Abstract
BACKGROUND:
The risks and benefits of pharmacologic treatment and operative closure of patent ductus arteriosus (O-PDA) in premature infants remain controversial. Recent series have demonstrated the feasibility of transcatheter PDA closure (TC-PDA) in increasingly small infants. The effect of this change on practice has not been evaluated.
METHODS:
A multicenter observational study of infants treated in neonatal intensive care units in hospitals contributing data to the Pediatric Health Information Systems Database from 1/2007–12/2017 was performed to study trends in the propensities for 1) mechanical closure of PDA and 2) TC-PDA vs. O-PDA, as well as inter-hospital variation in practice.
RESULTS:
A total of 6,214 subjects at 44 hospitals were studied (5% TC-PDA). Subject median gestational age was 25 weeks (IQR: 24–27 weeks). Median age at closure was 24 days (IQR: 14–36 days). The proportion of all NICU patients undergoing either O-PDA or TC-PDA decreased (3.1% in 2007 and 0.7% in 2017, p<0.001), while the proportion in which TC-PDA was used increased significantly (0.1% in 2007 to 29.0% in 2017). Case-mix-adjusted multivariable models similarly demonstrated increasing propensity to pursue TC-PDA (odds ratio (OR): 1.66 per year, p<0.001) with acceleration of the trend after 2014 (OR: 2.46 per year, p<0.001) as well as significant practice variation (p<0.001, median odds ratio: 4.6) across the study period.
CONCLUSION:
In the face of decreasing closure of PDA, the use of TC-PDA increased dramatically with significant practice variability. This demonstrates that there is equipoise for potential clinical trials.
Keywords: Pediatric cardiology, outcomes research, neonatology, transcatheter device
INTRODUCTION:
Patent ductus arteriosus (PDA) are nearly universally present in infants at birth, but most close within the first few weeks of life. Persistent patency of the ductus arteriosus is much more common in neonates born prematurely. In this population, hemodynamically significant PDA can result not only in significant left-to-right shunt aggravating respiratory distress syndrome and evolving bronchopulmonary dysplasia/chronic lung disease (BPD/CLD) but also diastolic runoff, compromised systemic perfusion, and increased risk of necrotizing enterocolitis, intraventricular hemorrhage, and renal insufficiency. Despite this, the optimal management of PDA in premature infants remains controversial. A number of pharmacological regimens (including acetaminophen, ibuprofen, or indomethacin) have been evaluated. These prophylactic and therapeutic regimen all have demonstrated superior closure rates to placebo, but clinical trials and subsequent meta-analyses1–4 have not demonstrated that this is accompanied by reduced risk of mortality, BPD/CLD, or other adverse outcomes. This may be because pharmacological therapy (either as prophylaxis or treatment dosing) is associated with renal insufficiency and altered cerebral perfusion1–3. Operative ligation of PDA (O-PDA) through a thoracotomy has been performed for 8 decades5. It is effective, but has been associated with hemodynamic and respiratory compromise (so-called post-ligation syndrome)6, as well as recurrent laryngeal nerve injury, hemi-diaphragm paresis, chylothorax, and scoliosis. As a result, a narrative review published by the American Academy of Pediatrics concluded that there was not sufficient evidence to suggest that routine closure of PDA improved long-term outcomes over conservative/supportive therapy (fluid restriction, diuretics, and respiratory support)7.
Until recently, transcatheter closure of PDA (TC-PDA) was restricted to larger infants and older patients. The development of smaller profile devices such as the Amplatzer vascular plug and plug II (Abbott Medical, Plymouth Minnesota) made TC-PDA more feasible8. Recently, TC-PDA in increasingly small premature infants9–15 have been reported, with high rates of technical success and low rates of adverse outcomes in selected populations16. Post-ligation syndrome in these series of TC-PDA is less pronounced and recovery is faster than after O-PDA10,15,17. Though there are ongoing trials to evaluate conservative therapy vs. a combination of pharmacologic regimens, e.g. Baby-OSCAR (EudraCT 2013-005336-23) in the United Kingdom, and Neonatal Research Network “Management of the PDA Trial” () in the United States, to our knowledge, none have included TC-PDA in studies of either short- or long-term outcomes.
The degree to which this controversy is reflected in real-world practice has not been evaluated. We performed a retrospective observational study to evaluate the trends in closure of PDA in a sample of primary pediatric hospitals in the United States, hypothesizing that the overall rate of PDA closure has decreased, the rate of TC-PDA has increased, and there is significant inter-hospital variation in the use of O-PDA and TC-PDA.
METHODS:
Data source:
The Pediatric Health Information Systems (PHIS) database contains administrative data from inpatient, emergency department, ambulatory surgery, and observation encounters from 45 not-for-profit, tertiary care pediatric hospitals in the United States. These hospitals are affiliated with the Children’s Hospital Association (CHA) (Overland Park, KS). Data quality and reliability are assured through a joint effort between CHA and participating hospitals. Participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures as well as utilization data (e.g. pharmacy products, radiologic studies, and laboratory studies). Data are de-identified at the time of data submission and are subject to a number of reliability and validity checks. The institutional review board of The Children’s Hospital of Philadelphia has determined that studies using PHIS data do not constitute human subjects and are not subject to review.
Study Population:
We studied infants (age at admission <1 year) born between 1/1/2007 and 12/31/2017 admitted to the NICU of a hospital submitting data to PHIS, with a diagnosis of a PDA who underwent O-PDA or TC-PDA. Diagnoses and procedures were identified using International Classification of Diseases version 9 and 10 (ICD9 and ICD10) codes. The study spanned periods in which both diagnosis code systems were used. ICD-9 codes for diagnoses and procedures were identified and converted to ICD-10 codes using United States Center for Medicare and Medicaid Services ICD-9 to ICD-10 crosswalk (cms.gov). Manual review of these tables was performed to insure that this was done accurately. As described previously18,19, ICD-9 codes for PDA closures do not differentiate O-PDA and TC-PDA. To address this, ICD-9 codes that uniquely identify cardiac catheterization were used to differentiate O-PDA and TC-PDA cases.
NICU admissions are identified in the PHIS database using a specific identifier. Cases from hospitals that do not identify their NICU admissions this way were excluded, as were hospitals with <25 total TC-PDA and/or O-PDA procedures over the course of the study period, and hospitals reporting data in <3/11 years of the study period. Hospital-level exclusion criteria were used to insure that only hospitals with stable data reporting practices were included, as described previously18,20–24. Individual subject-level exclusion criteria were concomitant congenital heart disease and/or combination of another cardiothoracic operation or transcatheter procedure during the same admission as the PDA closure. The goal of these exclusion criteria was to generate a study population of infants hospitalized in NICU’s who underwent either isolated transcatheter or operative PDA closure.
Study measures:
Data extracted from PHIS included sex, race (white, black, Asian, multiracial or other, or missing), age at admission, age at PDA closure, gestational age, insurance payer (private, Medicaid, other governmental insurance, or other), presence of known genetic syndromes, concomitant systemic disease (grouped by system as described previously25). Systems for which <1% of the study population had issues were grouped as “other medical conditions”18,20,21,23,26.
Analysis:
Descriptive statistics were calculated for both the TC-PDA and O-PDA cohorts. Continuous variables were expressed as mean ± standard deviation or median (inter-quartile range (IQR) and range) as appropriate. Categorical variables were expressed as percent (count). Student’s t-test, Wilcoxon rank sum test, and χ2 tests were used to evaluate differences in the distribution of these characteristics between the two cohorts.
The primary goal of the study was to evaluate the relative propensity to pursue TC-PDA or O-PDA, and how that changed over the study period. We also hoped to study how the propensity to intervene on PDA using either approach had changed. Identifying conservative management or the success/failure of pharmacological management of PDA was not possible in PHIS, so we measured the proportion of TC-PDA and O-PDA cases in the study sample each year relative to the total number of NICU patients treated at contributing hospitals over the same year. This also allows for meaningful comparisons as the number of hospitals and potential subjects at risk in each year varied over the study period. We evaluated whether there was a trend in the distribution of these characteristics using a Cochran-Armitage test.
Next, we sought to evaluate whether the propensity to pursue O-PDA vs. TC-PDA changed over time (adjusting for patient-characteristics). To do so, we calculated mixed effects logistic regression models with the primary outcome of TC-PDA vs. O-PDA. The primary exposure in the fixed effects of these models was date of procedure. Covariates included in the fixed effects included gestational age, age at PDA intervention, presence of other comorbid conditions, race, and insurance payer. A random intercept for hospital was added to account for clustering in preference by hospital. We hypothesized that the propensity to pursue TC-PDA would increase with time over the study period. There was no reason to believe that this association would be linear, so a number of secondary analyses were performed to evaluate other types of associations (e.g. an acceleration in the change or an inflection point in the study period). Model fit was assessed using Akaike information criteria22. Additional sensitivity analyses were performed to evaluate whether corrected gestational age performed better than the combination of post-natal age and gestational age in all models.
Modeling the propensity for an individual to receive O-PDA vs. TC-PDA has several advantages over simply counting the total number of O-PDA and TC-PDA procedures. It is not affected by changing numbers of hospitals in the study sample. It also has superior statistical power and greater ability to represent real behavior than analyses dividing the study period by year. This approach is also conducive to multivariable modeling using generalized linear models to adjust for subject characteristics and systematic differences between hospitals.
Inter-hospital variation was evaluated in two ways. First a likelihood ratio test was applied to the full model and the nested model without the random intercept, evaluating for heterogeneity and statistically significant between-hospital variation. To measure the magnitude of this variation, a median odds ratio (MOR) was calculated. As described previously22,27–33, the MOR represents the odds that two identical patients who were treated at randomly selected hospitals from the sample would receive different therapies. An MOR>1.2 is considered to be of clinically significant magnitude. Its statistical significance is also measured using a conventional p-value.
Two secondary analyses were performed after initial analysis of the data was performed and reviewed. First, the association between adjusted gestational age of TC-PDA and time was evaluated. Generalized linear models were calculated with both gamma and Gaussian links. This analysis was done for two time periods: 1) the entire study period and 2) from 1/1/2014 to 12/31/2017. The latter period is the period in which the majority of TC-PDA procedures were performed, and trends during this period are most informative about evolving practice. Second, we compared the adjusted gestational age of subjects undergoing TC-PDA at centers with the highest TC-PDA volume (top 10% programs). This comparison was done with Student’s t-test.
Missing data were rare (<1%) for most study variables. However, for both race and gestational age a larger proportion of subject data were missing. To ameliorate bias, a “missing” variable was introduced for race, gestational age, and corrected gestational age. Imputation was not used. A sensitivity analysis excluding subjects with missing gestational age was performed, and no differences in the primary association were seen (data not shown). Analyses were defined a priori with no model refinement after the fact. No adjustment was made for multiple comparisons. All data analyses were performed using STATA MP 13 (Statacorp, College Station, TX) and R v3.42 (R Development Core Team, Vienna, Austria).
RESULTS:
Study population:
After applying exclusion criteria, the analytic cohort was comprised of 6,214 infants from 44 hospitals (Figure 1). The patients were 53% male, 50% white, with median gestational age 25 weeks (IQR: 24–27 weeks). PDA closure was performed at median age of 24 days (IQR: 14–36 days) with resultant median corrected gestational age of 29 weeks (IQR: 27–31 weeks). Over the entire study period, 5% of PDA closure (from 34/44 hospitals) were TC-PDA procedures (Table 1). Subjects who underwent TC-PDA were older in terms of age, gestational age, and corrected gestational age (p<0.001 for all). They were also more likely to have a genetic syndrome (p=0.01), history of gastrointestinal condition (p<0.001), and respiratory condition (p<0.001).
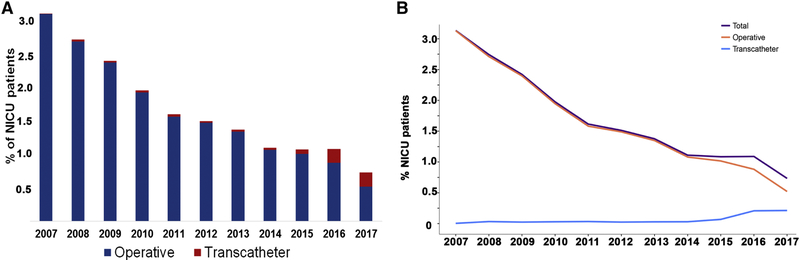
Figure 1: Study population.
Table 1:
Cohort Characteristics
| Transcatheter | Operative | p-value | |
|---|---|---|---|
| n=295 at 34 centers | n=5919 at 44 centers | ||
| Age at PDA closure (days) | 74 (IQR 44–105) | 23 (IQR 14–34) | <0.001 |
| Female sex | 51% (n=150) | 46% (n=2,750) | 0.14 |
| Race | 0.05 | ||
| White | 43% (n=126) | 50% (n=2,966) | |
| Black | 33% (n=98) | 27% (n=1,587) | |
| Other or mixed race | 4% (n=12) | 5% (n=279) | |
| Missing | 20% (n=59) | 18% (n=1,087) | |
| Payer | 0.04 | ||
| Private insurance | 39% (n=114) | 34% (n=1,985) | |
| Public insurance | 57% (n=167) | 57% (n=3,384) | |
| Non-public government | 2% (n=6) | 4% (n=259) | |
| Other | 2% (n=7) | 5% (n=274) | |
| Missing | <1% (n=1) | <1% (n=17) | |
| Gestational age (weeks) | 26 (IQR 25–30) | 25 (IQR 24–26) | <0.001 |
| Gestational age | <0.001 | ||
| 23–24 weeks | 17% (n=50) | 31% (n=1,829) | |
| 25–27 weeks | 30% (n=87) | 39% (n=2,310) | |
| 28–33 weeks | 16% (n=48) | 10% (n=581) | |
| 34–36 weeks | 4% (n=12) | 1% (n=66) | |
| ≥37 weeks | 8% (n=23) | 1% (n=87) | |
| Missing | 25% (n=75) | 18% (n=1,046) | |
| Adjusted gestational age (weeks) | 36 (30–38) | 29 (IQR 27–31) | <0.001 |
| Adjusted gestational age | <0.001 | ||
| 23–24 | <1% (n=1) | 4% (n=220) | |
| 25–27 | 4% (n=13) | 30% (n=1,782) | |
| 28–33 | 15% (n=43) | 39% (n=2,330) | |
| 34–36 | 10% (n=29) | 4% (n=251) | |
| ≥37 | 45% (n=134) | 5% (n=290) | |
| Missing | 25% (n=75) | 18% (n=1,046) | |
| Known genetic syndrome | 4% (n=11) | 2% (n=101) | 0.01 |
| History of gastrointestinal condition | 38% (n=112) | 19% (n=1,151) | <0.001 |
| History of hematologic condition | 3% (n=8) | 3% (n=188) | 0.66 |
| History of neurologic condition | 18% (n=53) | 14% (n=851) | 0.09 |
| History of respiratory condition | 29% (n=87) | 16% (n=951) | <0.001 |
| Other complex chronic condition | 84% (n=248) | 89% (n=5,286) | 0.48 |
Observed trends in closure of PDA:
Over the study period, the proportion of all NICU patients undergoing closure decreased (Beta=−0.22% per year, 95% CI:−0.27% to −0.18%, p<0.001) with a rate of 3.1% in 2007 and 0.7% in 2017 (Figure 2A). Over the same period the proportion of PDA closures performed using a TC-PDA technique increased relative to O-PDA (0.43% per year, 95% CI: 0.29 to 0.58%, p<0.001) (Figure 2B).
Figure 2: Proportion of NICU patients undergoing operative or transcatheter closure of patent ductus arteriosus.
A Bar graph depicts the percentage of NICU patients treated at study hospitals who undergo either operative (blue) or transcatheter (red) closure of their PDA. The decrease in total PDA closure and operative closure is statistically significant (p<0.001). At the same time there is a significant increase in the proportion of NICU patients undergoing transcatheter closure of PDA (p<0.001)
B Line graph depicting the change in proportion of NICU patients undergoing PDA closure in total (purple), as well as via operative ligation (orange) or transcatheter methods (light blue).
Trends in the decision to pursue O-PDA versus TC-PDA
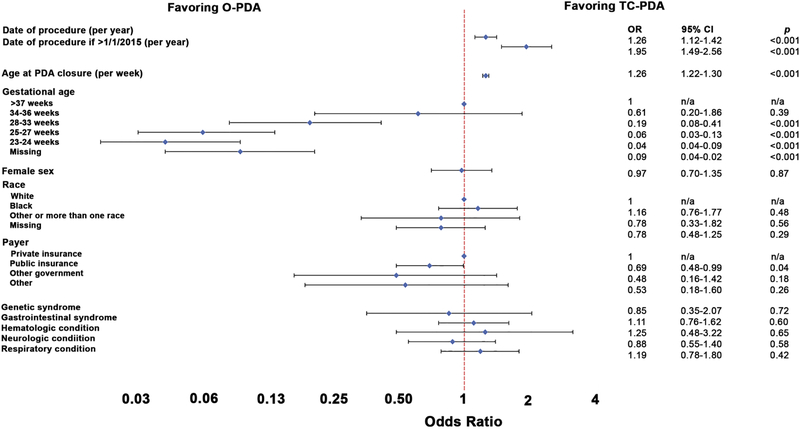
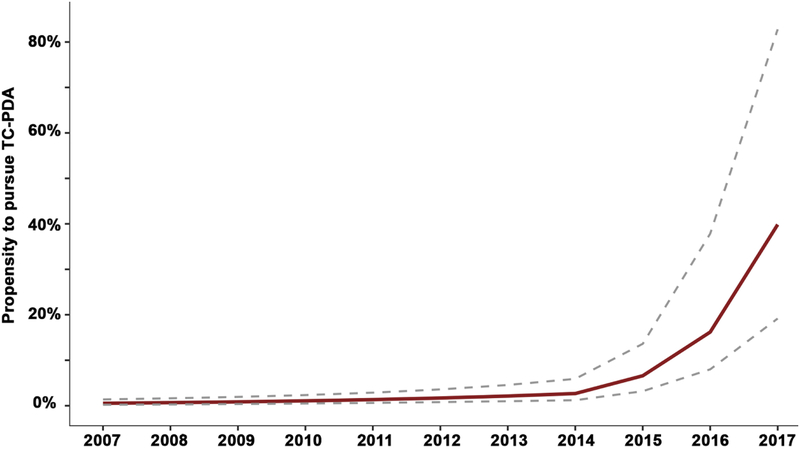
A series of multivariable models measuring the propensity to pursue O-PDA versus TC-PDA was calculated. The initial model included the date of procedure along with covariates (Supplementary Table 1). Later date of procedure was significantly associated with an increasing propensity to perform TC-PDA (OR: 1.63 per year, 95% CI: 1.52–1.76, p<0.001). Alternative models that modeled a non-linear change in the propensity uniformly demonstrated better fit than the initial model (Supplementary Table 2). The model with the best fit included an inflection point for cases after January 1, 2015 (Figure 3). In this model, later date of procedure was associated with an increased propensity to perform TC-PDA vs. O-PDA (OR: 1.26 per year, 95% CI: 1.12–1.42, p<0.001) that accelerated after 1/1/2015 (OR: 1.95 per year after 2015, 95% CI: 1.49–2.56, p<0.001). In other words from 2015 on, the OR is 2.46 (1.95 × 1.26) per year (95% CI: 2.04–2.96, p<0.001). To illustrate this pattern, the propensity to pursue TC-PDA (contingent on pursuing mechanical PDA closure) was calculated over the span of the study period for a hypothetical female infant with gestational age of 25–27 weeks, post-natal age of 74 days (median age for TC-PDA in our study sample), and no other risk factors (Figure 4). In 2007, the estimated propensity is 0.5% (95% CI: 0.2–1.4%) and relatively unchanged in 2014 (2.7%, 95% CI: 1.2–5.9%). However, the estimated propensity for that hypothetical patient in 2017 is 39.8% with broad confidence intervals (19.2–83%). Older postnatal age was associated with an increased propensity to pursue TC-PDA (OR: 1.26 per week, 95% CI: 1.22–1.30, p<0.001) and lower gestational age was associated with a reduced propensity to pursue TC-PDA. These associations were seen in all alternative models (Supplementary Tables 3–5).
Figure 3: Multivariable model of propensity to pursue TC-PDA vs. O-PDA with inflection point at 1/1/2015.
This Forest plot depicts the results of the multivariable hierarchical logistic regression model. The point estimate (diamonds) of odds ratio for TC-PDA vs. O-PDA and 95% confidence intervals depicted (brackets) are depicted. Variables for which both the point estimate and confidence intervals are to the right are associated with increased propensity to pursue TC-PDA and vice versa.
Figure 4. Estimated probability of TC-PDA versus O-PDA.
Conditional standardization as used to calculate an adjusted probability of O-PDA vs. TC-PDA for a hypothetical white female with gestational age of 25–27 weeks and age of procedure of 74 days (the median age for TC-PDA in the study population) with no other risk factors (red line, 95% CI are depicted with dashed gray lines). This was based on the mixed effects model depicted in Figure 3. The probability of TC-PDA increased from 2007–2014 (OR: 1.26 per year, 95% CI: 1.12–1.42) but the propensity to pursue TC-PDA accelerated after 2014 (OR: 2.46, 95% CI: 2.05–2.96).
Sensitivity analyses were performed to determine if including corrected gestational age provided superior model fit than age and gestational age considered separately. Use of corrected gestational age did not improve model fit, and the primary association seen between date of procedure and propensity to pursue TC-PDA was unchanged (Supplementary Table 6–10).
A post hoc secondary analysis was performed to evaluate whether a significant association was seen between date of TC-PDA and the adjusted gestational age of subjects at that time. When evaluated over the entire study period, no significant association was seen (p=0.53). However, when analysis was restricted to the period during which most TC-PDA procedures were performed (1/2014 to 12/2017), later date of TC-PDA was associated with lower adjusted gestational age (ratio: 0.97 per year, p=0.03).
A second post hoc analysis was performed to evaluate whether there were significant differences in the adjusted gestational age at TC-PDA at hospitals with higher TC-PDA volumes compared to lower volume hospitals. The five centers with the highest total TC-PDA volume during the study period performed 43% (99/231) of TC-PDA cases with known gestational age. The adjusted gestational age (37+/−8 weeks) of TC-PDA subjects at these hospitals was significantly lower than those at lower volume hospitals (42+/−9 weeks, p=0.0001).
Inter-hospital variation in the propensity to utilize TC-PDA
In these same models, inter-hospital practice variation was evaluated. A test of heterogeneity demonstrated that there is significant inter-hospital practice variation (p<0.001). After adjusting for measurable confounders, the MOR for TC-PDA in the NICU population was 4.5, which is consistent with very large magnitude variation in practice. MOR for other models were all of similar magnitude (data not shown).
DISCUSSION:
In this multicenter retrospective observational study of 44 United States primary pediatric hospitals, two significant trends were observed. Between 2007 and 2017, the overall propensity to pursue mechanical closure of PDA in NICU patients decreased. At the same time, both the observed number of TC-PDA and case-mix adjusted propensity to perform TC-PDA increased with acceleration of both trends after 2015. Use of TC-PDA in NICU patients has not been adopted uniformly, with large magnitude practice variation in the utilization of TC-PDA between different institutions in our study sample.
In a previous era, a study using data from the National Inpatient Sample Kid’s Inpatient Database demonstrated that between 2003 and 2009 there was a modest increase in the rate of pursuing operative ligation in premature infants34. To our knowledge, data about contemporary practice are limited. Observational studies of very low birth weight neonates in North American NICUs have demonstrated decreasing use of both O-PDA and pharmacological treatment of PDA35,36. The current study demonstrates a similar trend in mechanical closure across the entire range of patients seen in United States tertiary care NICU’s. At the same time it also demonstrates an accelerating expansion of the use of TC-PDA in premature infants. It is not possible in this study to determine the reason(s) for this acceleration but represents a trend that may reflect dissemination of early case series and use of novel devices such as the Microvascular plug (Medtronic, Minneapolis Minnesota)11 and the Amplatzer Ductal Occluder 2 Additional Sizes, now renamed the Amplatzer Piccolo Occluder, (Abbott Medical, Plymouth Minnesota)37, the latter of which was in the process of enrolling subjects for an open-label trial during the study period (ClinicalTrials.gov ).
The argument for conservative therapy of hemodynamically significant PDA is predicated on the limitations of pharmacological therapy and O-PDA. Both prophylactic and therapeutic pharmacological intervention are not uniformly effective and are associated with increased risk of early adverse outcomes without demonstrable benefit in terms of mortality, development of BPD, and neurodevelopment1–4,38. O-PDA is uniformly effective in closing the PDA but incurs both both acute (post-ligation syndrome) and long-term risks of a thoracotomy in patients with already compromised pulmonary development. The promise of TC-PDA is achieving the technical success of ligation while reducing the adverse outcomes due to its less invasive nature, and early studies have had promising results9–15. Use of transthoracic echocardiographic evaluation of the position of the device vis a vis the aorta allows interventional cardiologists to perform TC-PDA without arterial access, which was the source of the most common and severe adverse outcomes in the smallest patients14,16. A clinical trial comparing TC-PDA to other treatment modalities may be necessary to determine whether these theoretical benefits are borne out.
At this time several issues remain outstanding that are potential obstacles to planning such a trial and to guiding clinical practice. Specifically, further research is necessary to identify which PDA are 1) hemodynamically significant and 2) likely to remain so. Also, it is important to identify the critical period(s) when PDA closure provides the maximal benefit. These two concerns may be in conflict with one another, but may become moot as TC-PDA in smaller premature infants becomes more routine. At this early stage, younger age is associated with increased risk of major adverse events, and lower birth weight is associated with increased risk of device embolization14. Though published series report closure of PDA in the very smallest of premature infants, the current study shows that the majority (55%) of TC-PDA procedures in NICU patients still are performed in patients with a corrected gestational age ≥34 weeks with persistent PDA. This suggests that TC-PDA is being withheld except for older patients who may still be lingering. Centers with significant experience are already reporting series in young neonates whose interventions were performed much earlier9–15, demonstrating that it is technically feasible with existing technology to intervene earlier in small children. Secondary analyses demonstrated that TC-PDA is being utilized in lower adjusted gestational age subjects and that higher volume centers have been performing TC-PDA in children with lower adjusted gestational age than less experienced centers. There does appear to be potential for earlier intervention, and these trends suggest that as TC-PDA in premature infants continues to disseminate, intervention will continue to be performed in young and smaller premature infants. This underscores the importance of determining the optimal time to intervene is (both in terms of patient size, post-natal age, and corrected gestational age). Experience with other transcatheter interventions shows that real-world practice varies significantly even in relatively established interventions22,32,33. If these questions are not directly addressed in clinical trials, the status quo is likely to result in significant practice variability and the inability to determine the potential benefit of TC-PDA in infants born prematurely.
There are several limitations to the current study. As an observational study, we cannot infer the causes of the observed trends. In addition, the clinically relevant data about each subject are limited to that which is available in the database. It is also important to acknowledge that this study depicts a very early phase of adoption of TC-PDA in this population. Practice will likely evolve as techniques disseminate and are refined, and as purpose-built devices become available such as the Amplatzer Piccolo Occluder, which has recently received FDA approval39. We acknowledge that at the present time, the experience of TC-PDA in premature infants is concentrated at a few centers. It is reasonable to expect that the patterns of use and practice surrounding treatment of hemodynamically significant PDA will evolve. However, we felt that the benefits of reporting the state of current practice and trends in that practice in the current era outweighed the risks. There is significant variation in practice at this time, indicating the presence of equipoise. We hope that this data will motivate and inform clinical trial design to determine the optimal method for treating hemodynamically significant PDA in this population and avoid dissemination of the technique of closing PDA without answering which patients will benefit from the procedure. Well-designed trials, we hope, will guide practice and ultimately result in the best possible care for premature infants with hemodynamically significant PDA.
CONCLUSION:
Despite these limitations, we conclude that there is evidence that there are simultaneously decreasing overall use of mechanical PDA closure in NICU patients in our study sample and rapidly increasing utilization of TC-PDA in these patients. It is too early at this time to determine whether the latter trend will result in a reversal of the former trend. Important research regarding the optimal patient selection and timing of TC-PDA is still needed, specifically a clinical trial(s) addressing relative safety and efficacy.
Supplementary Material
Table 2:
Multivariable model of propensity to pursue TC-PDA vs. O-PDA
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Date of procedure (per year) | 1.63 | 1.52, 1.76 | <0.001 |
| Age at PDA closure (by week) | 1.26 | 1.22, 1.30 | <0.001 |
| Gestational age | |||
| ≥37 weeks | 1 (ref.) | - | - |
| 34–36 weeks | 0.68 | 0.22, 2.06 | 0.49 |
| 28–33 weeks | 0.22 | 0.10, 0.48 | 0.001 |
| 25–27 weeks | 0.07 | 0.03, 0.15 | <0.001 |
| 23–24 weeks | 0.05 | 0.02, 0.10 | 0.001 |
| Missing | 0.11 | 0.05, 0.25 | 0.001 |
| Female sex | 0.97 | 0.71, 1.34 | 0.87 |
| Race | |||
| White | 1 (ref.) | - | - |
| Black | 1.10 | 0.73, 1.67 | 0.64 |
| Other or mixed race | 0.81 | 0.35, 1.86 | 0.62 |
| Missing | 0.77 | 0.48, 1.22 | 0.26 |
| Payer | |||
| Private insurance | 1 (ref.) | - | - |
| Public insurance | 0.71 | 0.50, 1.02 | 0.06 |
| Other government | 0.46 | 0.16, 1.34 | 0.16 |
| Other | 0.60 | 0.20, 1.80 | 0.36 |
| Genetic syndrome | 0.87 | 0.36, 2.11 | 0.75 |
| Gastrointestinal condition | 1.13 | 0.78, 1.65 | 0.52 |
| Hematologic condition | 1.16 | 0.46, 2.93 | 0.76 |
| Neurologic condition | 0.89 | 0.56, 1.40 | 0.61 |
| Respiratory condition | 1.14 | 0.76, 1.73 | 0.53 |
Highlights.
Between 2007–2017, mechanical closure of PDA has been pursued less frequently in US NICUs.
The propensity to pursue transcatheter closure of PDA has increased, especially since 2015.
Significant inter-hospital variation in the choice between O-PDA and TC-PDA is seen.
ACKNOWLEDGEMENTS:
This project utilized resources from the Cardiac Center Clinical Research Core at The Children’s Hospital of Philadelphia.
FUNDING SOURCES: Dr. O’Byrne receives support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420–01). Mr. Grady received support from The Children’s Hospital of Philadelphia Research Institute Summer Scholars Program (CRISSP) program. The funding agencies had no role in the planning or execution of the study, nor did they edit the manuscript as presented. The manuscript represents the opinions of the authors alone.
Abbreviations:
- BPD/CLD
bronchopulmonary dysplasia/chronic lung disease
- NICU
neonatal intensive care unit
- O-PDA
operative ligation of PDA
- PHIS
Pediatric Health Information Systems Database
- PDA
patent ductus arteriosus
- TC-PDA
transcatheter closure of PDA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: Dr. O’Byrne has received honoraria from Gore Medical (Newark, Delaware). There are no other conflicts of interest to disclose.
References
- 1.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2018;9:CD003481. doi: 10.1002/14651858.CD003481.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohlsson A, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;4:CD010061. doi: 10.1002/14651858.CD010061.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitra S, Florez ID, Tamayo ME, et al. Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen With Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-analysis. JAMA. 2018;319(12):1221–1238. doi: 10.1001/jama.2018.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;(7):CD000174. doi: 10.1002/14651858.CD000174.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross RE, Hubbard JP. Surgical Ligation of a Patent Ductus Arteriosus: Report of First Successful Case. JAMA. 1939;112:729–731. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira LS, Shivananda SP, Stephens D, Van Arsdell G, McNamara PJ. Postoperative cardiorespiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J Perinatol. 2008;28(12):803–810. doi: 10.1038/jp.2008.101. [DOI] [PubMed] [Google Scholar]
- 7.Benitz WE, Committee on Fetus and Newborn, American Academy of Pediatrics. Patent Ductus Arteriosus in Preterm Infants. Pediatr. 2016;137(1). doi: 10.1542/peds.2015-3730. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz M, Glatz AC, Rome JJ, Gillespie MJ. The amplatzer vascular plug and amplatzer vascular plug II for vascular occlusion procedures in 50 patients with congenital cardiovascular disease. Cathet Cardiovasc Intervent. 2009;76(3):411–417. doi: 10.1002/ccd.22370. [DOI] [PubMed] [Google Scholar]
- 9.Zahn EM, Peck D, Phillips A, et al. Transcatheter Closure of Patent Ductus Arteriosus in Extremely Premature Newborns Early Results and Midterm Follow-Up. JACC Cardiovasc Interv. 2016;9(23):2429–2437. doi: 10.1016/j.jcin.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Abu Hazeem AA, Gillespie MJ, Thun H, et al. Percutaneous closure of patent ductus arteriosus in small infants with significant lung disease may offer faster recovery of respiratory function when compared to surgical ligation. Catheter Cardiovasc Interv. 2013;82(4):526–533. doi: 10.1002/ccd.25032. [DOI] [PubMed] [Google Scholar]
- 11.Sathanandam S, Justino H, Waller BR, Radtke W, Qureshi AM. Initial clinical experience with the Medtronic Micro Vascular Plug™ in transcatheter occlusion of PDAs in extremely premature infants. Catheter Cardiovasc Interv. 2017;89(6):1051–1058. doi: 10.1002/ccd.26878. [DOI] [PubMed] [Google Scholar]
- 12.Philip R, Waller BR, Agrawal V, et al. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv. 2016;87(2):310–317. doi: 10.1002/ccd.26287. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MC, Nykanen D, Winner LH, et al. Transcatheter Patent Ductus Arteriosus Occlusion in Small Infants. Congenit Heart Dis. 2016;11(6):647–655. doi: 10.1111/chd.12360. [DOI] [PubMed] [Google Scholar]
- 14.Backes CH, Kennedy KF, Locke M, et al. Transcatheter Occlusion of the Patent Ductus Arteriosus in 747 Infants. JACC Cardiovasc Interv. 2017;10(17):1729–1737. doi: 10.1016/j.jcin.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Sathanandam S, Balduf K, Chilakala S, et al. Role of Transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc Interv. 2019;93(1):89–96. doi: 10.1002/ccd.27808. [DOI] [PubMed] [Google Scholar]
- 16.Backes CH, Rivera BK, Bridge JA, et al. Percutaneous Patent Ductus Arteriosus (PDA) Closure During Infancy: A Meta-analysis. Pediatr. 2017;139(2):e20162927. doi: 10.1542/peds.2016-2927. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez Ogando A, Planelles Asensio I, la Blanca de ARS, et al. Surgical Ligation Versus Percutaneous Closure of Patent Ductus Arteriosus in Very Low-Weight Preterm Infants: Which are the Real Benefits of the Percutaneous Approach? Pediatr Cardiol. 2018;39(2):398–410. doi: 10.1007/s00246-017-1768-5. [DOI] [PubMed] [Google Scholar]
- 18.O’Byrne ML, Glatz AC, Shinohara RT, et al. Effect of center catheterization volume on risk of catastrophic adverse event after cardiac catheterization in children. Am Heart J. 2015;169(6):823–832.e825. doi: 10.1016/j.ahj.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Byrne ML, Shinohara RT, Mi L, et al. Inter-hospital Variation in Costs of Pediatric Cardiac Catheterization: An Analysis of the PHIS Database. Circ Cardiovasc Qual Outcomes. 2018;11(Suppl 1):A227. [Google Scholar]
- 20.O’Byrne ML, Glatz AC, Faerber JA, et al. Inter-hospital variation in the costs of pediatric/congenital cardiac catheterization procedures:. J Am Heart Assoc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Byrne ML, Glatz AC, Hanna BD, et al. Predictors of Catastrophic Adverse Outcomes in Children With Pulmonary Hypertension Undergoing Cardiac Catheterization: A Multi-Institutional Analysis From the Pediatric Health Information Systems Database. J Am Coll Cardiol. 2015;66(11):1261–1269. doi: 10.1016/j.jacc.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Byrne ML, Shinohara RT, Grant EK, et al. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: An observational study using data from the Pediatric Health Information Systems database. Am Heart J. 2017;192:85–97. doi: 10.1016/j.ahj.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Byrne ML, Glatz AC, Mercer-Rosa L, et al. Trends in pulmonary valve replacement in children and adults with tetralogy of fallot. Am J Cardiol. 2015;115(1):118–124. doi: 10.1016/j.amjcard.2014.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasquali SK, Hall M, Li JS, et al. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation. 2010;122(21):2123–2130. doi: 10.1161/CIRCULATIONAHA.110.948737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatr. 2001;107:1–5. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 26.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of Transcatheter and Operative Pulmonary Valve Replacement (from the Pediatric Health Information Systems Database). Am J Cardiol. 2016;117(1):121–126. doi: 10.1016/j.amjcard.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. AmJEpidemiol. 2005;161(1):81–88. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 28.Peterson PN, Chan PS, Spertus JA, et al. Practice-level variation in use of recommended medications among outpatients with heart failure: Insights from the NCDR PINNACLE program. Circ Heart Fail. 2013;6(6):1132–1138. doi: 10.1161/CIRCHEARTFAILURE.113.000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddox TM, Chan PS, Spertus JA, et al. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2014;63(6):539–546. doi: 10.1016/j.jacc.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hira RS, Kennedy K, Jneid H, et al. Frequency and practice-level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;63(25 Pt A):2876–2877. doi: 10.1016/j.jacc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am J Cardiol. 2011;108(8):1136–1140. doi: 10.1016/j.amjcard.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Byrne ML, Kennedy KF, Rome JJ, Glatz AC. Variation in practice patterns in device closure of atrial septal defects and patent ductus arteriosus: An analysis of data from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J. 2018;196:119–130. doi: 10.1016/j.ahj.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glatz AC, Kennedy KF, Rome JJ, O’Byrne ML. Variations in Practice Patterns and Consistency With Published Guidelines for Balloon Aortic and Pulmonary Valvuloplasty. JACC Cardiovasc Interv. 2018;11(6):529–538. doi: 10.1016/j.jcin.2018.01.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg JG, Evans FJ, Burns KM, Pearson GD, Kaltman JR. Surgical ligation of patent ductus arteriosus in premature infants: trends and practice variation. Cardiol Young. 2016;26(6):1107–1114. doi: 10.1017/S1047951115001869. [DOI] [PubMed] [Google Scholar]
- 35.Hagadorn JI, Brownell EA, Trzaski JM, et al. Trends and variation in management and outcomes of very low-birth-weight infants with patent ductus arteriosus. Pediatric Research. 2016;80(6):785–792. doi: 10.1038/pr.2016.166. [DOI] [PubMed] [Google Scholar]
- 36.Lokku A, Mirea L, Lee SK, Shah PS, Canadian Neonatal Network. Trends and Outcomes of Patent Ductus Arteriosus Treatment in Very Preterm Infants in Canada. Am J Perinatol. 2017;34(5):441–450. doi: 10.1055/s-0036-1593351. [DOI] [PubMed] [Google Scholar]
- 37.Bruckheimer E, Godfrey M, Dagan T, Levinzon M, Amir G, Birk E. The Amplatzer Duct Occluder II Additional Sizes device for transcatheter PDA closure: initial experience. Catheter Cardiovasc Interv. 2014;83(7):1097–1101. doi: 10.1002/ccd.25445. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt B, Roberts RS, Fanaroff A, et al. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP). J Pediatr. 2006;148(6):730–734. doi: 10.1016/j.jpeds.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 39.United States Food and Drug Administration. Premarket Approval: AMPLATZER Piccolo Occluder. accessdata.fda.gov. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.