Abstract
OBJECTIVE:
To assess the impact of early triggered palliative care consultation on the outcomes of high risk ICU patients.
DESIGN:
Single-center cluster randomized crossover trial.
SETTING:
Two medical ICUs at Barnes Jewish Hospital.
PATIENTS:
Patients (n=199) admitted to the medical ICUs from August 2017 to May 2018 with a positive palliative care screen indicating high risk for morbidity or mortality.
INTERVENTIONS:
The medical ICUs were randomized to intervention or usual care followed by washout and crossover, with independent assignment of patients to each ICU on admission. Intervention arm patients received a palliative care consultation from an interprofessional team led by board-certified palliative care providers within 48 hours of ICU admission.
MEASUREMENTS AND MAIN RESULTS:
97 patients (48.7%) were assigned to the intervention and 102 (51.3%) to usual care. Transition to do-not-resuscitate/do-not-intubate (DNR/DNI) occurred earlier and significantly more often in the intervention group than the control group (50.5% vs 23.4%, p<0.0001). The intervention group had significantly more transfers to hospice care (18.6% vs 4.9%; p<0.01) with fewer ventilator days (median 4 vs 6 days; p<0.05), tracheostomies performed (1% vs 7.8%; p<0.05), and post-discharge emergency department visits and/or readmissions (17.3% vs 38.9%; p<0.01). While total operating cost was not significantly different, medical ICU (p<0.01) and pharmacy (p<0.05) operating costs were significantly lower in the intervention group. There was no significant difference in ICU length of stay (median 5 vs 5.5 days), hospital length of stay (median 10 vs 11 days), in-hospital mortality (22.6% vs 29.4%), or 30-day mortality between groups (35.1% vs 36.3%) (p values>0.05).
CONCLUSIONS:
Early triggered palliative care consultation was associated with greater transition to DNR/DNI and to hospice care, as well as decreased ICU and post-ICU healthcare resource utilization. Our study suggests that routine palliative care consultation may positively impact the care of high risk, critically ill patients.
TRIAL REGISTRATION:
ClinicalTrials.gov Identifier:
Keywords: Palliative care, critical care, quality improvement, outcome assessment, cardiopulmonary resuscitation, utilization review
INTRODUCTION
In the last two decades, palliative care has emerged as a core component of ICU care. As a specialty dedicated to alleviation of symptoms and clarification of goals of care, palliative care has a logical place in the ICU. Critically ill patients frequently have pain, agitation, and delirium, and their mortality rates remain in excess of 20% in many institutions. 1,2 Current guidelines therefore advocate for the incorporation of palliative care into the ICU regardless of overall prognosis.3,4 However while palliative care has shown benefit across a wide spectrum of disease and healthcare settings, results in the ICU have been mixed.5-8 Multiple palliative care interventions have been trialed in the ICU without consistent statistical benefits, including informational packets for families, ethics consults, embedded palliative care teams, and others.9-11 Although the current body of literature suggests that palliative care interventions may reduce ICU admissions, length of stay (LOS), and resource utilization, these studies have been limited by significant clinical and methodological heterogeneity. Systematic reviews have identified at least thirty unique interventions, with few randomized controlled trials among them.12,13
As a result, despite increasing advocacy for integration of palliative care practices into the ICU, implementing this process in a meaningful way has been challenging. An analysis of 1440 patients across six ICUs found that only 10% of patients at high risk of dying had a palliative care consultation, and on average these consults occurred on the ninth hospital day.14 These late palliative care consults stand in contrast to previous studies showing that earlier palliative care interventions are more effective.15-19 Triggered palliative care consultations to identify and help high risk patients early in their clinical course have been described, however, no recent studies have examined the effect of such interventions in a randomized controlled ICU setting.20-24 Another challenge is quantifying the effect of palliative care consultation, as palliative care team composition and practice can vary by institution.
To that end, we identified an alternative outcome measure using data from a recent retrospective analysis showing that palliative care consultation in the ICU is strongly associated with de-escalation of code status to Do-Not-Resuscitate/Do-Not-Intubate (DNR/DNI).25 Discussions of patient preferences regarding resuscitation are often critically delayed and under-documented in the hospital.26-30 Aggressive care is frequently undesired at the end of life by both patients and healthcare professionals, and early goals of care discussions have been shown to reduce unwanted interventions and potentially futile care, and to improve quality of life.31-34 An appropriate transition to DNR/DNI may be beneficial, particularly in light of the consistently poor outcomes of in-hospital CPR, and is just one possible result of the larger discussions that palliative care teams focus on.35-38 We postulated that palliative care consultation led to earlier and more effective discussion of treatment options, including resuscitation preferences. De-escalation in code status following palliative care consultation therefore can serve as an easily-identified surrogate measure for effective goals of care discussions as well as a potential marker for decreased healthcare resource utilization.
The purpose of this study was to evaluate whether early triggered palliative care consultation in the ICU could improve patient outcomes and positively impact the care of high risk individuals as estimated through code status changes. We hypothesized that palliative care consultation for high-risk patients within the first two days of ICU admission would increase transition to DNR/DNI code status and discharge to hospice while decreasing ICU and post-ICU resource utilization.
METHODS
Trial Design and Setting
This study was designed as a single center cluster randomized crossover trial conducted between August 2017 and May 2018. The two medical ICUs (MICUs) of Barnes-Jewish Hospital (1250 beds) comprised of 16 and 18 beds respectively were randomly assigned to intervention or usual care. A washout period of six weeks occurred halfway through the study during which enrollment was halted and new admissions received usual care, followed by crossover of the MICUs to intervention or usual care. The two MICUs are geographically adjacent closed units staffed 24/7 by separate teams of physicians, nurses, pharmacists, and respiratory therapists. The teams are supervised by intensivists board-certified in critical care, and as previously reported, these intensivists have traditionally managed palliative care needs in the ICU, including symptom management and discussion regarding goals of care.39 The Washington University School of Medicine Human Studies Committee approved this investigation and waived the need for informed consent (Institutional Review Board Number: 201707067; ClinicalTrials.gov Identifier: ).
Patient Selection
Patients 18 years and older consecutively admitted on weekdays to the MICUs were screened for study enrollment using a tool comprised of nine pre-determined palliative care criteria to identify patients at high risk for morbidity and mortality based on severe or chronic organ dysfunction (Table 1). These criteria were informed by previous studies that had examined proactive palliative care interventions in the ICU.20-23,40-41 Patients who screened positive for at least one item were eligible for enrollment.
Table 1.
Palliative Care ICU Screening Tool and Exclusion Criteria
| Item # | Screening Criteria |
|---|---|
| 1 | Admitted from long term skilled nursing facility or acute care facility, or admitted from home with activities of daily living dependencies requiring skilled nursing |
| 2 | End stage dementia, amyotrophic lateral sclerosis, Parkinson’s disease, or multiple sclerosis |
| 3 | Advanced or metastatic cancer |
| 4 | Cardiac or respiratory arrest with neurological compromise |
| 5 | Multiple organ system failure |
| 6 | Known end-stage organ disease including cirrhosis, renal disease on dialysis, NYHA Class III or IV heart failure, or COPD on home oxygen |
| 7 | Acute shock requiring at least six hours of vasopressors or inotropic support |
| 8 | Acute respiratory failure requiring mask ventilation or intubation |
| 9 | Hospital stay greater than five days or ICU readmission for the same diagnosis within thirty days |
| Exclusion Criteria | |
| 1 | History of any stem cell transplant |
| 2 | Solid organ transplant within 1 year of transplant, or actively undergoing work-up for solid organ transplant |
| 3 | Non-English speaking patients for whom an interpreter was unavailable |
| 4 | Patients without capacity to participate in palliative care discussions with no identifiable surrogate |
| 5 | Patients who refused to had a surrogate refuse palliative care consultation |
| 6 | Patients who had received a palliative care consultation earlier during the same hospitalization |
| 7 | Patients already determined to be do-not-resuscitate/do-not-intubate |
A member of the research team who was independent of the ICU and palliative care teams screened the electronic medical records of MICU admissions within the previous 24 hours. Up to the first two consecutively screened patients meeting eligibility criteria were enrolled per MICU each weekday in both the intervention and control arms; identifying information of those enrolled to the intervention then was conveyed to the palliative care team. The patient limit was determined by the anticipated additional workload this study would place on the palliative care consultation service. Once the limit was reached on any given day, additional charts were not reviewed to prevent unintended selection bias among potentially eligible patients. Patients were excluded from enrollment in either arm if they met any of the exclusion criteria listed in Table 1.
Trial Procedures
Patients in the intervention arm received a palliative care consultation within 48 hours of MICU admission. This consultation was comprised of regular visits by an interprofessional palliative care team including a physician board-certified in palliative care, nurse practitioners, a palliative care clinical fellow, a social worker, and a chaplain. A palliative care consultation included: chart review of the patient’s hospitalization, meeting with the patient and available healthcare proxies, identification of physical and emotional needs of the patient and family, discussion with the primary team on how best to meet those needs, and communication between all parties with respect to goals, values, and treatment decisions. Formal meetings including the palliative care team, primary team, and the patient or healthcare proxies were encouraged but not mandatory. A board-certified palliative care physician or nurse practitioner performed the initial evaluation, and a care plan for each consultation was discussed by the entire palliative care team at rounds, with additional team members participating as appropriate. The palliative care team continued to follow the patient until discharge from the hospital. The control arm received standard of care: palliative care could be consulted at the discretion of the MICU clinicians.
Measurements
Two trained research study team members independently collected process and outcome data for each patient’s hospitalization, and a third team member reconciled any discrepancies. Physicians and study team members were not blinded because of inherent difficulties with blinding of the palliative care intervention. Sociodemographic data were collected from the electronic medical record, including age, sex, and self-reported race. Medical comorbidities and presence of a terminal illness, defined as any condition with life expectancy less than six months, as well as initial resuscitation preference and presence of an advance directive, were determined as of the time of study enrollment. To estimate disease severity, a modified Acute Physiology and Chronic Health Evaluation II (APACHE II) score was calculated based on initial clinical data at time of transfer to the MICUs.42 Clinical data collected included changes in resuscitation preferences during the hospitalization, MICU and hospital LOS, duration and use of mechanical ventilation and vasopressors, operating cost, and in-hospital and 30-day mortality. Post discharge metrics were collected on review of the electronic medical record, which encompasses all 14 acute care hospitals within the BJC Healthcare system as well as regional home health and hospice data.
Trial Outcomes
The primary outcome was the proportion of patients who transitioned to DNR/DNI resuscitation preference prior to hospital discharge, where DNR/DNI specifically refers to both Do-Not-Resuscitate and Do-Not-Intubate. Secondary exploratory outcomes included MICU LOS, hospital LOS, discharge to hospice, duration of mechanical ventilation, duration of vasopressors, tracheostomy, cardiopulmonary resuscitation, mortality, operating cost, post discharge emergency department visits, and hospital readmissions.
Statistical Analysis
Based on preliminary data from the first sixty days of the study, a generalized estimating equation model was used to determine that 96 patients would be required per arm to detect a threefold increase (54% versus 18%) in the primary outcome with 80% power and a type 1 error of 5%.43 This model estimated a within-cluster correlation of 0.03; interperiod correlation was assumed to be zero as new patients were enrolled after the washout period. This power analysis assumed the possibility of patient crossover between arms, which did not actually occur, leading to a conservative sample size. Results were analyzed by intention-to-treat.
Counts and frequencies were computed for categorical variables and compared using the χ2 test or Fisher’s exact test if the expected count was <5. Means and standard deviations were computed for normally distributed variables, otherwise medians and interquartile ranges were calculated for non-normal variables such as LOS. Monte Carlo simulations were performed to estimate 95% confidence intervals (CI) for differences in medians and operating costs. Survival probabilities were estimated for the transition time from full code to DNR/DNI starting from the day of study enrollment. Survival time was considered right-censored at the date of discharge or thirty days after enrollment, whichever was first. To examine the independent effect of arm assignment on the primary outcome, univariate Cox proportional hazards analysis was performed, both taking into account ICU clustering and using ICU assignment as a fixed effect in the model. The following potential confounders were tested in univariate analysis: age, gender, race, presence of an advance directive, medical comorbidities, enrollment criteria, presence of a terminal illness, mechanical ventilation, vasopressor requirement, and modified APACHE II score. Multivariable Cox proportional hazards analysis was then performed for the primary and secondary outcomes including variables with p<0.10 in univariate analysis. The proportional hazards assumption was examined using plots of the deviance residuals from the fit model and including a time-varying covariate. All statistical analyses were performed at significance level of 0.05 with two-sided tests using SAS version 9.4, SAS Institute Inc., Cary NC, USA.
RESULTS
242 screened patients meeting study entry criteria were enrolled (Supplemental Figure 1). Nine patients were excluded due to previous study inclusion. After randomization of the MICUs, there were 117 patients assigned to the intervention group and 116 patients assigned to the usual care group. Twenty patients in the intervention group were subsequently excluded for already having transitioned to DNR/DNI prior to MICU admission, while 14 patients in the usual care group were excluded for the same reason. There were 199 patients (104 [52.3%] men; mean [SD] age, 64 [12.8] years; modified APACHE II, 17.0 [5.8]) that were eligible for the primary outcome and analyzed. Most patients (77.9%) did not have documented advance directives at study inclusion. The baseline patient characteristics were well balanced between study groups (Table 2). All 97 patients in the intervention arm received a palliative care consultation within 48 hours of MICU admission, with 30 (30.9%) patients seen on the same day as admission, 62 (63.9%) on the day following admission, and 5 (5.1%) on the following day. The mean (SD) number of palliative care visits per patient in the intervention group was 3.9 (2.5). Seven (6.8%) patients in the usual care group had a palliative care consultation requested by their primary team occurring on average seven days after MICU admission, with a mean (SD) of 4.7 (2.1) palliative care visits per patient.
Table 2.
Baseline Characteristics
| Characteristic | Intervention Group (n = 97) |
Usual Care Group (n = 102) |
P value |
|---|---|---|---|
| Age, mean (SD) | 66 (14) | 62 (12) | 0.0497 |
| Male patients, No. (%) | 48 (49) | 56 (55) | 0.4444 |
| Caucasian, No. (%) | 45 (46) | 53 (52) | 0.6549† |
| African American, No. (%) | 48 (49) | 45 (44) | |
| APACHE II score, Mean (SD)@,25 | 17 (5) | 17 (6) | 0.9233 |
| Terminal Illness, No. (%) | 42 (43) | 37 (36) | 0.3144 |
| Enrollment Criteria, No. (%) | |||
| SNF/LTAC | 29 (30) | 20 (20) | 0.0922 |
| End-stage neurologic condition | 10 (10) | 5 (5) | 0.1487 |
| Advanced or Metastatic cancer | 22 (23) | 14 (14) | 0.1009 |
| Arrest with neurologic compromise | 5 (5) | 7 (7) | 0.6129 |
| Multiple organ system failure | 10 (10) | 18 (18) | 0.1368 |
| End-stage organ disease | 39 (40) | 36 (35) | 0.4748 |
| Shock | 18 (19) | 22 (22) | 0.5962 |
| Acute respiratory failure | 46 (47) | 45 (44) | 0.6399 |
| Prolonged length of stay or ICU readmission* | 7 (7) | 10 (10) | 0.5139 |
| Medical Comorbidities, No. (%) | |||
| Neurologic | 31 (32) | 30 (29) | 0.6969 |
| Congestive heart failure | 32 (33) | 25 (25) | 0.1860 |
| Coronary artery or peripheral vascular disease | 40 (41) | 50 (49) | 0.2702 |
| End-stage renal disease | 26 (27) | 24 (24) | 0.5945 |
| Solid organ transplant | 5 (5) | 3 (3) | 0.4895 |
| Cancer | 32 (33) | 26 (25) | 0.2446 |
| Human immunodeficiency virus | 2 (2) | 2 (2) | 1.0 |
| Chronic obstructive pulmonary disease | 23 (24) | 24 (24) | 0.9759 |
| Cirrhosis | 15 (15) | 17 (17) | 0.8174 |
| Advanced Directives and Family Data, No. (%) | |||
| No advanced directive | 71 (73) | 84 (82) | 0.2668† |
| Advanced directive not with patient | 14 (14) | 11 (11) | |
| Advanced directive with patient | 12 (12) | 7 (7) | |
| Family present | 94 (97) | 101 (99) | 0.3589 |
| Spouse/partner | 34 (35) | 45 (44) | 0.2334 |
| Adult children | 43 (44) | 37 (36) | 0.1962 |
| Sibling | 24 (25) | 19 (19) | 0.2581 |
| Parents | 9 (9) | 12 (12) | 0.6036 |
| Grandchildren | 2 (2) | 1 (1) | 0.6099 |
| 2nd degree relative | 7 (7) | 10 (10) | 0.5438 |
| Other, non-related friend | 5 (5) | 3 (3) | 0.4854 |
Abbreviations: APACHE, acute physiology and chronic health evaluation; SNF, skilled nursing facility; LTAC, Long term acute care; ICU, intensive care unit.
Range, 0-71 points; a higher score indicates an increased risk of mortality. A modified version of APACHE II was utilized in which all Glasgow Coma Scale (GCS) scores were assumed to be normal due to inherent difficulties in estimating GCS on chart review, e.g. for patients who were sedated on arrival
Patients enrolled for hospital length of stay greater than five days or ICU readmission within thirty days
P value provided for difference between all options for race and advanced directives
Primary Outcome: Change in Code Status
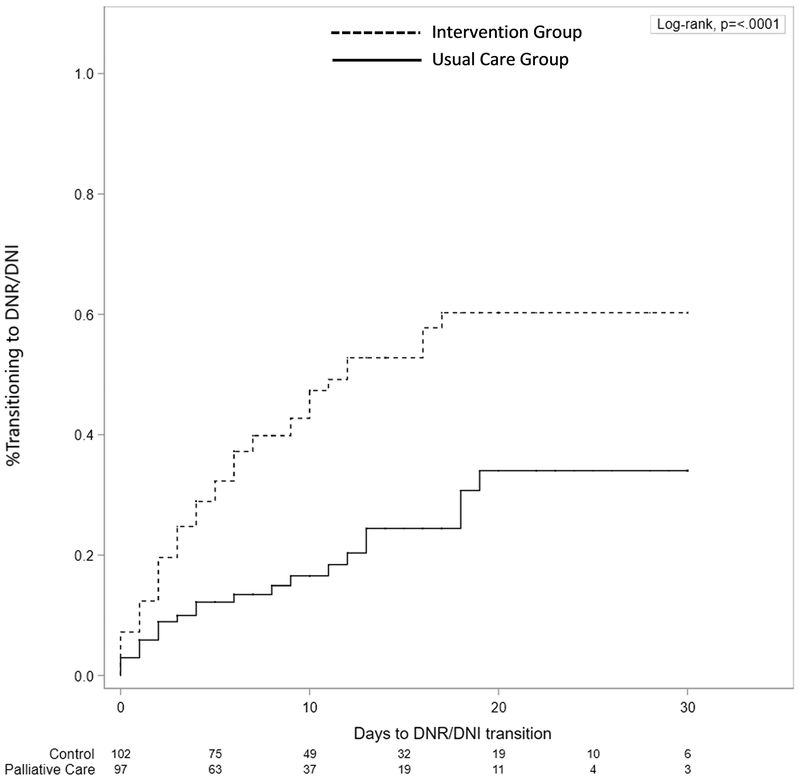
The number of patients transitioned to DNR/DNI was significantly higher in the intervention group (50.5%) compared to the usual care group (23.4%) with a risk difference (RD) of +27.0% (95% CI, 13.6% to 39.1%; p<0.0001). Kaplan Meier curves showed that transition to DNR/DNI occurred earlier and more frequently over the thirty days from study enrollment in the intervention group compared to the usual care group (p<0.0001, Log-Rank Test) (Figure 1). Intervention arm assignment was a significant predictor of the primary outcome in the univariate Cox proportional hazards analysis, whether adjusting for ICU clustering or treating ICU assignment as a fixed effect in the model (Supplemental Table 1). Subsequent multivariable analysis showed that intervention arm assignment was independently associated with the primary outcome even after adjusting for potential confounders. Age, admission from a skilled nursing or acute care facility, cardiac or respiratory arrest with neurologic compromise, and presence of a terminal illness were also found to independently predict the transition to DNR/DNI. The effect of crossover was not significant in any analysis.
Figure 1.
Kaplan Meier curves demonstrating that transition to do-not-resuscitate/do-not-intubate occurred earlier and more frequently in the intervention group compared to the usual care group over the thirty days from study enrollment (p<0.0001, Log-Rank Test).
Secondary Outcomes
Transfer to hospice care occurred significantly more often in the intervention group compared to the usual care group (18.6% vs 4.9%; p=0.0026) (Table 3). Median duration of mechanical ventilation was shorter by 2 days in the intervention group compared to the usual care group (4 vs 6 days; p=0.0415). Performance of a tracheostomy during the hospitalization was less in the intervention group compared to the usual care group (1.0% vs 7.8%; p=0.0354). There was no significant between-group difference for hospital mortality (21.7% vs 27.5%), 30-day mortality (35.1% vs 36.3%), ICU LOS (median 5 vs 5.5 days), or hospital LOS (median 10 vs 11 days) (p values>0.05). Of the 71 patients who passed away within 30 days of hospital discharge, a significantly higher proportion of decedents in the intervention group had been transitioned to DNR/DNI (88.2% vs 56.8%; p=0.0032) or to hospice (29.4% vs 10.8%; p=0.0491) during their hospital stay. Of the patients that survived until discharge, fewer patients in the intervention group compared to the control group presented to an emergency department within 30 days after discharge (1.3% vs 12.5%; p=0.0067), were admitted to the hospital (17.3% vs 33.3%, p=0.0236), or required either hospital readmission or an emergency department visit over the same time period (17.3% vs 38.9%; p=0.0028). Cox proportional hazards analysis showed that intervention arm assignment was a significant predictor of the following secondary outcomes: transfer to hospice (multivariate hazard ratio [MHR], 6.88; 95% CI, 3.30–14.31; p=0.0090), post-discharge emergency department visit (MHR, 8.94; 95% CI, 3.07–26.18; p=0.0403), and post-discharge emergency department visit or hospital readmission (MHR, 2.04; 95% CI, 1.45–2.88; p=0.0380).
Table 3.
Primary and Secondary Outcome Measures
| Intervention Group (n = 97) [95% CI] |
Usual Care Group (n = 102) [95% CI] |
Between Group Difference [95% CI] |
P Value | |
|---|---|---|---|---|
| Primary Outcome | ||||
| Transition to DNR/DNI, (%) | 49/97 (50.5) [40.2 to 60.8] | 24/102 (23.4) [15.7 to 33.0] | RD 27.0 (13.6 to 39.1) | <0.0001 |
| Secondary Outcomes | ||||
| Mechanical ventilation, (%) | 52/97 (53.6) [43.2 to 63.8] | 58/102 (56.9) [46.7 to 66.6] | RD −3.3 [−16.7 to 10.4] | 0.6444 |
| Vasopressors, (%) | 47/97 (48.5) [38.2 to 58.8] | 51/102 (50.0) [39.9 to 60.1] | RD −1.5 [−15.1 to 12.1] | 0.8274 |
| Hemodialysis, (%) | 15/97 (15.5) [8.9 to 24.2] | 24/102 (23.5) [15.7 to 33.0] | RD −8.0 [−18.9 to 3.1] | 0.1519 |
| Tracheostomy, (%) | 1/97 (1.0) [0.0 to 5.6] | 8/102 (7.8) [3.4 to 14.9] | RD −6.8 [−13.7 to −0.9] | 0.0354 |
| Cardiopulmonary resuscitation, (%) | 5/97 (5.2) [1.7 to 11.6] | 7/102 (6.9) [2.8 to 13.6] | RD −1.7 [−9.0 to 5.5] | 0.6129 |
| Transfer to hospice, (%) | 18/97 (18.6) [11.4 to 27.7] | 5/102 (4.9) [1.6 to 11.1] | RD 13.7 [4.8 to 23.0] | 0.0026 |
| Hospital mortality (%) | 22/97 (22.6) [14.8 to 32.3] | 30/102 (29.4) [20.8 to 39.3] | RD −6.8 [−18.9 to 5.4] | 0.2776 |
| 30-day mortality (%) | 34/97 (35.1) [25.6 to 44.5] | 37/102 (36.3) [26.9 to 45.6] | RD −1.2 [−15.5 to 13.1] | 0.8671 |
| ED visit, (%) | 1/75 (1.3%) [0.0 to 7.2] | 9/72 (12.5) [5.9 to 22.4] | RD −11.2 [−19.2 to −3.1] | 0.0067 |
| Hospital readmission, (%) | 13/75 (17.3) [9.6 to 27.8] | 24/72 (33.3) [22.7 to 45.4] | RD −16.0 [−29.9 to −2.2] | 0.0236 |
| ED visit or hospital readmission (%) | 13/75 (17.3) [9.6 to 27.8] | 28/72 (38.9) [27.6 to 51.1] | RD −21.6 [−35.7 to −7.4] | 0.0028 |
| Hospital duration, days (IQR) | 10 (6 to 15) | 11 (6 to 19) | MD −1 (−4.5 to 2) | 0.4258 |
| ICU duration, days (IQR) | 5 (3 to 8) | 5.5 (3 to 10) | MD −0.5 (−2 to 1) | 0.3800 |
| Mechanical ventilation days (IQR) | 4 (3 to 7) | 6 (3 to 13) | MD −2 (−6 to 0) | 0.0415 |
| Vasopressor days (IQR) | 3 (1 to 6) | 3 (2 to 6) | MD 0 (−1.5 to 1.5) | 0.9111 |
Abbreviations: DNR/DNI, do-not-resuscitate/do-not-intubate; ED, emergency department; ICU, intensive care unit; CI, confidence interval; IQR, interquartile range; RD, risk difference; MD, median difference.
Post-discharge ED visits and readmissions calculated for patients surviving until discharge.
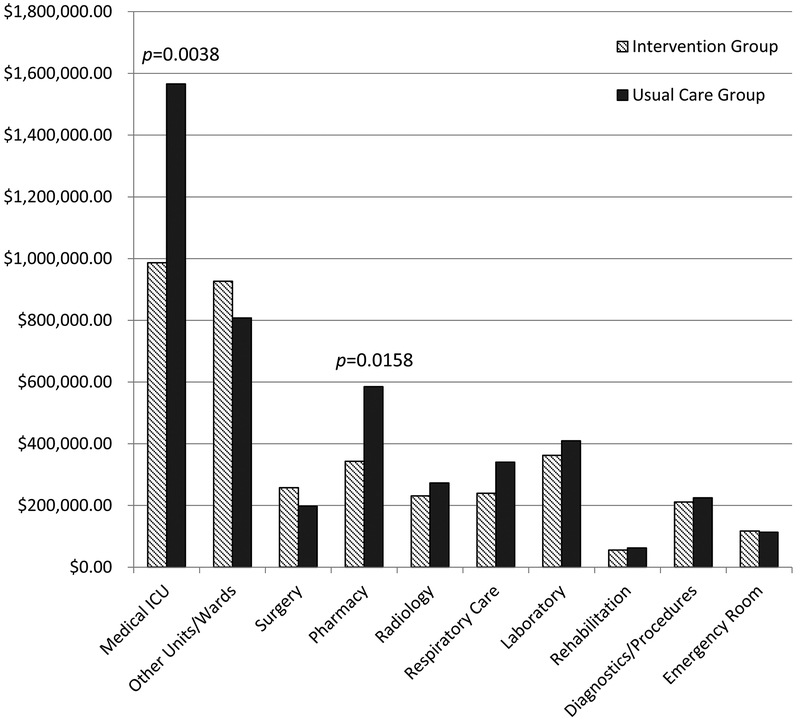
The total operating cost per patient in the intervention group was $37,310 compared to $45,790 in the control, which was not significantly different (p=0.1353). An estimated $880 of the intervention group’s per-patient total operating cost was due to the added cost of the palliative care consultation. When analyzing total operating cost by department, the intervention group had significantly lower MICU ($9,860 vs $15,660; p=0.0038) and pharmacy ($3,430 vs $5,850; p=0.0158) costs per patient compared to the control group (Figure 2, Supplemental Table 2).
Figure 2.
Total operating cost comparison by departments between the intervention and usual care groups scaled to 100 patients, with medical ICU and pharmacy operating costs significantly different between groups (p<0.01 and p<0.05 respectively).
DISCUSSION
Our study demonstrated that an early triggered palliative care intervention in the ICU was associated with greater transition to DNR/DNI resuscitation preference and hospice referrals. We also found that ICU and post-ICU resource utilization in the form of mechanical ventilation days, tracheostomies, and post discharge emergency department visits and/or readmissions decreased among patients receiving early palliative care consultation. Total operating cost was non-significantly lower by 18.5% in the intervention group, while MICU and pharmacy costs were significantly lower by 37% and 41% respectively. Mortality, ICU LOS, and hospital LOS were unchanged with the intervention.
The evidence leading up to this study strongly suggests that patients stand to benefit most from early palliative care interventions, and that clinical severity triggers can guide appropriate palliative care consultation in a variety of settings.15-16,20-23,25 However, past studies have also shown promising but inconsistent benefits of palliative care in the ICU, with conflicting results on LOS, patient/family satisfaction, and resource utilization.12-13,24,44-46 We have previously shown that an automated Early Warning System designed for non-ICU patients at high risk of mortality or ICU transfer could identify patients likely to benefit from palliative care interventions prior to transfer.47 This intervention resulted in increased documentation of patient preferences for level of care, and more importantly, a significant reduction in unwanted ICU transfers. Our current study examines whether a similar trigger-based approach after ICU transfer might improve clinical outcomes.
Our investigation is unique in being a prospective randomized study assessing an early ICU-based intervention led by experienced board-certified palliative care specialists. Our study builds upon past research by using a palliative care screening tool to identify newly admitted ICU patients at highest risk for morbidity and mortality followed by a full patient-centered palliative care consultation within 48 hours. The interprofessional palliative care team interacted with the patient, family, and primary team on multiple occasions throughout the admission to facilitate symptom relief, set appropriate expectations, and address goals of care. Code status changes were significantly increased in the intervention group, even after controlling for potential confounders, and as expected there were improvements in multiple other clinically important metrics likely driven by effective goals of care discussions. While the difference in total operating cost did not reach statistical significance, our study was not powered for the highly skewed nature of financial data. Despite this limitation, there were significant differences in MICU and pharmacy operating costs between groups, which may reflect a decrease in invasive procedures and salvage pharmaceutical therapies with the intervention. The use of a full palliative care consultation and the focus on high risk patients may explain why our study found decreased healthcare utilization not previously observed.
Our study has several important limitations. First, this study was performed at a single academic institution and the results may not be transferrable to other centers. Institutions that routinely integrate palliative care into the management of critically ill patients may not benefit from additional triggers for palliative care consultation. However our findings are in line with multiple systematic reviews and the recent multicenter retrospective analysis performed by Zalenski et al, suggesting that the benefits seen in our study could be applicable elsewhere.9,15,22 Second, the enrollment process and limit on the number of patients that could be seen by the palliative care service precluded screening every MICU admission over the study period for study eligibility. Even though the patient characteristics were similar in each arm, the absence of data on potentially eligible patients that were excluded due to staffing limitations is a potential source of bias in our results. Third, the crossover design of our trial raises the possibility of diffusion of our intervention in the two adjacent MICUs. The statistical analysis did not show any significant effect of clustering on the primary outcome, however, and notably we did not find any persistent effect of the intervention after crossover and washout. Fourth, the decrease in MICU and pharmacy costs with palliative care consultation may reflect shifts in costs to lower acuity settings rather than absolute reductions, although the decrease in post-discharge metrics and overall cost trend suggest otherwise. Lastly while DNR/DNI orders likely serve as a surrogate marker of goals of care discussions in addition to their inherent benefits, they are not a direct measure of quality of life or patient/family satisfaction, which remain a critical target for future palliative care interventions in the ICU.
In conclusion, an early directed multifaceted palliative care intervention led by experienced clinicians board-certified in palliative care significantly influenced code status, hospice referrals, and medical resource utilization. Further studies are required to determine the optimal application of palliative care services in the ICU setting focusing on clinical outcomes as well as patient and family satisfaction.
Supplementary Material
Supplemental Figure 1. 1643 patients were admitted to the medical intensive care units (MICUs) during the study period of which 242 consecutive patients met the screening criteria and were enrolled in the study, up to the limit of two patients per MICU per day due to workload limitations. DNR/DNI, do-not-resuscitate/do-not-intubate.
ACKNOWLEDGEMENTS
The authors thank Dr. Abigail Barker and the Center for Health Economics Policy at Washington University in Saint Louis for indispensable guidance in analyzing the financial data.
Financial Support: This publication in part was supported by The Foundation for Barnes-Jewish Hospital and their generous donors, and the Washington University Institute of Clinical and Translational Sciences which is, in part, supported by the NIH/National Center for Advancing Translational Sciences (NCATS), CTSA grant UL1TR002345.
Funding/Sponsor: This publication in part was supported by The Foundation for Barnes-Jewish Hospital and their generous donors, and the Washington University Institute of Clinical and Translational Sciences which is, in part, supported by the NIH/National Center for Advancing Translational Sciences (NCATS), CTSA grant UL1TR002345.
Copyright form disclosure: Dr. Ma and Dr. Chi disclosed that the study is supported by The Foundation for Barnes-Jewish Hospital and Washington University Institute of Clinical and Translational Sciences (ICTS) which is, in part, supported by the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences, CTSA grant UL1TR002345. Drs. Chi, Buettner, Al-Hammadi, and Kollef received support for article research from the NIH. Dr. Buettner’s institution received funding from ICTS Just In Time award. Drs. Buettner and Dans’ institutions received funding from Barnes Jewish Hospital Foundation. Dr. Chen disclosed work for hire. Dr. Dans’ institution also received funding from ICTS at Washington University School of Medicine and Barnes Jewish Hospital Foundation, and she received funding from National Comprehensive Cancer Network (NCCN) (Guidelines Panel Chair for the Palliative Care Guidelines. The NCCN reimburses travel & accommodation expenses for attending their annual conference). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Conflict of Interest Disclosures: Dr. Kollef’s effort was supported by the Barnes-Jewish Hospital Foundation.
Role of the Funder/Sponsor: The sponsor had no role in either the design of the study, analysis of the data, or writing and approval of the manuscript.
REFERENCES
- 1.Devlin JW, Skrobik Y, Gélinas C, et al. : Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2018; 46:e825–e873. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Barnato AE, Linde-Zwirble WT, et al. : Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med 2004; 32:638–643. [DOI] [PubMed] [Google Scholar]
- 3.Truog RD, Campbell ML, Curtis JR, et al. : Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med. 2008;36(3):953–963. [DOI] [PubMed] [Google Scholar]
- 4.Penrod JD, Pronovost PJ, Livote EE, et al. : Meeting standards of high-quality intensive care unit palliative care: clinical performance and predictors. Crit Care Med. 2012;40(4):1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chettiar A, Montez-Rath M, Liu S, et al. : Association of Inpatient Palliative Care with Health Care Utilization and Postdischarge Outcomes among Medicare Beneficiaries with End Stage Kidney Disease. Clin J Am Soc Nephrol 2018; 13:1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith S, Brick A, O’Hara S, et al. : Evidence on the cost and cost-effectiveness of palliative care: a literature review. Palliat Med 2014; 28:130–150. [DOI] [PubMed] [Google Scholar]
- 7.Kavalieratos D, Corbelli J, Zhang D, et al. : Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA 2016; 316:2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakitas M, Lyons KD, Hegel MT, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009; 302:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penrod JD, Luhrs CA, Livote EE, et al. : Implementation and evaluation of a network-based pilot program to improve palliative care in the intensive care unit. J Pain Symptom Manage 2011; 42:668–671. [DOI] [PubMed] [Google Scholar]
- 10.Schneiderman LJ, Gilmer T, Teetzel HD, et al. : Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: a randomized controlled trial. JAMA 2003; 290:1166–1172. [DOI] [PubMed] [Google Scholar]
- 11.Lamba S, Murphy P, McVicker S, et al. : Changing end-of-life care practice for liver transplant service patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage 2012; 44:508–519. [DOI] [PubMed] [Google Scholar]
- 12.Khandelwal N, Kross EK, Engelberg RA, et al. : Estimating the Effect of Palliative Care Interventions and Advance Care Planning on ICU Utilization: A Systematic Review. Crit Care Med 2015; 43:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslakson R, Cheng J, Vollenweider D, et al. : Evidence-Based Palliative Care in the Intensive Care Unit: A Systematic Review of Interventions. J Palliat Med 2014; 17:219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seaman JB, Barnato AE, Sereika SM, et al. : Patterns of Palliative Care Service Consultation in a Sample of Critically Ill ICU Patients at High Risk of Dying. Heart Lung 2017; 46:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maltoni M, Scarpi E, Dall’Agata M, et al. : Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 2016; 65:61–68. [DOI] [PubMed] [Google Scholar]
- 16.Amano K, Morita T, Tatara R, et al. : Association between early palliative care referrals, inpatient hospice utilization, and aggressiveness of care at the end of life. J Palliat Med 2015; 18:270–273. [DOI] [PubMed] [Google Scholar]
- 17.Salins N, Ramanjulu R, Patra L, et al. : Integration of Early Specialist Palliative Care in Cancer Care and Patient Related Outcomes: A Critical Review of Evidence. Indian J Palliat Care 2016; 22:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu FM, Newman JM, Lasher A, et al. : Effects of initiating palliative care consultation in the emergency department on inpatient length of stay. J Palliat Med 2013; 16:1362–1367. [DOI] [PubMed] [Google Scholar]
- 19.May P, Garrido MM, Cassel JB, et al. : Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients With Advanced Cancer: Earlier Consultation Is Associated With Larger Cost-Saving Effect. J Clin Oncol 2015; 33:2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell ML, Guzman JA: Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest 2003; 123:266–271. [DOI] [PubMed] [Google Scholar]
- 21.Nelson JE, Curtis JR, Mulkerin C, et al. : Choosing and using screening criteria for palliative care consultation in the ICU: a report from the Improving Palliative Care in the ICU (IPAL-ICU) Advisory Board. Crit Care Med 2013; 41:2318–2327. [DOI] [PubMed] [Google Scholar]
- 22.Norton SA, Hogan LA, Holloway RG, et al. : Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high-risk patients. Crit Care Med 2007; 35:1530–1535. [DOI] [PubMed] [Google Scholar]
- 23.Curtis JR, Nielsen EL, Treece PD, et al. : Effect of a Quality-Improvement Intervention on End-of-Life Care in the Intensive Care Unit: A Randomized Trial. American Journal of Respiratory and Critical Care Medicine 2011; 183:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carson SS, Cox CE, Wallenstein S, et al. : Effect of Palliative Care-Led Meetings for Families of Patients With Chronic Critical Illness: A Randomized Clinical Trial. JAMA 2016; 316:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zalenski RJ, Jones SS, Courage C, et al. : Impact of Palliative Care Screening and Consultation in the ICU: A Multihospital Quality Improvement Project. J Pain Symptom Manage 2017; 53:5–12.e3. [DOI] [PubMed] [Google Scholar]
- 26.Gieniusz M, Nunes R, Saha V, et al. : Earlier Goals of Care Discussions in Hospitalized Terminally Ill Patients and the Quality of End-of-Life Care: A Retrospective Study. Am J Hosp Palliat Care 2018; 35:21–27. [DOI] [PubMed] [Google Scholar]
- 27.Weinerman AS, Dhalla IA, Kiss A, et al. : Frequency and clinical relevance of inconsistent code status documentation. J Hosp Med 2015; 10:491–496. [DOI] [PubMed] [Google Scholar]
- 28.Mack JW, Weeks JC, Wright AA, et al. : End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 2010; 28:1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia HL, Patel NR, Choma NN, et al. : Code status and resuscitation options in the electronic health record. Resuscitation 2015; 87:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warren MB, Lapid MI, McKean AJ, et al. : Code status discussions in psychiatric and medical inpatients. J Clin Psychiatry 2015; 76:49–53. [DOI] [PubMed] [Google Scholar]
- 31.Celso BG, Meenrajan S: The triad that matters: palliative medicine, code status, and health care costs. Am J Hosp Palliat Care 2010; 27:398–401. [DOI] [PubMed] [Google Scholar]
- 32.Romano AM, Gade KE, Nielsen G, et al. : Early Palliative Care Reduces End-of-Life Intensive Care Unit (ICU) Use but Not ICU Course in Patients with Advanced Cancer. Oncologist 2017; 22:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrido MM, Balboni TA, Maciejewski PK, et al. : Quality of Life and Cost of Care at the End of Life: The Role of Advance Directives. J Pain Symptom Manage 2015; 49:828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahluwalia SC, Tisnado DM, Walling AM, et al. : Association of Early Patient-Physician Care Planning Discussions and End-of-Life Care Intensity in Advanced Cancer. J Palliat Med 2015; 18:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad AS, Mudasser S, Khan MN, et al. : Outcomes of Cardiopulmonary Resuscitation and Estimation of Healthcare Costs in Potential “Do Not Resuscitate” Cases. Sultan Qaboos Univ Med J 2016; 16:e27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolan JP, Soar J, Smith GB, et al. : Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation 2014; 85:987–992. [DOI] [PubMed] [Google Scholar]
- 37.van Gijn MS, Frijns D, van de Glind EMM, et al. : The chance of survival and the functional outcome after in-hospital cardiopulmonary resuscitation in older people: a systematic review. Age Ageing 2014; 43:456–463. [DOI] [PubMed] [Google Scholar]
- 38.Zafar W, Ghafoor I, Jamshed A, et al. : Outcomes of In-Hospital Cardiopulmonary Resuscitation Among Patients With Cancer. Am J Hosp Palliat Care 2017; 34:212–216. [DOI] [PubMed] [Google Scholar]
- 39.Kollef MH: Private attending physician status and the withdrawal of life-sustaining interventions in a medical intensive care unit population. Critical Care Medicine 1996; 24:968–975. [DOI] [PubMed] [Google Scholar]
- 40.Braus N, Campbell TC, Kwekkeboom KL, et al. Prospective study of a proactive palliative care rounding intervention in a medical ICU. Intensive Care Med. 2016;42(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalenski R, Courage C, Edelen A, et al. : Evaluation of screening criteria for palliative care consultation in the MICU: a multihospital analysis. BMJ Supportive & Palliative Care 2014; 4:254–262. [DOI] [PubMed] [Google Scholar]
- 42.Knaus WA, Draper EA, Wagner DP, et al. : APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–8299. [PubMed] [Google Scholar]
- 43.Giraudeau B, Ravaud P, Donner A: Sample size calculation for cluster randomized cross-over trials. Stat Med 2008; 27:5578–5585. [DOI] [PubMed] [Google Scholar]
- 44.Curtis JR, Treece PD, Nielsen EL, et al. : Randomized Trial of Communication Facilitators to Reduce Family Distress and Intensity of End-of-Life Care. American Journal of Respiratory and Critical Care Medicine 2016; 193:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martins BDCPCC, Oliveira RA, Cataneo AJM: Palliative care for terminally ill patients in the intensive care unit: Systematic review and metaanalysis. Palliat Support Care 2017; 15:376–383. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Dawod Y, Wonnaparhown A, et al. : Palliat Support Care Effects of hospital palliative care on health, length of stay, and in-hospital mortality across intensive and non-intensive-care units: A systematic review and metaanalysis. Palliat Support Care 2017; 15:741–752. [DOI] [PubMed] [Google Scholar]
- 47.Picker D, Dans M, Heard K, et al. : A Randomized Trial of Palliative Care Discussions Linked to an Automated Early Warning System Alert. Crit Care Med 2017; 45:234–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. 1643 patients were admitted to the medical intensive care units (MICUs) during the study period of which 242 consecutive patients met the screening criteria and were enrolled in the study, up to the limit of two patients per MICU per day due to workload limitations. DNR/DNI, do-not-resuscitate/do-not-intubate.