Highlights
-
•
Electroconvulsive therapy (ECT) induced enlargement in hippocampus volume contrast to pharmaceutical therapy in schizophrenia.
-
•
Both ECT responders and non-responders show hippocampal volume expansion.
-
•
Increased FC between hippocampus and brain cognitive networks only in ECT responders.
Keywords: Electroconvulsive therapy, Schizophrenia, Hippocampus, MRI, Functional connectivity, Resting-state fMRI
Abstract
Electroconvulsive therapy (ECT) is considered a treatment option in patients with drug-resistant schizophrenia (SZ). However, approximately one-third of patients do not benefit from ECT in the clinic. Thus, it is critical to investigate differences between ECT responders and non-responders. Accumulated evidence has indicated that one region of ECT action is the hippocampus, which also plays an important role in SZ pathophysiology. To date, no studies have investigated differences in ECT effects in the hippocampus between treatment responders and non-responders. This study recruited twenty-one SZ patients treated for four weeks with ECT (MSZ, n = 21) and twenty-one SZ patients who received pharmaceutical therapy (DSZ, n = 21). The MSZ group was further categorized into responders (MSR, n = 10) or non-responders (MNR, n = 11) based on treatment outcomes by the criterion of a 50% reduction in the Positive and Negative Syndrome Scale total scores. Using structural and resting-state functional MRI, we measured the hippocampal volume and functional connectivity (FC) in all SZ patients (before and after treatment) and 23 healthy controls. In contrast to pharmaceutical therapy, ECT induced bilateral hippocampal volume increases in the MSZ. Both the MSR and MNR exhibited hippocampal expansion after ECT, whereas a lower baseline volume in one of hippocampal subfield (hippocampus-amygdala transition area) was found in the MNR. After ECT, increased FC between the hippocampus and brain networks associated with cognitive function was only observed in the MSR. The mechanism of action of ECT in schizophrenia is complex. A combination of baseline impairment level, ECT-introduced morphological changes and post-ECT FC increases in the hippocampus may jointly contribute to the post-ECT symptom improvements in patients with SZ.
1. Introduction
Schizophrenia (SZ) is a serious mental disorder mainly characterized by multidimensional psychotic syndrome, such as positive and negative symptoms, as well as cognitive and affective impairments (van der Meer et al., 2010). In SZ, electroconvulsive therapy (ECT) is considered a treatment option, particularly in patients with drug-resistant symptoms or to resolve acute symptoms (Pompili et al., 2013). Although ECT has been a conventional technique in clinical treatment, its mechanisms of action on the brain have not been fully clarified.
Since ECT was introduced to clinical practice, several hypotheses, including monoamine neurotransmitter, neuroendocrine and anticonvulsant theories (Kellner et al., 2012), have been provided to interpret possible mechanisms of action. ECT-related neuroplasticity is one of the potential mechanisms, especially neuroplasticity occurring in the hippocampus (Bouckaert et al., 2016a; Oltedal et al., 2017; Redlich et al., 2016). Several studies found reproducible results that electroconvulsive shock induced neurogenesis in the dentate gyrus of the hippocampus in an animal model (Madsen et al., 2000; Perera et al., 2007). In humans, accumulated evidence has also indicated that ECT induced volume increases in the hippocampus (Abbott et al., 2014; Bouckaert et al., 2016a; Cano et al., 2017). More recently, Takamiy and colleagues systematically reviewed MRI studies investigating structural changes due to ECT in patients with depression and quantitatively analysed whether ECT induced hippocampal and other brain region structural changes through a meta-analytic approach (Takamiya et al., 2018). They found that both right and left hippocampal and amygdalar volumes increased after ECT. In addition, our previous study applied a data-driven method (voxel-based morphometry, VBM) to detect ECT-induced grey-matter alterations across the whole brain and found significant grey matter increases within the hippocampus (Wang et al., 2019). Although some studies have also reported ECT-induced changes in other regions, such as the striatum (Wade et al., 2016), cingulate cortex (Argyelan et al., 2016) and insula (Bouckaert et al., 2016a), the current hypothesis-driven study focused on ECT-induced hippocampal plasticity, compared hippocampal volume and FC between ECT responders and non-responders to link ECT-related hippocampal plasticity with clinical outcomes in SZ.
The hippocampus anatomically connects brain regions that mediate emotional and cognitive regulation, which supports the hippocampus as having a key role in SZ-related circuitry (Chen et al., 2017; Price and Drevets, 2010). In addition, structural and functional disruptions in the hippocampus have been implicated in the pathophysiology of SZ. On the one hand, hippocampal volume reduction has been widely reported in previous SZ studies (Adriano et al., 2012; Dietsche et al., 2017; Jiang et al., 2018b). However, several structural MRI studies have reported contrasting results with no differences in hippocampal volume between SZ and healthy subjects (Adriano et al., 2012; Niemann et al., 2000). These inconsistencies might be explained by illness duration or treatment strategy (Adriano et al., 2012). As there are these inconsistent results regarding the hippocampal volume reductions in SZ, it is meaningful for clinical research to clarify the relationship between the levels of hippocampal volume reduction and clinical responses to ECT. A substantial number of studies have indicated that there are FC alterations in the hippocampus in patients with SZ (Kraguljac et al., 2014; Schmitt et al., 2011). Moreover, studies have found associations between hippocampal FC changes and clinical symptoms in SZ patients (Duan et al., 2015a; Kraguljac et al., 2016). These findings not only suggest that hippocampal FC is highly relevant to the symptoms and pathobiology of SZ but also imply that clinical treatment outcomes (e.g., symptom remission) may be directly reflected in FC changes. However, to date, no study has investigated whether ECT causes differential FC changes in the hippocampus between responders and non-responders and assessed the relationship between FC changes and symptom remission.
Clinically, approximately one-third of patients do not benefit from ECT (Lally et al., 2016); thus, it is of high clinical significance to investigate differences between ECT responders and non-responders. Taking into account these issues, in this observational study, which was designed in a similar manner as a previous study (Redlich et al., 2016), we systematically assessed ECT effects on hippocampal structure and function in patients with SZ. Our specific aims were to clarify (1) whether the hippocampal volume changes induced by ECT were different than those treated with drug treatment alone. We hypothesized that the hippocampal volume would be increased after ECT treatment but would not change after drug treatment alone; (2) whether the hippocampal volume increase is an ECT effect common to both clinical responders and non-responders. We hypothesized that both responders and non-responders would show increased hippocampal volume after ECT; (3) whether the hippocampal FC changes are ECT effects that are specific to either the responders or non-responders. We hypothesized that ECT would increase hippocampal FC in the responders but decreased FC in the non-responders; and (4) whether there were hippocampal volume and FC differences between ECT responders and non-responders at baseline.
2. Materials and methods
2.1. Participants
In the present study, forty-two patients with acute SZ were divided into two groups according to treatment strategy. One group received a four-week ECT series in addition to antipsychotic drugs (MSZ group, n = 21); the other group received only antipsychotic drugs (DSZ group, n = 21). All inpatients were recruited from the Shanghai Mental Health Centre (SMHC) from October 2013 to January 2015. The patients were diagnosed with SZ by trained clinical psychiatrists using the SCID-I/P (Structural Clinical Interview for DSM-IV-TR, Patient edition) and met the indications for ECT. In addition, the patients had no history of ECT within the previous six months. Psychiatric symptom severity was assessed by the Positive and Negative Syndrome Scale (PANSS), and the total PANSS scores of all patients were greater than 60. All patients received antipsychotic medications, and the daily antipsychotic medication dosage was converted to chlorpromazine equivalents (mg/d) (Andreasen et al., 2010). Additional details regarding the antipsychotic medication for each patient are provided in Supplementary Information 1. A sample of healthy controls (HC, n = 23), which was matched to the patient groups by age, sex and education level (Table 1), was also recruited from the faculty in SMHC. All healthy controls did not have a lifetime psychiatric disorder or family history of psychosis in their first-degree relatives. Potential participants were excluded if they had brain injuries, organic mental disorders, neurological abnormalities, other serious physical illnesses, dementia, substance abuse or dependence, or contraindications to MRI. The Ethics Committee of SMHC approved the study protocol. Written informed consent was obtained from all subjects prior to study participation.
Table 1.
Demographic and clinical data of participants.
| Characteristic | MSZ: Mean (SD) | DSZ: Mean (SD) | HC (n = 23) | ||||
|---|---|---|---|---|---|---|---|
| MSR (n = 10) | MNR (n = 11) | P valuea | DR (n = 12) | DNR (n = 9) | P valuea | Mean (SD) | |
| Gender (M/F) | 5/5 | 5/6 | 0.835b | 3/9 | 6/3 | 0.056b | 11/12 |
| Age (years) | 30.4(7.7) | 28.1(6.7) | 0.472 | 31.0(6.6) | 30.2(9.5) | 0.827 | 31.2(5.9) |
| Education (years) | 13.8(3.5) | 10.9(2.9) | 0.052 | 12.9(3.3) | 12.1(2.5) | 0.546 | 13.5(2.5) |
| Handness (left/right) | 0/10 | 0/11 | – | 0/12 | 0/9 | – | 0/23 |
| Chinese Han nationality | 10 | 11 | – | 12 | 9 | – | 23 |
| Married/unmarried/divorced | 2/7/1 | 3/8/0 | – | 4/7/1 | 1/7/1 | – | 13/10/0 |
| Smoking/nonsmoking | 2/8 | 1/10 | – | 2/10 | 1/8 | – | 7/16 |
| Drinking/nondrinking | 0/10 | 0/11 | – | 0/12 | 0/9 | – | 3/20 |
| Family history of schizophrenia (yes/no) | 3/7 | 5/6 | – | 4/8 | 2/7 | – | 0/23 |
| Illness duration (months) | 70.3(53.8) | 88.4(56.1) | 0.462 | 51.3(76.7) | 115.3(75.0) | 0.071 | – |
| Chlopromazine equivalents (mg/d) | 333.8(218.5) | 850.8(675.9) | 0.032 | 406.2(420.5) | 701.1(482.4) | 0.151 | – |
| Baseline PANSS score | |||||||
| Total | 70.4(6.4) | 73.4(10.1) | 0.435 | 68.8(7.7) | 73.3(11.8) | 0.303 | – |
| Positive | 21.8(1.9) | 19.6(2.7) | 0.051 | 18.4(3.6) | 20.0(3.4) | 0.322 | – |
| Negative | 17.1(6.3) | 21.4(8.0) | 0.196 | 17.3(3.8) | 17.6(6.8) | 0.925 | – |
| General | 31.5(3.7) | 32.4(4.0) | 0.612 | 33.0(4.4) | 35.8(7.0) | 0.277 | – |
| 4-weeks PANSS score | |||||||
| Total | 41.6(5.9) | 57.1(5.2) | <0.001 | 42.0(5.3) | 61.9(10.3) | <0.001 | – |
| Positive | 9.3(1.9) | 12.3(3.1) | 0.017 | 8.5(1.4) | 16.6(3.4) | <0.001 | – |
| Negative | 10.8(3.4) | 18.0(6.1) | 0.004 | 12.6(4.0) | 16.0(6.4) | 0.152 | – |
| General | 21.5(2.0) | 26.8(1.9) | <0.001 | 20.9(1.7) | 29.2(4.9) | <0.001 | – |
Abbreviations: MSZ, schizophrenia patients treated by ECT; DSZ, schizophrenia patients treated by antipsychotic drugs; MSR, schizophrenia patients with symptom remission after ECT; MNR, schizophrenia patients without symptom remission after ECT; DR, schizophrenia patients with symptom remission after antipsychotic medications; DNR, schizophrenia patients without symptom remission after antipsychotic medications; HC, healthy controls; PANSS, Positive and Negative Syndrome Scale.
P values were obtained from the two sample t-test except where noted.
P values were obtained using the chi-square test.
2.2. Electroconvulsive therapy
Using a therapeutic apparatus Thymatron System IV (Somatics, Lake Bluff, IL, USA), ECT was administered 3 times weekly for 4 weeks and 12 sessions. Before ECT, succinylcholine chloride (1.0 mg/kg) was used to relax muscles, atropine (0.5 mg) was applied to reduce airway secretion, etomidate (0.21–0.3 mg/kg) and propofol (1.82–2.44 mg/kg) were conducted to keep anaesthesia. Two electrodes were placed at bilateral temporal scalps. The main parameters of ECT were similar for all patients (frequency, 10–70 Hz; maximum charge delivered, 504 mC; output current, 0.9 A; pulse width, 1.0 ms; maximum stimulus duration, 8 s). During ECT, we monitored motor convulsions and induced tachycardia, and also recorded electroencephalogram and electromyogram (when necessary). The antipsychotic therapy remained stable during ECT period except for the discontinuation of pharmacotherapy in the morning before ECT.
2.3. Symptom assessments
At pre- and post-treatment, we measured the positive (PANSS-P), negative (PANSS-N), general psychopathology (PANSS-G) subscales and total scores (PANSS-T). The PANSS reductive ratio was defined as percentage PANSS changes as: △PANSS-T% = (PANSS-Tt1−PANSS-Tt2) × 100 /(PANSS-Tt1−30). PANSS-Tt1−30 was used as baseline value instead of PANSS-Tt1 as 30 was the lowest possible value for PANSS total score (Hasan et al., 2017). Similarly, △PANSS-P%, △PANSS-N%, △PANSS-G% were also calculated. After ECT, the MSZ group was further classified as the ECT responder (MSR group, n = 10) and non-responder (MNR group, n = 11) groups, with the criterion of less than 50% individual symptom relief for non-response according to the PANSS total reductive ratio (Boter et al., 2009). Similarly, the DSZ group was also grouped to the drug responder (DR, n = 12) and non-responder groups (DNR, n = 9). The responders and non-responders did not differ in terms of gender, age, education, illness duration and baseline PANSS scores (Table 1).
2.4. Data acquisition
Whole-brain imaging data were acquired using a 3-T Siemens Magnetom veriosyngo MR B17 scanner. Functional MRI data were obtained by a gradient echo planar imaging (EPI) sequence (TR 2000 ms; TE, 30 ms; flip angle, 90°; FOV, 220 mm × 220 mm; matrix, 64 × 64; slice thickness, 4 mm; 30 slices; voxel size, 3.4 × 3.4 × 3.4 mm; 180 vol). In addition, high-resolution T1-weighted structural images were collected using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence (TR, 2530 ms, TE, 2.56 ms, flip angle, 7°, inversion time, 1100 ms, FOV, 256 mm × 256 mm, matrix, 256 × 256, 224 slices, slice thickness, 1 mm; voxel size, 1.0 × 1.0 × 1.0 mm). The patients were scanned twice before (baseline) and after 4-week therapy. The HC underwent scanning only at baseline. The first MRI scan of patients was obtained within 24 h before the first ECT, and the final MRI scan was collected 24–48 h after the last session of ECT. Participants were instructed to keep their eyes closed, relax, but not to fall sleep.
2.5. Data processing
2.5.1. Hippocampal structural analysis
A longitudinal segmentation of hippocampal substructures was performed using FreeSurfer (version 6.0, http://surfer.nmr.mgh.harvard.edu/). Specifically, a longitudinal processing stream (Reuter et al., 2012) including skull stripping, tissue segmentation, surface reconstruction, registration and parcellation, was applied to all subjects' T1 images in Freesurfer. This stream created an unbiased within-subject template space by using robust, inverse consistent registration across scanning images (Reuter and Fischl, 2011). A longitudinal segmentation of hippocampal subfields tool in Freesurfer was also applied to segmented hippocampal regions to obtain twelve hippocampal subfields for each hemisphere (Iglesias et al., 2016). The longitudinal algorithm uses a subject-specific atlas and treats all scanning time points the same way to avoid processing bias, and thus increases the robustness of segmentation (Iglesias et al., 2016). Whole hippocampal volume and twelve subfields volumes were measured for each hemisphere, including the hippocampal tail, subiculum, presubiculum, parasubiculum, cornu ammonis area 1 (CA1), CA3, CA4, hippocampal fissure, granular cells layer of the dentate gyrus (GC-ML-DG), molecular layer, hippocampus-amygdala transition area (HATA) and fimbria. Illustrations of hippocampal subfield segmentation for each subject are provided in Supplementary Information 2.
2.5.2. Hippocampal resting-state functional connectivity analysis
Resting-state fMRI processing was performed in SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and DPABI (http://rfmri.org/dpabi) software. The fMRI data preprocessing pipeline was similar with previous study (Gong et al., 2019; Huang et al., 2018; Jiang et al., 2018a) and was only briefly described here. Firstly, the first 10 time points were removed for signal equilibrium and to allow the subjects’ adaptation to the scanning noise; secondly, we did the slice-timing correction, realignment correction, normalization and resampling to 3 × 3 × 3 mm3; thirdly, the nuisance covariates including 24 motion parameters, white matter and cerebrospinal fluid signals and linear trending were regressed out; then, we performed temporally scrubbing (Power et al., 2015) and temporal filtering (0.01–0.1 Hz); finally, the data was smoothed (FWHM= 6 mm). Following the preprocessing, seed-based FC analysis was used to evaluate the hippocampal FC. Four regions of bilateral rostral hippocampus (RosHIP) and caudal hippocampus (CauHIP) were defined as the seed ROIs according to the Human Brainnetome Atlas (Fan et al., 2016). Pearson's correlation coefficients between the time series of each seed and that of the other voxels in the whole brain were calculated and then Fisher's z-transformed to z-scores. For each subject and each seed, a FC z-score map was obtained and used for following statistical analysis.
2.6. Statistical analysis
2.6.1. Hippocampal volume comparisons
To determine whether the hippocampal volume changes were a specific effect induced by ECT rather than drug, repeated measured two-way ANOVA was performed on the volumes of the whole hippocampus and each subfield with the between-subject factor (treatment strategy: MSZ vs. DSZ) and the within-subject factor (time: t1 vs. t2). Post-hoc paired t-tests were performed in the MSZ and DSZ groups to indentify longitudinal changes after controlling for the overall intracranial volume.
To further examine whether hippocampal volume changes were different between the ECT responders and non-responders, repeated measured two-way ANOVA was conducted with the between-subject factor (MSR vs. MNR) and the within-subject factor (time: t1 vs. t2). Two post-hoc paired t-tests were separately performed in the MSR and MNR groups to characterize the longitudinal changes in hippocampal volume after the ECT when controlling the effect of the overall intracranial volume.
Additionally, ANOVA and post-hoc tests were used to compare the baseline differences among the MSR, MNR and HC groups.
2.6.2. Hippocampal FC comparisons
To verify the hypothesis that ECT induced specific changes in hippocampal FC in the ECT responders, repeated measured two-way ANOVA was conducted with the between-subject factor (outcome: MSR vs. MNR) and the within-subject factor (time: t1 vs. t2); the interaction effect of outcome and time was used to investigate the specific changes observed in the ECT responders. Two post-hoc paired t-tests were separately performed in the MSR and MNR groups to detect the longitudinal changes in hippocampal FC after the ECT. In addition, ANOVA and post-hoc tests were applied to the comparisons amongststststst the MSR, MNR and HC groups at the baseline. For these analyses on the voxel-wise FC z-score maps, a multiple comparison correction was performed using a height threshold (z > 2.7) for individual voxels and a cluster size based on the Gaussian random field theory, which corresponds to p < 0.05 after correction (Huang et al., 2018).
2.7. . Relationship between hippocampal changes and symptom improvements
The hippocampal volume and FC values that exhibited significant longitudinal changes were extracted. The hippocampal volume change was defined as the difference in volume (Volumet2 - Volumet1). Similarly, the FC changes were computed as difference values (FCt2 - FCt1). As these values did not conform to a normal distribution according to the Kolmogorov-Smirnov tests, Spearman rank correlations were used to assess the relationships between the hippocampal volume or FC changes and the reductive ratios of the symptoms (△PANSS-P%, △PANSS-N%, △PANSS-G%, and △PANSS-T%) in the MSR group.
2.8. Power analysis on the longitudinal changes in hippocampal volume and FC
Finally, to estimate the statistical power for these longitudinal changes in hippocampal volume and functional connectivity in the MSR and MNR groups, power analyses were conducted using the G*Power 3.1.9.2 (http://www.gpower.hhu.de/).
2.9. Additional analysis
2.9.1. Baseline comparisons between MSZ and DSZ
To investigate the differences in hippocampal volume and FC between the MSZ and DSZ groups at baseline, we performed two-sample t-tests to compare the differences between the MSZ and DSZ in the volume of each hippocampal subfield and FC.
2.9.2. Comparisons between responders and non-responders
In addition, we divided all the SZ patients into responders (SZR group: N = 22; 14 female; 30.72±7.38 years of age) and non-responders (SZNR group: N = 20; 9 female; 29.05±7.24 years of age) to further compare the baseline volume and FC across groups and the group-by-time interactions using repeated measured ANOVA.
2.9.3. FC changes in the DR and DNR groups
To further investigate the FC changes due to pharmacological treatment, paired t-tests were used to separately compare the differences between t1 and t2 in the DR and DNR groups.
3. Results
3.1. Hippocampal volume changes
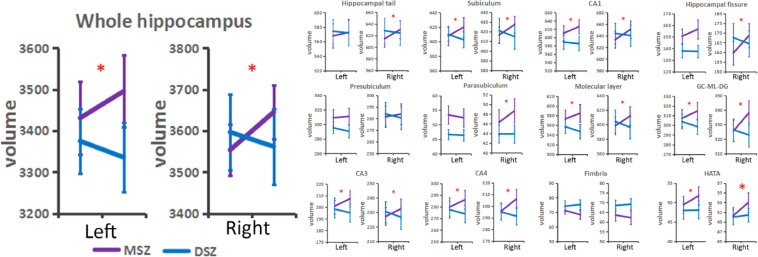
Repeated measured ANOVA showed a significant interaction effect between the treatment strategy (MSZ vs. DSZ) and the time (t1 vs. t2) in bilateral hippocampal volumes (left hippocampus, F = 12.76, p<0.001; right hippocampus, F = 23.70, p<0.001) and certain subfields (Fig. 1). Post-hoc paired t-test analysis showed significant increases in bilateral whole hippocampal volumes in the MSZ group when controlling for the effect of overall intracranial volume (left, t = 3.97, p<0.001; right, t = 4.53, p<0.001). However, a volume increase was not observed in the DSZ group. Details of changes in all hippocampal subfields are shown in Supplementary Information 3.
Fig. 1.
Different changes of hippocampus volume between MSZ group and DSZ group. * represents that repeated measured ANOVA showed a significant interaction effect between the treatment strategy (MSZ vs. DSZ) and the time (t1 vs. t2) in bilateral hippocampal whole volumes and certain subfields. Abbreviation: MSZ, schizophrenia patients treated by ECT; DSZ, schizophrenia patients treated by antipsychotic drugs; CA, cornu ammonis area; GC-ML-DG, granular cells layer of the dentate gyrus; HATA, hippocampus-amygdala transition area.
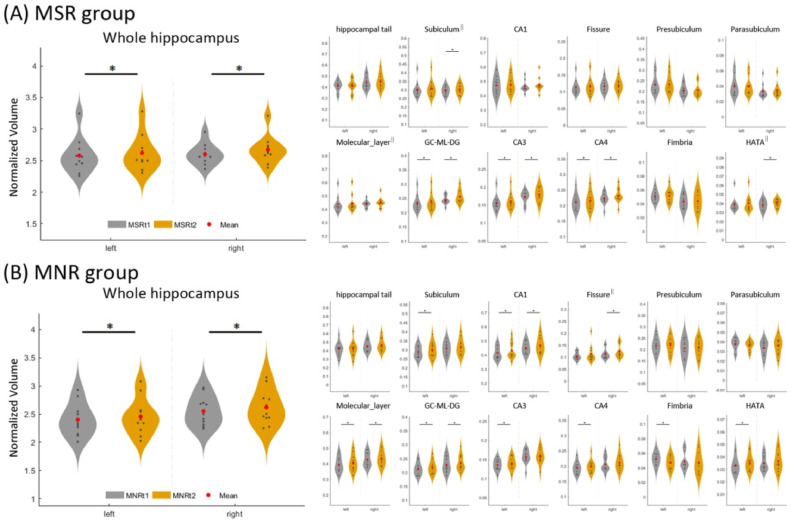
According to their remission status, patients in the MSZ group were divided into MSR and MNR groups. Repeated measured ANOVA (Group effect: MSR vs. MNR; Time effect: t2 vs. t1) showed a significant time effect in bilateral hippocampal volumes (left, F = 17.65, p<0.001; right, F = 29.57, p<0.001). Further paired t-tests showed that ECT induced significant volume increases in the bilateral hippocampus and certain subfields for both the MSR group and MNR group even after controlling for the overall intracranial volume (Fig. 2 and Supplementary Information 4). Moreover, a power analysis exhibited a high statistical power (> 0.8) for these longitudinal changes (Supplementary Information 5).
Fig. 2.
Longitudinal changes of hippocampus and subfields volumes in MSR and MNR after ECT. * represents that paired t-tests indicate a significant increased volume in bilateral hippocampus and certain subfields in the MSR group and MNR group. Abbreviation: MSR, schizophrenia patients with symptom remission after ECT; MNR, schizophrenia patients without symptom remission after ECT; CA, cornu ammonis area; GC-ML-DG, granular cells layer of the dentate gyrus; HATA, hippocampus-amygdala transition area. The || represents that outliers (>mean±2*SD) dots were removed when performing the statistical analyses.
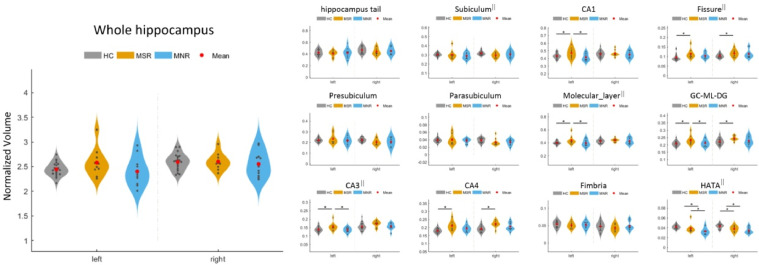
Baseline comparisons showed significant group differences amongststststst the MSR, MNR and HC groups in the left CA1, left CA3, left molecular_layer, bilateral fissure, GC-ML-DG, CA4 and HATA (Fig. 3 and Supplementary Information 6). However, after multiple comparison corrections by Bonferroni correction, only the bilateral HATA (left, F = 8.26, p<0.001; right, F = 10.88, p<0.001) and CA4 (left, F = 9.19, p<0.001; right, F = 8.23, p = 0.001) remained significant. Post-hoc tests showed that in the left HATA, the MNR group had lower volume than the MSR and HC groups; in the right HATA, both the MNR and MSR groups showed lower volumes than the HC group; in the bilateral CA4, the MSR group showed higher volumes than the HC group (Fig. 3 and Supplementary Information 6).
Fig. 3.
Baseline comparisons in hippocampal and subfields volume among MSR, MNR and HC. * represents that ANOVA and post-hoc tests indicate a significant difference among the three groups. Abbreviation: MSR, schizophrenia patients with symptom remission after ECT; MNR, schizophrenia patients without symptom remission after ECT; CA, cornu ammonis area; GC-ML-DG, granular cells layer of the dentate gyrus; HATA, hippocampus-amygdala transition area. The || represents that outliers (>mean±2*SD) dots were removed when performing the statistical analyses.
3.2. Hippocampal FC changes
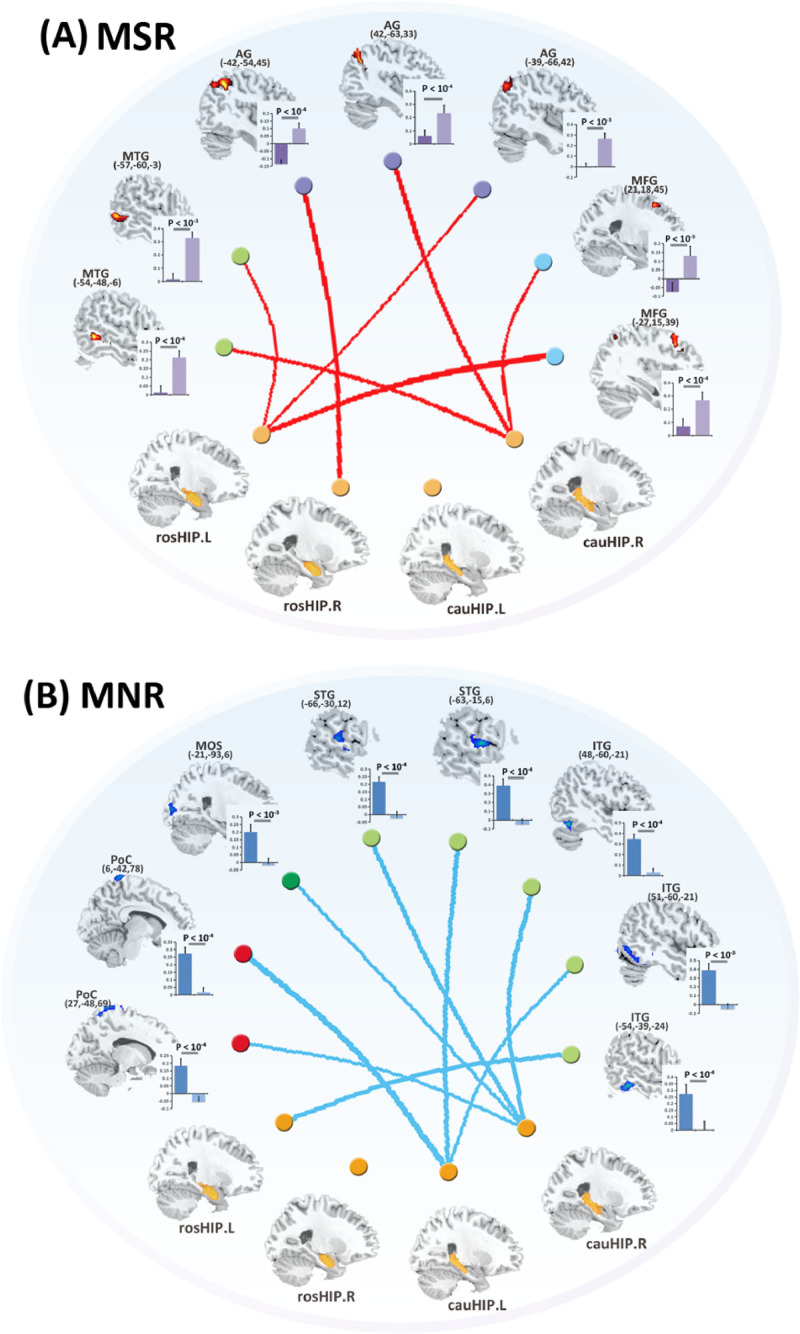
Paired t-tests showed that after ECT, the MSR exhibited significantly increased FC between the hippocampus and prefrontal cortex as well as between the hippocampus and regions in default mode network (DMN). No decreases in FC were observed in the MSR group. However, in the MNR group, the post-ECT patients showed decreased FC in the hippocampus and primary sensory network. No increases in FC were observed in the MNR group. Detailed information is provided in Fig. 4 and Supplementary Information 7. Moreover, a power analysis exhibited a high statistical power (> 0.8) for these longitudinal changes (Supplementary Information 5).
Fig. 4.
Longitudinal FC changes between pre-ECT and post-ECT in MSR group and MNR group.
Abbreviations: MSR, schizophrenia patients with symptom remission after ECT; MNR, schizophrenia patients without symptom remission after ECT; RosHIP, rostral hippocampus; CauHIP, caudal hippocampus; MTG, middle temporal gyrus; MFG, middle frontal gyrus; AG, angular gyrus; ITG, inferior temporal gyrus; STG, superior temporal gyrus; PoC, postcentral gyrus; MOS, middle occipital cortex.
Consistent with the above findings, significant interaction effects between the outcomes (MSR vs. MNR) and time (t1 vs. t2) were observed for FC between the hippocampus and DMN as well as between the hippocampus and primary sensory network. Detailed information is shown in Supplementary Information 8.
In addition, baseline comparisons among the MSR, MNR and HC groups found significant group differences in FC between the left cauHIP and the right middle frontal gyrus (MFG), between the right cauHIP and the right MFG, and between the left rosHIP and right putamen (Supplementary Information 9).
3.3. Relationship between hippocampal changes and symptom improvements
In the MSR group, a significant association was observed between the left CA4 increase and the general psychopathology reduction ratio (Rho = 0.697, p = 0.025). In addition, a significant correlation was observed between the change in FC (left cauHIP and right angular gyrus) and the general psychopathology reduction ratio (Rho = 0.721, p = 0.019).
3.4. Additional analysis results
3.4.1. Baseline comparisons between MSZ and DSZ
The baseline comparisons between the MSZ and DSZ groups showed that there was no significant difference between the two patient groups in bilateral hippocampal volumes. The MSZ group exhibited higher FC between the bilateral superior temporal gyrus and bilateral cauHIP than the DSZ group. Details of baseline comparisons between the MSZ and DSZ groups are shown in Supplementary Information 10.
3.4.2. Comparisons between responders and non-responders
By dividing all the SZ patients into SZR and SZNR, we found that at baseline, the SZR group had higher volumes in the left CA1, left molecular layer, left GC-ML-DG, left CA3 and left CA4 than the SZNR group. Post-hoc paired t-tests showed that in the SZR group, there were increased volumes in the right hippocampus and subfields after treatment. Details of baseline comparisons between the SZR and SZNR groups and longitudinal changes between t1 and t2 are shown in Supplementary Information 11.
3.4.3. FC changes in the DR and DNR groups
Paired t-tests showed that the DR group had increased FC between the left rosHIP and right insula as well as between the left rosHIP and right inferior frontal gyrus (IFG) after pharmacological treatment. However, the DNR group exhibited decreased FC between the left rosHIP and right middle frontal gyrus (MFG) and between the left cauHIP and right MFG after pharmacological treatment. Detailed information on FC changes in the DR and DNR groups is shown in Supplementary Information 12.
4. Discussion
To our knowledge, this is the first study investigating hippocampal volume and FC changes between ECT responders and non-responders in SZ. As expected, four main findings are as follows: (1) Hippocampal volume increases were only observed in patients with SZ treated by ECT. This suggested that the hippocampal volume increase was a specific ECT effect rather than a drug effect. (2) Both the MSR and MNR groups exhibited increased hippocampal volume following ECT, which further indicated that hippocampal volume increases were an inherent effect of ECT. (3) Interestingly, in the MSR group, we found increased FC between the hippocampus and higher-order cognitive networks; however, in the MNR group, we observed reduced FC between the hippocampus and primary sensory networks, including visual, sensorimotor and auditory networks. These findings suggested that ECT-induced changes in hippocampal FC with higher-order cognitive networks might be associated with clinical symptoms. (4) Finally, baseline comparisons showed that compared with the HC group, the MNR group had lower baseline volume in the HATA and higher FC between the hippocampus and putamen, which contributed to the prediction for ECT treatment outcomes.
Our investigation corroborated previous findings of hippocampal volume increases following ECT in psychiatric disorders (Abbott et al., 2014; Bouckaert et al., 2016a, 2016b; Nordanskog et al., 2010, 2014; Thomann et al., 2017) and further compared the differences between ECT and drug-only samples. Our study revealed increased volumes in the bilateral hippocampus only in the ECT sample, whereas evidence for such structural changes was absent in the drug-only sample. Furthermore, the current study divided the ECT samples into MSR and MNR groups and observed a hippocampal volume increase common to both groups. This finding indicated that these changes in hippocampal volume induced by ECT were not unique to patients with improved symptoms. In this study, the ECT-related brain changes in the hippocampus observed in patients with SZ were similar to those observed in patients with MDD, which is consistent with previous studies (Thomann et al., 2017; Wolf et al., 2016). Thomann and colleagues reported a similar pattern of brain volume changes in the hippocampus of individuals with SZ and MDD (Thomann et al., 2017; Wolf et al., 2016). In addition, hippocampal deficits have frequently been reported to be robust in patients with SZ (Chen et al., 2017; Li et al., 2018), and previous studies provided evidence that such abnormalities also exist in those with MDD (Chen et al., 2018; Sheline et al., 2019). This suggests that ECT modulates neural effects that are not diagnosis-specific but are critical for both affective and non-affective psychoses.
Accumulated evidence has indicated that SZ is related to aberrant functional interactions between large-scale brain networks and cortical-subcortical pathways (Dong et al., 2019; Duan et al., 2015b; He et al., 2019; Lyu et al., 2015; Ma et al., 2016). To date, several neuroimaging studies have investigated FC changes induced by ECT using resting-state fMRI (Abbott et al., 2014; Huang et al., 2018; Jiang et al., 2019). Abbott reported that hippocampal FC increased after ECT in MDD patients and correlated with depressive symptom reduction (Abbott et al., 2014). Our recent study found increased functional integration in the DMN in patients with SZ following ECT using a data-driven FC density analysis (Huang et al., 2018). However, very few neuroimaging studies have addressed whether the brain FC affected by ECT differs between responders and non-responders. To our knowledge, only one study used baseline resting-state FC networks to predict ECT clinical responsiveness, although they did not investigate the longitudinal alterations that may have contributed to symptom improvement (van Waarde et al., 2015). These findings supported the hypothesis that FC networks are specifically altered in patients who respond to ECT. Interestingly, in the current study, we found increased FC between the hippocampus and higher-order cognitive networks, especially the default mode network (DMN), in the MSR group. The hippocampus is a key region for memory encoding and retrieval functions (Tamminga et al., 2010). The DMN has been widely implicated in self-referential processes related to internal mental states (Dong et al., 2018b; Menon, 2011). The dorsolateral prefrontal cortex, as an important region of the central executive network, is responsible for higher-order cognitive function and is crucial for interfacing with the external environment (Liao et al., 2019; Menon, 2011). A possible ECT mechanism could be reinforcing the connectivity between the hippocampus and higher-order cognitive networks to manage the information integration between the internal- and external-based mental landscapes and thus influence the clinical symptoms. In addition, an association between FC changes and symptom reductions was observed, which further demonstrated the relationship between ECT effects and clinical treatment outcomes. In addition, we found reduced FC between the hippocampus and primary sensory networks in the non-responders. Notably, after ECT, these so-called "non-responders" exhibited an incomplete remission relative to the "responders", rather than a worsening of clinical symptoms. These FC reductions may be another ECT mechanism that weakens the connectivity of the hippocampus and primary sensory networks to block access of the primary information and achieve mild symptom remission. In this manner, the lack of bottom-up primary information may give rise to abnormalities in higher-order information processing. Therefore, these patients failed to attain complete symptom remission. However, these interpretations are speculative; thus, future studies should test this hypothesis by task-based fMRI.
Baseline comparisons indicated that compared with the HCs, the SZ patients in the MSR and MNR groups showed lower hippocampal subfield volumes in the HATA. This finding is also consistent with previous meta-analysis studies (Adriano et al., 2012). Furthermore, our study found a lower baseline volume in the HATA in the MNR group. To our knowledge, this is the first study reporting that a lower baseline hippocampal subfield volume correlated with poorer ECT outcomes. In addition, FC baseline comparisons also found higher hippocampus-putamen FC in the MNR group than in the MSR and HC groups. However, the baseline differences between responders and non-responders may have also been influenced by age, disease duration, episode duration, medication and other random factors. Thus, the baseline differences should be interpreted with caution. Despite these potential confounds, the results from the current study suggest that pre-existing or more serious reductions in volume in the hippocampus may have a negative impact on clinical outcomes to ECT, which could help psychiatrists, clinicians and patients make better treatment decisions.
Despite these encouraging findings, several limitations must be acknowledged. First, the current sample size is relatively modest. Although sufficient statistical power was provided by the power analysis in this study, a larger sample size would be more useful to increase the reliability and sensitivity. Second, the SZ participants included medicated and chronic patients. Antipsychotic medication and illness duration may have confounding effects on brain structure and function (Gong et al., 2016). Although we included a matched pharmacotherapy group as the treatment control, the effects of concomitant antipsychotics during the ECT course cannot be completely ruled out. Third, neuropsychological assessments were not evaluated; thus, we did not assess the associations between the changes in cognitive function and brain changes. Fourth, we assumed that the volume and function of the normal brain would not significantly change over the course of one month; thus, healthy controls were only scanned at baseline. Finally, as patients were not randomized to each group, some potential bias may have been introduced into the analyses.
In conclusion, this study demonstrated that ECT induced a hippocampal volume increase in patients with SZ that was common to both responders and non-responders. However, we observed hippocampal FC increases in the clinical responders, while we observed decreases in non-responders. Furthermore, the FC changes correlated with symptom improvements. These findings identified ECT-induced effects in the hippocampus. In addition, the ECT-induced improvements in both structure and function in the hippocampus might imply an important mechanism of action of ECT in patients with SZ.
Declaration of Competing Interest
There is no conflict of interest.
Acknowledgments
This study was funded by grants from the National Natural Science Foundation of China (grant number: 61933003, 81861128001, 61761166001, 81471638, 81771822, 81571759, 81660233, 81671332), the ‘111′ Project (B12027) and grants from Ministry of Science and Technology of China (2016YFC1306800) and SHSMU-ION Research Center for Brain Disorders (2015NKX001). We gratefully acknowledge the participation of the study subjects and investigators.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102081.
Contributor Information
Jijun Wang, Email: jijunwang27@163.com.
Cheng Luo, Email: chengluo@uestc.edu.cn.
Appendix. Supplementary materials
References
- Abbott C.C., Jones T., Lemke N.T., Gallegos P., McClintock S.M., Mayer A.R., Bustillo J., Calhoun V.D. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl. Psychiatry. 2014;4:e483. doi: 10.1038/tp.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriano F., Caltagirone C., Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18:180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M., Lencz T., Kaliora S., Sarpal D.K., Weissman N., Kingsley P.B., Malhotra A.K., Petrides G. Subgenual cingulate cortical activity predicts the efficacy of electroconvulsive therapy. Transl. Psychiatry. 2016;6:e789. doi: 10.1038/tp.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boter H., Peuskens J., Libiger J., Fleischhacker W.W., Davidson M., Galderisi S., Kahn R.S., Grp E.S. Effectiveness of antipsychotics in first-episode schizophrenia and schizophreniform disorder on response and remission: an open randomized clinical trial (EUFEST) Schizophr. Res. 2009;115:97–103. doi: 10.1016/j.schres.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Bouckaert F., De Winter F.L., Emsell L., Dols A., Rhebergen D., Wampers M., Sunaert S., Stek M., Sienaert P., Vandenbulcke M. Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study. J. Psychiatry Neurosci. 2016;41:105–114. doi: 10.1503/jpn.140322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert F., Dols A., Emsell L., De Winter F.L., Vansteelandt K., Claes L., Sunaert S., Stek M., Sienaert P., Vandenbulcke M. Relationship between hippocampal volume, serum BDNF, and depression severity following electroconvulsive therapy in late-life depression. Neuropsychopharmacology. 2016;41:2741–2748. doi: 10.1038/npp.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M., Martinez-Zalacain I., Bernabeu-Sanz A., Contreras-Rodriguez O., Hernandez-Ribas R., Via E., de Arriba-Arnau A., Galvez V., Urretavizcaya M., Pujol J., Menchon J.M., Cardoner N., Soriano-Mas C. Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment-resistant depression: a longitudinal neuroimaging study. Transl. Psychiatry. 2017;7:e1023. doi: 10.1038/tp.2016.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang Y., Niu C., Zhong S., Hu H., Chen P., Zhang S., Chen G., Deng F., Lai S., Wang J., Huang L., Huang R. Common and distinct abnormal frontal-limbic system structural and functional patterns in patients with major depression and bipolar disorder. Neuroimage Clin. 2018;20:42–50. doi: 10.1016/j.nicl.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Jiang Y., Chen L., He H., Dong L., Hou C., Duan M., Yang M., Yao D., Luo C. Altered hippocampo-cerebello-cortical circuit in schizophrenia by a spatiotemporal consistency and causal connectivity analysis. Front. Neurosci. 2017;11:25. doi: 10.3389/fnins.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietsche B., Kircher T., Falkenberg I. Structural brain changes in schizophrenia at different stages of the illness: a selective review of longitudinal magnetic resonance imaging studies. Aust. N. Z. J. Psychiatry. 2017;51:500–508. doi: 10.1177/0004867417699473. [DOI] [PubMed] [Google Scholar]
- Dong D., Duan M., Wang Y., Zhang X., Jia X., Li Y., Xin F., Yao D., Luo C. Reconfiguration of dynamic functional connectivity in sensory and perceptual system in schizophrenia. Cereb. Cortex. 2019;29:3577–3589. doi: 10.1093/cercor/bhy232. [DOI] [PubMed] [Google Scholar]
- Dong D., Wang Y., Chang X., Luo C., Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44:168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H.F., Gan J.L., Yang J.M., Cheng Z.X., Gao C.Y., Shi Z.J., Zhu X.Q., Liang X.J., Zhao L.M. A longitudinal study on intrinsic connectivity of hippocampus associated with positive symptom in first-episode schizophrenia. Behav. Brain Res. 2015;283:78–86. doi: 10.1016/j.bbr.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Duan M., Chen X., He H., Jiang Y., Jiang S., Xie Q., Lai Y., Luo C., Yao D. Altered basal ganglia network integration in schizophrenia. Front. Hum. Neurosci. 2015;9:561. doi: 10.3389/fnhum.2015.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Li H., Zhuo J., Zhang Y., Wang J., Chen L., Yang Z., Chu C., Xie S., Laird A.R., Fox P.T., Eickhoff S.B., Yu C., Jiang T. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Luo C., Li X., Jiang S., Khundrakpam B.S., Duan M., Chen X., Yao D. Evaluation of functional connectivity in subdivisions of the thalamus in schizophrenia. Br. J. Psychiatry. 2019;214:288–296. doi: 10.1192/bjp.2018.299. [DOI] [PubMed] [Google Scholar]
- Gong Q., Lui S., Sweeney J.A. A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am. J. Psychiatry. 2016;173:232–243. doi: 10.1176/appi.ajp.2015.15050641. [DOI] [PubMed] [Google Scholar]
- Hasan A., Wobrock T., Guse B., Langguth B., Landgrebe M., Eichhammer P., Frank E., Cordes J., Wolwer W., Musso F., Winterer G., Gaebel W., Hajak G., Ohmann C., Verde P.E., Rietschel M., Ahmed R., Honer W.G., Dechent P., Malchow B., Castro M.F.U., Dwyer D., Cabral C., Kreuzer P.M., Poeppl T.B., Schneider-Axmann T., Falkai P., Koutsouleris N. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol. Psychiatry. 2017;22:857–864. doi: 10.1038/mp.2016.161. [DOI] [PubMed] [Google Scholar]
- He H., Luo C., Luo Y., Duan M., Yi Q., Biswal B.B., Yao D. Reduction in gray matter of cerebellum in schizophrenia and its influence on static and dynamic connectivity. Hum. Brain Mapp. 2019;40:517–528. doi: 10.1002/hbm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Jiang Y., Xia M., Tang Y., Zhang T., Cui H., Wang J., Li Y., Xu L., Curtin A., Sheng J., Jia Y., Yao D., Li C., Luo C., Wang J. Increased resting-state global functional connectivity density of default mode network in schizophrenia subjects treated with electroconvulsive therapy. Schizophr Res. 2018;197:192–199. doi: 10.1016/j.schres.2017.10.044. [DOI] [PubMed] [Google Scholar]
- Iglesias J.E., Van Leemput K., Augustinack J., Insausti R., Fischl B., Reuter M., Alzheimer’s Disease Neuroimaging I. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. Neuroimage. 2016;141:542–555. doi: 10.1016/j.neuroimage.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Luo C., Gong J., Peng R., Ma S., Tan S., Ye G., Dong L., Yao D. Aberrant thalamocortical connectivity in juvenile myoclonic epilepsy. Int. J. Neural Syst. 2018;28 doi: 10.1142/S0129065717500344. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Luo C., Li X., Duan M., He H., Chen X., Yang H., Gong J., Chang X., Woelfer M., Biswal B.B., Yao D. Progressive reduction in gray matter in patients with schizophrenia assessed with mr imaging by using causal network analysis. Radiology. 2018;287:633–642. doi: 10.1148/radiol.2017171832. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Xia M., Li X., Tang Y., Li C., Huang H., Dong D., Jiang S., Wang J., Xu J., Luo C., Yao D. Insular changes induced by electroconvulsive therapy response to symptom improvements in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;89:254–262. doi: 10.1016/j.pnpbp.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Kellner C.H., Greenberg R.M., Murrough J.W., Bryson E.O., Briggs M.C., Pasculli R.M. ECT in treatment-resistant depression. Am. J. Psychiatry. 2012;169:1238–1244. doi: 10.1176/appi.ajp.2012.12050648. [DOI] [PubMed] [Google Scholar]
- Kraguljac N.V., White D.M., Hadley J., Reid M.A., Lahti A.C. Hippocampal-parietal dysconnectivity and glutamate abnormalities in unmedicated patients with schizophrenia. Hippocampus. 2014;24:1524–1532. doi: 10.1002/hipo.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac N.V., White D.M., Hadley N., Hadley J.A., Ver Hoef L., Davis E., Lahti A.C. Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr. Bull. 2016;42:1046–1055. doi: 10.1093/schbul/sbv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally J., Tully J., Robertson D., Stubbs B., Gaughran F., MacCabe J.H. Augmentation of clozapine with electroconvulsive therapy in treatment resistant schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 2016;171:215–224. doi: 10.1016/j.schres.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Li W.B., Li K.M., Guan P.J., Chen Y., Xiao Y., Lui S., Sweeney J.A., Gong Q.Y. Volume alteration of hippocampal subfields in first-episode antipsychotic-naive schizophrenia patients before and after acute antipsychotic treatment. Neuroimage-Clin. 2018;20:169–176. doi: 10.1016/j.nicl.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Fan Y.-S., Yang S., Li J., Duan X., Cui Q., Chen H. Preservation effect: cigarette smoking acts on the dynamic of influences among unifying neuropsychiatric triple networks in schizophrenia. Schizophr. Bull. 2019;45:1242–1250. doi: 10.1093/schbul/sby184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu H., Hu M., Eyler L.T., Jin H., Wang J., Ou J., Guo X., He Z., Liu F., Zhao J., Guo W. Regional white matter abnormalities in drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Aust. N. Z. J. Psychiatry. 2015;49:246–254. doi: 10.1177/0004867414554268. [DOI] [PubMed] [Google Scholar]
- Ma X., Wang D., Zhou Y., Zhuo C., Qin W., Zhu J., Yu C. Sex-dependent alterations in resting-state cerebral blood flow, amplitude of low-frequency fluctuations and their coupling relationship in schizophrenia. Aust. N. Z. J. Psychiatry. 2016;50:334–344. doi: 10.1177/0004867415601728. [DOI] [PubMed] [Google Scholar]
- Madsen T.M., Treschow A., Bengzon J., Bolwig T.G., Lindvall O., Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol. Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Niemann K., Hammers A., Coenen V.A., Thron A., Klosterkotter J. Evidence of a smaller left hippocampus and left temporal horn in both patients with first episode schizophrenia and normal control subjects. Psychiatry Res. 2000;99:93–110. doi: 10.1016/s0925-4927(00)00059-7. [DOI] [PubMed] [Google Scholar]
- Nordanskog P., Dahlstrand U., Larsson M.R., Larsson E.M., Knutsson L., Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT. 2010;26:62–67. doi: 10.1097/YCT.0b013e3181a95da8. [DOI] [PubMed] [Google Scholar]
- Nordanskog P., Larsson M.R., Larsson E.M., Johanson A. Hippocampal volume in relation to clinical and cognitive outcome after electroconvulsive therapy in depression. Acta Psychiatr. Scand. 2014;129:303–311. doi: 10.1111/acps.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltedal L., Bartsch H., Sorhaug O.J., Kessler U., Abbott C., Dols A., Stek M.L., Ersland L., Emsell L., van Eijndhoven P., Argyelan M., Tendolkar I., Nordanskog P., Hamilton P., Jorgensen M.B., Sommer I.E., Heringa S.M., Draganski B., Redlich R., Dannlowski U., Kugel H., Bouckaert F., Sienaert P., Anand A., Espinoza R., Narr K.L., Holland D., Dale A.M., Oedegaard K.J. The global ECT-MRI research collaboration (GEMRIC): establishing a multi-site investigation of the neural mechanisms underlying response to electroconvulsive therapy. Neuroimage Clin. 2017;14:422–432. doi: 10.1016/j.nicl.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera T.D., Coplan J.D., Lisanby S.H., Lipira C.M., Arif M., Carpio C., Spitzer G., Santarelli L., Scharf B., Hen R., Rosoklija G., Sackeim H.A., Dwork A.J. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili M., Lester D., Dominici G., Longo L., Marconi G., Forte A., Serafini G., Amore M., Girardi P. Indications for electroconvulsive treatment in schizophrenia: a systematic review. Schizophr. Res. 2013;146:1–9. doi: 10.1016/j.schres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R., Opel N., Grotegerd D., Dohm K., Zaremba D., Burger C., Munker S., Muhlmann L., Wahl P., Heindel W., Arolt V., Alferink J., Zwanzger P., Zavorotnyy M., Kugel H., Dannlowski U. Prediction of individual response to electroconvulsive therapy via machine learning on structural magnetic resonance imaging data. JAMA Psychiatry. 2016;73:557–564. doi: 10.1001/jamapsychiatry.2016.0316. [DOI] [PubMed] [Google Scholar]
- Reuter M., Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A., Hasan A., Gruber O., Falkai P. Schizophrenia as a disorder of disconnectivity. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261(Suppl 2):S150–S154. doi: 10.1007/s00406-011-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Liston C., McEwen B.S. Parsing the hippocampus in depression: chronic stress, hippocampal volume, and major depressive disorder. Biol. Psychiatry. 2019;85:436–438. doi: 10.1016/j.biopsych.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Takamiya A., Chung J.K., Liang K.C., Graff-Guerrero A., Mimura M., Kishimoto T. Effect of electroconvulsive therapy on hippocampal and amygdala volumes: systematic review and meta-analysis. Br. J. Psychiatry. 2018;212:19–26. doi: 10.1192/bjp.2017.11. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Stan A.D., Wagner A.D. The hippocampal formation in schizophrenia. Am. J. Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Thomann P.A., Wolf R.C., Nolte H.M., Hirjak D., Hofer S., Seidl U., Depping M.S., Stieltjes B., Maier-Hein K., Sambataro F., Wustenberg T. Neuromodulation in response to electroconvulsive therapy in schizophrenia and major depression. Brain Stimul. 2017;10:637–644. doi: 10.1016/j.brs.2017.01.578. [DOI] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A.S. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- van Waarde J.A., Scholte H.S., van Oudheusden L.J., Verwey B., Denys D., van Wingen G.A. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol. Psychiatry. 2015;20:609–614. doi: 10.1038/mp.2014.78. [DOI] [PubMed] [Google Scholar]
- Wade B.S., Joshi S.H., Njau S., Leaver A.M., Vasavada M., Woods R.P., Gutman B.A., Thompson P.M., Espinoza R., Narr K.L. Effect of electroconvulsive therapy on striatal morphometry in major depressive disorder. Neuropsychopharmacology. 2016;41:2481–2491. doi: 10.1038/npp.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.J., Tang Y.Y., Curtin A., Xia M.Q., Tang X.C., Zhao Y.Q., Li Y., Qian Z.Y., Sheng J.H., Zhang T.H., Jia Y.P., Li C.B., Wang J.J. ECT-induced brain plasticity correlates with positive symptom improvement in schizophrenia by voxel-based morphometry analysis of grey matter. Brain Stimul. 2019;12:319–328. doi: 10.1016/j.brs.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Wolf R.C., Nolte H.M., Hirjak D., Hofer S., Seidl U., Depping M.S., Stieltjes B., Maier-Hein K., Sambataro F., Thomann P.A. Structural network changes in patients with major depression and schizophrenia treated with electroconvulsive therapy. Eur. Neuropsychopharmacol. 2016;26:1465–1474. doi: 10.1016/j.euroneuro.2016.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.