Abstract
Background:
Hemostatic complications are commonly encountered in blood or marrow transplantation (BMT) recipients, increasing their morbidity and mortality and are well described in the immediate post-transplant period. The risk of venous thromboembolism (VTE) in long term autologous BMT survivors has not been studied previously.
Methods:
Patients who underwent autologous BMT between January 1, 1974 and December 31, 2010 for a hematologic malignancy, lived 2 years or more after a BMT and were ≥18 years were surveyed for long-term outcomes. Median follow up was 9.8 years (IQR, 6.4-14.3y). We analyzed the risk of VTE in 820 autologous BMT recipients who survived ≥2 years, as compared to 644 siblings as well as among the BMT recipients.
Results:
BMT survivors were at a 2.6-fold higher risk of VTE as compared to siblings (95%CI:1.6-4.4, p<0.0004), after adjusting for sociodemographic characteristics. Conditional on surviving ≥2 years after BMT, the cumulative incidence of VTE was 3.9±0.8% at 5 years and 6.1±1.1% at 10 years. A diagnosis of plasma cell disorder (HR=2.37, 95%CI: 1.3-4.2, p=0.004) and annual house hold income ≤$50,000 (HR=2.02, 95%CI 1.2-3.6, p=0.015) were associated with increased VTE risk.
Conclusions:
Autologous BMT survivors are at risk for developing late-occurring VTE. Development of risk prediction models to identify autologous BMT survivors at highest risk for VTE and thromboprophylaxis may help decrease the morbidity and mortality associated with VTE.
Introduction
Blood or marrow transplantation (BMT) is associated with an acquired hypercoagulable state characterized by inflammation, endothelial damage and activation of endothelium-dependent coagulation factors, increase in von Willebrand factor and platelet adhesion, increased thrombin generation, decreased levels of anticoagulant proteins such as anti-thrombin and protein C.1–3 Previous reports of VTE in BMT recipients have focused on the early posttransplant period, with a widely variable incidence (0.5%-23.5%) depending on the population characteristics and the methods used for diagnosing VTE.4 Majority of these studies are limited by brief post-BMT follow-up and/or relatively small samples.5 Catheter related thrombosis occurs frequently in lymphoma and myeloma patients undergoing autologous BMT, and majority of these events occur within first 100 days of BMT.6 A comprehensive assessment of the incidence and risk factors for VTE in long term autologous BMT survivors remains unstudied. This is important to understand since VTE is associated with decreased survival in BMT recipients.7 We addressed this gap by using the resources offered by the Blood or Marrow Transplant Survivor Study (BMTSS).
Materials and Methods
BMTSS is a collaborative effort between City of Hope (COH), University of Minnesota (UMN) and University of Alabama at Birmingham (UAB) and is examining long-term outcome of individuals who have lived 2 years or more after undergoing BMT between 1974 and 2010 at one of these three institutions. Comparison with a non-cancer population has been made possible by asking participating survivors to invite a nearest-age sibling to the study. The Human Subjects Committee at participating institutions approved the protocol; informed consent was provided according to the Declaration of Helsinki.
A BMTSS survey was administered to eligible patients and covered the following: diagnosis by a healthcare provider of specific chronic health conditions, relapse of primary cancer and development of subsequent neoplasms, age at diagnosis of these health conditions, medication use, height/weight at the time of survey completion, and sociodemographic characteristics (sex, race/ethnicity, education, employment, household income and health insurance).8 We have previously shown that BMT survivors are able to report their outcomes with a high degree of accuracy.9 Information regarding primary cancer diagnosis, transplant preparative regimens, and graft type (bone marrow or peripheral blood stem cells [PBSC]) was obtained from institutional databases.
The current study aimed to describe the risk of VTE in long term autologous BMT survivors. We hypothesized that the risk of VTE will be high several years after BMT due to endothelial damage from prior treatments including chemotherapy and radiation, continued inflammatory state and new on set co-morbidities in this patient population. Patients who were alive and 18 years or older at time of study were included. The underlying hematologic malignancies included acute myeloid leukemia/myelodysplasia (AML/MDS), chronic myeloid leukemia (CML), plasma cell disorders (PCD), or non-Hodgkin lymphoma (NHL). We excluded patients who underwent a second BMT. Self-report of VTE diagnosed by a healthcare provider was used to identify patients with VTE. Patients with arterial thrombosis or thrombotic microangiopathy were not included as a VTE outcome.
A total of 1,556 patients had undergone autologous BMT for hematologic malignancies at COH, UMN or UAB between 1974 and 2010, survived ≥2 years, were 18 years or older, and alive at study participation. Of these, 119 (7.6%) were lost to follow-up. Of the 1,437 BMT recipients approached, 455 did not participate (185 [11.8%] refused participation; 270 [17.3%] did not respond to the survey request), yielding 982 participants (68.3% participation rate). Of the 982 study participants, history of VTE was not available for 109 participants. Since we were interested in studying the risk of post-BMT VTE, patients who developed VTE prior to BMT or with missing age at VTE diagnosis were excluded (53). In total, 820 patients were included in the final analysis.
Statistical Analysis
Logistic regression was used to study the risk of VTE in BMT survivors compared to siblings, adjusting for age at study participation, sex, race/ethnicity, education, annual household income, insurance, body mass index (BMI) and comorbidities. Cumulative incidence of VTE conditional on surviving 2 years or more after BMT was calculated using competing risk methods. Since we were interested in studying the risk of VTE in BMT recipients who survived 2 years or more after BMT, we took 2 years after transplant as the starting point to calculate cumulative incidence. If the date of onset of VTE occurred within the first 2 years after BMT, the condition was considered as present 2 years after BMT. For the purposes of analysis, the onset date was shifted forward to that time point. Data on long term complications in cancer survivors was presented in a similar way in previous publications.8,10 Cox regression analysis was used for identifying predictors of VTE risk among autologous BMT survivors.11 Risk factors evaluated for association with VTE included age at BMT, sex, race/ethnicity, education, income, insurance status, BMI, primary hematologic malignancy, stem cell source, dyslipidemia, hypertension, diabetes, smoking and hormone replacement therapy. Relapse of primary hematologic malignancy and development of subsequent neoplasms were analyzed as time-varying variables. Parsimonious models were obtained using backward variable selection, keeping variables with p <0.1 in the model. Two-sided tests with p <0.05 were considered statistically significant. All analyses were performed with SAS software version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Patient Characteristics
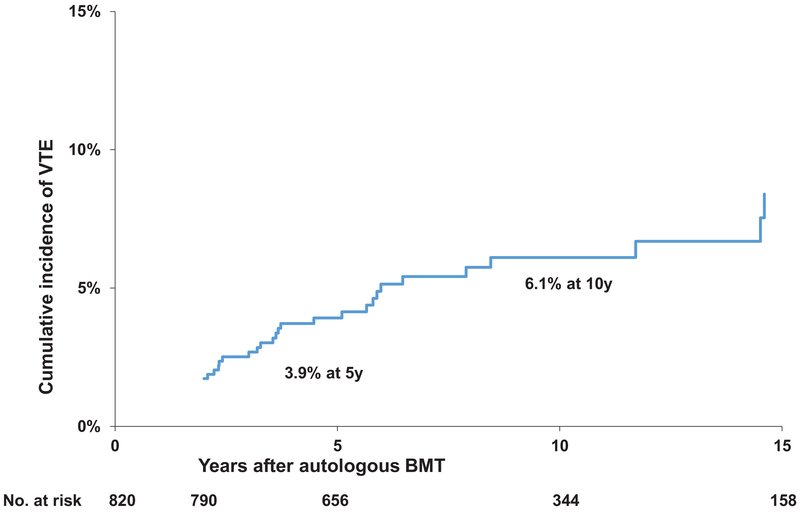
Demographic and clinical characteristics of the BMTSS cohort and siblings are summarized in Table 1. Mean age at BMT was 49.5 years (standard deviation ±12.6 years), whereas the mean age at survey participation was 60.5 years (±10.6) for BMT survivors compared to 53.1 (±12.6) years in siblings. The cohort included 441 (53.8%) males and 648 (79%) non-Hispanic whites. Median duration of follow up from BMT was 9.8 years (inter quartile range: 6.4-14.3y). Primary diagnoses included NHL in 420 patients (51.2%), PCD in 296 (36.1%), AML/MDS in 95 (11.6%), and CML in 9 (1.1%). The majority of patients (781, 95.2%) were transplanted with PBSC. Cyclophosphamide was used as part of conditioning in 443 (54%) patients, etoposide in 449 (54.8%), melphalan in 376 (45.9%) and total body irradiation (TBI) in 362 (44.2%). One hundred and seventeen (14.3%) patients developed a subsequent malignancy or relapse of primary cancer post-BMT. A total of 60 (7.3%) patients developed VTE after BMT; 50% of these occurred ≥2 years after BMT. Median time to VTE development was 2.04 years from the time of BMT (inter quartile range: 2.0-6.22 years). Conditional on surviving ≥2 years after BMT, the cumulative incidence of VTE was 3.9±0.8% at 5 years and 6.1±1.1% at 10 years (Figure 1).
Table 1.
Demographic and clinical characteristics of the autologous BMTSS survivors and siblings
| Variable | BMT survivors | Siblings | P | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| VTE | |||||
| Yes | 60 | 7.3 | 19 | 3.0 | 0.0002 |
| Sex | |||||
| Male | 441 | 53.8 | 259 | 40.2 | <0.0001 |
| Race/Ethnicity | |||||
| White | 648 | 79.0 | 552 | 85.7 | 0.0004 |
| Hispanic | 71 | 8.7 | 49 | 7.6 | |
| Asian | 32 | 3.9 | 23 | 3.6 | |
| Black | 54 | 6.6 | 13 | 2.0 | |
| Other | 15 | 1.8 | 7 | 1.1 | |
| Education | |||||
| ≤High School | 158 | 19.3 | 80 | 12.4 | 0.0007 |
| Some college | 293 | 35.7 | 227 | 35.3 | |
| College Graduate | 367 | 44.8 | 336 | 52.2 | |
| Missing | 2 | 0.2 | 1 | 0.2 | |
| Household income | |||||
| ≤$50k | 250 | 30.5 | 126 | 19.6 | <0.0001 |
| $50–100k | 247 | 30.1 | 200 | 31.1 | |
| >$100k | 232 | 28.3 | 252 | 39.2 | |
| Missing | 91 | 11.1 | 66 | 10.3 | |
| Health insurance | |||||
| Yes | 804 | 98.1 | 628 | 97.5 | 0.4885 |
| History of smoking (ever) | |||||
| Yes | 326 | 39.8 | 204 | 31.7 | 0.001 |
| Diabetes | |||||
| Yes | 140 | 17.1 | 50 | 7.8 | <0.0001 |
| Hypertension | |||||
| Yes | 354 | 43.2 | 190 | 29.5 | <0.0001 |
| Dyslipidemia | |||||
| Yes | 312 | 38.1 | 147 | 22.8 | <0.0001 |
| Female Hormone replacement | |||||
| Yes | 101 | 12.3 | 106 | 16.5 | 0.02 |
| Testosterone replacement | |||||
| Yes | 138 | 16.8 | 28 | 4.4 | <0.0001 |
| Primary diagnosis | |||||
| AML/MDS | 95 | 11.6 | |||
| CML | 9 | 1.1 | |||
| NHL | 420 | 51.2 | |||
| PCD | 296 | 36.1 | |||
| Stem cell source | |||||
| PBSC | 781 | 95.2 | |||
| Bone Marrow | 39 | 4.8 | |||
| Conditioning regimen | |||||
| Busulfan | 76 | 9.3 | |||
| Carmustine | 198 | 24.2 | |||
| Cytoxan | 443 | 54.0 | |||
| Etoposide | 449 | 54.8 | |||
| Melphalan | 376 | 45.9 | |||
| Other | 112 | 13.7 | |||
| Any radiation | 362 | 44.2 | |||
| Relapse/SMN | |||||
| Yes | 117 | 14.27 | |||
Abbreviations: BMTSS-2, Blood or Marrow Transplant Survivor Study-2; BMT, blood or marrow transplant; VTE, venous thromboembolism; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; PCD, plasma cell disorder; GvHD, graft versus host disease; PBSC, peripheral blood stem cells.
Figure 1.
Cumulative incidence of venous thromboembolism in autologous BMT survivors
Risk of VTE – BMT survivors vs. siblings:
Logistic regression analysis after adjusting for age, sex, socioeconomic status, health insurance, comorbidities (diabetes, hypertension, dyslipidemia, BMI), smoking and hormone replacemen therapy, showed that the odds of developing a VTE were significantly higher in BMT survivors (odds ratio [OR]=2.62, 95% confidence interval [CI]: 1.55-4.43, p<0.0004) as compared with siblings (Table 2).
Table 2.
Risk of Venous Thromboembolism in autologous BMT survivors compared with a sibling cohort
| Variable | Univariate | Multivariate | Parsimonious* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Cohort | |||||||||
| Siblings | 1.00 | ||||||||
| BMT cohort | 2.60 | (1.5,4.4) | 0.000 | 2.37 | (1.4,4.2) | 0.003 | 2.62 | (1.6,4.4) | 0.0004 |
| Age at survey | 1.02 | (1.0,1.1) | 0.021 | 1.01 | (1.0,1.0) | 0.221 | |||
| BMI | 0.99 | (1.0,1.0) | 0.525 | 0.98 | (0.9,1.0) | 0.375 | |||
| Sex | |||||||||
| Females | 1.00 | ||||||||
| Male | 1.19 | (0.8,1.9) | 0.455 | 1.06 | (0.6,1.8) | 0.834 | |||
| Race/Ethnicity | |||||||||
| Other | 1.00 | 1.00 | |||||||
| Non-Hispanic white | 1.38 | (0.7,2.7) | 0.331 | 1.45 | (0.7,2.9) | 0.288 | |||
| Education | |||||||||
| ≤High School | 1.00 | 1.00 | |||||||
| Some college | 1.30 | (0.6,2.7) | 0.490 | 1.54 | (0.7,3.3) | 0.266 | |||
| College Graduate | 1.41 | (0.7,2.9) | 0.340 | 1.84 | (0.9,3.9) | 0.114 | |||
| Household Income | |||||||||
| ≤$50K | 1.00 | 1.00 | |||||||
| >$50K | 0.69 | (0.4,1.1) | 0.133 | 0.66 | (0.4,1.1) | 0.122 | |||
| Missing | 0.43 | (0.2,1.1) | 0.085 | 0.41 | (0.2,1.1) | 0.080 | |||
| Health insurance | |||||||||
| Yes | 0.56 | (0.1,4.2) | 0.571 | 0.61 | (0.1,4.7) | 0.631 | |||
| History of smoking | |||||||||
| Yes | 1.14 | (0.7,1.8) | 0.590 | 1.01 | (0.6,1.7) | 0.954 | |||
| Diabetes | |||||||||
| Yes | 1.34 | (0.7,2.5) | 0.345 | 1.25 | (0.6,2.5) | 0.512 | |||
| Hypertension | |||||||||
| Yes | 1.30 | (0.8,2.1) | 0.267 | 1.21 | (0.7,2.0) | 0.467 | |||
| Hyperlipidemia | |||||||||
| Yes | 1.01 | (0.6,1.7) | 0.954 | 0.80 | (0.5,1.4) | 0.403 | |||
| Female hormone replacement | |||||||||
| Yes | 0.87 | (0.4,1.7) | 0.698 | 0.91 | (0.4,1.9) | 0.791 | |||
| Testosterone replacement | |||||||||
| Yes | 1.28 | (0.7,2.5) | 0.457 | 0.98 | (0.5,2.0) | 0.951 | |||
Parsimonious model was obtained using backward variable selection, keeping variables with p<0.1 in the model.
Abbreviations: BMT, blood or marrow transplant; OR, odds ratio; CI, confidence interval; BMI, body mass index.
Risk of VTE among autologous BMT survivors:
Diagnosis of plasma cell disorder (HR=2.37, 95%CI: 1.3-4.2, p=0.004) and annual house hold income ≤$50,000 (HR=2.02, 95%CI 1.2-3.6, p=0.015) were associated with increased VTE risk (Table 3).
Table 3.
Risk factors for Venous Thromboembolism in BMT survivors
| Category | Univariate | Multivariate | Parsimonious* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Sex | |||||||||
| Female | 1.00 | 1.00 | |||||||
| Male | 0.99 | (0.6,1.6) | 0.96 | 0.99 | (0.6,1.8) | 0.96 | |||
| Race/ethnicity | |||||||||
| Other | 1.00 | 1.00 | |||||||
| Whites | 1.42 | (0.7,2.9) | 0.34 | 1.41 | (0.7,3.0) | 0.36 | |||
| Age at diagnosis | 1.01 | (1.0,1.0) | 0.20 | ||||||
| Age at transplant | 1.02 | (1.0,1.0) | 0.06 | 1.01 | (1.0,1.0) | 0.28 | |||
| BMI | 0.98 | (0.9,1.03) | 0.34 | 0.96 | (0.9,1.0) | 0.08 | 0.96 | (0.9,1.0) | 0.10 |
| Education | |||||||||
| ≤High School | 1.00 | 1.00 | |||||||
| Some college | 1.09 | (0.5,2.4) | 0.83 | 1.25 | (0.6,2.8) | 0.60 | 1.25 | (0.6,2.8) | 0.59 |
| College Graduate | 1.59 | (0.8,3.3) | 0.22 | 2.08 | (0.9,4.6) | 0.07 | 2.04 | (0.9,4.5) | 0.07 |
| Household income | |||||||||
| >$50k | 1.00 | 1.00 | |||||||
| ≤$50k | 1.60 | (1.0,2.7) | 0.08 | 1.91 | (1.1,3.4) | 0.029 | 2.02 | (1.2,3.6) | 0.015 |
| Missing | 0.31 | (0.1,1.03) | 0.06 | 0.27 | (0.1,0.9) | 0.036 | 0.27 | (0.1,0.9) | 0.03 |
| Health insurance | |||||||||
| Yes | 0.92 | (0.1,6.6) | 0.93 | 0.87 | (0.1,6.5) | 0.89 | |||
| Ever smoked | |||||||||
| Yes | 1.03 | (0.6,1.7) | 0.92 | 1.08 | (0.6,1.9) | 0.77 | |||
| Diabetes | |||||||||
| Yes | 1.23 | (0.7,2.3) | 0.52 | 1.45 | (0.7,2.9) | 0.30 | |||
| Hypertension | |||||||||
| Yes | 1.19 | (0.7,2.0) | 0.50 | 1.21 | (0.7,2.1) | 0.50 | |||
| Hyperlipidemia | |||||||||
| Yes | 0.74 | (0.4,1.3) | 0.27 | 0.72 | (0.4,1.3) | 0.27 | |||
| Female hormone replacement | |||||||||
| Yes | 0.84 | (0.4,1.9) | 0.67 | 0.78 | (0.3,1.9) | 0.58 | |||
| Testosterone replacement | |||||||||
| Yes | 0.78 | (0.4,1.6) | 0.48 | 0.84 | (0.4,1.8) | 0.66 | |||
| Primary diagnosis | |||||||||
| NHL | 1.00 | 1.00 | |||||||
| AML/MDS | 1.51 | (0.7,3.3) | 0.30 | 1.82 | (0.8,4.2) | 0.15 | 1.58 | (0.7,3.5) | 0.26 |
| CML | 1.49 | (0.2,11.1) | 0.70 | 1.76 | (0.2,14.0) | 0.59 | 1.50 | (0.2,11.3) | 0.69 |
| PCD | 2.25 | (1.3,4.0) | 0.005 | 2.04 | (1.1,3.7) | 0.02 | 2.37 | (1.3,4.2) | 0.004 |
| Stem cell source | |||||||||
| Bone Marrow | 1.00 | 1.00 | |||||||
| PBSC | 1.51 | (0.5,4.9) | 0.49 | 1.34 | (0.4,4.8) | 0.65 | |||
| Relapse/SMN | |||||||||
| No | 1.00 | 1.00 | |||||||
| Yes | 1.93 | (0.8, 4.9) | 0.16 | 1.96 | (0.8, 5.0) | 0.17 | |||
Parsimonious model was obtained using backward variable selection, keeping variables with p<0.1 in the model.
Abbreviations: BMT, blood or marrow transplant; HR, Hazard ratio; CI, Confidence interval; BMI, body mass index; CML, chronic myeloid leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; PCD, plasma cell disorder; GvHD, graft versus host disease; PBSC, peripheral blood stem cells; SMN, secondary malignant neoplasms.
Discussion
We found the risk of VTE to be 2.6-fold higher among autologous BMT survivors when compared with a sibling cohort without cancer. Conditional on surviving ≥2 years after BMT, the 10 year cumulative incidence of VTE was 6.1% after autologous BMT. These findings provide evidence for ongoing vigilance regarding this complication.
Long term BMT survivors are at increased risk of developing atherosclerosis, arterial vascular events and new onset cardiovascular risk factors such as diabetes, hypertension and dyslipidemia.12,13 There had been a paucity of information regarding the risk of VTE among long term autologous BMT survivors; our study is the first to address this issue. We found that an underlying diagnosis of PCD and low income was associated with an increased risk of VTE. Previous studies have shown that patients with PCD are at increased risk of VTE, either due to the primary disease or treatment.14 Multiple mechanisms such as clonality, inhibition of natural anticoagulants or hypercoagulability due to inflammatory cytokines, increased von Willebrand factor, factor VIII, fibrinogen levels, decreased protein S levels, acquired activated protein C resistance, or interference of fibrin structure by paraprotein may contribute to this increased risk.15,16 This risk increases several fold in patients treated with thalidomide, lenalidomide or pomalidomide in combination with high dose dexamethasone, doxorubicin or multi-agent chemotherapy and in patients with ≥2 individual or PCD risk factors, and hence thromboprophylaxis is recommended.15 Due to the increased risk, several clinical trials have added thromboprophylaxis to the induction regimens of multiple myeloma, with subsequent decrease in VTE incidence.17,18 Our study shows that the risk of VTE remains elevated several years after autologous BMT in patients with PCD. Some of these patients are likely being treated with maintenance therapies which may contribute to continued increased risk of VTE after BMT. We were unable to abstract the post-transplant maintenance therapy for myeloma patients in our study and could not assess this as a risk factor for VTE. Thus, the increased risk of VTE in patients with PCD could be because of post-transplant exposure to maintenance therapy or the underlying diagnosis of PCD, or a combination of both. The increasing use of post-transplant maintenance therapy with lenalidomide in the current era may add to the VTE risk after autologous BMT in PCD patients. It is important to thoroughly investigate the risk factors and identify high risk PCD patients who may benefit from thromboprophylaxis after BMT, and should be explored further in prospective studies.
Relapse of primary cancer or development of second malignancy was not associated with increased risk of VTE. Co-morbidities such as diabetes, hypertension, dyslipidemia, obesity, history of smoking and use of oral contraception and hormonal therapy are known to contribute to the risk of VTE in the general population.19–21 However, they were not associated with VTE risk in autologous BMT survivors in our study. There is also increasing knowledge about clonal hematopoiesis of indeterminate potential (CHIP) and cardiovascular disease, and it would be important to study the association between CHIP and VTE in future studies.
The association between socioeconomic status and arterial cardiovascular disease is well established. A large population based study from Netherlands showed that high neighborhood socioeconomic status is associated with a lower risk of first VTE.22 In another prospective study from Sweden, low income and lower level of education were independently associated with increased risk of VTE.23 It is possible that access to health care, physical activity, and general health awareness are lower in BMT survivors with low socioeconomic status and may be contributing to the increased risk of VTE.
Our study needs to be placed in the context of its limitations. First, the study relied on self-report for identifying patients with VTE. However, the validity of the BMTSS questionnaire has been examined previously, showing that survivors are able to report the occurrence of adverse medical conditions with accuracy.9 Second, since our study was based on patient surveys, we could not capture complete details regarding clinical presentation and laboratory abnormalities at the time of VTE development. Future studies aimed at identifying biomarkers associated with VTE risk in BMT recipients are warranted. Though some patients provided details regarding the site of VTE, this information was not available for several patients. Hence, we were not able to categorize the findings based on the site of VTE. Third, we did not have information regarding family history of VTE, level of physical activity, corticosteroid use and hospitalizations at the time of VTE development. Fourth, the risk of VTE in BMT recipients was conditional on surviving the first 2 years after BMT. BMT recipients who died within the first 2 years were not included in the analysis, likely resulting in an underestimation of VTE risk after BMT. The incidence of VTE in the peri-transplant period is likely higher than what we found in our study patients, as a significant number of patients with VTE may have died within the first 2 years after transplant. Our intention was to determine the risk of VTE in long term BMT survivors. We found that this risk remains high several years after transplant, necessitating continued risk assessment in these patients. These limitations notwithstanding, our study provides a comprehensive analysis of the long-term risk of VTE in autologous BMT recipients and the associated risk factors.
In conclusion, autologous BMT survivors have a 2.6-fold higher risk of VTE when compared with siblings without cancer. The risk continues to increase for at least 10y post-BMT. Patients with PCD and lower socioeconomic status are particularly at high risk. In light of these observations, it is important to delve further in identifying vulnerable subpopulations among PCD patients treated with autologous BMT who may benefit from thromboprophylaxis.
Highlights.
Long term survivors of Blood or marrow transplantation are at increased risk of venous thromboembolism compared to their siblings.
A diagnosis of plasma cell disorder and lower socioeconomic status were associated with increased venous thrombosis risk.
Acknowledgements:
Funding/Support: This study was supported in part by grants from the National Cancer Institute (R01 CA078938), U01 CA213140 and the Leukemia and Lymphoma Society (R6502-16) (S Bhatia).
Funding/Support: This study was supported in part by grants from the National Cancer Institute (NIH) (R01 CA078938), and the Leukemia and Lymphoma Society (R6502-16) for Dr. Smita Bhatia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References:
- 1.Kaufman PA, Jones RB, Greenberg CS, Peters WP. Autologous bone marrow transplantation and factor XII, factor VII, and protein C deficiencies. Report of a new association and its possible relationship to endothelial cell injury. Cancer. 1990;66:515–521. [DOI] [PubMed] [Google Scholar]
- 2.Verheij M, Dewit LG, Boomgaard MN, Brinkman HJ, van Mourik JA. Ionizing radiation enhances platelet adhesion to the extracellular matrix of human endothelial cells by an increase in the release of von Willebrand factor. Radiat Res. 1994;137:202–207. [PubMed] [Google Scholar]
- 3.Vannucchi AM, Rafanelli D, Longo G, et al. Early hemostatic alterations following bone marrow transplantation: a prospective study. Haematologica. 1994;79:519–525. [PubMed] [Google Scholar]
- 4.Zahid MF, Murad MH, Litzow MR, et al. Venous thromboembolism following hematopoietic stem cell transplantation-a systematic review and meta-analysis. Annals of hematology. 2016;95:1457–1464. [DOI] [PubMed] [Google Scholar]
- 5.Gonsalves A, Carrier M, Wells PS, McDiarmid SA, Huebsch LB, Allan DS. Incidence of symptomatic venous thromboembolism following hematopoietic stem cell transplantation. J Thromb Haemost. 2008;6:1468–1473. [DOI] [PubMed] [Google Scholar]
- 6.Hegerova L, Bachan A, Cao Q, et al. Catheter-Related Thrombosis in Patients with Lymphoma or Myeloma Undergoing Autologous Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018;24:e20–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangaraju R, Chen Y, Hageman L, et al. Late mortality in blood or marrow transplant survivors with venous thromboembolism: report from the Blood or Marrow Transplant Survivor Study. Br J Haematol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139; quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. [DOI] [PubMed] [Google Scholar]
- 10.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. [DOI] [PubMed] [Google Scholar]
- 11.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological). 1972;34:187–220. [Google Scholar]
- 12.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatia S Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up. Expert Rev Hematol. 2011;4:437–452; quiz 453–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:4848–4857. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. [DOI] [PubMed] [Google Scholar]
- 16.Leebeek FW. Update of thrombosis in multiple myeloma. Thromb Res. 2016;140 Suppl 1:S76–80. [DOI] [PubMed] [Google Scholar]
- 17.Cavo M, Zamagni E, Tosi P, et al. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologica. 2004;89:826–831. [PubMed] [Google Scholar]
- 18.Baz R, Li L, Kottke-Marchant K, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin Proc. 2005;80:1568–1574. [DOI] [PubMed] [Google Scholar]
- 19.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. [DOI] [PubMed] [Google Scholar]
- 20.Grady D, Hulley SB, Furberg C. Venous thromboembolic events associated with hormone replacement therapy. Jama. 1997;278:477. [PubMed] [Google Scholar]
- 21.Beyer-Westendorf J, Bauersachs R, Hach-Wunderle V, Zotz RB, Rott H. Sex hormones and venous thromboembolism - from contraception to hormone replacement therapy. Vasa. 2018;47:441–450. [DOI] [PubMed] [Google Scholar]
- 22.Kort D, van Rein N, van der Meer FJM, et al. Relationship between neighborhood socioeconomic status and venous thromboembolism: results from a population-based study. J Thromb Haemost. 2017;15:2352–2360. [DOI] [PubMed] [Google Scholar]
- 23.Isma N, Merlo J, Ohlsson H, Svensson PJ, Lindblad B, Gottsater A. Socioeconomic factors and concomitant diseases are related to the risk for venous thromboembolism during long time follow-up. J Thromb Thrombolysis. 2013;36:58–64. [DOI] [PubMed] [Google Scholar]