Abstract
Objective:
Though bleeding frequently occurs in critical illness, no published definition to date describes the severity of bleeding accurately in critically ill children. We sought to develop diagnostic criteria for bleeding severity in critically ill children.
Design:
Delphi consensus process of multidisciplinary experts in bleeding/hemostasis in critically ill children, followed by prospective cohort study to test internal validity.
Setting:
Pediatric intensive care unit.
Patients:
Children at risk of bleeding in pediatric intensive care units.
Interventions:
None.
Measurements and Main Results:
Twenty-four physicians world-wide (10 on a steering committee and 14 on an expert committee) from disciplines related to bleeding participated in development of a definition for clinically relevant bleeding. A provisional definition was created from 35 descriptors of bleeding. Using a modified online Delphi process and conference calls, the final definition resulted after seven rounds of voting. The Bleeding Assessment Scale in critically Ill Children (BASIC) definition categorizes bleeding into severe, moderate and minimal, using organ dysfunction, proportional changes in vital signs, anemia and quantifiable bleeding. The criteria do not include treatments such as red cell transfusion or surgical interventions performed in response to the bleed. The definition was prospectively applied to forty critically ill children with forty-six distinct bleeding episodes. The kappa statistic between the two observers was 0.74 (95% CI 0.57-0.91) representing substantial inter-rater reliability.
Conclusion:
The BASIC definition of clinically relevant bleeding severity is the first physician-driven definition applicable for bleeding in critically ill children derived via international expert consensus. The BASIC definition includes clear criteria for bleeding severity in critically ill children. We anticipate that it will facilitate clinical communication among pediatric intensivists pertaining to bleeding, and serve in the design of future epidemiologic studies if it is validated with patient outcomes.
Keywords: child, critical illness, hemorrhage, critical care outcomes, Delphi technique, consensus
INTRODUCTION
Bleeding is a frequent complication of critical illness that is associated with significant mortality and morbidity. In certain adult populations, bleeding is independently associated with mortality [1,2], longer duration of mechanical ventilation, and longer intensive care unit (ICU) stays [3,4]. Similarly, in critically ill children, bleeding has been associated with increased mortality, increased duration of vasopressor support, and longer ICU stays [5, 6].
There are numerous definitions pertaining to the clinical significance of bleeding, but none are designed for critically ill children [7]. Current definitions contain descriptors that are subjective, lack bleeding typically seen in critically ill children (e.g. in endotracheal tube), include transfusions as criteria despite variability in clinical decision-making, often include vital sign changes pertinent to adults but not children, and are rarely correlated and validated to clinical outcomes. This lack of an operational definition prevents a clear understanding of the epidemiology of bleeding in clinical practice and limits clinical research on strategies to prevent, or stop bleeding. Additionally, the absence of validated bleeding scales in critically ill children leads to heterogeneity in how clinicians perceive bleeding severity. The National Institute of Health (NIH), National Heart, Lung and Blood Institute (NHLBI) and the Biomedical Excellence for Safer Transfusion (BEST) Collaborative have identified development of a validated bleeding measurement scale as a high priority [8,9].
A validated operational definition of clinically relevant bleeding can have many applications. It can provide common language to study the epidemiology of bleeding in particular populations of critically ill patients. It can help clinicians assess bleeding events and decide whether such events require specific interventions. We sought to develop a multi-disciplinary, physician-driven definition of clinically relevant bleeding for critically ill children, known as the Bleeding Assessment Scale in critically Ill Children (BASIC), and test the reproducibility of the definition in a cohort of critically ill children with bleeding.
METHODS
Definition Development
A steering committee was constituted for the study program comprised of the executive members of an international research consortium focused on transfusion medicine, hemostasis and blood management (BloodNet). Utilizing results of our recently published international survey of pediatric intensivists on clinically relevant bleeding [10], the steering committee developed a list of possible criteria to include within the definition. The majority of surveyed pediatric intensivists identified 35 clinical scenarios as “definitely clinically relevant”, as described previously. These items were incorporated into an initial proposed definition. The initial proposed definition grouped the elements into “severe”, “moderate” and “minimal” as it related to the clinical relevance of the bleeding event.
An independent panel of experts was constituted that did not include any of the members of the steering committee in order to allow for a non-biased Delphi process; each expert had previously published in the field of pediatric transfusion medicine and/or hemostasis. Before beginning the Delphi process, the expert committee was given a document summarizing the background, including the lack of current applicable definitions, and the purpose, including the use of the definition in clinical assessments and epidemiologic studies.
Using the Research and Development/UCLA Appropriateness Methods [11], adapted for an online-only Delphi process, each of the proposed elements (grouped into severe, moderate and minimal) were scored by the expert committee via an online survey tool on Research Electronic Data Capture (REDCap) tools hosted at Virginia Commonwealth University. A Likert scale of 1-9 was used with 1 representing “strongly disagree” and 9 representing “strongly agree” with inclusion of the element within both the definition itself, as well as its characterization of severity as it related to clinical relevance. For any item given a score less than 7, the member of the expert committee was asked why the element should not be included in the definition (or placed in a different category of severity) and alternatives were requested that were likely to achieve consensus. During the first Delphi round, members of the expert committee also had the opportunity to add new elements to the definition that had not previously been considered by the steering committee. Agreement within the expert committee was defined a priori as 80% of the experts rating the element as a score of 7, 8 or 9. A response rate of 100% was required for each round of voting. Items that were not accepted by at least 80% of the experts were deleted or reworded according to the experts’ suggestions, with subsequent votes. Items that could not be resolved through two rounds of the voting process by the expert committee were discussed via conference call prior to the next round of voting. Before the definition was finalized, the steering committee provided feedback with suggestions that were considered and voted upon by the expert committee.
Testing of Definition
To test the inter- and intra-rater reliability of the definition, children aged 1 month to 16 years of age within a single center, academic PICU were screened for bleeding and enrolled following parental consent. Basic demographic information and bleeding information that were a part of the BASIC definition but not routinely recorded in the electronic medical record (EMR) were collected and recorded in a REDCap database. Two separate pediatric ICU (PICU) physicians, neither of whom were involved in the development of the definition, reviewed the EMR, as well as details recorded in the database, and scored the bleeding event as minimal, moderate or severe, using the BASIC definition. Two weeks following their initial assessment, they again scored the bleeding based on the EMR and database. The study was approved by the Institutional Review Board at Weill Cornell Medicine.
Demographic and clinical outcomes were described as N (%) or median and interquartile range (IQR) as appropriate. The inter-rater and intra-rater reliability was assessed by calculation of the free marginal kappa [12]. The interpretation of the value for strength of agreement was: ≤0 = poor, 0.01–0.20 = slight, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial, and 0.81–1 = almost perfect [13]. All descriptive statistical analyses were conducted using SPSS version 25 (IBM Corp, Armonk, NY).
RESULTS
Development of Definition
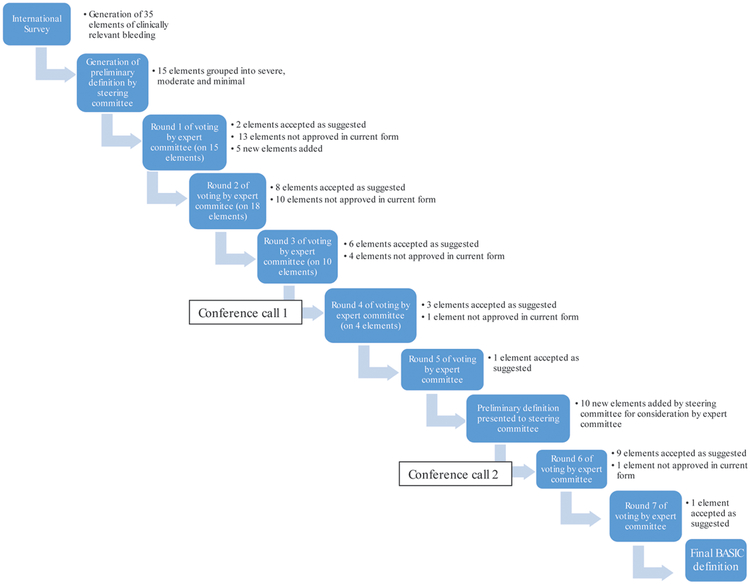
Eight pediatric intensivists, one pediatric hematologist/oncologist and one transfusion medicine specialist participated in the steering committee. Fourteen physicians (six pediatric intensivists, one pediatric cardiac intensivist, two pediatric surgeons, two pediatric anesthesiologists and three transfusion medicine specialists) participated in the expert committee from North America (8/14), Europe (5/14) and Australasia (1/14). Figure 1 outlines the process of item development and agreement. The steering committee initially presented fifteen elements (grouped into severe, moderate and minimal) to the expert committee. An additional five elements were added by the expert committee who then undertook five Delphi rounds to arrive at a preliminary definition which was presented to the steering committee. Upon review of the preliminary definition, the steering committee suggested the addition of ten elements to the expert committee for consideration before the final voting process. In total, seven rounds of suggestion/revision/voting and two conference calls were required to achieve a consensus definition.
Figure 1.
Development of BASIC definition. Each box represents an action by either the steering committee or expert committee. Each arrow represents revisions made to the elements of the definition based on feedback from the expert committee
The consensus definition obtained for Bleeding Assessment Scale in critically Ill children (BASIC) is presented in Table 1. Bleeding severity was categorized into severe (5 elements), moderate (5 elements) and minimal (6 elements). Separate descriptions were developed for “progressive” and “fatal” bleeding. The expert panel concluded that the presence of any one element listed in the criteria for severe or minimal bleeding is sufficient to define bleeding as that stipulated in each of these categories of bleeding. Any sites of bleeding that do not meet physiologic or anatomic criteria for “severe” bleeding, and are not specifically mentioned in the criteria for “minimal” bleeding, such as menorrhagia, are considered “moderate” bleeding.
Table 1.
Consensus Definition of Clinically Relevant Bleeding
| Bleeding Assessment Scale in critically Ill Children (BASIC) |
|---|
Any of the following criteria define severe bleeding:
|
All of the following criteria must be present to define moderate bleeding:
|
Any of the following criteria define minimal bleeding:
|
| Progressive bleeding: Progressive bleeding is bleeding that either progresses to a higher severity category (e.g. from minimal to moderate bleeding, or from moderate to severe bleeding) or to a higher number of criteria within the same category (e.g. hemodynamic instability progressing to organ failure, or streaks of blood in the ETT and subsequent slightly blood-tinged urine). |
| Fatal Bleeding: Bleeding that is the direct cause leading to death will be characterized as Fatal Bleeding. |
Organ dysfunction, as measured by the PEdiatric Logistic Organ Dysfunction-2 (PELOD-2) [14] score, was incorporated into elements of the BASIC definition. All vital signs and laboratory values (primarily hemoglobin level) are expressed in percentage change from baseline values. Though the proposed definition focuses on the physiologic effects of bleeding events, bleeding in three specific anatomic locations (intraspinal, intraarticular and intraocular) also defined severe bleeding. It was deemed necessary to consider anatomic location because the physiologic effects of bleeding in these specific locations may not affect overall measures of organ dysfunction or cause changes in vital signs. The volume of blood loss selected to describe severe bleeding (> 5 ml/kg/hr for 1 hour or more) was derived directly from results of the previously published international survey [10]. After significant debate, members of the expert committee agreed that interventions used to control bleeding (i.e. transfusion of RBCs or procedures to stop bleeding) would be excluded from the definition, because these were believed to be physician-dependent.
Testing of Definition
Forty children with 46 distinct bleeding episodes were enrolled. The children were 27/40 (68%) male with a median (IQR) age of 6.1 (0.8-12.3) years. Paired assessments were assessed for inter-rater reliability. The percent overall agreement was 83%. The free marginal kappa between the two observers was 0.74 (95% CI 0.57-0.91) representing substantial inter-rater reliability. Assessments completed at two different time points (at time of bleeding event and two weeks after) were evaluated for intra-rater reliability. The free marginal kappa between the two time points was 0.74 (95% CI 0.61-0.86) representing substantial intra-rater reliability.
DISCUSSION
The Bleeding Assessment Scale in critically Ill Children (BASIC) definition represents the first multidisciplinary, international, physician-driven, consensus definition for clinically relevant bleeding applicable to critically ill children [7]. It was designed with rigorous methodology that involved a preliminary extensive review of the medical literature [7], the input of 225 surveyed clinicians [10], opinion from a multi-disciplinary expert committee, and guidance from a steering committee with substantial expertise in transfusion research. The goal of the process was to create a definition of bleeding severity that would be specifically applicable across populations of critically ill children. BASIC is the first definition with bleeding criteria that includes volume of blood loss and complications of bleeding. No criteria in the definition are linked to therapies or interventions such as red cell transfusion or surgery because decisions to proceed with these are often based on subjective clinical decision-making. Evaluation of the internal validity of the definition demonstrates both good inter- and intra-rater reliability.
Defining clinically relevant bleeding can be challenging. In its simplest form, bleeding could be defined as “blood exiting from an intravascular space.” However, the clinical relevance of such a definition is vague. Unlike the definition of other clinical entities such as acute respiratory distress syndrome (ARDS) [15], which is based on diagnostic criteria not related to clinical consequences, the definition of bleeding severity must consider its clinical impact, just as the most recent definition of sepsis which includes resultant tissue hypoperfusion and tachycardia [16]. BASIC attempts to relate the severity of bleeding with clinical consequences, such as organ dysfunction or hemodynamic instability, which makes this definition both useful and pertinent in critically ill children with bleeding. The definition allows for a common language to be used amongst caregivers when caring for a critically ill child who is bleeding. Moreover, we believe this consensus definition will allow us to clearly identify the severity of bleeding events and describe their epidemiology.
Organ dysfunction, captured by the PELOD-2 score, was selected as a marker of severity for the clinical relevance of bleeding by the steering and expert committees. The PELOD-2 score has been validated as an accurate predictor of in-hospital mortality in general populations of critically ill children in both developed [14] and developing countries [17], and has also been validated among children with infection [18], acute respiratory failure [19], as well as those requiring prophylactic/therapeutic plasma transfusions [20]. More importantly, in critically ill children, an increase by one point in the PELOD-2 score during PICU stay has also been independently associated with a 30% increased risk of mortality[20,21].
The association between a quantified percent drop in hemoglobin and mortality is less clear. Some studies in adults have reported an association between the severity of hemoglobin drop and mortality after endovascular repair surgery [22] and in adults with decompensated heart failure [23]. To our knowledge, no similar studies have been reported for a pediatric population. However, anemia acquired in the PICU, which admittedly can be attributed to bleeding as well as other causes, has been associated with worse clinical outcome [24].
Some studies in children have attempted to establish an association with amount of bleeding and outcome. In a small retrospective cohort of children following cardiopulmonary bypass, Bercovitz and colleagues demonstrated that daily chest tube bleeding of >84 mL/kg/day was independently associated with longer hospital length of stay [25]. In a more recent evaluation of children supported by extracorporeal life support (ECLS), quantifiable bleeding from a chest tube >60 mL/kg/day was independently associated with increased risk of in-hospital mortality and longer PICU length of stay [26], although it was impossible to assess bleeding on an hourly basis. The BASIC definition assesses the effect of bleeding by considering both amount of blood loss and the complications of bleeding.
The definition was developed from physician experience and is thereby physician-driven, as opposed to data-driven. However, in developing a common language, it is important to have physician input. Some elements of the definition may elicit criticism. Criteria for minimal bleeding may be somewhat subjective, which may decrease the reproducibility of the definition. However, these elements were retained in the definition as they are commonly reported by nurses and may impact physician thresholds for interventions. As demonstrated by an evaluation of previous bleeding assessments as they related to platelet transfusion trials [27], the education and training of bleeding assessors, especially in the assessment of minor and moderate bleeding events, is necessary.
The implied causality between the bleeding and the change in vital signs or PELOD-2 might also be subjective, although this imputation is also used in the Sepsis-3 definition [16]. Additionally, the criteria are grouped into severity categories and involve numerical cut-off values (such as 5 mL/kg/hr). Whereas severity categories are easier to use, defining thresholds introduces bias as such thresholds are often subjective. In reality, the clinical relevance of a bleeding event is likely a continuous variable. However, binary outcomes and numerical thresholds have been used in the case of other clinical definitions including sepsis [16] and ARDS [15].
BASIC may be applied broadly in diverse pediatric pathologies. However, the clinical relevance may vary in certain patient populations. For example, an infant supported by extracorporeal life support may not tolerate the loss of >5 mL/kg/hr of blood for a prolonged time and his/her mortality be significantly affected by that blood loss whereas a teenager who sustained a gunshot wound might tolerate the same amount of blood loss without significant clinical impact. It may be difficult to discriminate if organ dysfunction (or other physiologic changes) are due to bleeding alone given the large number of interventions that each patient may receive. By grouping both bleeding amount and bleeding consequences, the ability of the definition to accurately discriminate meaningful clinical differences may be compromised. However, by combining both bleeding amount and bleeding consequences, we believe the definition can address the situations described above in which very different blood volumes and causes of bleeding could have very different clinical impacts.
With these limitations in mind, the following steps must be taken to further develop our understanding of the clinical relevance of bleeding in critically ill children. The internal and external validity of the BASIC definition must be further tested. If strong intra- and interrater reliability can be verified, cutoff variables can be compared to outcomes in specific patient populations. Additionally, the BASIC criteria can be applied to certain patient populations to define the epidemiology of bleeding. A clearer understanding of the epidemiology will allow researchers to power large observational and interventional studies not only to develop a numerical score of bleeding that can be validated to patient outcomes, but also decide on the most appropriate treatments in different clinical settings including when they should initiated.
CONCLUSIONS
The BASIC definition of clinically relevant bleeding is the first physician-driven diagnostic criteria for bleeding derived via expert consensus applicable to critically ill children. If it is validated, the BASIC definition can be used by intensivists as common descriptors for clinical communication, as well as criteria for epidemiologic studies.
Acknowledgments
Financial Support: CTSA award No. UL1TR000058 from the National Center for Advancing Translational Sciences (for access to REDCap).
Footnotes
Copyright form disclosure: Drs. Nellis and Josephson received support for article research from the National Institutes of Health. Dr. Zantek disclosed that she is an Executive Board member of the North American Specialized Coagulation Laboratory Association, and that her spouse is an employee of Boston Scientific and owns stock in Boston Scientific and ENDO International PLC. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Nevo S, Swan V, Enger C, et al. Acute bleeding after bone marrow transplantation (BMT) - incidence and effect on survival. A quantitative analysis in 1,402 patients. Blood 1998, 91(4):1469–1477. [PubMed] [Google Scholar]
- 2.Eikelboom JW, Mehta SR, Anand SS, et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006, 114(8):774–782. [DOI] [PubMed] [Google Scholar]
- 3.Arnold DM, Donahoe L, Clarke FJ, et al. Bleeding during critical illness: a prospective cohort study using a new measurement tool. Clinical and investigative medicine 2007, 30(2):E93–102. [DOI] [PubMed] [Google Scholar]
- 4.Christensen MC, Krapf S, Kempel A, et al. Costs of excessive postoperative hemorrhage in cardiac surgery. J Thorac Cardiovasc Surg 2009, 138(3):687–693. [DOI] [PubMed] [Google Scholar]
- 5.White LJ, Fredericks R, Mannarino CN, et al. Epidemiology of Bleeding in Critically Ill Children. J Pediatr 2017, 184:114–119.e116. [DOI] [PubMed] [Google Scholar]
- 6.Dalton HJ, Garcia-Filion P, Holubkov R, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med 2015, 16(2):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nellis ME, Levasseur J, Stribling J, et al. Bleeding Scales Applicable to Critically Ill Children: A Systematic Review. Pediatr Crit Care Med 2019. March 28. doi: 10.1097/PCC.0000000000001943. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Heddle NM, Cook RJ, Webert KE, et al. Methodologic issues in the use of bleeding as an outcome in transfusion medicine studies. Transfusion 2003, 43(6):742–752. [DOI] [PubMed] [Google Scholar]
- 9.Koreth R, Weinert C, Weisdorf DJ, et al. Measurement of bleeding severity: a critical review. Transfusion 2004, 44(4):605–617. [DOI] [PubMed] [Google Scholar]
- 10.Karam O, Nellis ME, Zantek ND, et al. Criteria for Clinically Relevant Bleeding in Critically Ill Children: An International Survey. Pediatr Crit Care Med. 2019. March;20(3):e137–e144. [DOI] [PubMed] [Google Scholar]
- 11.Fitch K, Bernstein S, Aguilar M. The RAND/UCLA Appropriateness Method User’s Manual. In. Arlington, VA; 2001. [Google Scholar]
- 12.Brennan RL, Prediger DJ. Coefficient Kappa: Some uses, misuses, and alternatives. Educational and Psychological Measurement 1981, 41:687–699. [Google Scholar]
- 13.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977, 33(1):159–174. [PubMed] [Google Scholar]
- 14.Leteurtre S, Duhamel A, Salleron J, et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med 2013, 41(7):1761–1773. [DOI] [PubMed] [Google Scholar]
- 15.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015, 16(5 Suppl 1):S23–40. [DOI] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Nawawy A, Mohsen AA, Abdel-Malik M, et al. Performance of the pediatric logistic organ dysfunction (PELOD) and (PELOD-2) scores in a pediatric intensive care unit of a developing country. Eur J Pediatr 2017, 176(7):849–855. [DOI] [PubMed] [Google Scholar]
- 18.Schlapbach LJ, Straney L, Bellomo R, et al. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive care medicine 2018, 44(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclerc F, Duhamel A, Deken V, et al. Nonrespiratory pediatric logistic organ dysfunction-2 score is a good predictor of mortality in children with acute respiratory failure. Pediatr Crit Care Med 2014, 15(7):590–593. [DOI] [PubMed] [Google Scholar]
- 20.Karam O, Demaret P, Duhamel A, et al. Performance of the PEdiatric Logistic Organ Dysfunction-2 score in critically ill children requiring plasma transfusions. Annals of intensive care 2016, 6(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leteurtre S, Duhamel A, Deken V, et al. Daily estimation of the severity of organ dysfunctions in critically ill children by using the PELOD-2 score. Critical care (London, England) 2015, 19:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorla R, Tsagakis K, Horacek M, et al. Impact of Preoperative Anemia and Postoperative Hemoglobin Drop on the Incidence of Acute Kidney Injury and In-Hospital Mortality in Patients With Type B Acute Aortic Syndromes Undergoing Thoracic Endovascular Aortic Repair. Vascular and endovascular surgery 2017, 51(3):131–138. [DOI] [PubMed] [Google Scholar]
- 23.Damluji AA, Macon C, Fox A, et al. The association between in-hospital hemoglobin changes, cardiovascular events, and mortality in acute decompensated heart failure: Results from the ESCAPE trial. International journal of cardiology 2016, 222:531–537. [DOI] [PubMed] [Google Scholar]
- 24.Bateman ST, Lacroix J, Boven K, et al. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med 2008, 178(1):26–33. [DOI] [PubMed] [Google Scholar]
- 25.Bercovitz RS, Shewmake AC, Newman DK, et al. Validation of a definition of excessive postoperative bleeding in infants undergoing cardiac surgery with cardiopulmonary bypass. J Thorac Cardiovasc Surg 2018, 155(5):2112–2124.e2112. [DOI] [PubMed] [Google Scholar]
- 26.Nellis ME, Dalton H, Karam O. Quantifiable bleeding in children supported by ECMO and outcome. Crit Care Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estcourt LJ, Heddle N, Kaufman R, et al. The challenges of measuring bleeding outcomes in clinical trials of platelet transfusions. Transfusion 2013, 53(7):1531–1543. [DOI] [PubMed] [Google Scholar]