Abstract
Introduction
Pregnancy-induced increases in nicotine metabolism may contribute to difficulties in quitting smoking during pregnancy. However, the time course of changes in nicotine metabolism during early and late pregnancy is unclear. This study investigated how pregnancy alters the nicotine metabolite ratio (NMR), a common biomarker of nicotine metabolism among nonpregnant smokers.
Methods
Urinary NMR (trans-3′-hydroxycotinine [3HC]/cotinine [COT]) was assessed using total (free + glucuronide) and free compounds among women (N = 47) from a randomized controlled trial for smoking cessation who self-reported smoking and provided a urine sample during early pregnancy (M ± SD = 12.5 ± 4.5 weeks’ gestation), late pregnancy (28.9 ± 2.0 weeks’ gestation), and 6 months postpartum (24.7 ± 1.2 weeks since childbirth). Urine samples were analyzed using liquid chromatography–tandem mass spectrometry and NMR were calculated as Total 3HC/Free COT, Free 3HC/Free COT, and Total 3HC/Total COT.
Results
NMR was significantly higher during early and late pregnancy compared to postpartum and significantly increased from early to late pregnancy as measured by Total 3HC/Free COT (0.76, 0.89, 0.60; all p’s < .05) and Free 3HC/Free COT (0.68, 0.80, 0.51; all p’s < .05). Total 3HC/Total COT did not vary over time (p = .81).
Conclusions
Total 3HC/Free COT and Free 3HC/Free COT increased in the first trimester and continued to increase throughout pregnancy, suggesting a considerable increase in nicotine metabolism over gestation. Future analyses are needed to interpret the changes in NMR in the context of nicotine pharmacokinetics, as well as its impact on changes in smoking behavior and cessation outcomes.
Implications
We observed that the NMR was significantly higher as early as 12 weeks’ gestation and increased further as a function of gestational age. Among nonpregnant smokers, elevated NMR is associated with smoking phenotypes such as smoking more cigarettes per day and poorer response to nicotine patch; therefore, pregnancy-induced increases in the NMR may contribute to smoking during the first trimester of pregnancy and reducing or quitting smoking may become more challenging as the rate of nicotine metabolism accelerates over the course of pregnancy.
Introduction
Despite known health risks, approximately 14% of US women continue to smoke while pregnant, which translates to nearly half a million pregnant cigarette smokers each year.1 One factor that may contribute to continued smoking antepartum is pregnancy-induced increases in nicotine metabolism and clearance. In a key study evaluating nicotine pharmacokinetics, 10 pregnant smokers received intravenous infusions of labeled nicotine-d2 (NIC-d2) and cotinine-d4 (COT-d4), and concentrations of parent compound and metabolites were measured in sessions conducted 1–2 times antepartum (16–40 weeks’ gestation) and/or 1 time postpartum (≥12 weeks postpartum).2 The clearance rates of nicotine and COT were 60% and 140% higher, respectively, during pregnancy compared to postpartum. No differences were observed within the antepartum period, although only three women received infusions during both the second and third trimesters.
A less invasive approach widely used to estimate the rate of the nicotine clearance among nonpregnant smokers is the nicotine metabolite ratio (NMR), which is the ratio of trans-3′-hydroxycotinine (3HC) to COT. The hepatic liver enzyme cytochrome P450 2A6 (CYP2A6) is primarily responsible for converting nicotine to COT and entirely responsible for converting COT to its proximate metabolite 3HC.3,4 The ratio of 3HC to COT serves as a surrogate for CYP2A6 activity and is strongly correlated with total nicotine clearance.5,6 The NMR, which can be measured in plasma, saliva, and urine, varies considerably from person to person due to extensive genetic variation in CYP2A6.7 Higher NMRs are associated with numerous smoking phenotypes including smoking more cigarettes per day, greater nicotine dependence, and poorer response to nicotine patch, making it a potentially useful biomarker for individualizing smoking cessation treatment.6,8 However, little is known about how the NMR is altered over the course of pregnancy.
To the best of our knowledge, a recent cohort study is the only study to date to examine the NMR during and following pregnancy.9 Salivary NMR was assessed during the first, second, and/or third trimesters of pregnancy and at 4 and/or 12 weeks postpartum; not all participants provided a sample at every time point. Overall, the NMR varied significantly over time, appearing higher antepartum compared to postpartum. Statistical analyses using the NMR at 12 weeks postpartum as the reference indicated NMR levels were 15%, 26%, 23%, and 9% higher in the first, second, and third trimesters, and 4 weeks postpartum, although only NMR assessed during the second and third trimesters were significantly higher; the substantial interindividual variation in NMR makes assessment of this data challenging to interpret.
Overall, previous studies2,9 provide evidence of increased nicotine and COT clearance and higher NMR levels during antepartum compared to postpartum, but leave uncertain when significant increases in the NMR first appear in pregnancy and whether the NMR increases significantly within the antepartum period. Additionally, although prior studies examining the NMR among pregnant smokers have measured metabolites in saliva and plasma,9,10 metabolite concentrations are considerably higher in urine, making it easier to detect and measure both Free (nonconjugated) and Total (sum of nonconjugated and conjugated) compounds.5 Among nonpregnant smokers, NMR described as Total 3HC/Free COT is considered the most biologically relevant measure of CYP2A6 activity, accounting for all 3HC metabolites converted from its precursor, Free COT (total product/free substrate).11,12 Studies have also used the Free 3HC/Free COT ratio13,14 and the Total 3HC/Total COT ratio15,16 as they are less expensive to assess. However, the Free 3HC/Free COT ratio does not incorporate all 3HC metabolites in the numerator; potential pregnancy-induced alterations in glucuronidation of 3HC could alter the numerator (ie, Free 3HC) and the resulting ratio in ways that are unrelated to CYP2A6 or nicotine clearance. Likewise the Total 3HC/Total COT ratio includes COT glucuronide in the denominator. Because pregnancy may directly influence urinary COT glucuronide levels,2 the relationship between the Total 3HC/Total COT ratio and estimation of CYP2A6 activity may be altered. Whether pregnancy differentially influences these ratios is unknown and was also a focus of this study.
This study used a rigorous within-subject design with complete NMR data at three assessment points: early and late pregnancy and 6 months postpartum. The primary aims were to examine (1) changes in the urinary NMR during pregnancy and postpartum, and (2) the relative impact of pregnancy on the biologically relevant urinary ratio in contrast to the other two NMR formulations.
Methods
This secondary analysis examined urine nicotine metabolite concentrations within a subset of pregnant smokers participating in a randomized clinical trial examining the effects of financial incentives on smoking abstinence during antepartum and postpartum periods.17 The University of Vermont and University of TorontoInstitutional Review Boards approved that study and all participants provided written informed consent. Details pertinent to the current investigation are described next.
Participants
Participants were recruited from obstetric practices and the Women, Infants, and Children (WIC) office in Burlington, Vermont. Main trial inclusion criteria were self-reported smoking in the past 7 days, urine COT specimen greater than 80 ng/mL, and gestational age at least 25 weeks. Exclusion criteria included self-reported use of prescribed opioid, psychomotor stimulant, or antipsychotic medications. A subset of 47 women who reported smoking and provided a urine specimen at each of the three time points of interest (see later) were included in this secondary analysis. On average, participants reported smoking 20 cigarettes/day before pregnancy and reduced to approximately 10 cigarettes/day by the early pregnancy assessment (Table 1).
Table 1.
Demographic and Smoking Characteristics at Early Pregnancy Assessment (N = 47)
| Demographics: | |
|---|---|
| Age (years) | 24.4 ± 5.4 |
| Education (years) | 12.2 ± 1.5 |
| % Caucasian | 91 |
| % married | 26 |
| % private insurance | 17 |
| % work for pay | 53 |
| % primigravida | 64 |
| Smoking characteristics: | |
| Age started smoking (years) | 15.1 ± 3.3 |
| % attempted to quit before learning of pregnancy | 77 |
| Cigarettes per day pre-pregnancy | 19.3 ± 8.8 |
| Cigarettes per day | 10.3 ± 7.2 |
| Minnesota Nicotine Withdrawal Scale score | 1.4 ± 0.7 |
| % living with other smoker(s) | 76 |
| % with none or few friends and/or family who smoke | 24 |
| % with no smoking allowed in home | 57 |
Values represent mean ± SD, unless otherwise specified.
Study Protocol
Urine samples were collected at trial assessments conducted during early pregnancy (mean ± standard deviation = 12.5 ± 4.5 weeks’ gestation), late pregnancy (28.9 ± 2.0 weeks’ gestation), and at 6 months postpartum (24.7 ± 1.2 weeks since childbirth). Urine samples were collected in specimen cups with temperature strips and tested for adulterants to help ensure their veracity. A portion of each sample was aliquoted into a specimen tube and stored frozen at −20°C until all samples were shipped to the University of Toronto in Ontario, Canada, for analysis. Urinary concentrations of nicotine and metabolites were directly analyzed using liquid chromatography–tandem mass spectrometry as described by Taghavi et al. (in press).18 In brief, urine samples were diluted and prepared using solid-phase extraction adapted from a previously established method.19 The limit of quantification was 1 ng/mL for all compounds. The accuracy and precision of this analytical method were within ±15% of the expected amount in both spiked nonsmokers’ and smokers’ urine samples, and the nicotine metabolic profile determined by this method closely matched known estimates in the literature.20–22
Data Analysis
Prior studies have shown that CYP2A6 activity can be measured by various other metabolite ratios (eg, 3HC/COT and COT/NIC) in plasma or urine depending on smoking status.23–25 In urine, three different metabolite ratios are used: Free 3HC/Free COT, Total 3HC/Total COT, and Total 3HC/Free COT. Considering that both CYP2A6 and UGT2B10 enzymatic activities change during pregnancy,26 all three ratios that are influenced by these enzymes were examined. As such, the NMR was calculated as Total 3HC/Free COT, Free 3HC/Free COT, and Total 3HC/Total COT, where total was the sum of the free compound and the glucuronide. All NMR values were logarithmically transformed. Statistical Analysis Software (SAS), version 9.4, PROC MIXED procedure, was used for all analyses. Repeated-measures analysis of covariance (see later) was used to determine whether urinary nicotine metabolite concentrations and each NMR differed over time. Significant main effects were followed by pairwise comparisons. Previous reports suggest that pregnancy may increase urine pH, which can alter reabsorption of nicotine and its metabolites.27 Urinary pH levels in the current sample differed significantly over time (F(2,45) = 4.93, p < .01; mean ± standard error = 6.94 ± 0.15 for early pregnancy, 7.11 ± 0.14 for late pregnancy, and 6.51 ± 0.14 for 6 months postpartum) and pairwise tests indicated that urinary pH was significantly higher during early and late pregnancy compared to 6 months postpartum (p’s < .05) but did not differ between early and late pregnancy. To control for these differences, urinary pH was entered as a time-varying covariate in each of the NMR models. Significance for all tests was set at p < .05.
Results
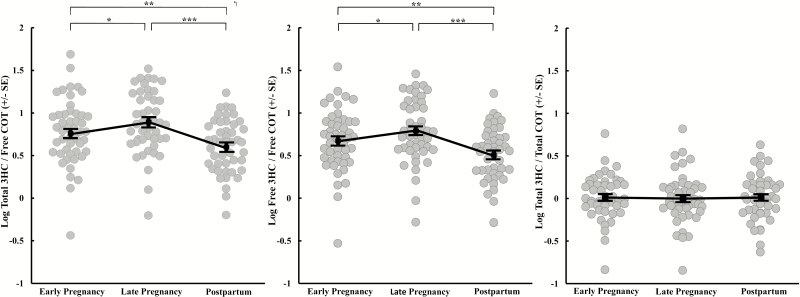
Free COT metabolite concentrations were significantly altered during pregnancy and postpartum (Table 2). Adjusting for changes in urine pH during early and late pregnancy and postpartum, the NMR measured by the ratio Total 3HC/Free COT differed significantly over time (F(2,93) = 15.02, p < .001; Figure 1, left panel). Specifically, the Total 3HC/Free COT ratio was significantly higher at both early and late pregnancy assessments compared to 6 months postpartum (22% and 33% higher, respectively; p’s < .01). Within the antepartum period, the Total 3HC/Free COT ratio was significantly higher at the late pregnancy compared to early pregnancy assessment (15% higher; p < .05). NMR calculated as the Free 3HC/Free COT ratio also differed significantly over time (F(2,93) = 15.09, p < 0.001; Figure 1, center panel) adjusting for urine pH. Like the Total 3HC/Free COT ratio, the Free 3HC/Free COT ratio was significantly higher at both early and late pregnancy assessments compared to 6 months postpartum (25% and 37% higher, respectively; p’s < .01) and was higher at the late pregnancy compared to early pregnancy assessment (15% higher; p < .05). In contrast, the Total 3HC/Total COT ratio did not change significantly over time (p = .81; Figure 1, right panel) adjusting for urine pH.
Table 2.
Nicotine Metabolite Levels During Pregnancy and Postpartum
| Analyte | Early pregnancy | Late pregnancy | Postpartum | Main effect of time |
|---|---|---|---|---|
| Free 3HC | 5376 ± 6856 | 4581 ± 5661 | 6006 ± 5427 | p = .183 |
| 3HC glucuronide | 1271 ± 1767 | 1074 ± 1490 | 1181 ± 1120 | p = .481 |
| Total 3HC | 6648 ± 8455 | 5656 ± 7092 | 7188 ± 6214 | p = .204 |
| Free COT | 834 ± 788a | 638 ± 581b | 1376 ± 848c | p < .001 |
| COT glucuronide | 5836 ± 10233 | 4765 ± 4827 | 5346 ± 4932 | p = .791 |
| Total COT | 6671 ± 10717 | 5403 ± 5236 | 6723 ± 5522 | p = .248 |
Mean (± SD) urinary nicotine metabolites (ng/mL). Statistical analysis conducted on log-transformed values. p values represent the main effect of time using repeated-measures analysis of covariance with pH as a time-varying covariate. When the overall F-statistic for the main effect of time was significant pairwise comparisons were examined. Means that do not share a common letter differed at p < .05.
3HC = trans-3′-hydroxycotinine; COT = cotinine.
Figure 1.
Mean (± SEM) log-transformed Total trans-3′-hydroxycotinine (3HC)/Free cotinine (COT) ratio (left), Free 3HC/Free COT ratio (middle), and Total 3HC/Total COT ratio (right) during early (M ± SD = 12.5 ± 4.5 weeks’ gestation) and late pregnancy (28.9 ± 2.0 weeks’ gestation) and postpartum (24.7 ± 1.2 weeks after childbirth). Significant differences between pregnancy and postpartum time points were derived from pairwise comparisons following repeated-measures analysis of covariance. *p < .05; **p < .01; ***p < .001.
Conclusions
This is the first systematic within person evaluation of changes in urinary NMR during antepartum and postpartum. Results indicate that the Total 3HC/Free COT and Free 3HC/Free COT ratios, but not Total 3HC/Total COT ratio, were significantly elevated during early and late pregnancy compared to postpartum and were significantly higher during late pregnancy relative to early pregnancy. The observation that the Total 3HC/Free COT and Free 3HC/Free COT ratios were elevated during pregnancy compared to postpartum extends the findings of previous studies that reported faster nicotine clearance and higher NMR during the second and third trimesters compared to postpartum.2,9 These results suggest that pregnancy may significantly alter the NMR earlier in pregnancy than previously reported (the 15% first trimester increase reported by Bowker et al.9 did not reach statistical significance) and the NMR may increase as a function of gestational age.
The observation that the Total 3HC/Free COT and Free 3HC/Free COT ratios were elevated during pregnancy suggests that pregnancy may induce CYP2A6. Consistent with this finding, the small pharmacokinetic study by Dempsey et al.2 reported that metabolic clearance of nicotine via the CYP2A6-mediated COT pathway was increased by 54% at approximately 25 weeks’ gestation compared to postpartum. In addition, a subsequent more detailed evaluation of metabolic markers of CYP2A6 activity within these subjects is consistent with an induction of CYP2A6 during pregnancy.26 Future studies evaluating concordance between the NMR and nicotine and COT clearance among pregnant smokers will be important for validating the NMR as proxy of CYP2A6 and a biomarker of nicotine metabolism and clearance among pregnant smokers.
Pregnancy did not appear to influence the Total 3HC/Total COT ratio. The denominator of the Total 3HC/Total COT ratio includes COT glucuronide and it is feasible that pregnancy may have increased COT glucuronidation, thus impacting the effect of pregnancy on the Total 3HC/Total COT ratio. In the later, more in-depth analyses of these samples, the UGT2B10 phenotype ratio (nicotine glucuronide/nicotine) was higher at early and late pregnancy compared with postpartum (p < .07 and < .05, respectively) and correlated with a second UGT2B10 phenotype ratio (COT glucuronide/COT) (p’s <.001), suggesting UGT2B10 activity is induced during pregnancy, altering COT glucuronidation and impacting the Total 3HC/Total COT ratio.26
These results should be considered in light of some limitations. Among nonpregnant smokers, the NMR is associated with several lifestyle and demographic factors such as ethnicity and oral contraceptive use.28, 29 Our sample was primarily composed of Caucasian women, who have relatively faster rates of nicotine clearance compared to other ethnicities, which may have affected the degree of change observed during pregnancy. We also did not assess oral contraceptive use during postpartum. Benowitz et al.29 reported that women taking oral contraceptives metabolized nicotine faster than women not using oral contraceptives. Thus, increases in the NMR during pregnancy compared to postpartum may be even larger than observed in this study if some number of women were using oral contraceptives at the 6–month postpartum assessment. Several pharmacokinetic parameters may also influence the NMR, such as renal clearance of nicotine and COT as well as urinary distribution, flow rate, and pH.16 In the current study, pregnancy increased the Total 3HC/Free COT and Free 3HC/Free ratios even after controlling for pregnancy-induced increases in urinary pH. Little is known about how pregnancy may influence other pharmacokinetic parameters in relation to the NMR, thus some caution is warranted regarding pregnancy-induced increases in the NMR directly reflecting greater CYP2A6 activity. In the more detailed analyses, the significant correlation of NMR and nicotine C-oxidation, also mediated by CYP2A6, throughout pregnancy and at postpartum (p’s < .001) strongly supports that CYP2A6 is induced during pregnancy, resulting in higher NMR.26
Our study expands on Dempsey et al.2 findings by examining additional time points comparing early and late stages of pregnancy longitudinally in a substantially larger within-subject sample set. We also extend the findings of Bowker et al.9 of overall higher salivary NMR during pregnancy by providing specific estimates for the timing and magnitude of change in urinary NMR ratios using a within-subject design, which has substantial advantages due to the large interindividual variation in NMR. To the best of our knowledge, this is also the first study to evaluate urinary NMR calculated using both total and free compounds. Previous data in nonpregnant smokers suggest that a faster rate of nicotine clearance, indicated by a higher NMR, is associated with higher levels of smoking. Although pregnant women tend to report decreases in cigarettes per day during pregnancy, subsequent evaluation of total nicotine equivalents in this population indicates an increase in total nicotine equivalents per cigarette per day during late pregnancy, which is consistent with induced CYP2A6.26,30 Of note, a related study by some of the present authors compared smoking topography between pregnant and nonpregnant women and found no differences in any of the parameters examined.31 Collectively, these results suggest increases in nicotine intake per cigarette may be due to other changes in smoking behavior (eg, smoking a greater portion of each cigarette) and/or to underreporting of cigarettes per day. Higher NMR in nonpregnant smokers is also associated with poorer response to nicotine patch and behavioral counseling; it will be important to test if more rapid nicotine clearance induced by pregnancy may make it more difficult for women to quit smoking as pregnancy progresses.
Funding
This work was supported by National Institute on Drug Abuse (NIDA) award R01DA014028, National Institute of Child Health and Human Development and Centers for Disease Control and Prevention award R01HD075669, NIDA and Food and Drug Administration Tobacco Centers of Regulatory Science (TCORS) award P50DA036114, and National Institute of General Medical Sciences Center of Biomedical Research Excellence (COBRE) award P20GM103644 (all to STH) as well as Canadian Institutes of Health Research award TMH-109787, Canadian Foundation for Innovation awards 20289 and 16014, a Canada Research Chair in Pharmacogenomics (all to RFT), and the Campbell Family Mental Health Research Institute of Centre for Addiction and Mental Health (CAMH), the CAMH Foundation, and the Ontario Ministry of Research and Innovation.
Declaration of Interests
RFT has served as paid consultant to Apotex and received unrestricted research funding from Pfizer.
Acknowledgments
We thank the research assistants and support staff at the University of Vermont and the University of Toronto for their efforts in making this study possible. We also thank Maria Novalen for analyzing urinary nicotine and metabolites.
References
- 1. Kurti AN, Redner R, Lopez AA, et al. Tobacco and nicotine delivery product use in a national sample of pregnant women. Prev Med. 2017;104:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dempsey D, Jacob P III, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–598. [DOI] [PubMed] [Google Scholar]
- 3. Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282(3):1608–1614. [PubMed] [Google Scholar]
- 4. Mwenifumbo JC, Zhou Q, Benowitz NL, Sellers EM, Tyndale RF. New CYP2A6 gene deletion and conversion variants in a population of Black African descent. Pharmacogenomics. 2010;11(2):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hukkanen J, Jacob P III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 6. Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob PIII. Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5(5):621–624. [DOI] [PubMed] [Google Scholar]
- 7. Tyndale RF, Sellers EM. Variable CYP2A6-mediated nicotine metabolism alters smoking behavior and risk. Drug Metab Dispos. 2001;29(4 Pt 2):548–552. [PubMed] [Google Scholar]
- 8. Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600–608. [DOI] [PubMed] [Google Scholar]
- 9. Bowker K, Lewis S, Coleman T, Cooper S. Changes in the rate of nicotine metabolism across pregnancy: a longitudinal study. Addiction. 2015;110(11):1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaz LR, Coleman T, Cooper S, Aveyard P, Leonardi-Bee J; SNAP trial team The nicotine metabolite ratio in pregnancy measured by trans-3′-hydroxycotinine to cotinine ratio: characteristics and relationship with smoking cessation. Nicotine Tob Res. 2015;17(11):1318–1323. [DOI] [PubMed] [Google Scholar]
- 11. St Helen G, Novalen M, Heitjan DF, et al. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gubner NR, Kozar-Konieczna A, Szoltysek-Boldys I, et al. Cessation of alcohol consumption decreases rate of nicotine metabolism in male alcohol-dependent smokers. Drug Alcohol Depend. 2016;163:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanner JA, Novalen M, Jatlow P, et al. Nicotine metabolite ratio (3-hydroxycotinine/cotinine) in plasma and urine by different analytical methods and laboratories: implications for clinical implementation. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Binnington MJ, Zhu AZ, Renner CC, et al. CYP2A6 and CYP2B6 genetic variation and its association with nicotine metabolism in South Western Alaska Native people. Pharmacogenet Genomics. 2012;22(6): 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Derby KS, Cuthrell K, Caberto C, et al. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3526–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swan GE, Lessov-Schlaggar CN, Bergen AW, He Y, Tyndale RF, Benowitz NL. Genetic and environmental influences on the ratio of 3′hydroxycotinine to cotinine in plasma and urine. Pharmacogenet Genomics. 2009;19(5):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins ST, Washio Y, Heil SH, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012;55:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taghavi T, Novalen M, Lerman C, George TP, Tyndale RF. A Comparison of Direct and Indirect Analytical Approaches to Measuring Total Nicotine Equivalents in Urine. Cancer Epidemiol Biomarkers Prev. 2018;27(8):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller EI, Norris HR, Rollins DE, Tiffany ST, Wilkins DG. A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(9-10):725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benowitz NL, Jacob PIII. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56(5):483–493. [DOI] [PubMed] [Google Scholar]
- 21. Benowitz NL, Jacob P III, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268(1):296–303. [PubMed] [Google Scholar]
- 22. Gubner NR, Kozar-Konieczna A, Szoltysek-Boldys I, et al. Cessation of alcohol consumption decreases rate of nicotine metabolism in male alcohol-dependent smokers. Drug Alcohol Depend. 2016;163: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bloom J, Hinrichs AL, Wang JC, et al. The contribution of common CYP2A6 alleles to variation in nicotine metabolism among European-Americans. Pharmacogenet Genomics. 2011;21(7):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strasser AA, Benowitz NL, Pinto AG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20(2):234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lerman C, Schnoll RA, Hawk LW Jr, et al. ; PGRN-PNAT Research Group Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taghavi T, Arger CA, Heil SH, Higgins ST, Tyndale RF. Longitudinal influence of pregnancy on nicotine metabolic pathways. J Pharmacol Exp Ther. 2018;364(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sefa R, Cetin EH, Gurkan K, Metin K. Are changes in urinary parameters during pregnancy clinically significant?Urol Res. 2006;34(4):244–248. [DOI] [PubMed] [Google Scholar]
- 28. Chenoweth MJ, Novalen M, Hawk LW Jr, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob PIII. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–488. [DOI] [PubMed] [Google Scholar]
- 30. Taghavi T, Arger CA, Heil SH, Higgins ST, Tyndale RF. Cigarette consumption and biomarkers of nicotine exposure during pregnancy and postpartum. Addiction. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bergeria CL, Heil SH, Bunn JY, Sigmon SC, Higgins ST. Comparing smoking topography and subjective measures of usual brand cigarettes between pregnant and non-pregnant smokers. Nicotine Tob Res. 2018;20(6):775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]