bolger, 2017). Finally, this study was conducted with a racially and ethnically diverse sample which potentially provides more generalizable findings to other populations.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers F32CA232796, R01DA014818, P30CA042014, R00MD010468, and P30CA016672. Additional support was provided by the Huntsman Cancer Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Huntsman Cancer Foundation.
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review, 111(1), 33–51. [DOI] [PubMed] [Google Scholar]
- Bolger N, & Laurenceau JP (2013). Intensive longitudinal methods: An introduction to diary and experience sampling research. New York, NY: Guilford Press. [Google Scholar]
- Bolman C, Verboon P, Thewissen V, Boonen V, Soons K, & Jacobs N (2018). Predicting smoking lapses in the first week of quitting: an ecological momentary assessment study. Journal of addiction medicine, 12(1), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle MS, Ma P, Kendzor DE, Frank SG, Vidrine DJ, & Wetter DW (2016a). An ecological momentary intervention for smoking cessation: evaluation of feasibility and effectiveness. Journal of Medical Internet Research, 18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle MS, Ma P, Kendzor DE, Frank SG, Wetter DW, & Vidrine DJ (2016b). Using intensive longitudinal data collected via mobile phone to detect imminent lapse in smokers undergoing a scheduled quit attempt. Journal of Medical Internet Research, 18(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle MS, Ma P, Kendzor DE, Reitzel LR, Chen MX, Lam CY, … Wetter DW (2014). Predicting Quit Attempts Among Homeless Smokers Seeking Cessation Treatment: An Ecological Momentary Assessment Study. Nicotine & Tobacco Research, 16(10), 1371–1378. doi: 10.1093/ntr/ntu088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro Y, Cano MA, Businelle MS, Correa-Fernandez V, Heppner WL, Mazas CA, & Wetter DW (2014). A cross-lagged path analysis of five intrapersonal determinants of smoking cessation. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 385–396. [PubMed] [Google Scholar]
- Curran PJ, & Bauer DJ (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology, 62, 583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertin E, Stohs N, Kumar S, Raij A, al’Absi M, Kwon T, … Jeong J (2011). AutoSense: Unobtrusively wearable sensor suite for inferencing of onset, causality, and consequences of stress in the field. Paper presented at the Proceedings of the 9th ACM Conference on Embedded Networked Sensor Systems (SenSys). [Google Scholar]
- Farris SG, Aston ER, Leyro TM, Brown LA, & Zvolensky MJ (2018). Distress Intolerance and Smoking Topography in the Context of a Biological Challenge. Nicotine & Tobacco Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Jaen C, & Baker T (2008). Treating Tobacco Use and Dependence: 2008 Update Clinical Practice Guidelines. Retrieved from Rockville, MD: http://www.surgeongeneral.gov/tobacco/default.htm [Google Scholar]
- Gwaltney CJ, Metrik J, Kahler CW, & Shiffman S (2009). Self-efficacy and smoking cessation: a meta-analysis: American Psychological Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, & Paty JA (2005). Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. Journal of Abnormal Psychology, 114(4), 661–675. [DOI] [PubMed] [Google Scholar]
- Hamaker EL, & Grasman RP (2015). To center or not to center? Investigating inertia with a multilevel autoregressive model. Frontiers in psychology, 5, 1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert ET, Stevens EM, Frank SG, Kendzor DE, Wetter DW, Zvolensky MJ, … Businelle MS (2018). An ecological momentary intervention for smoking cessation: The associations of just-in-time, tailored messages with lapse risk factors. Addictive Behaviors, 78, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner WL, Spears CA, Correa-Fernández V, Castro Y, Li Y, Guo B, … Cofta-Woerpel L (2016). Dispositional mindfulness predicts enhanced smoking cessation and smoking lapse recovery. Annals of Behavioral Medicine, 50(3), 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, & Paronis CA (2003). Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull, 129(2), 270–304. [DOI] [PubMed] [Google Scholar]
- Kendzor DE, Costello TJ, Li Y, Vidrine JI, Mazas CA, Reitzel LR, … Wetter DW (2008). Race/ethnicity and multiple cancer risk factors among individuals seeking smoking cessation treatment. Cancer Epidemiology, Biomarkers, & Prevention, 17(11), 2937–2945. doi:17/11/2937 [pii] 10.1158/1055-9965.EPI-07-2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CY, Businelle MS, Aigner CJ, McClure JB, Cofta-Woerpel L, Cinciripini PM, & Wetter DW (2014). Individual and combined effects of multiple high-risk triggers on postcessation smoking urge and lapse. Nicotine Tob Res, 16(5), 569–575. doi: 10.1093/ntr/ntt190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless MH, Harrison KA, Grandits GA, Eberly LE, & Allen SS (2015). Perceived stress and smoking-related behaviors and symptomatology in male and female smokers. Addictive Behaviors, 51, 80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc VR (2009). The effects of acute stress on performance: implications for health professions education. Academic Medicine, 84(10), S25–S33. [DOI] [PubMed] [Google Scholar]
- Maciejewski PK, Prigerson HG, & Mazure CM (2000). Self-efficacy as a mediator between stressful life events and depressive symptoms: Differences based on history of prior depression. The British Journal of Psychiatry, 176(4), 373–378. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, & Pirlott AG (2015). Statistical approaches for enhancing causal interpretation of the M to Y relation in mediation analysis. Personality and Social Psychology Review, 19(1), 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, & Shaham Y (2016). Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology, 41(1), 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt G (1985). Relapse prevention: Theoretical rationale and overview of the model In Marlatt G & Gordon JR (Eds.), Relapse prevention: Maintenance strategies in the treatment of addictive behaviors (pp. 3–70). New York: Guilford Press. [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, & Wanzer J (2011). Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of psychopharmacology, 25(4), 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami H, McCarthy DE, Jorenby DE, & Baker TB (2011). An ecological momentary assessment analysis of relations among coping, affect and smoking during a quit attempt. Addiction, 106(3), 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, & Asparouhov T (2012). Bayesian structural equation modeling: A more flexible representation of substantive theory. Psychological Methods, 17(3), 313. [DOI] [PubMed] [Google Scholar]
- Muthen L, & Muthen B (2017). Mplus. Los Angeles: Muthen & Muthen. [Google Scholar]
- Ng DM, & Jeffery RW (2003). Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychology, 22(6), 638. [DOI] [PubMed] [Google Scholar]
- Oberleitner L, Moore KE, Verplaetse T, Roberts W, & McKee SA (2018). Developing a laboratory model of smoking lapse targeting stress and brief nicotine deprivation. Experimental and Clinical Psychopharmacology, 26(3), 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Zhang Z, & Zyphur MJ (2011). Alternative methods for assessing mediation in multilevel data: The advantages of multilevel SEM. Structural Equation Modeling, 18(2), 161–182. [Google Scholar]
- Preacher KJ, Zyphur MJ, & Zhang Z (2010). A general multilevel SEM framework for assessing multilevel mediation. Psychological Methods, 15(3), 209–233. doi: 10.1037/a0020141 [DOI] [PubMed] [Google Scholar]
- Richards JM, Stipelman BA, Bornovalova MA, Daughters SB, Sinha R, & Lejuez C (2011). Biological mechanisms underlying the relationship between stress and smoking: state of the science and directions for future work. Biological Psychology, 88(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Denis C, Swendsen J, & Auriacombe M (2018). Predictors of craving and substance use among patients with alcohol, tobacco, cannabis or opiate addictions: Commonalities and specificities across substances. Addictive Behaviors, 83, 123–129. [DOI] [PubMed] [Google Scholar]
- Shadel WG, & Mermelstein RJ (1993). Cigarette smoking under stress: The role of coping expectancies among smokers in a clinic-based smoking cessation program. Health Psychology, 12(6), 443. [DOI] [PubMed] [Google Scholar]
- Shiffman S (2006). Reflections on smoking relapse research. Drug and Alcohol Review, 25(1), 15–20. [DOI] [PubMed] [Google Scholar]
- Shiffman S (2009). Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment, 21(4), 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Richards T, & Kassel JD (1997). Individual differences in the context of smoking lapse episodes. Addictive Behaviors, 22(6), 797–811. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, & Hickcox M (1996). First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology, 64(2), 366–379. [DOI] [PubMed] [Google Scholar]
- Shiffman S, & Waters AJ (2004). Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol, 72(2), 192–201. [DOI] [PubMed] [Google Scholar]
- Sinha R (2007). The role of stress in addiction relapse. Current psychiatry reports, 9(5), 388–395. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences, 1141(1), 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears CA, Hedeker D, Li L, Wu C, Anderson NK, Houchins SC, … Cinciripini PM (2017). Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. Journal of Consulting and Clinical Psychology, 85(11), 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi D, West SG, & MacKinnon DP (2013). Multilevel mediation analysis: The effects of omitted variables in the 1–1–1 model. British Journal of Mathematical and Statistical Psychology, 66(2), 290–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. (2014). The Health Consequences of Smoking-50 Years of Progress A Report of the Surgeon General. Retrieved from Atlanta, CA: [Google Scholar]
- Vangeli E, Stapleton J, Smit ES, Borland R, & West R (2011). Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction, 106(12), 2110–2121. [DOI] [PubMed] [Google Scholar]
- Volz AR, Dennis PA, Dennis MF, Calhoun PS, Wilson SM, & Beckham JC (2014). The role of daily hassles and distress tolerance in predicting cigarette craving during a quit attempt. Nicotine & Tobacco Research, 16(6), 872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorre M, & Bolger N (2017). Within-subject mediation analysis for experimental data in cognitive psychology and neuroscience. Behav Res Methods, 1–19. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, & Marlatt GA (2004). Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am Psychol, 59(4), 224–235. doi: 10.1037/0003-066X.59.4.224 [DOI] [PubMed] [Google Scholar]
- Wu P, Wilson K, Dimoulas P, & Mills EJ (2006). Effectiveness of smoking cessation therapies: a systematic review and meta-analysis. BMC Public Health, 6(1), 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Madden PA, Lyons MJ, Tsuang M, True WR, & Eisen SA (2005). Latent class typology of nicotine withdrawal: genetic contributions and association with failed smoking cessation and psychiatric disorders. Psychological Medicine, 35(3), 409–419. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, & West R (2009). Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addictive Behaviors, 34(4), 365–373. [DOI] [PubMed] [Google Scholar]