Abstract
Eimeria species are intestinal protozoan parasites that cause lack of production, malabsorption and mortality in floor raised chickens. Administering an oral antibody to interleukin 10 (aIL-10) reduces the symptoms of coccidiosis in broilers, indicating interleukin 10 (IL-10) is key to Eimeria pathology. IL-10 is an anti-inflammatory cytokine and acts as a stand down signal to reduce inflammation and host pathology during disease. Related protozoan parasites exploit IL-10 to reduce pathogen-damaging host inflammatory responses. We hypothesize that IL-10 is increased during Eimeria infection through an unknown host-pathogen interaction, and by feeding aIL-10 to neutralize excess IL-10 the bird is allowed to mount an effective immune response to Eimeria. To determine the effects of aIL-10 during the intestinal immune response, intestinal pathology and the relationship between IL-10, interferon gamma (IFNγ) and Eimeria infection were evaluated in this study.
In both experiments, broilers were administered either a 10x dose of Advent® Eimeria vaccine or saline. Duodenum, jejunum and cecum samples were collected, processed, stained and examined under a microscope. Evaluation of intestinal histomorphology during aIL-10 administration showed minimal differences in birds fed aIL-10 during infection compared to animals fed a control antibody during Eimeria infection. To further evaluate aIL-10’s positive effect during infection, immunofluorescent histochemistry was performed on chicken intestines days 3-7 post Eimeria infection for IL-10 and IFNγ presence in intestinal mucosa in control and infected birds, in regions with and without visible Eimeria burden. IL-10 and IFNγ had significant changes between days 4.5-7 post-infection in birds fed aIL-10 compared to animals fed a control antibody. Overall we found that the duodenum had increased IL-10 presence and increased IFNγ presence, and the jejunum and cecum had decreased IL-10 presence and decreased IFNγ presence. These differences in spatial regulation of IL-10 and IFNγ may indicate Eimeria species induce slightly different cytokine responses.
Keywords: Eimeria, broiler chicken, Interleukin-10, Interferon Gamma, coccidiosis
INTRODUCTION
Eimeria spp. are a genus of intestinal parasitic pathogens that cause a disease termed coccidiosis, highly prevalent in floor raised poultry. Eimeria infection causes intestinal cell rupture, which leads to reduced production and mortality in both layer and broiler chickens. Eimeria infection causes an increase in host intestinal and systemic interleukin 10 (IL-10) levels (Hong et al. 2006a; Hong et al. 2006b; Rochell et al. 2016; Arendt et al. 2016). IL-10 is a potent anti-inflammatory cytokine that acts as a ‘stand down’ signal for cells of myeloid origin. IL-10 controls the host immune response to limit off-target host cell damage during inflammation by, for example, inhibiting pro-inflammatory cytokines such as IL-12 and interferon gamma (IFNγ), preventing major histocompatibility II expression and suppressing nitric oxide species production (Moore et al. 2001). Protozoan parasites can use IL-10 to downregulate host immunity and reduce pathogen-damaging inflammatory responses (Cyktor and Turner 2011). We previously found oral IgY antibodies to IL-10 (aIL-10) are effective at alleviating the negative effect of coccidiosis on body weight in broiler chickens (Sand et al. 2016). The benefit of aIL-10 during Eimeria infection suggests that Eimeria also use host IL-10 to dampen the chicken immune response. The direct relationship between Eimeria hijacking host IL-10 to benefit its pathogenesis has not been established.
To delve into the mechanism of aIL-10 as a therapeutic for coccidiosis, more needs to be known about IL-10’s role during Eimeria infection. Specifically, when and where in the intestine IL-10 is increased during infection and how does this affect inflammation. If IL-10 is increased for succinct periods of time after exposure to Eimeria, poultry producers can shorten the time aIL-10 is administered after Eimeria vaccination to reduce costs associated with treatment. Intestinal luminally-secreted IL-10 levels during Eimeria infection were previously evaluated by ELISA, and an increase was found on days 4 in the cecum and 7 in the jejunum compared to uninfected chickens (Arendt et al. 2016).
In this study, we use immunofluorescent histochemistry (IHC) to measure IL-10 and IFNγ in intestinal mucosal microenvironments where Eimeria are or are not present compared to uninfected chickens. We chose to use IHC because reviewing our past studies, which used an ELISA to quantify luminal IL-10 protein, as well as related literature in which IFNγ and IL-10 were quantified using qPCR and ELISA, we noted the precise intestinal locations and timing of Eimeria induction of host intestinal cytokines was missing. IHC provided us qualitative insight to answer the question: Does Eimeria interact with host cells to create a pathogen-beneficial microenvironment enriched with anti-inflammatory cytokines, such as IL-10 and depleted of pro-inflammatory cytokines, such as IFNγ? By evaluating intestinal microenvironments with IHC rather than quantitative measures on whole intestine, we are able to determine if IL-10 production is homogeneously affected throughout the intestinal mucosa or only where Eimeria is present.
IL-10 antagonizes the inflammatory response by acting on macrophages and dendritic cells to inhibit macrophage activation and the production of proinflammatory cytokines such as IL-12 and IFNγ (Moore et al. 2001). IFNγ has previously been shown to be important in the protective immune response against Eimeria; IFN-γ levels and fecal oocyst shedding were inversely correlated (Dimier-Poisson et al. 2004; Kogut and Lange 1989; Lillehoj and Choi 1998, Yun et al. 2000). Therefore, we used IFNγ as a marker to measure the intestinal pro-inflammatory response. We hypothesize that Eimeria upregulate the production of IL-10 in surrounding microenvironments to allow for evasion of the host immune response and down regulation of IFNγ.
The increase of IL-10 during Eimeria infection and subsequent reduced host immunity hypothetically may aid in parasite replication. However, using aIL-10 as an anti-coccidial does not decrease Eimeria oocyst shedding in feces (Sand et al. 2016). The lack of an effect on oocyst shedding provides evidence that Eimeria are able to cycle and replicate normally. We hypothesize that aIL-10 allows the chicken to mount an adequate immune response to Eimeria and improves intestinal barrier integrity. In this study, measurements of intestinal histomorphology are used to determine if aIL-10 reduces Eimeria-associated intestinal damage, such as widened and shortened villi and increased crypt depth (Kim et al. 2017).
MATERIALS AND METHODS
Experiments involving chickens were approved by the College of Agricultural and Life Sciences Animal Care Committee at the University of Wisconsin-Madison.
Intestinal IL-10 and IFNγ mucosal microenvironments during Eimeria infection
Chick Experimentation
Sixty-three, one-day-old straight run Cornish rock Cobb broiler chicks from Welp Hatchery (Bancroft, Iowa) were raised on a commercial chick diet until 19 days of age to ensure the presence of mature secondary lymphoid organs. At 19 days of age, chicks were divided into two treatment groups; chicks were randomly selected to be orally administered either Eimeria challenge by oral gavage with 10x dose of Advent® coccidia vaccine [infected group, n=36] (Huvepharma, Sofia, Bulgaria) or phosphate buffered saline [control group, n=27]. The Advent® coccidia vaccine consists of three Eimeria species, E. acervulina, maxima and tenella, which each have tropism to a distinct intestinal section, the duodenum, jejunum and cecum, respectively. Each section of the intestine was collected at 12 hour intervals due to the short, 60 minute half-life of IL-10 and to accurately assess the three Eimeria species (Le et al. 1997). At 12 hour intervals between 3-7 days post infection, three uninfected birds and four infected birds were randomly selected for sample collection. Samples were taken from the duodenal loop, jejunum proximal to Meckel’s diverticulum, and the blind end of the cecum. The ileum was not collected because Eimeria strains used do not infect the ileum. Intestinal segments were fixed in 10% formalin solution before embedded into paraffin wax and sectioned.
Immunohistochemistry Staining
Paraffin sections were incubated overnight at 60°C before being deparaffinized with three changes of Xylene for 10 minutes each, and rehydrated with isopropyl alcohol at two changes of 100% alcohol, two changes of 95% alcohol, one change of 75% alcohol, and one change of distilled water for 1 min each change. Slides underwent heat induced epitope-retrieval in Tris Urea solution. After rinsing with Tris Buffered Saline solution three times, an ImmEdge ™ Hydrophobic Barrier pen (Vector Laboratories, Inc., Burlingame, CA) was used to isolate tissue sections and slides were submerged with blocking buffer for 1 hour in a humidified chamber (Bobeck et al. 2015).
To stain IL-10, tissues were coated in rabbit aIL-10 polyclonal antibody (Bioss Inc., Boston, MA) at 1:300 dilution in blocking buffer overnight. To stain IFNγ, a contiguous intestinal section was coated in rabbit anti- chicken IFNγ polyclonal antibody (My Biosource Inc., San Diego, CA) at 1:100 dilution in blocking buffer overnight at 4°C in a humidified dark enclosure. Slides were stained with 1:100 diluted Donkey anti-rabbit Dylight®594 (Bethyl, Montgomery, TX) for one hour in a humidified chamber. Nuclei were highlighted by 4',6-diamidino-2-phenylindole (DAPI) in Fluoro-Gel with tris buffer solution (Electron Microscopy Sciences, Hatfield, PA).
Slide imaging and analysis
Slides were imaged at 200 X with a Nikon Eclipse E600 microscope with Y-FL fluorescence attachment (Nikon Instruments Inc., Melville, NY). Eimeria gamete and oocyst wall autofluorescence at 495 nm was used to consistently identify Eimeria infected regions (Beer et al. 2018). Three images per intestinal section per bird were taken systematically; one image of the tip, one image of the middle and one image of the crypt, each from three different random villi in a sample. When imaging slides from birds infected with Eimeria, three images were taken in a similar manner from villi not containing visible Eimeria (null) and three images were taken from villi where Eimeria were visible and easily identified (Eimeria visible) to evaluate if the microenvironment interaction between Eimeria and intestinal cells undergo differing cytokine interactions compared to the surrounding mucosal environment. Image J quantified the percent tissue area of IL-10 and IFNγ presence (Schindelin et al. 2015).
Statistical Analysis
The experimental design is a completely randomized design with subsampling. Data analysis was done by a linear mixed effects model on SAS 9.4. For data that had a significant main effect the differences of least squared means were used to determine significant differences between infection vs. non-infection values within time points. Statistical significance was declared at a P-value of ≤0.05.
Intestinal histomorphology during coccidiosis with aIL-10 treatment
Chick Experimentation
The experiment was set up in a 2 × 2 factorial design. Chicks were either orally gavaged with saline or a 10x dose of coccidia vaccine (Advent ®, consisting of a proprietary blend of live non-attenuated Eimeria acervulina, Eimeria maxima, and Eimeria tenella oocysts, Huvepharma, Sofia, Bulgaria), on day 3 of life and fed control antibody or aIL-10 throughout the study. Control and IL-10 egg yolk antibody was prepared as previously described, lyophilized and fed at 0.341g/Kg feed (Arendt et al. 2016; Sand et al. 2016). Thirty-six chicks were randomly separated into nine chicks per treatment, and randomly selected for intestinal sampling on 4, 7 and 13 days post infection (DPI). Intestinal sections were collected from the duodenum, jejunum and ceca and promptly placed in formalin. Intestines underwent paraffin sectioning and hematoxylin and eosin staining to evaluate intestinal morphology.
Intestinal Histology Measurements
Each sample had measurements taken of villi height, villi width, crypt depth, and muscularis height on an Axio Vert.A1 microscope (Zeiss, Oberkochen, Germany) with QCapture (QImaging, Surrey, British Columbia, Canada). Samples were blinded, and all measurements were taken by the same individual. To obtain an accurate mean, villi height, villi width, and crypt depth were measured three times per sample, and the muscularis height was measured five times per sample.
Statistical Analysis
The trial was a single-blinded randomized controlled trial, and collected data were analyzed using ANOVAs PROC MIXED of SAS 9.4 (SAS Institute Inc., Cary, NC). The least significant difference test was used for multiple treatment comparisons using the least squares means statement of SAS 9.4 with letter grouping obtained using the SAS pdmix800 macro (pairwise mean comparisons). For the different statistical tests, significance was declared at a P-value of <0.05.
RESULTS
Intestinal IL-10 and IFNγ mucosal microenvironments during Eimeria infection
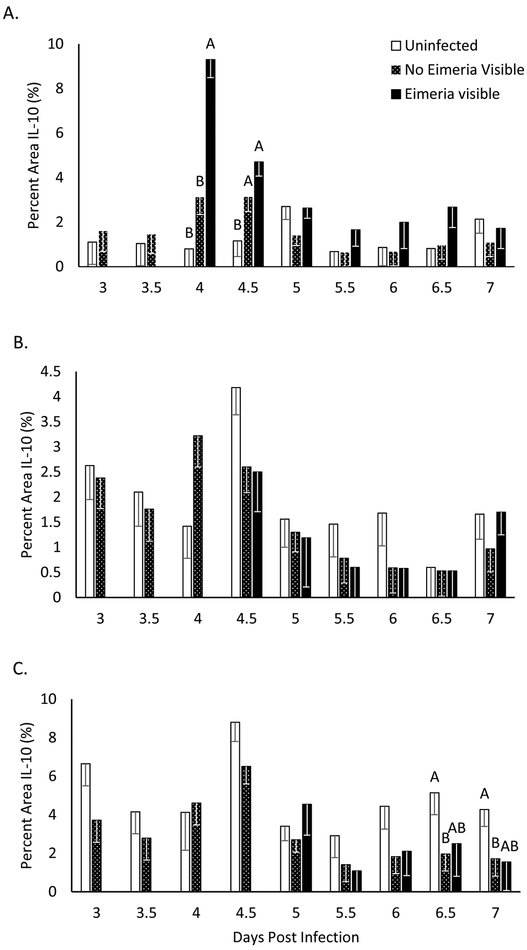
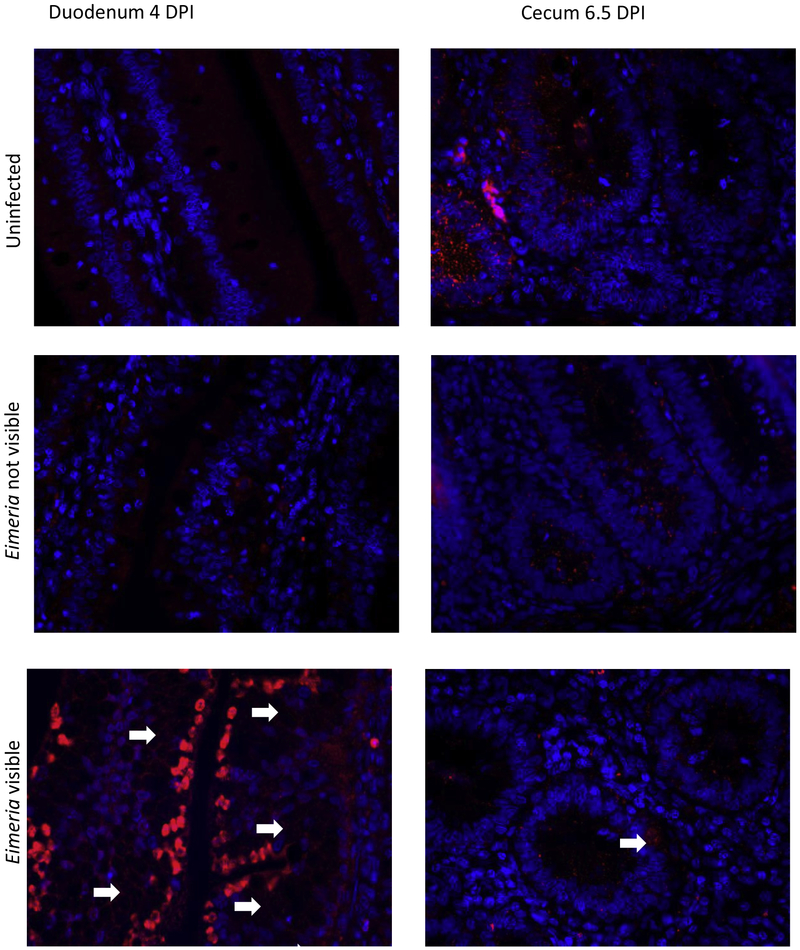
After infection and immunofluorescent staining, we measured the percentage of red pixels for each intestinal section for infected and uninfected birds. In the duodenum, uninfected birds had a narrow range of 0.7 to 2.7 percent area of IL-10 fluorescence. For infected birds, duodenal IL-10 presence remains within this range except for 4 and 4.5 days post infection (DPI). IL-10 presence significantly peaked at 9.3% in microenvironments where Eimeria infection was visible on 4 DPI compared to uninfected birds at 0.8% and infected birds where Eimeria were not visible at 3.1% (p<0.05, figure 1A). Immunofluorescent imaging shows the increased IL-10 appears to be cellular-associated and located along the apical surface of intestinal epithelial cells in regions heavily infected with Eimeria (figure 2). On 4.5 DPI in the duodenum, IL-10 presence is increased in Eimeria infected birds both in regions where Eimeria are and are not visible, at 4.7% and 3.1% respectively, compared to uninfected birds at 1.16% (p<0.05, figure 1A). In the jejunum, no significant differences in IL-10 presence are observed (figure 1B); however, there was greater variation in IL-10 percent area between days, ranging from 0.6 to 4.2%. Similarly, the cecum had a wide range of percent area of IL-10 in uninfected birds from 2.91% on 5.5 DPI and 8.8% on 4.5 DPI. The increase of IL-10 presence in the duodenal mucosa is in contrast to the effect of Eimeria infection in the cecum. IL-10 presence is significantly decreased in infected birds where no Eimeria are visible on days 6.5 and 7 in the cecum (p<0.05, figure 1C). IL-10 presence in the intestinal epithelial cells of uninfected birds is concentrated intracellularly at the crypt (figure 2).
Figure 1: Interleukin 10 Quantification in Intestinal Sections.
Interleukin 10 quantified with Image J as a percent area of fluorescence of the imaged intestinal section. Chickens were infected at day 19 of age and intestinal sections were collected on every 12 hours days 3-7 post infection. Eimeria visible samples are not present early in the infection because only gametogony stages of Eimeria infection were assessed. Each data point shows the average percent area of IL-10 fluorescence and error bars show SEM, n= 3 uninfected chicks or 4 infected chicks. For intestinal sections that had a significant main effect of Eimeria infection on IL-10 presence, the differences of least squared means were used to determine significant differences between infection vs. non-infection values within time points. A,B Indicates significant difference at P<0.05.
Figure 2: Interleukin 10 Immunofluorescent Histochemistry Images.
Immunofluorescent histochemistry sections demonstrating IL-10 presence in uninfected and Eimeria infected chicks. Chicks at 19 days of age were infected with an avirulent cocci-vaccine (10X dose) that contained E. acervulina, E. maxima and E. tenella. Images shown are at time points in each section of the intestine when the largest difference of IL-10 presence was evident. Immunohistochemical staining of IL-10 protein (red) and cellular nuclei (blue) in control (top), null (middle) and infected (bottom) chicken mucosa. The intestinal lumen is located at the top of each photo. White arrows indicate areas where Eimeria are present.
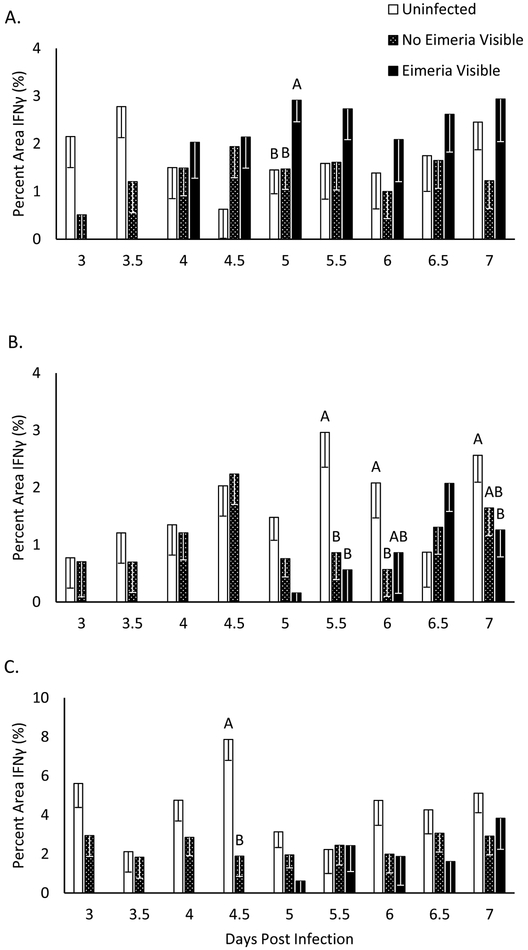
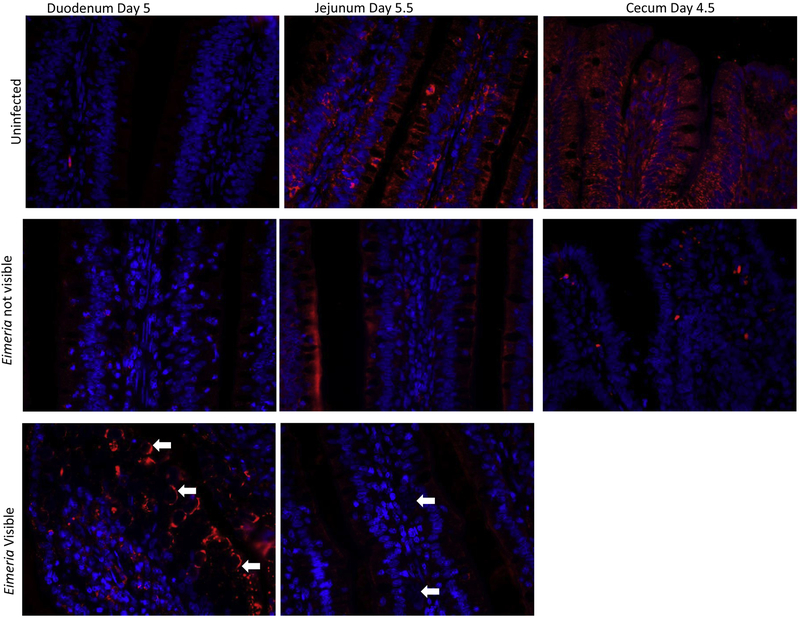
IFNγ is increased in Eimeria infected duodenal mucosa at 5 DPI in microenvironments where Eimeria are visible (p<0.05, figure 3A). Immunofluorescent images of Eimeria infected regions of the intestine demonstrate IFNγ surrounding Eimeria gametocytes and zygotes (figure 4). However, regions where Eimeria are not visible have an IFNγ presence equivalent to that of an uninfected bird. In the jejunum, IFNγ presence is decreased on days 5.5, 6 and 7 (p<0.05, figure 3B). In the cecum, IFNγ presence is significantly decreased only on day 4.5 post infection (p<0.05, figure. 3C). Similar to IL-10, IFNγ presence in uninfected birds is located within intestinal epithelial cells (figure 4). The range of IFNγ percent area of uninfected birds is 0.6-2.8% in the duodenum, 0.8-2.9% in the jejunum and 2.1-7.9% in the cecum. No other significant differences were observed.
Figure 3: Interferon Gamma Quantification in Intestinal Sections.
IFNγ quantified with Image J as a percent area of fluorescence of the imaged intestinal section. Chickens were infected at day 19 of age and intestinal sections were collected on every 12 hours days 3-7 post infection. Eimeria visible samples are not present early in the infection because only gametogony stages of Eimeria infection were assessed. Each data point shows the average percent area of IFNγ fluorescence for 3 uninfected chicks or 4 infected chicks. A, B * Indicates significant difference at P<0.05.
Figure 4: Interferon Gamma Immunofluorescent Histochemistry Images.
IFNγ presence is quantified using image J to calculate the percent staining of the imaged intestinal section. Chicks were orally gavaged with a 10X dose of an avirulent coccidia vaccine that contained E. acervulina, E. maxima, and E. tenella. Images shown are at time points in each section of the intestine when the largest difference of IFNγ presence was evident. Immunohistochemical staining of IFNγ protein (red) and cellular nuclei (blue) in control (top), null (middle) and infected (bottom) chicken mucosa. White arrows indicate areas where Eimeria are present. The intestinal lumen is located at the top of each photo. On day 4.5, identifiable Eimeria stages were not visualized in the cecum, therefore only uninfected and null images are shown.
Intestinal histomorphology during coccidiosis with aIL-10 treatment
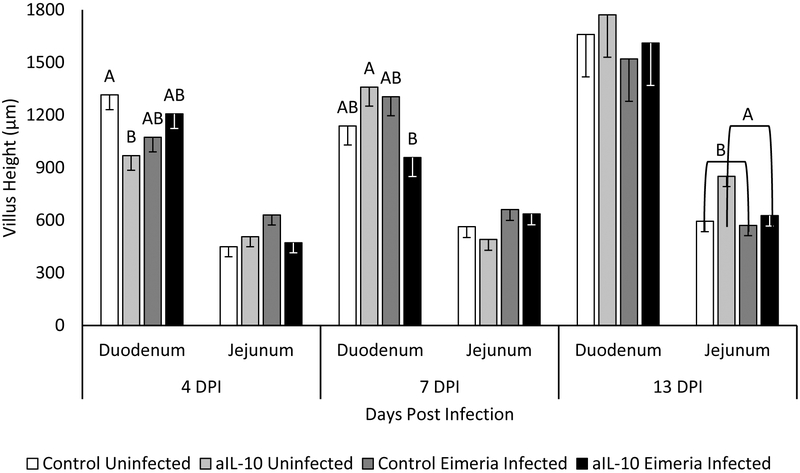
As a measure of intestinal pathology, we measured villi height, width, crypt depth and muscularis (figure 5). Villi height was significantly decreased on 4 DPI in aIL-10 fed, uninfected chicks (figure 6), but this decrease in villi height is recovered by 7 DPI. While aIL-10 fed uninfected chicks had decreased villi height, aIL-10 fed infected chicks had an even greater decreased villi height (figure 6). No significant differences were observed on villi height in the jejunum; however, aIL-10 diet had a positive effect on jejunal villi height on 13 DPI with a mean height of 738.5 μm compared to 582.5 μm in control fed birds (p<0.05). No significant changes were observed in intestinal villi width among any of the treatments (supplemental figure 1).
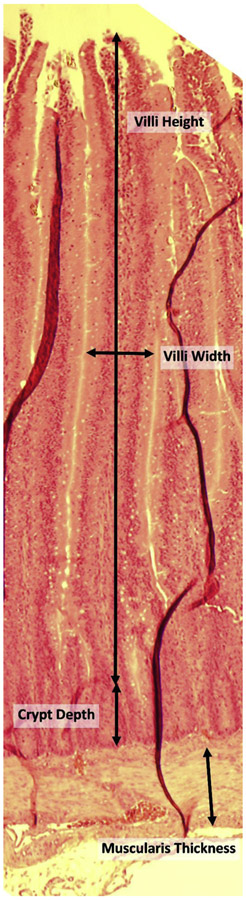
Figure 5: Intestinal Histomorphology Measurement Diagram.
Intestinal histomorphology measures were taken on villi where the red blood cells could be visualized in the lamina propria and epithelial cell presence at the tip of the villus to ensure measurement of a complete villi. Measurements taken included villus height, villus width, crypt depth, and muscularis thickness.
Figure 6: Villus Height.
Chickens were infected with Eimeria at 3 days of age and samples were taken at 4, 7 and 13 DPI. Villus height is measured from tip of villi to start of crypt in micrometers. Cecal measurements are not included because by definition, the ceca lack villi. Each bar represents the mean villus height of 3 chicks. Error bars indicate SEM. A,B Indicates significant difference, P<0.05 for each intestinal section and day post infection.
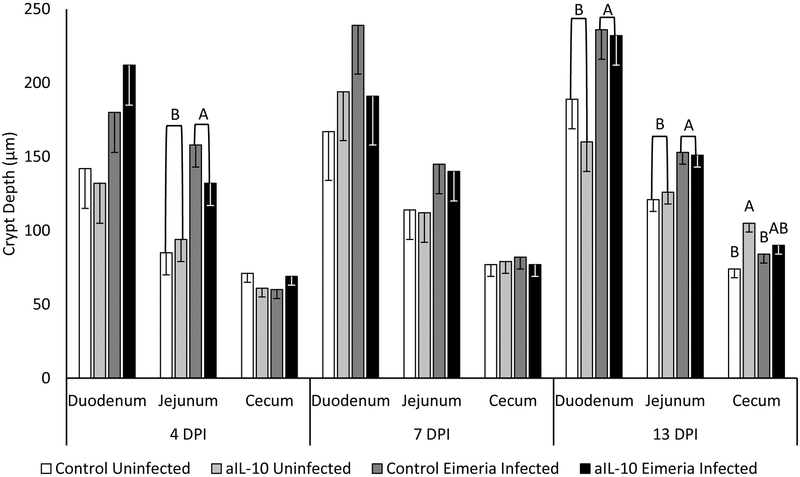
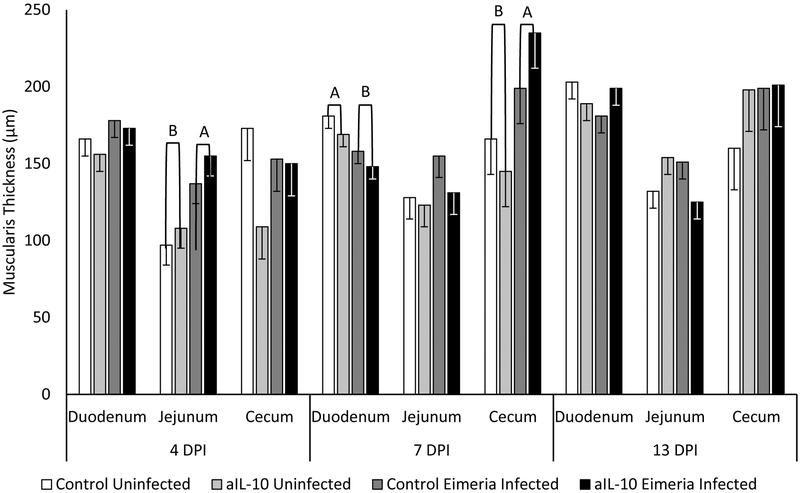
Eimeria infection increases crypt depth in the duodenum and jejunum at 4 and 13 DPI (figure 7). No significant change was seen on 7 DPI. On 13 DPI, the crypt depth of aIL-10, uninfected chicks is 105 μm, which is significantly greater than the crypt depth of control fed chicks at 74 μm (p<0.05). Jejunal muscularis thickness was significantly increased by 43% in Eimeria infected chicks regardless of diet treatment on 4 DPI (figure 8). On 10 DPI, the duodenal muscularis decreased from 175 μm in uninfected chicks to 153 μm in infected chicks. The cecal muscularis of infected chicks significantly increased in size by 40% compared to uninfected birds. No other significant changes were observed.
Figure 7: Crypt Depth.
Chickens were infected with Eimeria at 3 days of age and samples were taken at 4, 7 and 13 DPI. Crypt depth is measured from start of crypt to muscularis in micrometers. Each bar represents the mean crypt depth of 3 chicks. Error bars indicate SEM. A,B Indicates significant difference, P<0.05 for each intestinal section and day post infection.
Figure 8: Muscularis Thickness.
Chickens were infected with Eimeria at 3 days of age and samples were taken at 4, 7 and 13 DPI. Muscularis thickness is measured as the thickness of both muscular layers that make up the wall of the intestine. Each bar represents the mean muscularis thickness of 3 chicks. Error bars indicate SEM. A,B Indicates significant difference, P<0.05 for each intestinal section and day post infection.
DISCUSSION
The purpose of this study was to determine the effects of Eimeria infection on intestinal IL-10 and IFNγ. We found that IL-10 and IFNγ were both present during infection and both increased in the duodenum compared to uninfected birds. In the jejunum, IFNγ was decreased and both IL-10 and IFNγ were decreased in the cecum at various time points. The decrease in cytokine presence compared to uninfected birds in the jejunum and cecum was not expected; however, it may indicate why there were minimal effects on intestinal histomorphology with aIL-10 treatment during Eimeria infection. IFNγ and IL-10 levels increased or decreased in conjunction and were not inversely related as we had hypothesized. Furthermore, our hypothesis that aIL-10 works by increasing IFNγ does not align with the lack of impact of aIL-10 on oocyst production. If aIL-10 supported an increase in intestinal IFNγ, our past production studies would show a decrease in Eimeria oocyst production as observed by Yun et al. (2000), but no effect of aIL-10 on oocyst excretion was observed (Sand et al. 2016). If aIL-10 targets luminal IL-10 and not mucosal IL-10, it may have more of an effect on microbes within the lumen rather than intestinal pathology in the jejunum and the cecum.
We previously found an increase in IL-10 on day 7 in the jejunal luminal contents and day 4 in the cecal luminal contents (Arendt et al. 2016). The increase of cytokines in the luminal contents and decrease of cytokines in the mucosa suggests that enterocytes secrete cytokines into the lumen of the intestine in the jejunum and the cecum. The IL-10 receptor is expressed on the apical side of intestinal epithelial cells, and the presence of luminal IL-10 suggests IL-10 has an extracellular role in the lumen of the intestine (Kominsky et al. 2014). The interaction between the microbiome and the mucosal immune system is an emerging field of study, and the effect of luminal cytokines are largely unknown (Shi et al. 2017). Our uninfected samples had variability in cytokine percent area fluorescence day to day despite uniform sampling and processing techniques. The normal variation in uninfected bird intestinal mucosal cytokine presence may be due to differing microbiome interactions.
IL-10 and IFNγ mRNA presence during Eimeria infection have been evaluated in previous literature. Each species has been evaluated individually in their respective section of intestine. Eimeria acervulina infection of the duodenum results in an increase in IL-10 mRNA on 5 and 6 DPI, and in IFNγ mRNA on 6, 7 and 13 DPI (Rochell et al. 2016, Hong et al. 2006a, Hong, et al. 2006b). E. maxima infection of the jejunum causes an increase in IL-10 mRNA on 4, 6 and 9 DPI and in IFNγ on 4, 6 and 9 DPI (Hong et al. 2006a; Rothwell et al. 2004). E. tenella infection of the cecum resulted in an increase in IL-10 mRNA on 6, 9 and 10 DPI and in IFNγ on 6, 7, 8 DPI (Hong et al. 2006b; Yun et al. 2000). We found an increase in cytokine presence in the jejunum and cecum in uninfected compared to infected birds. Our results may be different because we measured IL-10 protein in the intestinal epithelial cells, whereas the other studies measured mRNA in intestinal epithelial lymphocytes or intestinal tissue which would include luminal mucus and secretions. Additionally, our research has shown that IL-10 presence is not homogenous in the lumen, mucosa and lamina propria (Arendt et al. 2016).
Intestinal epithelial cell expression of IL-10 is important in maintaining intestinal homeostasis and barrier integrity (Hyun et al. 2015). In uninfected birds IL-10 increased in the distal intestine, which correlates with increased bacterial presence. These differences in baseline epithelial IL-10 levels could play a role in the variation in Eimeria species specific immune response, pathology or intestinal section specificity. Differences in cytokine presence observed in each section of the intestinal tract, may be due to either the Eimeria species specific immune response as the three Eimeria species used in this study have a tropism for different sections of the gut. Differences in cytokine response to each Eimeria species were explored by Hong et al. (2006a, 2006b). Another explanation may be that the E. acervulina strain in the Advent® vaccine may be more virulent than the E. maxima and E. tenella vaccine strains and elicits the increase in IL-10 and IFNγ seen in the duodenum. The different cytokine responses observed in distinct regions of the intestine suggests that the intestinal immune response may be compartmentalized to microenviroments of the gut surrounding infection.
Known pathology associated with Eimeria infection include shorter villi due to increased intestinal epithelial cell sloughing and widened villi with increased crypt depth due to leukocyte infiltration (Kim, 2017). However, we did not see a significant difference in villi height and width due to Eimeria infection, because of the use of less pathogenic vaccine Eimeria strains to avoid bird mortality. Eimeria infection caused an expected increase in crypt depth, which was observed in the duodenum on day 13 post infection and in the jejunum on days 4 and 13 post infection. The increase in crypt depth due to Eimeria infection indicates leukocyte infiltration and increased intestinal epithelial cell turnover. aIL-10 had no effect on crypt depth in the duodenum and the jejunum. IL-10 reduces the recruitment of leukocytes, such as neutrophils. We hypothesized that aIL-10 would result in increased leukocyte presence and increased crypt depth, which was seen on day 13 post infection in the cecum, aIL-10 increased crypt depth in uninfected birds when compared to control fed birds (Sun et al. 2009).
aIL-10 had a negative impact on duodenal villi height in uninfected birds at 7 days of age or 4 DPI. This result is likely due to an overdose of aIL-10 because the chicks consume two times their body weight during the first week of life. The decrease in villi height may be due to the effect of aIL-10 on the intestinal microbiota or gut associated lymphoid tissue development. Germ free chickens have shorter and wider villi, while chickens fed beneficial probiotics have longer villi (Pan and Yu 2014). The mild negative effect in the duodenum of aIL-10 during the first week of life is recovered by day 10 in uninfected birds or at 7 DPI. At 2 weeks of age aIL-10 has a positive impact on villi height in the jejunum. The positive effect of aIL-10 on jejunal villi height on 16 days of age regardless of Eimeria infection offers support for a beneficial effect of aIL-10 on the gut microbiome. IL-10 is vital to intestinal homeostasis, in fact, IL-10−/− mice are used as a model for inflammatory bowel disease and have decreased villus height to crypt depth ratio compared to wild type mice (Gomes-Santos et al. 2012). In a healthy intestine, IL-10 promotes homeostasis by reducing the presence of pro-inflammatory cytokines, such as Tumor Necrosis Factor alpha (TNFα). TNFα increases intestinal epithelial shedding during intestinal inflammation. An increase in TNFα due to the inactivation of IL-10 by aIL-10, may be why we see a decrease in villi height in the duodenum on day 7 of life in aIL-10 treated, uninfected chicks (Blander, 2016, Oliveira et al. 2014). Overall aIL-10 had limited positive effects on intestinal pathology during Eimeria infection, but the increase in villi height at 14 days of age may indicate aIL-10 has beneficial effects on the intestinal microbiome during disease. aIL-10 may aid to prevent the growth of Clostridium perfringens, a key organism in Eimeria infection sequelae, necrotic enteritis. Clostridium perfringens stimulates an increase in IL-10 production in chicken intestinal epithelial cells (Lee et al. 2018).
This study found that aIL-10 has minimal effects on the intestinal histopathology during Eimeria infection. Results from this study, in addition to our previous work, indicate the role of IL-10 during Eimeria infection is likely more important in the mucosa in E. acervulina as its presence is increased in the mucosa rather than the luminal contents of the duodenum (Arendt et al. 2016). In contrast, during E. maxima and E. tenella infection, IL-10 presence is increased in luminal contents, rather than mucosal contents. This distinction is important as it may be related to the pathogenicity of each strain. The demonstrated difference in quantity of cytokine in mucosal and luminal samples indicates that cytokines are secreted into the lumen of the intestine and act on apical receptors. This difference underlines the importance of collecting luminal and mucosal samples to gain an accurate understanding of cytokine presence during intestinal infections. Moving forward, the findings presented in this study indicate future research on the microbiome during aIL-10 Eimeria infection and the role of intestinal luminal cytokines is needed to improve the understanding of intestinal immunity.
Supplementary Material
Supplemental Graph: Villus Width.
Chickens were infected with Eimeria at 3 days of age and samples were taken at 4, 7 and 13 DPI. Villus width is measured at the middle of the villi. Cecal measurements are not included because by definition, the ceca lack villi. Each bar represents the mean muscularis thickness of 3 chicks. Error bars indicate SEM. A,B Indicates significant difference, P<0.05 for each intestinal section and day post infection.
HIGHLIGHTS:
Intestinal cytokine responses differ by section during a mixed Eimeria infection.
Duodenal intestinal epithelial cell IL-10 and IFNγ increase during Eimeria infection.
Oral antibody to IL-10 improves jejunum villi height.
ACKNOWLEDGEMENTS:
The authors would like to thank Jessica Muhlenbeck, Zhouzheng Ren, Christopher Nguyen, Zachary Simons, Jordan Sand and Dan Butz for aiding in sample collection and analysis, as well as the UW Animal Care Staff (Dawn Irish, John Kemper and Terry Jobsis) for animal care. The authors would also like to thank the Chad Vezina laboratory for use of the Nikon Eclipse E600 microscope and the Laura Hernandez lab for use of the Axio Vert A1 microscope. Laying hens used for making antibodies in these studies were kindly donated by S&R Farms, Whitewater, WI. Eimeria vaccine was donated by Huvepharma, Sofia, Bulgaria.
FUNDING:
This work was supported by the NIH T32OD010423 training grant, Poultry Science Association Foundation Merck Animal Health Graduate Poultry Research Fellowship, the University of Wisconsin - Madison Food Research Institute and the University of Wisconsin School of Veterinary Medicine Summer Research Scholars Program. These funding sources had no involvement in the study design, data collection and analysis, or manuscript preparation or submission.
Footnotes
DECLARATION OF INTEREST
M. Cook had a patent registration for aIL-10 and had an ownership interest in AbE Discovery, LLC, which has licensed technology reported in this publication. All of the remaining authors have no declarations of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arendt MK, Sand JM, Marcone TM, and Cook ME, 2016. Interleukin-10 neutralizing antibody for detection of intestinal luminal levels and as a dietary additive in Eimeria challenged broiler chicks. Poult. Sci 95, 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer LC, Bielke LR, Barta JR, Faulkner OB, Latorre JD, Briggs WN, Wilson KM, Baxter MFA, Tellez G, and Hargis BM, 2018. Evaluation of autofluorescent Eimeria maxima oocysts as a potential indicator of non-viability when enumerating oocysts. Poult. Sci 97, 2684–2689. [DOI] [PubMed] [Google Scholar]
- Blander JM, 2016. Death in the intestinal epithelium—basic biology and implications for inflammatory bowel disease. The FEBS journal, 283, 2720–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EA, Hellestad EM, Sand JM, Piccione ML, Bishop JW, H. C, Petkovich M, and Cook ME, 2015. Oral peptide specific egg antibody to intestinal sodium-dependent phosphate co-transporter-2b is effective at altering phosphate transport in vitro and in vivo 1. Poult. Sci 94, 1128–1137. [DOI] [PubMed] [Google Scholar]
- Cyktor JC, and Turner J, 2011. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect. Immun 79, 2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimier-Poisson IH, Bout DT, and Quere P, 2004. Chicken primary enterocytes: inhibition of Eimeria tenella replication after activation with crude interferon-γ supernatants. Avian Dis. 48, 617–624. [DOI] [PubMed] [Google Scholar]
- Gomes-Santos A, Moreira TG, Castro-Junior A, Horta BC, Lemos L, Cruz DN, Guimarães MAF, Cara DC, McCafferty D-M, and Faria A, 2012. New Insights into the Immunological Changes in IL-10-Deficient Mice during the Course of Spontaneous Inflammation in the Gut Mucosa. Clin. Dev. Immunol 2012, 560817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YH, Lillehoj HS, Lillehoj EP, and Lee SH, 2006a. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol 114, 259–272. [DOI] [PubMed] [Google Scholar]
- Hong YH, Lillehoj HS, Lee SH, Dalloul RA, and Lillehoj EP, 2006b. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. and Immunopathol 114, 209–223. [DOI] [PubMed] [Google Scholar]
- Hyun J, Romero L, Riveron R, Flores C, Kanagavelu S, Chung KD, Alonso A, Sotolongo J, Ruiz J, Manukyan A, Chun S, Singh G, Salas P, Targan SR, and Fukata M, 2015. Human intestinal epithelial cells express interleukin-10 through Toll-like receptor 4-mediated epithelial-macrophage crosstalk. J. Innate Immun 7, 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Leung H, Akhtar N, Li J, Barta JR, Wang Y, Yang C, and Kiarie E, 2017. Growth performance and gastrointestinal responses of broiler chickens fed corn-soybean meal diet without or with exogenous epidermal growth factor upon challenge with Eimeria. Poult. Sci 96, 3676–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut MH, and Lange C, 1989. Interferon-γ-mediated inhibition of the development of Eimeria tenella in cultured cells. J. Parasitol 313–317. [PubMed] [Google Scholar]
- Kominsky DJ, Campbell EL, Ehrentraut SF, Wilson KE, Kelly CJ, Glover LE, Collins CB, Bayless AJ, Saeedi B, Dobrinskikh E, Bowers BE, MacManus CF, Müller W, Colgan SP, and Bruder D, 2014. IFN-γ–Mediated Induction of an Apical IL-10 Receptor on Polarized Intestinal Epithelia. J. Immunol 192, 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T, Leung L, Carroll WL, and Schibler KR, 1997. Regulation of interleukin-10 gene expression: possible mechanisms accounting for its upregulation and for maturational differences in its expression by blood mononuclear cells. Blood 89, 4112–4119. [PubMed] [Google Scholar]
- Lee Y, Kim WH, Lee S, and Lillehoj HS, 2018. Detection of chicken interleukin-10 production in intestinal epithelial cells and necrotic enteritis induced by Clostridium perfringens using capture ELISA. Vet. Immunol. Immunopathol 204, 52–58. [DOI] [PubMed] [Google Scholar]
- Lillehoj HS, and Choi KD, 1998. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 307–314. [PubMed] [Google Scholar]
- Moore K, deWaalMalefyt R, Coffman RL, and O’Garra A, 2001. Interleukin-10 and the interleukin-10 receptor. Annul. Rev. Immunol 19, 683–765. [DOI] [PubMed] [Google Scholar]
- Oliveira WN, Ribeiro LE, Schrieffer A, Machado P, Carvalho EM and Bacellar O, 2014. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of human tegumentary leishmaniasis. Cytokine 66:2, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, and Yu Z, 2014. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochell SJ, Helmbrecht A, Parsons CM, and Dilger RN, 2016. Influence of dietary amino acid reductions and Eimeria acervulina infection on growth performance and intestinal cytokine responses of broilers fed low crude protein diets. Poult. Sci 95, 2602–2614. [DOI] [PubMed] [Google Scholar]
- Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, and Kaiser P, 2004. Cloning and Characterization of Chicken IL-10 and Its Role in the Immune Response to Eimeria maxima. J. Immunol 173, 2675–2682. [DOI] [PubMed] [Google Scholar]
- Sand JM, Arendt MK, Repasy A, Deniz G, and Cook ME, 2016. Oral antibody to interleukin-10 reduces growth rate depression due to Eimeria spp. infection in broiler chickens. Poult. Sci 95, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Rueden CT, Hiner MC, and Eliceiri KW, 2015. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev 82, 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N, Li N, Duan X, and Niu H, 2017. Interaction between the gut microbiome and mucosal immune system. Mil. Med 4, 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Guo R-F, Newstead MW, Standiford TJ, Macariola DR, and Shanley TP, 2009. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. Am. J. Respir. Cell Mol. Bio 41, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun CH, Lillehoj HS, and Choi KD, 2000. Eimeria tenella Infection Induces Local Gamma Interferon Production and Intestinal Lymphocyte Subpopulation Changes. Infect. Immun 1282–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Graph: Villus Width.
Chickens were infected with Eimeria at 3 days of age and samples were taken at 4, 7 and 13 DPI. Villus width is measured at the middle of the villi. Cecal measurements are not included because by definition, the ceca lack villi. Each bar represents the mean muscularis thickness of 3 chicks. Error bars indicate SEM. A,B Indicates significant difference, P<0.05 for each intestinal section and day post infection.