Abstract
The benefits and harms of lung cancer (LC) screening with low-dose computed tomography (LDCT) are debatable. Positive results from the US National Lung Screening Trial were not evident in the European trials, possibly due to their smaller sample sizes. To address this issue, we conducted a patient-level pooled analysis of two Italian randomized controlled trials. Data from DANTE and MILD trials were combined for a total of 3640 individuals in the LDCT arm and 2909 in the control arm. LC and overall mortality were analyzed using multivariate hazard ratios (HRs) and log-rank tests stratified by study. The median follow-up was 8.2 years, with a total of 30 480 person-years in the LDCT arm and 22 157 in the control arm. A total of 192 patients developed LC in the LDCT arm and 105 in the control arm. Half of the LC cases in the LDCT arm had stage IA or IB cancer, as compared with 21% in the control arm. Overall mortality rates/100 000 person-years were 925 in the LDCT arm and 1074 in the control arm, and LC mortality rates were 299 and 357, respectively. The multivariate pooled overall mortality HR was 0.89 (95% confidence interval: 0.74–1.06) and the LC mortality HR was 0.83 (95% confidence interval: 0.61–1.12) for the LDCT arm as compared with the control arm. The present pooled analysis shows a nonsignificant 11% reduction in overall mortality in individuals undergoing LDCT screening as compared with the control arm. A pooled analysis of all European trials would be a useful contribution to assess the real benefit of LDCT screening.
Keywords: lung cancer, meta-analysis, mortality, screening
Introduction
Lung cancer (LC) is the leading cause of cancer mortality, accounting for 28% of all cancer-related deaths in men and 26% in women in the USA (Siegel et al., 2014). The corresponding proportions in the European Union were 25 and 15% in 2015 (Malvezzi et al., 2015). According to projections of global mortality, in 2030 LC will be the third leading cause of death in high-income countries and the sixth worldwide (Mathers and Loncar, 2006). LC mortality has shown a downward trend in both US men and women, but only in men in most of the European countries. This reflects a pattern of tobacco consumption in subsequent generations of American (Meza et al., 2015) and European (Malvezzi et al., 2013; Rosso et al., 2015) men and women, and confirms that tobacco control is essential for reducing LC incidence (Bray and Weiderpass, 2010) in low-dose computed tomography (LDCT) screening volunteers (Pozzi et al., 2015). Even if heavy smokers remain at high risk of LC after quitting, the overwhelming mortality for cardiovascular disease rapidly drops.
Despite important advances in clinical care and diagnostic imaging (De Angelis et al., 2014), most LC patients are diagnosed with advanced-stage disease, with poor prognosis. Conversely, in early stage (IA) LC, 5-year survival is over 70%, and hence advances in early detection are crucial to enable curative surgery (Crino et al., 2010; Vansteenkiste et al., 2013).
To date, the benefits, harms, and challenges of potential practical implementation of larger-scale LC screening with LDCT, including financial burden, remain at least in part undefined (Pastorino, 2010; Bach et al., 2012; Manser et al., 2013; Morere et al., 2015).
The National Lung Screening Trial (NLST) based on either LDCT (n = 26 722 participants) or single-view posteroanterior chest radiography (n = 26 732) reported a 7% overall mortality reduction with LDCT screening in high-risk individuals, as compared with the radiography control group (Aberle et al., 2011). However, three small European trials showed no reduction in LC mortality through screening (Pastorino et al., 2012; Infante et al., 2015; Wille et al., 2015).
Plausible reasons for such inconsistent results have been previously discussed in several reviews (Humphrey et al., 2013; Pastorino, 2013; Tammemagi and Lam, 2014; Cui et al., 2015), and include differences in the characteristics of individuals enrolled in European studies, and the limited power of each single trial to detect a real benefit.
We therefore conducted, for the first time, a patient-level pooled analysis of the two Italian randomized controlled trials (RCTs) with a long-term follow-up.
Patients and methods
The present pooled analysis includes patient-level data derived from the Detection And screening of early LC with Novel imaging TEchnology and molecular assays (DANTE) study and the Multicentric Italian Lung Detection (MILD) study, for a total of 3640 participants in the LDCT arm and 2909 in the control arm. Details of these screening programs have been reported elsewhere (Infante et al., 2008, 2009, 2015; Pastorino et al., 2012) and are described here in brief.
The DANTE study started in 2001, coordinated by the Humanitas Research Hospital of Milan (Infante et al., 2008, 2009, 2015). Based on a reduction in mortality by 50% in the LDCT arm, a sample size of 2400 volunteers was planned. A total of 2450 participants were prospectively enrolled and randomized to the control arm (undergoing yearly clinical review only, with chest radiography in case of respiratory complaints and/or abnormal findings, n = 1186) or the LDCT arm (receiving LDCT every year, n = 1264) between March 2001 and February 2006. Eligibility criteria included being male, aged 60–74 years, being a current or former heavy smoker with a smoking history of at least 20 pack-years, and no history of cancer within the previous 10 years. The follow-up cutoff date for the present analysis was 15 May 2013.
The MILD study started in 2005 at the ‘Istituto Nazionale dei Tumori’ of Milan (Pastorino et al., 2012). Initially, the study protocol was based on a multicentric recruitment and included a sample size of 10 000 volunteers with an active screening program of 10 years corresponding to a total target of 100 000 person-years to detect a reduction by 30% in LC mortality in the intervention arm. However, several practical issues arose, including limited funding and support from local authorities, which restricted the recruitment to a few centers only. Therefore, this analysis is based on a total of 4099 participants (2717 men and 1385 women) prospectively enrolled and randomized to the control arm (receiving smoking cessation advice only, n = 1723) or the LDCT arm (n = 2376) between September 2005 and January 2011 at Istituto Nazionale Tumori of Milan. The participants of the LDCT arm were initially randomized to receive annual (n = 1190) or biennial (n = 1186) LDCT. After 6 months of debate, the ethics committee approved the randomization to an observational arm, and this delay accounts for the excess of 653 participants in the two LDCT arms compared with the control group. Eligibility criteria included age 49 years and above, being current or former smokers with a smoking history of at least 20 pack-years, and no history of cancer within the previous 5 years. Active follow-up was conducted through telephone and record linkage with national and regional administrative databases, which blindly trace the status of all participants. For the present analysis, all participants were followed up until February 2015. Both annual and biennial LDCT arms of the MILD trial were included in the present pooled analysis, as the overall mortality and LC mortality at 8 years were similar (P for log-rank test equal to 0.23 and 0.70, respectively; data not shown). Both studies were approved by the competent ethics committees.
Statistical analysis
We considered three different endpoints: LC incidence; LC mortality; and all-cause mortality. For the first endpoint, time to event was calculated until the date of diagnosis of LC (failure), the date of death (censored), or the date of last follow-up (censored). For the second endpoint, time to event was calculated until the date of LC-related death (failure), the date of death from causes other than LC (censored), or the date of last follow-up (censored). For overall mortality, the survival time of patients was censored at the date of last follow-up.
Survival curves were estimated using the Kaplan–Meier method and were compared using the log-rank test, also stratified by study (Xie and Liu, 2005). Hazard ratios (HRs) of LC and overall mortality, and the corresponding 95% confidence intervals (CIs), were estimated using Cox’s proportional hazard models, including terms for study, sex, age, pack-years of cigarette smoking, and forced expiratory volume in 1 s (FEV1) (Cox, 1972). As participants were independently randomized in each separate study, all the analyses were adjusted for study, as in any pooled analysis, to account for any possible modifying effect. All tests were two-sided and a P-value less than 0.05 was taken as statistically significant. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina, USA) and the figures were obtained using STATA 11.0 (StataCorp LP, College Station, Texas, USA) statistical software.
Results
The baseline characteristics of 6549 LC screening participants are reported in Table 1. There were 3640 participants in the LDCT arm and 2909 in the control group. Most of them were male (79% in the LDCT arm and 78% in the control group), 52% of participants in both arms were 60–69 years old, and 64% in the LDCT arm and 74% in the control group were current smokers, with a median of 40 pack-years in the LDCT arm (interquartile range = 22) and 39 pack-years in the control arm (interquartile range = 24). The median follow-up period was 8.2 years (8.4 in the LDCT arm and 7.8 in the control group), for a total of 52 637 person-years of observation (30 480 in the LDCT arm and 22 157 in the control group).
Table 1.
Baseline characteristics of lung cancer-screening participants in the pooled analysis (Italy, 2001–2011)
| Pooled analysis | |||
|---|---|---|---|
| LDCT arm | Control arm | All | |
| Number of participants | 3640 | 2909 | 6549 |
| Age (years) [N (%)] | |||
| ≤ 60 | 1766 (48.5) | 1392 (47.8) | 3158 (48.2) |
| > 60 | 1874 (51.5) | 1571 (52.2) | 3391 (51.8) |
| Median (IQR) | 61 (10) | 61 (10) | 61 (10) |
| Male [N (%)] | 2890 (79.4) | 2277 (78.3) | 5167 (78.9) |
| Current smokers [N (%)] | 2344 (64.4) | 2164 (74.4) | 4508 (68.8) |
| Pack-years [median (IQR)] | 40 (21.8) | 39 (24.5) | 39.1 (22.8) |
IQR, interquartile range; LDCT, low-dose computed tomography.
Table 2 gives LC and total mortality in the LDCT group and control group in strata of selected covariates. A total of 297 patients developed LC, 192 (65%) in the LDCT arm and 105 (35%) in the control group. The corresponding 8-year cumulative LC probability was 5.2% (95% CI: 4.5–5.9) and 3.7% (95% CI: 3.0–4.5), respectively. Overall, 136/192 (71%) LC cases in the LDCT arm were diagnosed by the LC screening program (Table 2). Half of the LC cases in the LDCT arm had stage IA or IB cancer, as compared with 21% in the control group. Adenocarcinoma was the most frequently detected LC histotype in both arms, 49% in the LDCT arm and 28% in the control group. Corresponding information from the two Italian studies is reported separately in Supplementary Table 1 (Supplemental digital content 1, http://links.lww.com/EJCP/A73).
Table 2.
Lung cancer detection modality, stage and histology, cause of death, and corresponding all-cause and lung cancer-specific mortality rates (/100 000 person-years) in the pooled analysis (Italy, 2001–2011)
| Pooled analysis | |||
|---|---|---|---|
| LDCT arm (%) | Control arm (%) | All (%) | |
| Number of participants | 3640 | 2909 | 6549 |
| Person-years FU | 30 480 | 22 157 | 52 637 |
| Total patients with LC | 192 | 105 | 297 |
| Total deaths | 282 | 238 | 520 |
| Mode of detection | |||
| Screen detected | 136 (70.8) | 10 (9.5) | 146 (49.2) |
| Interval cancer | 56 (29.2) | 95 (90.5) | 151 (50.8) |
| Stage | |||
| IA | 68 (35.4) | 12 (11.4) | 80 (26.9) |
| IB | 23 (12.0) | 10 (9.5) | 33 (11.1) |
| II | 11 (5.7) | 7 (6.7) | 18 (6.1) |
| Othera | 90 (46.9) | 76 (72.4) | 166 (55.9) |
| Histotype | |||
| Adenocarcinoma | 95 (49.5) | 29 (27.6) | 124 (41.8) |
| Squamous cell carcinoma | 41 (21.4) | 22 (21.0) | 63 (21.2) |
| Othera | 56 (29.1) | 54 (51.4) | 110 (37.0) |
| Cause of death | |||
| Cancer of the lung | 91 (32.3) | 79 (33.2) | 170 (32.7) |
| Cancer of other organs | 95 (33.7) | 75 (31.5) | 170 (32.7) |
| Otherb | 96 (34.0) | 84 (35.3) | 180 (34.6) |
| All-cause mortality | |||
| Rate/100 000 person-years | 925 | 1074 | 988 |
| Lung cancer mortality | |||
| Rate/100 000 person-years | 299 | 357 | 323 |
FU, follow-up; LC, lung cancer; LDCT, low-dose computed tomography.
Including missing values (22 stage and 33 histotype).
One patient died of disseminated cancer of unknown origin, and one died of unknown causes in a foreign country.
Causes of death, all-cause mortality, and LC mortality rates of the pooled analysis are reported in Table 2. A total of 520 deaths from any cause were observed in the pooled analysis, 282 (54%) in the LDCT arm and 238 (46%) in the control group. More than 60% of deaths were due to neoplastic conditions, in both study arms. A total of 170 LC deaths were observed and the corresponding overall proportion, around 30%, was similar in the pooled LDCT and control arms. Similar results were observed, separately, in the two Italian studies (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJCP/A73). The pooled all-cause mortality rates were 988/100 000 in overall participants, 925/100 000 in the LDCT arm, and 1074/100 000 in the control group. Corresponding rates for LC mortality were 323/100 000, 299/100 000, and 357/100 000, respectively.
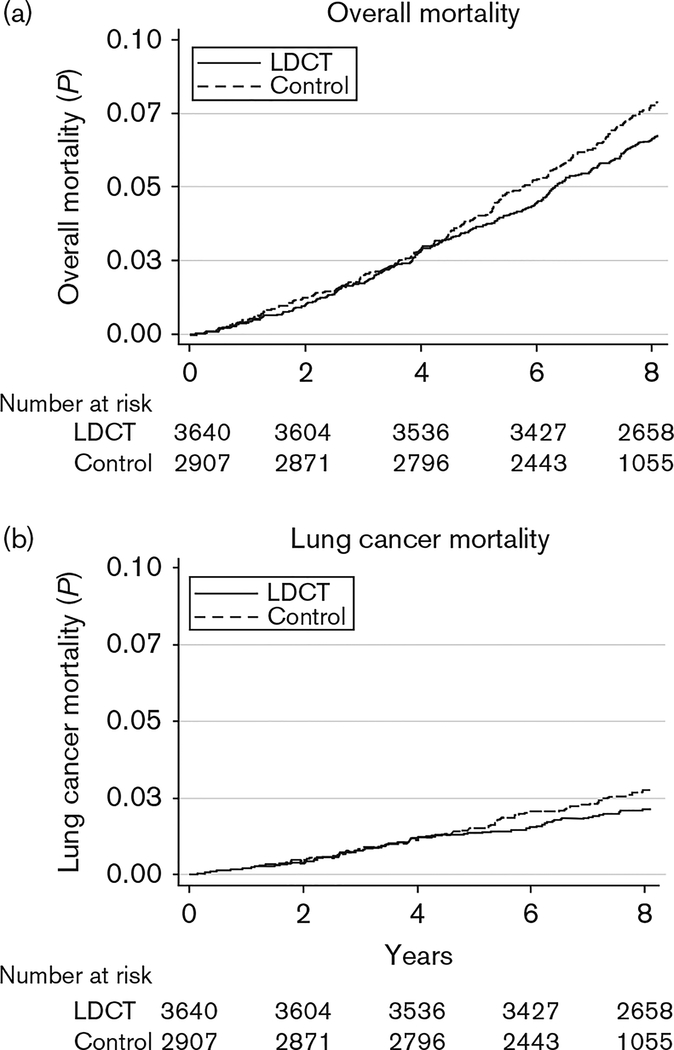
Figure 1 gives the cumulative probability of death from all causes (a) and from LC (b). The Supplementary Figure (Supplemental digital content 2, http://links.lww.com/EJCP/A74) gives the cumulative probability of death from all causes separately in former and current smokers at baseline. In current smokers, the reduction in overall mortality was apparently somewhat stronger, but the difference was not significant.
Fig. 1.
Cumulative probability of death from all causes (a) and from lung cancer (b) (Italy, 2001–2011). LDCT, low-dose computed tomography.
The multivariate pooled HR of overall mortality was 0.89 (95% CI: 0.74–1.06) in the LDCT group as compared with the control group (Table 3). The corresponding HR of LC mortality was 0.83 (95% CI: 0.61–1.12). Results were not materially different across strata of selected covariates, including age, FEV1, pack-years, and smoking status at baseline (Table 4).
Table 3.
Results from the Cox proportional hazard models on overall mortality and lung cancer-specific mortality (Italy, 2001–2011)
| Overall mortality | Lung cancer-specific mortality | ||||
|---|---|---|---|---|---|
| N | HRa | 95% CI | HRa | 95% CI | |
| Male | 5167 | 1.46 | 1.01–2.10 | 3.51 | 1.50–8.21 |
| Age (years) | |||||
| ≥ 55 to ≤ 60 | 1727 | 1.42 | 0.88–2.28 | 1.12 | 0.47–2.66 |
| > 60 | 3391 | 3.92 | 2.53–6.10 | 3.98 | 1.84–8.59 |
| FEV1 (< 80%) | 759 | 1.79 | 1.44–2.22 | 2.67 | 1.90–3.75 |
| Pack-years | |||||
| ≥ 30 to < 40 | 1478 | 1.08 | 0.76–1.52 | 1.39 | 0.72–2.67 |
| ≥ 40 | 3743 | 1.31 | 1.01–1.70 | 1.74 | 1.04–2.90 |
| LDCT group | 3640 | 0.89 | 0.74–1.06 | 0.83 | 0.61–1.12 |
CI, confidence interval; FEV1, forced expiratory volume in 1 s; HR, hazard ratio; LDCT, low-dose computed tomography.
Model adjusted for study and mutually adjusted for all the covariates listed above.
Table 4.
Results from the Cox proportional hazard models on overall mortality and lung cancer-specific mortality in the low-dose computed tomography group versus the control group, across strata of selected covariates (Italy, 2001–2011)
| Overall mortality | Lung cancer-specific mortality | ||||
|---|---|---|---|---|---|
| N | HRa | 95% CI | HRa | 95% CI | |
| Age (years) | |||||
| ≤ 60 | 3158 | 0.86 | 0.55–1.33 | 0.52 | 0.23–1.21 |
| > 60 | 3391 | 0.90 | 0.74–1.08 | 0.88 | 0.64–1.23 |
| FEV1 (%) | |||||
| < 80 | 759 | 0.99 | 0.65–1.50 | 1.03 | 0.56–1.89 |
| ≥ 80 | 5790 | 0.87 | 0.71–1.05 | 0.78 | 0.54–1.11 |
| Pack-years | |||||
| < 40 | 2806 | 1.03 | 0.73–1.46 | 0.53 | 0.27–1.04 |
| ≥ 40 | 3743 | 0.85 | 0.69–1.04 | 0.94 | 0.66–1.33 |
| Current smokers | 4515 | 0.87 | 0.70–1.07 | 0.78 | 0.54–1.11 |
| Ex-smokers | 1846 | 1.21 | 0.84–1.74 | 1.32 | 0.63–2.75 |
CI, confidence interval; FEV1, forced expiratory volume in 1 s; HR, hazard ratio.
Model adjusted for study, sex, age (<55, 55 to ≤60, >60 years old), FEV1 (<80%, ≥80%), pack-years (<30, 30 to <40, ≥40), as appropriate.
Overall mortality and LC-specific mortality were also evaluated in selected subsets of individuals at high risk, according to age (≥55 years old), pack-years (≥30), and FEV1 (<80%) (Supplementary Table 2, Supplemental digital content 3, http://links.lww.com/EJCP/A75). In particular, the multivariate pooled HR of overall mortality in individuals fulfilling the NLST criteria (i.e. of age ≥55 years and pack-years ≥30) was 0.80 (95% CI: 0.66–0.97).
Discussion
The present pooled analysis shows a nonsignificant 11% reduction in overall mortality at 8-year follow-up in individuals undergoing LDCT screening, compared with individuals receiving only usual care. The overall mortality reduction is close to that observed in the NLST study (Aberle et al., 2011). With reference to LC mortality, we found a nonsignificant reduction by 17%, similar to the effect size observed in the NLST study versus a chest radiography-screened control group. The number of LC cases screened to prevent one LC death was 464, again nonsignificant. The reduction in LC and total mortality started after year 4. It is therefore possible that a follow-up longer than 8 years shows additional advantages. Thus, continuing additional follow-up is planned for both studies. A slightly larger (though not significant) effect of LC screening in current smokers is interesting and somewhat counterintuitive, as the reduction in mortality from cardiovascular diseases after smoking cessation, already present in the relatively short term, would in theory make an effect on mortality more evident in former smokers. Further insight might come from analyzing such subgroups in other trials.
In addition to these Italian LC screening programs, there are five other ongoing RCTs being conducted in Europe: the NELSON trial in the Netherlands (including 15 822 participants) (van den Bergh et al., 2008); the Danish Lung Cancer Screening (DLCST) trial in Denmark (including 4104 participants) (Wille et al., 2015); the Lung Cancer Screening Intervention (LUSI) trial in Germany (including 4052 participants) (Becker et al., 2015); the Italian Lung (ITALUNG) trial (including 3206 participants) (Lopes Pegna et al., 2009); and the UK Lung Screen (UKLS) trial (including 28 000 participants) (Baldwin et al., 2011), all comparing annual LDCT versus pure observation, without chest radiography screening.
To date, none of the published results (Pastorino et al., 2012; Infante et al., 2015; Wille et al., 2015) have confirmed the LC mortality reduction found in the NLST study. A possible reason for the inconsistent results may be the limited power to detect a true benefit in each single trial. Hence, a solution would be to meta-analyze data from all such studies at patient level. The present pooled analysis is the first effort in this direction, by pooling two RCTs, for a total of 6549 participants, reaching 52 637 person-years of observation.
Over the last two decades, various epidemiological consortia have been established to pool and analyze data at patient level using an interdisciplinary approach involving clinicians, epidemiologists, and biostatisticians, and they have contributed with new relevant evidence from diagnosis to prognosis for several cancer sites (Hung et al., 2008; Peto et al., 2012; McGale et al., 2014; Winn et al., 2015). For these reasons, as in the past (Field et al., 2013), we advocate a consortium of RCTs on LDCT to combine all European trials and provide further critical evidence on the efficacy of LC screening in a relatively short period. The pooled results, based on information on about 35 000 European individuals already enrolled, could validate the reduction in overall and LC mortality reported in the NLST study.
Smoking characteristics at baseline differ between the European and NLST trials, as the median smoking intensity of individuals included in the LDCT arm of the European studies was about 40 pack-years as compared with 56 pack-years in the NLST investigation (Pastorino, 2013). Also, the various European studies differ in terms of patient recruitment (volunteers in the DANTE, DLCST, and MILD investigations; registry-based in the NELSON trial; identified through general practitioners’ lists in the ITALUNG trial; population-based in the LUSI study), age groups, total number of LDCTs per individual, and duration of follow-up (the DANTE trial started during 2001; NELSON during 2003; ITALUNG and DLCST during 2004; MILD during 2005; LUSI during 2007) (Pastorino, 2013). The establishment of a Consortium of European trials would also improve the knowledge on efficacy of LC screening in subgroups of participants (i.e. high-risk vs. low-risk population; different methodologies of accrual; and smoking status) that might contribute to the debate on the benefits, harms, and potential practical implementation of LC screening on a large scale.
Last, the efficacy of LDCT screening in current smokers could be better investigated with the aim of implementing pharmacological intervention for tobacco consumption in future screening policies, as shown by the recent demonstration of reduced mortality for all causes in smoking quitters during LDCT screening (Pastorino et al., 2016).
Supplementary Material
Acknowledgements
The authors thank all the DANTE and MILD participants and their families. With respect to the MILD study, the authors thank Claudio Jacomelli and Claudio Citterio for data management as well as the MILD staff (Elena Bertocchi, Carolina Ninni, Annamaria Calanca, Paola Suatoni).
With respect to the DANTE study, they thank Dr Ilaria Malvezzi, E. Canetoli and the staff of the Italian Association for the Fight against Cancer (Lega Italiana per la Lotta contro i Tumori) - Milan Section for their administrative support, and Dr E. Morenghi for her kind advice.
Financial support for the DANTE study was provided by the Italian Association for the Fight against Cancer (Lega Italiana per la Lotta contro i Tumori) - Milan Section, the Cariplo Foundation and the Italian Association for the Fight against Cancer (Lega Italiana per la Lotta contro i Tumori) - Head Office in Rome. The three Institutions at which the research was performed provided logistic support, telephone lines, IT support, an office and a project assistant free of charge. Financial support for the MILD study was provided by the Italian Association for Cancer Research (AIRC), the Italian Ministry of Health, the Lombardy Region and the Cariplo Foundation.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.eurjcancerprev.com).
References
- Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. (2011). Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. (2012). Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 307:2418–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK (2011). UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax 66:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. (2015). Randomized study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow-up after randomization. J Thorac Oncol 10:890–896. [DOI] [PubMed] [Google Scholar]
- Bray FI, Weiderpass E (2010). Lung cancer mortality trends in 36 European countries: secular trends and birth cohort patterns by sex and region 1970–2007. Int J Cancer 126:1454–1466. [DOI] [PubMed] [Google Scholar]
- Cox DR (1972). Regression models and life-tables. J R Stat Soc B 34:187–220. [Google Scholar]
- Crino L, Weder W, van Meerbeeck J, Felip E (2010). Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21 (Suppl 5):v103–v115. [DOI] [PubMed] [Google Scholar]
- Cui JW, Li W, Han FJ, Liu YD (2015). Screening for lung cancer using low-dose computed tomography: concerns about the application in low-risk individuals. Transl Lung Cancer Res 4:275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. (2014). Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5 – a population-based study. Lancet Oncol 15:23–34. [DOI] [PubMed] [Google Scholar]
- Field JK, van Klaveren R, Pedersen JH, Pastorino U, Paci E, Becker N, et al. (2013). European randomized lung cancer screening trials: post NLST. J Surg Oncol 108:280–286. [DOI] [PubMed] [Google Scholar]
- Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, et al. (2013). Screening for lung cancer with low-dose computed tomography: a systematic review to update the US preventive services task force recommendation. Ann Intern Med 159:411–420. [DOI] [PubMed] [Google Scholar]
- Hung RJ, Christiani DC, Risch A, Popanda O, Haugen A, Zienolddiny S, et al. (2008). International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol Biomarkers Prev 17:3081–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante M, Lutman FR, Cavuto S, Brambilla G, Chiesa G, Passera E, et al. (2008). Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer 59:355–363. [DOI] [PubMed] [Google Scholar]
- Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, et al. (2009). A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med 180:445–453. [DOI] [PubMed] [Google Scholar]
- Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. (2015). Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med 191:1166–1175. [DOI] [PubMed] [Google Scholar]
- Lopes Pegna A, Picozzi G, Mascalchi M, Maria Carozzi F, Carrozzi L, Comin C, et al. (2009). Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 64:34–40. [DOI] [PubMed] [Google Scholar]
- Malvezzi M, Bosetti C, Rosso T, Bertuccio P, Chatenoud L, Levi F, et al. (2013). Lung cancer mortality in European men: trends and predictions. Lung Cancer 80:138–145. [DOI] [PubMed] [Google Scholar]
- Malvezzi M, Bertuccio P, Rosso T, Rota M, Levi F, La Vecchia C, et al. (2015). European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol 26:779–786. [DOI] [PubMed] [Google Scholar]
- Manser R, Lethaby A, Irving LB, Stone C, Byrnes G, Abramson MJ, et al. (2013). Screening for lung cancer. Cochrane Database Syst Rev 6:CD001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Loncar D (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, et al. (2014). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza R, Meernik C, Jeon J, Cote ML (2015). Lung cancer incidence trends by gender, race and histology in the United States, 1973–2010. PLoS One 10:e0121323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morere JF, Viguier J, Touboul C, Pivot X, Blay JY, Coscas Y, et al. (2015). Lung cancer risks, beliefs and healthcare access among the underprivileged. Eur J Cancer Prev 24 (Suppl):S82–S86. [DOI] [PubMed] [Google Scholar]
- Pastorino U (2010). Lung cancer screening. Br J Cancer 102:1681–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino U (2013). Current status of lung cancer screening. Thorac Surg Clin 23:129–140. [DOI] [PubMed] [Google Scholar]
- Pastorino U, Rossi M, Rosato V, Marchiano A, Sverzellati N, Morosi C, et al. (2012). Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 21:308–315. [DOI] [PubMed] [Google Scholar]
- Pastorino U, Boffi R, Marchiano A, Sestini S, Munarini E, Calareso G, et al. (2016). Stopping smoking reduces mortality in low-dose computed tomography screening participants. J Thorac Oncol (In press). [DOI] [PubMed] [Google Scholar]
- Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. (2012). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi P, Munarini E, Bravi F, Rossi M, La Vecchia C, Boffi R, et al. (2015). A combined smoking cessation intervention within a lung cancer screening trial: a pilot observational study. Tumori 101:306–311. [DOI] [PubMed] [Google Scholar]
- Rosso T, Bertuccio P, La Vecchia C, Negri E, Malvezzi M (2015). Cancer mortality trend analysis in Italy, 1980–2010, and predictions for 2015. Tumori 101:664–675. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A (2014). Cancer statistics, 2014. CA Cancer J Clin 64:9–29. [DOI] [PubMed] [Google Scholar]
- Tammemagi MC, Lam S (2014). Screening for lung cancer using low dose computed tomography. BMJ 348:g2253. [DOI] [PubMed] [Google Scholar]
- van den Bergh KA, Essink-Bot ML, Bunge EM, Scholten ET, Prokop M, van Iersel CA, et al. (2008). Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial). Cancer 113:396–404. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, Lim E, Senan S, Felip E, et al. (2013). Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24 (Suppl 6):vi89–vi98. [DOI] [PubMed] [Google Scholar]
- Wille MM, Dirksen A, Ashraf H, Saghir Z, Bach KS, Brodersen J, et al. (2015). Results of the randomized Danish lung cancer screening trial with focus on high-risk profiling. Am J Respir Crit Care Med 193:542–551. [DOI] [PubMed] [Google Scholar]
- Winn DM, Lee YC, Hashibe M, Boffetta P (2015). The INHANCE consortium: toward a better understanding of the causes and mechanisms of head and neck cancer. Oral Dis 21:685–693. [DOI] [PubMed] [Google Scholar]
- Xie J, Liu C (2005). Adjusted Kaplan–Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 24:3089–3110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.