Highlights
-
•
Largest controlled pediatric OCD symptom provocation study.
-
•
Novel standardized symptom provocation picture set for pediatric OCD.
-
•
Behavioral group differences strongest for ‘Just Right’ factor.
-
•
Temporal pole recruited by OCD group.
Keywords: Obsessive-compulsive disorder, Child, Adolescent, Symptom provocation, Neuroimaging
Abstract
Obsessive-compulsive disorder
(OCD)-affected adults and children exhibit three to four symptom dimensions with distinct but overlapping neural correlates. No symptom provocation behavioural or imaging study has examined all symptom dimensions in a pediatric OCD sample.
Method
Clinically diagnosed pediatric OCD-affected participants (n = 25) as well as age, gender and Tanner pubertal stage-matched healthy controls (HCs; n = 24) (total sample: mean age = 14.77 ± 2.93 years; age range = 9–18 years; 35% male) viewed alternating blocks of OCD symptom provocation (Contamination, Bad Thoughts, and Just Right symptom dimensions), Fear, Neutral and Rest (i.e. fixation) conditions during functional magnetic resonance imaging. A region-of-interest analysis used seeds based upon results of an adult OCD meta-analysis
Results
OCD participants found OCD symptom-related stimuli bothersome, particularly when compared to controls in the “Just Right” symptom dimension. Pediatric OCD patients exhibited greater recruitment of the left superior temporal gyrus (STG) than healthy controls during combined symptom provocation versus neutral conditions.
Conclusion
Findings suggest involvement of the temporal poles rather than in classic cortico-striatal-thalamico-cortical circuits in pediatric OCD during symptom provocation.
1. Introduction
Pediatric obsessive-compulsive disorder (OCD) is a common (1–3% lifetime prevalence rate; Flament et al., 1988) and debilitating neuropsychiatric illness characterized by intrusive thoughts and rituals (American Psychiatric Association, 2013). OCD-affected individuals exhibit a wide array of symptoms, with factor analyses identifying three to four dimensions: (1) a ‘Contamination’ factor comprised of obsessions around contagions and washing or cleaning compulsions; (2) a taboo, forbidden, or ‘Bad Thoughts’ factor comprised of aggressive, religious, sexual, and somatic obsessions, as well as checking compulsions; and (3) a symmetry or ‘Just Right’ factor comprised of symmetry obsessions as well as ordering, counting, repeating, and hoarding compulsions. In some factor analyses hoarding symptoms separate out onto a forth distinct factor (Højgaard et al., 2017). Additionally, pediatric studies sometimes differ from adult studies with respect to factor loading: somatic symptoms can load onto the Contamination factor and checking symptoms can load onto the Just Right factor in children (for review see Bloch et al., 2008).
Almost 30 adult and two pediatric OCD studies have used a symptom provocation task (SPT) during functional neuroimaging to probe the neural correlates of OCD symptom factors, primarily focusing on washing and checking subfactor symptoms. These SPT studies have been instrumental in solidifying the cortico-striato-thalamo-cortical (CSTC) neurobiological model of OCD (Brennan and Rauch, 2017), and have demonstrated that symptoms likely emerge from five distinct but overlapping brain circuits including the CSTC (van den Heuvel et al., 2009): the dorsal cognitive circuit, the ventral cognitive circuit, the affective circuit, the frontolimbic circuit, and the sensorimotor circuit (van den Heuvel et al., 2016).
Most SPT studies have not, however, studied the full range of OCD symptom dimensions (Brooks et al., 2018). For instance, only one adult study has examined the sexual obsession category of the Bad Thoughts symptom dimension (Thiel et al., 2014). An examination across dimensions is necessary, particularly in children, given the known associations between specific symptom dimensions and clinical outcomes in this population. Published research has recently shown that Just Right symptoms may predict treatment response (Højgaard et al., 2018) and individuals treated in their youth are more likely to reach full remission (Mancebo et al., 2014), whereas children who go untreated have poorer long-term prognoses (Stewart et al., 2004). A better understanding of dimension-specific neural correlates in pediatric OCD could illuminate paths to more informed treatment options for the childhood-onset form of this disorder.
Structural and functional imaging studies have reported differences in adult- versus child-onset OCD patients. The Enhancing Neuro-Imaging Genetics through Meta-Analysis (ENIGMA) consortium has recently published a number of large-scale structural studies uncovering distinct group differences between OCD and controls in adult versus childhood OCD with respect to cortical thickness, surface area (Boedhoe et al., 2018) and symmetry (Kong et al., 2019), as well as subcortical volumes (Boedhoe et al., 2017). Additionally, non-SPT functional imaging studies of pediatric OCD have identified opposite directions of activation in similar CSTC circuits as those in the adult OCD population (Brem et al., 2012).
With respect to neuroimaging studies of SPT in OCD, two adult meta-analyses have been conducted (Rotge et al., 2008; Thorsen et al., 2018), neither of which incorporated child studies. Rotge and colleagues identified a number of cortical and subcortical regions with activation differences during symptom provocation, including those in anterior cingulate cortices (ACC), inferior and middle frontal gyri, precentral gyrus, precuneus, insula and superior temporal gyrus.
To the authors’ knowledge, only two pediatric OCD symptom provocation imaging studies have been published to date, with contradictory results. In the first study (Gilbert et al., 2009), 18 OCD-affected and 18 matched healthy control (HC) participants, aged 10–17 years old, viewed blocks of pictures designed to either provoke symptoms (i.e., cleaning or symmetry subfactor stimuli) or not (i.e., neutral stimuli). Similar to most adult studies, no Bad Thoughts factor (i.e. aggression, religious or sexual thoughts) stimuli and few Just Right subfactor (e.g., just ordering, repeating) stimuli were included in the study. Whole brain analysis found that the OCD group deactivated right insula and dorsal cognitive CSTC network areas [e.g., thalamus and dorsolateral prefrontal cortex (dlPFC)] in comparison to controls. Region-of-interest (ROI) analyses in the cleaning subfactor condition found reduced activity in the right insula, right putamen, right thalamus, right dlPFC, and left orbitofrontal cortex (OFC) in OCD versus HC groups. ROI analyses in the symmetry subfactor condition found relative deactivation of the right thalamus and right insula. These findings are opposite the increased recruitment of these areas typically reported in adult OCD studies (Rotge et al., 2008). Gilbert suggested these opposite direction findings may have been due to the stimuli selected for the neutral condition used for comparison.
The second pediatric OCD imaging study of symptom provocation focused primarily on a repetitive transcranial magnetic stimulation (rTMS) manipulation (Pedapati et al., 2015). No control group was included in the study, and the symptom dimensions were not described in detail. The Provocation > Neutral stimuli contrast was reported, however, and revealed increased activity in left supplementary motor area (SMA; BA6), left inferior parietal lobule (BA7/40), bilateral inferior frontal gyrus/insula (BA47/13) extending to left superior temporal gyrus (BA38), as well as the left middle temporal gyrus (BA39), and bilateral cerebellum. These results better align with adult studies (Rotge et al., 2008), possibly due to the symptom dimension and control stimuli used. To optimize research progress in this domain, creating a standardized pediatric OCD symptom provocation picture set (POSPPS) for cross-study comparison is imperative.
Brooks and colleagues (2018), noted that no stimulus set has been developed to provoke all OCD symptom factors (and subfactors) in either adults or children, postulating that this may be due to the fact that some obsessions are harder to provoke than others via exposure to pictures or tactile stimuli. This hypothesis deserves testing. Furthermore, while a number of symptom provocation picture sets have been created for adult OCD populations (more information is available in Supplement 1), none have been created or tested for use specifically in children and youth, and this gap should also be closed.
The goals of this study were to: (1) develop and test a pediatric OCD symptom provocation picture set (POSPPS) that triggers each primary symptom dimension factor (Contamination, Bad Thoughts, and Just Right), and also includes Fear and Neutral pictures for comparison; and (2) define the neural correlates associated with OCD symptom provocation in pediatric patients versus healthy controls.
2. Materials and methods
2.1. Participants
Children and youth with OCD (n = 25) were recruited following intake and assessment by a tertiary care OCD subspecialty program. As such, most were on psychotropic medications and/or had begun cognitive behavioural therapy (CBT) at the time of study entry. Healthy control (n = 24) participants were recruited from the community through advertisements. Groups were matched by age, gender, and Tanner pubertal stage scores. The parents or guardians and all participants gave informed consent before beginning the study. All aspects of the study were approved by our institutional research ethics board and were in compliance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Exclusionary criteria for participants in both groups included any magnetic resonance imaging (MRI) contraindication (e.g., metal in the body or claustrophobia), major medical condition (e.g., seizure disorder), or head injury (with associated loss of consciousness for five minutes or more). In the HC group, participants with a DSM-V psychiatric disorder history, or a first-degree relative with a history of OCD were excluded. In the OCD group, participants with a history of bipolar disorder, psychosis, intellectual disability, autism spectrum disorders, or substance use disorder were excluded. As many pediatric OCD patients present with comorbid anxiety, major depression, attention deficit hyperactivity disorder (ADHD) or tics disorders (Ivarsson et al., 2008), these comorbidities were not excluded to improve the generalizability of findings. Comorbidities in the final OCD sample are reported below.
2.2. Clinical and diagnostic measures
A screening questionnaire was administered over the phone by trained research staff. This questionnaire recorded age, gender, handedness, eye glass/contact prescription, and MRI-contraindications.
Diagnoses were established via the Anxiety Disorder Interview Schedule for DSM-IV (ADIS-P; Silverman and Albano, 1996), a validated semi-structured diagnostic interview used to establish lifetime psychiatric diagnoses. Diagnoses were subsequently confirmed via assessment by a child and adolescent psychiatrist. For the OCD group the ADIS-P was administered by PhD-trained and certified psychologists, and for the HC group the ADIS-P was administered by Masters-level trained interviewers (supervised by PhD-trained and certified psychologists).
Additional measures included the Florida Obsessive-Compulsive Inventory (FOCI; Storch et al., 2007), the Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS; Scahill et al., 1997), and the Tanner pubertal staging scales (Marshall and Tanner, 1969; 1970). The FOCI is usually administered as a 20-item self-report checklist querying current (within the past week) and previously experienced common obsessions and compulsions. We also added extension items to the FOCI for this study, asking participants to rate their distress level (range = 0–8) for each current symptom they endorsed on the FOCI.
For the OCD participants the clinician-rated CY-BOCS (CY-BOCS-CR) was administered by a PhD-level registered psychologist at clinic intake. A self-report version (CY-BOCS-SR) was completed by all participants after scanning. The CY-BOCS has 10 items, yielding a total severity score (0–7 subclinical; 8–15 mild; 16–23 moderate; 24–31 severe; 32–40 extreme). The CY-BOCS-SR has good reliability as well as satisfactory convergent and divergent validity with the clinician-rated version (Storch et al., 2006).
The Tanner staging scales each depict five sequential diagrams of primary or secondary sexual characteristics. Diagrams that participants considered closest to their current stage of development were used in the scale averages . For use as a covariate in the fMRI analyses, a composite Tanner score was calculated by averaging the primary and secondary rating scales.
2.3. fMRI paradigm
2.3.1. Stimuli selection and validation
To create a picture set for symptom provocation in children and adolescents, pictures were selected from multiple sources including: an adult OCD SPT picture set (de Wit et al., 2015), the International Affective Picture System (IAPS; Lang et al., 2008) and the internet. A subset of pictures was piloted on an independent group of OCD-affected and healthy control children and youth who did not take part in the imaging study, and then all pictures used in the final picture set were rated according to “symptom triggering likelihood” and developmental appropriateness by five clinicians with expertise in pediatric OCD (two PhD-trained psychologists, two child and adolescent psychiatrists and a clinical psychology PhD candidate) (more information is available in Supplement 1). In total, 24 pictures were chosen for each of the five conditions: ‘Contamination’, ‘Bad Thoughts’, ‘Just Right’, ‘Fear’, and ‘Neutral’. During the Rest condition a small white fixation cross was displayed in the middle of a black screen.
2.3.2. The symptom provocation task (SPT)
Blocks consisted of picture triads, with each picture presented for 4 s, resulting in a total exposure time of 12 s per block (see Fig. 1). All stimuli within a block were from the same condition but were randomly selected from within the image set for that condition.
Fig. 1.
The Symptom Provocation Task (SPT). Each block was comprised of a symptom intensifying message (2000 ms), three pictures (1200 ms total), and a rating screen for response collection (2000 ms). There were six block types in the study: Contamination (contamination and somatic subfactors), Bad Thoughts (aggression, sexual, and religious subfactors), Just Right (counting, ordering, repeating, and symmetry subfactors), as well as Fear, Neutral and Rest (i.e. fixation) blocks. Please note that the neutral image above is in place of the International Affective Picture Set (IAPS; Lang et al., 2008) image used (IAPS slide no. 5800). IAPS pictures cannot be published in any domain, including research articles.
Similar to previous adult OCD symptom provocation studies (Mataix-Cols et al., 2003, 2004) a message was shown for two seconds at the beginning of each block, to intensify the condition as follows: Contamination- “Imagine being bothered that you have to touch the things in these pictures.” Bad Thoughts- “Imagine being upset and inside of these pictures.” Just Right- “Imagine being bothered that you can't correct the problem in these pictures.” Fear-“Imagine being afraid and inside of these pictures.” Neutral-“Imagine being relaxed and inside of these pictures”, and fixation-“Please relax and do not move.”
After exposure to the pictures participants rated how bothered they felt (1 = not bothered, 2 = a little bothered, or 3 = very bothered). Eight blocks were presented for each condition, resulting in a total of 48 blocks. Block order was optimally pseudo-randomized using fMRIsim (http://www.cabi.gatech.edu/CABI/archives/resources/guide/fmrisim).
2.4. Data acquisition
The symptom provocation task (SPT) was completed over two 13.75-min runs while functional magnetic images were recorded. The task was presented using E-prime (2.0, Psychological Software Tools, Pittsburgh, PA), back-projected to a screen within the MRI scanning room and viewed via a mirror affixed to the head coil. Responses were captured using a crescent-shaped response box, with button presses by the right index, middle and ring fingers. When needed, MR-compatible glasses were used.
The following sequences were collected on a 3T Discovery MR750 MRI scanner (GE Medical Systems) with a 12-channel head coil: a three-dimensional fast SPRG structural scan (TR = 8.184 ms; TE = 3.192 ms; matrix = 256 × 256 mm, 182 slices, voxel-size = 1 × 1 × 1 mm,), a field map to account for B0 distortions, and functional gradient echo-planar images (TR = 2000 ms; TE = 25 ms; FOV = 256; matrix = 96 × 96; 41–43 interleaved slices per volume, 3 × 3 mm in-plane resolution, slice thickness = 3 mm, interslice gap = 1 mm, 412 vol). Sagittal acquisition was used for all scans as it displayed the least amount of signal dropout over ventromedial prefrontal cortical structures during piloting.
2.5. Behavioural analyses
Behavioural responses were analyzed in R (Version 3.5.3). Group differences on bothersome ratings recorded in the scanner (range: 1–3) were tested using an ordinal logistic regression model over all the data, such that the response on item “X" for subject “J” was determined by group (OCD vs HC), condition (i.e., Contamination, Bad Thoughts, Just Right, Fear, and Neutral), and the interaction of group and condition. Further the model contained a random subject effect to account for the expectation that there would be random variation across subjects in average responses. After the model was created, specific contrasts of interest (e.g., the comparison of OCD vs HC for each symptom dimension factor minus neutral) were reviewed. The statistical threshold was set at α = 0.05, Bonferroni corrected.
Relationships between the bothersome ratings for each symptom dimension factor and age, sex, composite Tanner score, and OCD severity were examined via Pearson's correlation coefficients. Again, the statistical threshold was set at α = 0.05, Bonferroni corrected.
2.6. Image processing and analyses
A pediatric neuroradiologist (MKMH) reviewed all of the structural scans for incidental clinically relevant findings. None were noted in the final sample. Scans were preprocessed in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK), with the Fieldmap toolbox to correct for inhomogeneities in the B0 field, and the ArtRepair toolbox for high motion pediatric and clinical populations (version 4.0; Mazaika et al., 2004). In brief, all functional scans underwent slice repair, slice-time correction, unwarping, realignment, and co-registration. Images were then normalized to the Montreal Neurological Institute (MNI) T1 template provided by SPM8, and smoothed (Gaussian kernel with FWHM of 6 × 6 × 6 mm). ArtRepair was then used for volume repair and to de-weight volumes to improve 1st-level estimations (more information regarding this pediatric preprocessing pipeline is provided in Supplement 2). One healthy control participant was dropped entirely from the analyses due to poor functional scan quality, resulting in a final HC group with n = 23.
At the first level, data were analyzed using the general linear model (GLM), with all conditions entered as regressors in the following order: Contamination, Bad Thoughts, Just Right, Fear, and Neutral. The Rest condition was assigned to the implicit baseline.
Then, for each participant, data from the two runs were pooled and four contrasts were created for each experimental condition minus the neutral condition (i.e., Contamination-Neutral: 1 0 0 0 −1; Bad Thoughts-Neutral: 0 1 0 0 −1; Just Right-Neutral: 0 0 1 0 −1; Fear-Neutral: 0 0 0 1 −1).
These 1st-level contrasts (48 participants x 4 conditions = 192) were then entered into a mixed measures ANCOVA using the SwE toolbox (v2.01.0) (Guillaume et al., 2014), which overcomes known issues with the standard group-level repeated measures analysis in SPM (McFarquhar, 2019) by selecting the correct error terms. Variance and covariance estimates were pooled over all subjects. The default small sample adjustment (type C2) and degree of freedom type (approx. III) were used and all conditions (Contamination-Neutral, Bad Thoughts-Neutral, Just Right-Neutral and Fear-Neutral) and covariates (age and gender) were defined for model estimation. An F contrast was used to test for group differences between the Symptom Provocation-Neutral blocks, with the voxel-wise significance threshold was set at p ≤ 0.001, FWE corrected using wild boot-strapping (Guillaume and Nichols, 2015).
Additionally, 10 cortical regions of interest (ROIs) were explored. ROIs were defined using a 5 mm sphere around seeds from the cortical areas reported by Rotge and colleagues in their OCD symptom provocation Activation Likelihood Estimation (ALE) meta-analysis in adults (2008).
Group differences were analyzed using a linear mixed effects model in R (Version 3.5.3) and the package ‘nlme’. The model included fixed effects of ROI, condition, and group, and interactions among these variables. The model also included fixed effects of age and gender to account for their effects on brain activation. A random intercept modeled between-subject variation, with heteroskedasticity in variation allowed between ROIs. Significant interaction effects (based on an uncorrected α = 0.05) were followed by pairwise comparisons with Benjamini-Hochberg FDR correction for multiple pairwise testing (Benjamini and Hochberg, 1995).
In a follow-up model, the analysis was re-run replacing age with the composite Tanner score, to study the effect of pubertal development on the results (given individual variability of pubertal onset and related hormonal brain influences). In a second follow-up, data were “winsorized” (i.e., extreme values were attenuated by setting them to either the value of the 1st or 99th percentile) to determine the effect of outliers on the results.
Finally, a separate linear mixed effects model was created, using the OCD group only, with CY-BOCS as an additional covariate to examine potential associations between symptom severity (CY-BOCS) and brain activity.
3. Results
3.1. Demographics
The pediatric OCD group (n = 25) was comprised of 10 males and 15 females, 9 to 18 years old (mean = 15.17 years), with an average OCD onset at 9.14 years of age (SD = 3.20). OCD and HC groups were well-matched on demographic variables, with no between group differences (see Table 1).
Table 1.
Demographic characteristics of the OCD (n = 25) and HC (n = 24) groups.
| OCD | HC | Statistical test | P value | |
|---|---|---|---|---|
| Average age, years (SD) | 15.17 (2.68) | 14.37 (3.11) | 0.880a | 0.383 |
| Gender,% male | 40.0% | 33.3% | 0.235b | 0.769 |
| Handedness,% right handed | 88.0% | 87.5% | 0.003b | 1.000 |
| Primary Tanner Stage, mean (SD) | 3.50 (1.22) | 3.26 (1.14) | 0.696a | 0.490 |
| Secondary Tanner Stage, mean (SD) | 3.50 (1.22) | 3.17 (1.44) | 0.864a | 0.404 |
Note: HC = Healthy control; OCD = Obsessive-compulsive disorder.
Independent samples t-test, two-tailed.
Fisher's Exact Test, two-tailed.
OCD-affected youth reported severe range symptoms (mean CY-BOCS-CR = 24.48, SD = 8.18) at initial clinic intake and milder range symptoms on scan day (average CY-BOCS-SR = 12.88, SD = 6.53). Of note, clinical intervention was initiated in the interval between clinic assessment and scan day in all but two cases. Based on the FOCI (Storch et al., 2007) ratings given by OCD participants on scan day, 60% (n = 15) were experiencing Contamination symptoms [with the added FOCI extension item showing these current symptoms had a mean distress rating of 3.76 out of 8.00 (SD = 1.65)]. Eighty-four percent (n = 21) were experiencing Bad Thoughts [mean distress = 4.27/8.00 (1.83)] and 72% (n = 18) were experiencing Just Right symptoms [mean distress ratings = 4.31/8.0 (1.56)]. Most reported having experienced each symptom dimension at some point in their lives (see Supplement 3, Table S5).
Current comorbidities included anxiety disorders (n = 9), dysthymia (n = 1), ADHD (n = 4) and tic disorders (n = 4). Three-quarters of OCD participants were taking at least one psychotropic medication (n = 19; 76%). All 19 medicated participants were taking a serotonin-reuptake inhibitor (n = 19), and some were also taking additional agents, such as benzodiazepines (n = 3), stimulants (n = 2), atypical antipsychotics (n = 1), α2- adrenergic agonists (n = 1), and l-Tryptophan (n = 1).
3.2. Behavioral results
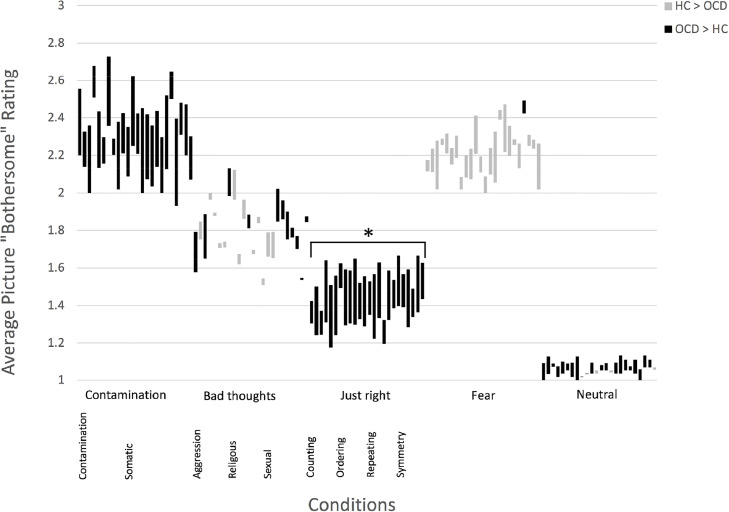
All participants reported ‘bothersome’ ratings for pictures throughout the scanning session. Across groups, the Fear and Contamination pictures were reported to be most bothersome, the Neutral and Just Right pictures were least bothersome, and the Bad Thoughts pictures elicited intermediate responses (see Fig. 2).
Fig. 2.
Average OCD and HC bothersome ratings (range: 1–3) for each picture rated in the scanner. There were 24 pictures per condition. Averages were calculated for each picture while ignoring the other pictures presented in the triad. In the figure above, the mean OCD and HC rating scores are connected by a line: Black lines represent pictures with higher average OCD ratings (i.e. top point = OCD mean, bottom point = HC mean); Grey lines represent pictures with higher average HC ratings. * Significant difference at p ≤ 0.05, two-tailed, corrected.
All Contamination and Just Right pictures produced ratings in the anticipated direction (i.e., more bothersome to the OCD versus HC group), but only the Just Right condition exhibited a significant difference between groups [Z = 2.735, p = 0.006] (see Fig. 2 and Supplement 4, Table S6). In the Bad Thoughts condition only eleven of the twenty-four pictures were given ratings in the anticipated direction, and this category did not show a significant group difference [Z = −0.613, p = 0.540]. Almost all Fear condition pictures (23 out of 24) were rated as more bothersome by the HC participants, but this did not lead to a significant group difference [Z = −1.719, p = 0.086].
Within the OCD group, there were no significant correlations between OCD severity and bothersome ratings. Regardless of group, boys reported significantly lower average bothersome ratings (1.70/3.00) than girls (1.97/3.00) [t(46) = 2.70, p = 0.01] in response to the OCD-related stimuli. There were no significant correlations between bothersome ratings and age [r(47) = 0.104, p = 0.479] or composite Tanner score [r(47) = 0.081, p = 0.583].
3.3. Imaging results
Whole brain analysis found no significant group differences at the chosen significance threshold (p < 0.001, FWE corrected). Results at p < 0.001, uncorrected, are presented in Supplement 5, but are not discussed here further.
Region-of-interest (ROI) analysis of the 10 cortical regions suggested by Rotge and colleagues (2008) revealed a significant Group by Condition interaction [F (2, 1352) = 5.82, p = 0.003] and Group by ROI interaction [F (9, 1352) = 2.18, p = 0.02]. Sensitivity analysis using composite Tanner score rather than age as a covariate produced the same pattern of results (Supplement 6, Table S8).
The post-hoc pairwise analysis of Group by ROI revealed a significant between-group difference in activation in the left STG BA38 (x, y, z = −44, 24, −18), collapsing across the three symptom provocation conditions. There was greater activation among the OCD group (mean = 0.58, SE = 0.06) compared to the HC group (mean = 0.29, SE = 0.07; p = 0.003) (see Fig. 3 and Supplement 6, Table S9).
Fig. 3.
ROI analysis of neural activity in OCD (n = =25) versus HC (n = =23) participants. A significant difference was found such that the pediatric OCD group demonstrated greater activation in a region of the left superior temporal pole (STG) during symptom provocation blocks than HC participants. Abbreviations: ACC = Anterior Cingulate Cortex; BA = Brodmann Area; IFG = Inferior Frontal Gyrus; L = Left; Med.FG = Medial Frontal Gyrus; Mid.FG = Middle Frontal Gyrus; R = Right; ROI = Region of Interest; STG = Superior Temporal Gyrus.
Follow-up pairwise analysis found no significant Group by Condition effect that survived FDR correction (see Supplement 6 Table S10). Attenuating extreme scores by winsorizing the data did not change these findings.
Examining OCD children only, there was no main effect for the relationship between brain activation across the 10 ROIs and symptom severity, and no relationship between any individual ROI and symptom severity (all p values > 0.05).
4. Discussion
This is the first neuroimaging study to use a symptom provocation task (SPT) to examine all OCD symptom dimensions and subdimensions, and the largest pediatric OCD SPT imaging study to date. Two of the three pediatric OCD symptom provocation picture set (POSPPS) symptom dimensions worked well with respect to reported group differences. In particular, the Just Right subset, which included pictures related to symmetry, ordering, counting, and repeating subfactors, showed a strong differential effect between groups with respect to how bothersome they were reported to be. This is notable, as a recent review of behavioural studies using OCD or subclinical OCD participants (de Putter et al., 2017) did not identify any studies examining the provocation with the symmetry subdimension. Additionally, although previous OCD imaging studies have included Just Right dimension stimuli (Adler et al., 2000; Banca et al., 2015; de Wit et al., 2015; Simon et al., 2014, 2013, 2010) they often focus on the counting, ordering or symmetry subdimensions, and miss the repeating subdimension. Given that Just Right symptoms may predict treatment response (Højgaard et al., 2018), all of the subdimensions of the Just Right factor deserve further exploration.
In the Contamination condition, with respect to provoking reported distress or ‘bothersomeness’, stimuli did not show a significant difference between groups. This may be due to the high level of disgust evoked as well in the healthy control group. A previous neuroimaging study in adults (Phillips et al., 2000) found that contamination stimuli only elicited differential insular activity in the OCD group compared to the HC group when 'non-disgusting' pictures were used. As such, future studies should be careful to select stimuli that are not perceived as disgusting by healthy control participants. Additionally, in future, the use of a 5-point instead of 3-point Likert scale during scanning may allow for a wider range of answers and increase the probability of finding significant behavioural effects in the Contamination condition.
The Bad Thoughts pictures, although validated by clinicians with expertise in childhood OCD, did not perform well in the scanner with respect to evoking differential subjective ‘bothersomeness’ compared the the control group. This result lends support to Brooks and colleagues’ (2018) hypothesis that some OCD symptom dimensions may be harder to provoke than others through picture exposure. De Putter and colleagues’ meta-analysis (2017) revealed only a small to medium sized effect for a Bad Thoughts subdimension (i.e., checking: g = 0.58), and the current study suggests none of the other subdimensions elicit strong behavioural group differences either. If true, this phenomenon might explain why only one neuroimaging study has reported on OCD provocation using sexual stimuli (Thiel et al., 2014). Other studies using Bad Thought stimuli may have produced null results and not been published (i.e., become part of the “file drawer problem”; Rosenthal, 1979).
Despite these difficulties, the authors believe that future imaging studies should attempt to incorporate all OCD symptom dimensions to help develop and refine biologically-informed brain networks and in turn potentially fine-tune treatment approaches across the heterogeneous presentations of adult and pediatric OCD. Noting our results, interested readers may request a copy of the POSPPS by contacting the senior author (SES) directly.
4.1. Temporal pole implicated in pediatric symptom provocation
The current imaging study results highlight the involvement of the temporal poles in pediatric OCD, perhaps modulating the canonical cortico-striato-thalamo-cortical (CSTC) network model as described in adult OCD (van den Heuvel et al., 2016).
The most anterior portion of the temporal cortex, the temporal poles (BA 38) exhibit dense connections to fronto-limbic CSTC circuit areas (e.g., amygdala), and may be involved in the binding of complex perceptual inputs to visceral emotional responses (Pehrs et al., 2017). Furthermore, recent research suggests the temporal poles may be part of a control mechanism that down-regulates emotional saliency by reducing the recruitment of the ventral visual stream during the processing of emotional stimuli (Pehrs et al., 2017).
Entering the temporal pole seed (x,y,z = −44, 24, −18) and a 5 mm diameter into Neurosyth, a website-based, automated meta-analysis of fMRI studies (Yarkoni et al., 2011), a number of studies have found this area to be involved in emotion, particularly disgust (e.g., Shapira et al., 2003; Schienle et al., 2005). This fits well with the OCD literature suggesting exaggerated and inappropriate disgust reactions may drive some symptoms (Bhikram et al., 2017), particularly with respect to Contamination symptoms (Rickelt et al., 2019).
The temporal poles have not been a focus of great interest in previous adult OCD symptom provocation studies, although the recruitment of this area has often been reported (Rotge et al., 2008). This activity may have been overlooked given that the temporal pole is not included in most described CSTC models. Temporal pole activity was negatively correlated with OCD illness duration in a recent meta-analysis (Thorsen et al., 2018), suggesting that the temporal pole may play a larger role in pediatric versus adult OCD or in earlier stages of the natural history of illness. By recruiting temporal poles, OCD-affected children and youth with OCD may be able to regulate the emotional saliency of triggering events, particularly early on in their disease course. With age, and a longer disease course, patients may lose this mechanism. If true, this again underlines the need to begin intervention as soon as possible to improve long term outcomes.
4.2. Limitations
This study was cross-sectional, comprised of participants at various stages of treatment. As such, it is not possible to discuss if group differences were impacted by treatment. The primary limitation of this study was the inclusion of OCD participants taking psychotropic medications. In a recent meta-analysis of emotional processing studies in adult OCD participants (Thorsen et al., 2018) the use of psychotropic medication, particularly selective serotonin reuptake inhibitors (SSRIs), was negatively correlated with activation of amygdala and the left inferior occipital gyrus in OCD > HC contrasts. This is a potential explanation for why group differences in these areas were not found in the current study's whole brain analysis.
Another limitation of this study was the inclusion of participants with comorbidities. OCD often presents with comorbid anxiety, depression, ADHD and tic disorders (Ivarsson et al., 2008) and as such these diagnoses were included to improve the generalizability of our findings. However, in their meta-analysis, Thorsen and colleagues (2018) found that comorbidities were associated with increased insular and decreased amygdala activity in OCD > HC contrasts. As such, the presence of comorbidities may have influenced the involvement of fronto-limbic circuit areas in response to this paradigm.
Finally, the current paper reports a SwE whole brain analysis (Guillaume et al., 2014), with no voxels surviving the statistical threshold currently recommended by field experts (e.g., Eklund et al., 2016). It should be noted, however, that determining how to set an appropriate statistical threshold for whole-brain analyses is complicated (Chen et al., 2019); and 0.001 FWE may be too strict with respect to the current sample size. As such, any negative findings reported here should be interpreted with caution. To balance the requirement for a stringent statistical threshold for whole-brain analyses, we utilized an ROI approach, with carefully selected ROIs as informed by previous research and using a less strict FDR correction.
New statistical techniques allow for the study of functional connectivity, and have moved the field from “region”-based models to “network”-based models of neurobiology (van den Heuvel et al., 2016). Future work that pursues functional connectivity analysis methods, such as constrained principal component analyses for fMRI (fMRI-CPCA; Lavigne et al., 2015; Woodward et al., 2013) may be more fruitful in determining which brain areas work together, and to determine when different compensatory networks are brought online in response to symptom provoking stimuli.
Acknowledgments
The authors are grateful to Odile van den Heuvel M.D., Ph.D, of the Amsterdam Universitair Medische Centra and her colleagues, for sharing their adult OCD symptom provocation stimulus set and for advising on the study. Locally at the BC Children's Hospital (BCCH), we would like to thank Tamara Vanderwal, M.D., for comments on an early draft of the manuscript and helpful guidance on figure design. Within the Provincial OCD program (POP) we would like to thank the five clinicians who rated our stimuli: Annie Simpson, Ph.D., Katherine McKenney, Ph.D., Clare Bleakley, M.D., Kourosh Edalati, M.D., and David Schuberth (Ph.D. candidate). Also within POP, we would' like to thank the research assistants and volunteers who helped with MRI data collection: Diana Franco Yamin, M.A., Yayuk Joffres, M.Sc., Daniel Lebovitz, B.Sc., Rachel Ho, B.A., and He “Ann” Sheng, B.Sc.; and the research assistants and volunteers who helped with image manipulation and data entry: Cynthia Lu, B.A., Sang “Jerry” Hun Han, B.Sc., Hannah Seo, B.Sc., James Gibbons, B.Sc., and Boyee Lin, B.Sc. Finally, we are greatly appreciative of the time and effort given by our participants and their families.
This work was supported by the British Columbia Children's Hospital(BCCH) Foundation, the Michael Smith Foundation for Health Research (MSFHR), the BC Provincial Health Services Authority (PHSA), and the Canadian Institutes of Health Research (CIHR). No funding source was involved in study design, data collection, analysis, interpretation, writing, or the decision to submit this work for publication. The authors have no other financial relationships or declarations of interest to disclose.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102034.
Appendix. Supplementary materials
References
- Adler C.M., McDonough-Ryan P., Sax K.W., Holland S.K., Arndt S., Strakowski S.M. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J. Psychiatr. Res. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. American Psychiatryic Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Banca P., Voon V., Vestergaard M.D., Philipiak G., Pocinho F. Imbalance in habitual versus goal directed neural systems during symptom provocation in obsessive-compulsive disorder. Brain. 2015;138:798–811. doi: 10.1093/brain/awu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate : a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. Ser. B (Methodol.) 1995;57(1):289–300. [Google Scholar]
- Bhikram T., Abi-Jaoude E., Sandor P. OCD: obsessive-compulsive … disgust? The role of disguest in obessive-compulsive disorder. J. Psychiatry Neurosci. 2017;42(5):300–306. doi: 10.1503/jpn.160079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M.H., Landeros-Weisenberger A., Rosario M.C., Pittenger C., Leckman J.F. Meta-Analysis of the symptom structure of obsessive-compulsive disorder. Am. J. Psychiatry. 2008;165(12):1532–1542. doi: 10.1176/appi.ajp.2008.08020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedhoe P.S.W., Schmaal L., Abe Y., Alonso P., Ameis S.H., Anticevic A., van den Heuvel O.A. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the enigma obsessive-compulsive disorder working group. Am. J. Psychiatry. 2018;175(5):453–462. doi: 10.1176/appi.ajp.2017.17050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedhoe P.S.W., Schmaal L., Abe Y., Ameis S.H., Arnold P.D., Batistuzzo M.C., Van Den Heuvel O.A. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. Am. J. Psychiatry. 2017;174(1):60–70. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S., Hauser T.U., Iannaccone R., Brandeis D., Drechsler R., Walitza S. Neuroimaging of cognitive brain function in paediatric obsessive compulsive disorder: a review of literature and preliminary meta-analysis. J. Neural Transm. 2012;119(11):1425–1448. doi: 10.1007/s00702-012-0813-z. [DOI] [PubMed] [Google Scholar]
- Brennan B., Rauch S. Functional neuroimaging studies in obsessive-compulsive disorder: overview and synthesis. In: Pittenger C., editor. Obsessive-compulsive disorder: Phenomenology, Pathophysiology and Treatment. 2017. pp. 213–230. [Google Scholar]
- Brooks H., Kichuk S.A., Adams T.G., Koller W.N., Eken H.N., Rance M., Hampson M. Developing image sets for inducing obsessive-compulsive checking symptoms. Psychiatry Res. 2018;265:249–255. doi: 10.1016/j.psychres.2018.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Xiao Y., Taylor P.A., Rajendra J.K., Riggins T., Geng F., Cox R.W. Handling multiplicity in neuroimaging through Bayesian lenses with multilevel modeling. Neuroinformatics. 2019:1–31. doi: 10.1007/s12021-018-9409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Putter L.M., van Yper L., Koster E.H. Obsessions and compulsions in the lab: a meta-analysis of procedures to induce symptoms of obsessive-compulsive disorder. Clin. Psychol. Rev. 2017;52:137–147. doi: 10.1016/j.cpr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- de Wit S.J., Van Der Werf Y.D., Mataix-Cols D., Trujillo J.P., Van Oppen P., Veltman D.J., Van Den Heuvel O.A. Emotion regulation before and after transcranial magnetic stimulation in obsessive compulsive disorder. Psychol. Med. 2015;45(14):3059–3073. doi: 10.1017/S0033291715001026. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure : why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U.S.A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament M.F., Whitaker A., Rapoport J.L., Davies M., Berg C.Z., Kalikow K., Shaffer D. Obsessive compulsive disorder in adolescence: an epidemiological study. J. Am. Acad. Child Adolesc. Psychiatry. 1988;27(6):764–771. doi: 10.1097/00004583-198811000-00018. [DOI] [PubMed] [Google Scholar]
- Gilbert A.R., Akkal D., Almeida J.R.C., Mataix-Cols D., Kalas C., Devlin B., Phillips M.L. Neural correlates of symptom dimensions in pediatric obsessive-compulsive disorder: a functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(9):936–944. doi: 10.1097/CHI.0b013e3181b2163c. [DOI] [PubMed] [Google Scholar]
- Guillaume B., Nichols T.E. Poster presented at the Organization for Human Brain Mapping (OHBM) in Hawai, 14–18, 2015. 2015. Non-parametric inference for longitudinal and repeated-measures neuroimaging data with the wild bootstrap (p. 2) [Google Scholar]; https://warwick.ac.uk/fac/sci/statistics/staff/academicresearch/nichols/presentations/ohbm2015/Guillaume-LongRepeatMeasWB-OHBM2015.pdf.
- Guillaume B., Hua X., Thompson P.M., Waldorp L., Nichols T.E. NeuroImage fast and accurate modelling of longitudinal and repeated measures neuroimaging data. Neuroimage. 2014;94:287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Højgaard D.R.M.A., Hybel K.A., Mortensen E.L., Ivarsson T., Nissen J.B., Weidle B., Thomsen P.H. Obsessive-compulsive symptom dimensions: association with comorbidity profiles and cognitive-behavioral therapy outcome in pediatric obsessive-compulsive disorder. Psychiatry Res. 2018;270:317–323. doi: 10.1016/j.psychres.2018.09.054. [DOI] [PubMed] [Google Scholar]
- Højgaard D.R.M.A., Mortensen E.L., Ivarsson T., Hybel K., Skarphedinsson G., Nissen J.B., Thomsen P.H. Structure and clinical correlates of obsessive–compulsive symptoms in a large sample of children and adolescents: a factor analytic study across five nations. Eur. Child Adolesc. Psychiatry. 2017;26(3):281–291. doi: 10.1007/s00787-016-0887-5. [DOI] [PubMed] [Google Scholar]
- Ivarsson T., Melin K., Wallin L. Categorical and dimensional aspects of co-morbidity in obsessive-compulsive disorder (OCD) Eur. Child Adolesc. Psychiatry. 2008;17(1):20–31. doi: 10.1007/s00787-007-0626-z. [DOI] [PubMed] [Google Scholar]
- Kong X., Boedhoe P.S.W., Abe Y., Alonso P., Stephanie H., Arnold P.D., Francks C. Mapping cortical and subcortical asymmetry in obsessive-compulsive disorder: findings from the enigma consortium. Biol. Psychiatry. 2019 doi: 10.1016/j.biopsych.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lavigne K.M., Metzak P.D., Woodward T.S. Functional brain networks underlying detection and integration of disconfirmatory evidence. Neuroimage. 2015;112:138–151. doi: 10.1016/j.neuroimage.2015.02.043. [DOI] [PubMed] [Google Scholar]
- Mancebo M.C., Boisseau C.L., Garnaat S.L., Eisen J.L., Greenberg B.D., Sibrava N.J., Rasmussen S.A. Long-term course of pediatric obsessive-compulsive disorder: 3 years of prospective follow-up. Compr. Psychiatry. 2014;55(7):1498–1504. doi: 10.1016/j.comppsych.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-cols D., Cullen S., Lange K., Zelaya F., Andrew C., Amaro E., Phillips M.L. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol. Psychiatry. 2003;56(6):482–493. doi: 10.1016/s0006-3223(02)01504-4. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D., Wooderson S., Lawrence N., Brammer M.J., Speckens A., Phillips M.L. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2004;61(6):564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Mazaika P.K., Whitfield S., Cooper J.C. Detection and repair of transient artifacts in fMRI data. Neuroimage. 2004;26(Suppl 1):S36. [Google Scholar]
- McFarquhar M. Modeling group-level repeated measurements of neuroimaging data using the univariate general linear model. Front. Neurosci. 2019 doi: 10.3389/fnins.2019.00352. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedapati E., DiFrancesco M., Wu S., Giovanetti C., Nash T., Mantovani A., Harris E. Neural correlates associated with symptom provocation in pediatric obsessive compulsive disorder after a single session of sham-controlled repetitive transcranial magnetic stimulation. Psychiatry Res. Neuroimag. 2015;233(3):466–473. doi: 10.1016/j.pscychresns.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Pehrs C., Zaki J., Schlochtermeier L.H., Jacobs A.M., Kuchinke L., Koelsch S. The temporal pole top-down modulates the ventral visual stream during social cognition. Cerebral Cortex. 2017;27(1):777–792. doi: 10.1093/cercor/bhv226. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Marks I.M., Senior C., Lythgoe D., O’Dwyer A.M., Meehan O., McGuire P.K. A differential neural response in obsessive-compulsive disorder patients with washing compared with checking symptoms to disgust. Psychol. Med. 2000;30(5):1037–1050. doi: 10.1017/s0033291799002652. [DOI] [PubMed] [Google Scholar]
- Rickelt J., de Wit S.J., van der Werf Y.D., Schruers K.R.J., Marcelis M., de Vries F.E., van den Heuvel O.A. Emotional processing and disgust sensitivity in OCD patients with and without contamination-type obsessive-compulsive symptoms - An fMRI study. J. Obsessive Compuls. Relat. Disord. 2019;22:1–11. [Google Scholar]
- Rosenthal R. File drawer problem and tolerance for null results. Psychol. Bull. 1979;86(3):638–641. [Google Scholar]
- Rotge J.Y., Guehl D., Dilharreguy B., Cuny E., Tignol J., Bioulac B., Aouizerate B. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analsysis of functional neuroimaging studies. J. Psychiatry Neurosci. 2008;33(5):405–412. [PMC free article] [PubMed] [Google Scholar]
- Scahill L., Riddle M.A., McSwiggin-Hardin M., Ort S.I., King R.A., Goodman W.K., Leckman J.F. Children’s yale-brown obsessive compulsive scale: reliability and validity. J. Am. Acad. Child. Adolesc. Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Schienle A., Schäfer A., Stark R., Walter B., Vaitl D. Neural responses of OCD patients towards disorder-relevant, generally disgusting-inducing and fear-inducing pictures. Int. J. Psychophysiol. 2005;57(1):67–77. doi: 10.1016/j.ijpsycho.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Shapira N.A., Liu Y., He A.G., Bradley M.M., Lessig M.C., James G.A., Goodman W.K. Brain activation by disgust-inducing pictures in obessive-compulsive disorder. Biol. Psychiatry. 2003;54(7):751–756. doi: 10.1016/s0006-3223(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Silverman W.K., Albano A.M. Oxford University Press; 1996. Anxiety disorders interview schedule for DSM-IV: Child version. [Google Scholar]
- Simon D., Adler N., Kaufmann C., Kathmann N. Amygdala hyperactivation during symptom provocation in obsessive-compulsive disorder and its modulation by distraction. NeuroImage Clin. 2014;4:549–557. doi: 10.1016/j.nicl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Kaufmann C., Kniesche R., Kischkel E., Kathmann N. Autonomic responses and neural-cardiac coupling during individually tailored symptom provocation in obsessive-compulsive disorder. J. Anxiety Disord. 2013;27(7):635–644. doi: 10.1016/j.janxdis.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Simon D., Kaufmann C., Müsch K., Kischkel E., Kathmann N. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology. 2010;47(4):728–738. doi: 10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- Stewart S.E., Geller D.A., Jenike M., Pauls D., Shaw D., Mullin B., Faraone S.V. Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr. Scand. 2004;110(1):4–13. doi: 10.1111/j.1600-0447.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- Storch E.A., Bagner D., Merlo L.J., Shapira N.A., Geffken G.R., Murphy T.K., Goodman W.K. Florida obsessive-compulsive inventory: development, reliability, and validity. J. Clin. Psychol. 2007;63(9):851–859. doi: 10.1002/jclp.20382. [DOI] [PubMed] [Google Scholar]
- Storch E.A., Murphy T.K., Adkins J.W., Lewin A.B., Geffken G.R., Johns N.B., Goodman W.K. The children’s Yale-Brown obsessive-compulsive scale: psychometric properties of child- and parent-report formats. J. Anxiety Disord. 2006;20(8):1055–1070. doi: 10.1016/j.janxdis.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Thiel A., Thiel J., Oddo S., Langnickel R., Brand M., Markowitsch H.J., Stirn A. Obsessive-compulsive disorder patients with washing symptoms show a specific brain network when confronted with aggressive, sexual, and disgusting stimuli. Neuropsychoanalysis. 2014;16(2):83–96. [Google Scholar]
- Thorsen A.L., Hagland P., Radua J., Mataix-Cols D., Kvale G., Hansen B., van den Heuvel O.A. Emotional processing in obsessive-compulsive disorder: a systematic review and meta-analysis of 25 functional neuroimaging studies. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2018;3(6):563–571. doi: 10.1016/j.bpsc.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Heuvel O.A., Remijnse P.L., Mataix-Cols D., Vrenken H., Groenewegen H.J., Uylings H.B.M., Veltman D.J. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132(4):853–868. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- van den Heuvel O.A., van Wingen G., Soriano-Mas C., Alonso P., Chamberlain S.R., Nakamae T., Veltman D.J. Brain circuitry of compulsivity. Eur. Neuropsychopharmacol. 2016;26(5):810–827. doi: 10.1016/j.euroneuro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Woodward T.S., Feredoes E., Metzak P.D., Takane Y., Manoach D.S. Epoch-specific functional networks involved in working memory. Neuroimage. 2013;65:529–539. doi: 10.1016/j.neuroimage.2012.09.070. [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T., van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Method. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.