Abstract
The bacterium Helicobacter pylori (H. pylori) infects the stomachs of approximately 50% of all humans. With its universal occurrence, high infectivity and virulence properties it is considered as one of the most severe global burdens of modern humankind. It has accompanied humans for many thousands of years, and due to its high genetic variability and vertical transmission, its population genetics reflects the history of human migrations. However, especially complex demographic events such as the colonisation of Europe cannot be resolved with population genetic analysis of modern H. pylori strains alone. This is best exemplified with the reconstruction of the 5300-year-old H. pylori genome of the Iceman, a European Copper Age mummy. Our analysis provided precious insights into the ancestry and evolution of the pathogen and underlined the high complexity of ancient European population history. In this review we will provide an overview on the molecular analysis of H. pylori in mummified human remains that were done so far and we will outline methodological advancements in the field of ancient DNA research that support the reconstruction and authentication of ancient H. pylori genome sequences.
Keywords: Helicobacter pylori, Ancient DNA, Evolution, Iceman, Ancient gut contents, Coprolites
Core tip: The molecular analysis of ancient human remains holds the potential to reconstruct Helicobacter pylori genomes dating to various time periods from all over the world. This will provide precious insights into the virulence evolution and population history of this important stomach pathogen.
INTRODUCTION
One of the oldest and most common members of the human stomach microbial community is the pathogen Helicobacter pylori (H. pylori). This spiral shaped bacterium colonizes the stomachs of approximately half the world’s population and is the primary cause of peptic ulcers and gastric cancer[1]. Modern H. pylori strains cluster into multiple, distinct populations based on their geographic origin[2,3]. During H. pylori evolution, founder effects, introgression and geographic separation significantly shaped modern populations and despite very high within-population genetic diversity, genetic signals for more ancient admixture events still persist. Interestingly, the ancestry proportions in H. pylori correlate well with those in associated human populations, indicating that the pathogen accompanied its host on migrations over the last 100000 years[4]. The distinct phylogeographic pattern observed for H. pylori renders this bacterium a powerful marker for tracing complex demographic events in human prehistory, especially those that occurred in hybrid zones where isolates from several ancestral populations admixed[5]. One of these complex demographic histories are still visible in the modern European H. pylori population (hpEurope), which was shown to have arisen through large-scale admixture after secondary contact between two ancestral populations: Ancestral Europe (AE) 1 and AE2[6]. Today, hpEurope is indigenous to Europe, North Africa and western Asia, but one or other of its ancestral components are found at highest frequency in areas that geographically fringe this range. Thus, strains with a high AE1 component have been isolated in Finland, Kazakhstan and northern India belonging to the modern hpAsia2 population[6], whereas highly AE2 strains are predominantly found in North-East Africa and the Sahel, and belong to the modern hpNEAfrica population[7]. However, since several waves of migrations are known to have shaped the population structure of European people[8-10], it remains controversial which colonizing human group brought with it AE1 and which AE2. Given that modern day humans living in and around the Fertile Crescent are all infected with hpEurope, it would seem even more likely that the AE1-AE2 admixture occurred somewhere in the western Asia, and later colonized Europe in the stomachs of Neolithic farmers[4].
The analysis of ancient H. pylori genome sequences adds new precious mosaic pieces to the understanding of these complex demographic events. Our recent study of H. pylori in the Iceman, a 5300-year-old Copper age individual, provided important details on a possible disease manifestation in the mummy and the origin of the stomach pathogen in Europe. In this review we will give an overview on the H. pylori diagnostics that have been performed so far on mummified human remains and we will outline methodological and analytical advancements in the field of ancient DNA research that support the reconstruction and authentication of ancient H. pylori genome sequences.
DETECTION OF H. PYLORI IN ANCIENT HUMAN REMAINS
Currently, there exist only few studies that provided evidence for the presence of H. pylori biomolecules (DNA, proteins) in ancient human remains (Table 1). In contrast to other pathogens such as Yersina pestis or Mycobacterium tuberculosis[11,12], H. pylori cells or biomolecules seem not to become distributed during infection via the blood stream into different skeletal parts (e.g., tooth, vertebra). Therefore, all studies so far diagnosed ancient H. pylori biomolecules at the actual site of infection and its surroundings, in mummified stomach tissues and intestinal contents.
Table 1.
Mummified human remains diagnosed Helicobacter pylori positive
| Individ-uals | Country | Dating | Analysed material | No. of analysed individ-uals/H. pylori positive |
Diagnostics |
Ref. | |||||
| AB |
PCR |
Full genome | |||||||||
| 16S rRNA | UreB | VacA | CagA | ||||||||
| Iceman | Italy | 5300 BP | Stomach content | 1 | X | X | X | [20] | |||
| Andean mummies | Chile | 300 AD | Stool sample | > 2/1 | X | [13] | |||||
| Mexican pre-Columb-ian mummies | Mexico | 1350 AD | Gastric tissue | 5/1 | X | X | [14] | ||||
| Joseon Mummies | South Korea | 1622 to 1800 AD | Stomach tissue | 8/2 | X | X | [16] | ||||
| Kwäday Dän Ts´inchi | Alaska | 1670 to 1850 AD | Stomach tissue | 1 | X | X | [15] | ||||
AD: Anno domini; BC: Before present; AB: Antibody; UreB: Urease subunit beta; VacA: Vacuolating cytotoxin A; CagA: Cytotoxin-associated gene A; H. pylori: Helicobacter pylori.
The first evidence for the existence of H. pylori biomolecules in ancient human remains came from a study that analysed 1700-year-old stool samples from South America[13]. By using a commercial ELISA kit (Helicobacter pylori stool assay, Meridian Diagnostics) Correa and colleagues unambiguously detected H. pylori antigens in two out of 16 stool specimens. The two H. pylori positive specimens came from a 25-year-old mummified male that belonged to the Cabuza culture in Chile. Another study of four pre-Columbian mummies from Mexico using a PCR-hybridisation assay targeting the 16S rRNA and ureB gene confirmed the presence of H. pylori DNA in gastric tissues of a 50 to 60-year-old male dated to approx. 1350 AD[14].
The next two studies not only diagnosed H. pylori DNA in ancient specimen but also characterized two major virulence factors of the stomach pathogen by targeting the cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) using a PCR-based assay. Molecular analysis on stomach tissue of the Kwäday Dan Tsi’nchi individual, a 200 to 300-years-old frozen male mummy discovered in a glacier in British Colombia, Canada, revealed the presence of an ancient H. pylori strain carrying a hybrid vacA m2a/m1d middle (m) region allele and a vacA s2 signal (s) region allele[15]. This unusual allele combination, having a hybrid middle allele clustering to Asian allele variants and a s2 signal region more common to Western strains, suggests that the ancient strain is a possible hybrid between Asian and European strains. This observation would support the assumption that this first nation individual is a descendent of the first humans who migrated into the New World by crossing over the Bering Strait from Asia and that he already had early European contact. A second study screened stomach specimens from eight excellently well preserved 17th century Joseon Korean mummies for the presence of H. pylori[16]. The applied PCR-assay identified the vacA allele type of two mummies, the Cheongdo mummy (vacA s1/m2) and the Dangjin mummy (vacA s1). While the vacA s1 allele is one of the most frequently detected subtypes in the modern South Korean populations, the m2 subtype is only found in 12% of H. pylori patients in South Korea[17,18].
Notably, both studies on the Kwäday Dan Tsi’nchi individual and on the Korean mummies report the absence of the cagA gene, that encodes one of the major effector proteins in the H. pylori cag pathogenicity island. It is known that cagA is present in only approximately 70% of modern H. pylori strains worldwide. Interestingly, this rate varies geographically from approximately 90% in East Asian countries to only about 40% in Western Countries[19]. Whether this observation in the ancient H. pylori strains is true or only an artefact due to the highly degraded state of the ancient DNA remains to be determined.
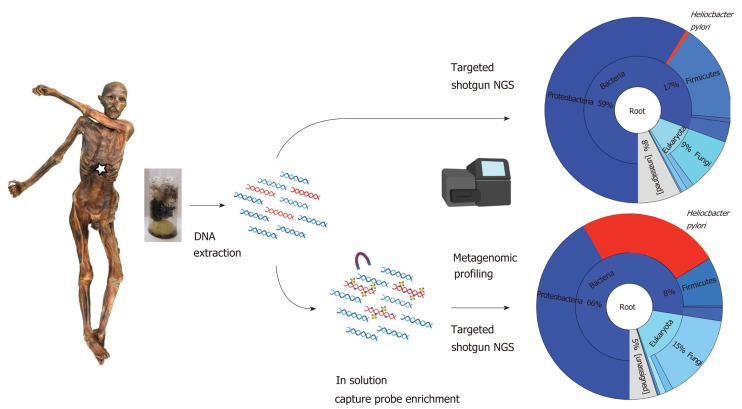
The most comprehensive study of H. pylori in ancient human remains was the reconstruction and analysis of the 5300-year-old H. pylori genome of the Iceman[20]. The Tyrolean Iceman, commonly referred to as “Ötzi”, is one of the oldest human mummies discovered (Figure 1). His body was preserved for more than five millennia in an Italian Alpine glacier before he was discovered by two German mountaineers at an altitude of 3,210 m above sea level in September 1991[21,22]. The discovery of the Iceman is extremely valuable for scientists, not only because of his historical age and the range of objects he was carrying when he died (clothing, hunting equipment such as an axe, dagger, a bow and quiver of arrows), but also the way he was preserved over time. The Iceman is a so-called “ice mummy”, i.e., humidity was retained in his cells while he was naturally mummified by freeze-drying[23]. The body tissues and intestines are therefore still well preserved, and this feature makes them suitable for various modern scientific investigations[24-29]. During a recent radiological re-examination, the Iceman´s stomach was identified and shown to be completely filled[30]. Endoscopy-guided biopsy samples of the Iceman’s stomach wall and content were taken with subsequent microscopic and molecular analysis to identify the nature of the Iceman´s last meal[31] and to determine the presence of H. pylori[20]. First histological analysis of the Iceman´s stomach mucosa revealed only remnants of connective tissue without any further structural details. No human cellular structures or attached bacterial cells were present in the ancient tissue. Further PCR based diagnostics targeting a fragment of the vacA gene revealed traces of H. pylori DNA only in the stomach content material and not in the stomach mucosa tissue. Therefore, we hypothesize that the bacterial cells and biomolecules most presumably detached post-mortem from the mucosa surface and accumulated in the stomach content.
Figure 1.
Molecular workflow applied to the Iceman intestinal content samples. Both “untargeted” shotgun metagenomic next generation sequencing and “targeted” capture-enrichment have been used to identify endogenous Helicobacter pylori DNA and to reconstruct the ancient pathogen genome.
Since we could not exclude the possibility that this first PCR-based positive result may have also come from an external contamination with modern H. pylori cells we decided to extend our molecular approach to a metagenomics diagnostic assay. As outlined by Spyrou and colleagues[32] in their excellent review on ancient pathogen genomics includes this assay two major steps, first a shotgun-based “untargeted” metagenomic screening for the presence of authentic pathogen DNA and second a “targeted” capture-sequencing based enrichment approach aiming to reconstruct ancient pathogen genomes (Figure 1). Importantly, this approach allows not only an appropriate authentication of the detected H. pylori DNA but also provides the unique opportunity to perform comparative sequence analysis on a whole genome level (as outlined in the next two chapters). First metagenomic analysis of 12 biopsy samples from the gastrointestinal tract of the Iceman yielded endogenous ancient H. pylori DNA in all gastrointestinal tract contents. Importantly, the H. pylori reads did not only cover most of the reference genome sequence, but also followed the expected physiological distribution pattern, with abundance steeply decreasing as samples move away from the stomach towards the lower large intestinal tract. Thus, even after 5300 years, the distribution of H. pylori in the Iceman´s gastrointestinal tract is homologous to the distribution found in modern H. pylori-positive humans. In modern patients with peptic ulcers, H. pylori can account for > 93% of the bacterial reads of the stomach microbiome[33]. Due to leakage from the stomach into the intestines the depleted H. pylori antigens and DNA can still be used for diagnostics in stool samples[34]. After having confirmed the presence of authentic endogenous H. pylori DNA we subjected Iceman stomach content biopsies to a capture-sequencing based enrichment approach. To account for the high genomic variability between H. pylori strains, we implemented the genomes of nine different H. pylori multi locus sequence types (MLSTs), representing the pathogen´s known global diversity, in the RNA based in solution target enrichment assay. Using this capture design, we successfully enriched H. pylori sequences up to 216-fold which finally allowed us to reconstruct 92.2% of the 1.6-Mb H. pylori reference genome with an 18.9-fold average coverage.
AUTHENTIFICATION OF ANCIENT H. PYLORI GENOME SEQUENCES
The advent of high-throughput sequencing (HTS) has greatly improved the field of ancient DNA research[35]. In contrast to PCR based methods, where single DNA regions are targeted, HTS has the possibility to sequence whole genomes and metagenomes. By using HTS it is also possible to target shorter fragments of DNA, which is one of the typical features of ancient DNA[36], as well as authenticate ancient endogenous sequences and distinguish them from modern or environmental contamination[37].
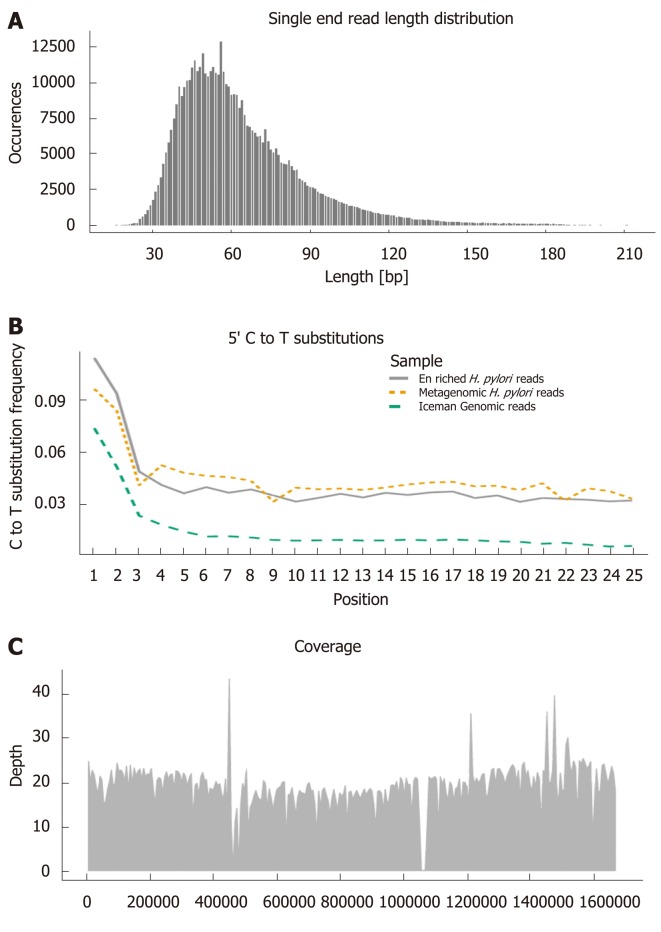
There are several features of ancient DNA which can be used to distinguish between endogenous and contaminant DNA. Firstly, in contrast to modern DNA, ancient DNA has been degraded and typically has a very short sequence length[38], as can be seen in the H. pylori sequences from the Iceman gut content (Figure 2A). Secondly, the degradation of DNA also result in specific damage patterns at the ends of the sequence reads. In comparison to the reference genome, ancient DNA have an accumulation of C to T substitutions toward the 5’ end and G to A substitutions toward the 3’ end[39]. These characteristic C to T substitutions are present in the H. pylori sequences from the Iceman gut, as well as the human sequences from the Iceman himself (Figure 2B), indicating their ancient origin.
Figure 2.
Ancient DNA characteristics used for authenticating endogenous sequences. Visualised with Helicobacter pylori (H. pylori) sequences enriched from the gut of the Iceman and aligned to the H. pylori 26695 reference genome[20]: A: Read length distribution. B: C to T substitutions towards the 5’ end of sequence reads (Enriched H. pylori reads, metagenomic H. pylori reads, human Iceman genomic reads). C: Mean genome coverage and distribution of the aligned sequences. H. pylori: Helicobacter pylori.
Further validation of ancient sequences can be done by studying the distribution patterns of species assigned sequences, i.e., how well distributed the sequences are across the reference genome[37]. The even distribution of the Iceman H. pylori sequences across the H. pylori 26696 reference genome (Figure 2C), suggests that most parts of the genome are still present. Clustering of sequences in a few regions of the genome (stacking effect) would instead have suggested an incorrect assignment due to unspecific homology only[37]. In summary, the above-mentioned characteristics allow the proper authentication of ancient endogenous pathogen DNA which opens the possibility for further comparative sequence analysis on a whole genome level.
COMPARATIVE SEQUENCE ANALYSIS USING ANCIENT H. PYLORI DNA
First, the Iceman H. pylori virulence factors cagA and vacA were subjected to comparative sequence analysis with modern H. pylori allele types. Depending on the presence or absence of cagA and vacA in the genome and based on different allele types, H. pylori strains can be associated with varying host tropism and virulence. Based on the current understanding of the allele types and the role of the H. pylori virulence factors CagA and VacA, the Iceman H. pylori can be classified as cagA-positive vacA s1a/i1/m1 type strain associated with a significantly increased inflammation within the gastric mucosa[18,40]. Further paleo-proteomic analysis revealed the presence of an intestinal inflammation marker protein indicating active inflammatory host response in the Iceman stomach strongly associated with H. pylori infection.
Next, we assigned the ancient strain to a modern H. pylori population. For this, we extracted the seven (MLST) loci from the ancient genome and compared the gene fragments, that are used for population and subpopulation differentiation among H. pylori[2,6], with a MLST database of 1603 H. pylori strains using the Bayesian population assignment software STRUCTURE[41]. The comparison to a global set of modern H. pylori strains assigned the 5300-year-old bacterium to the modern population hpAsia2 which is today commonly found in Central and South Asia (Figure 3A). This result was further confirmed by a more focused analysis of the strains belonging to the populations hpAsia2, hpEurope and hpNEAfrica (Figure 3B). The assignment to hpAsia2 was rather surprising since stomachs of modern Europeans are pre-dominantly colonized by recombinant hpEurope strains originating from the two ancestral populations AE1 and AE2. By applying the STRUCTURE linkage model[42], that was designed to determine levels of ancient admixture, we could show that 95% of the nucleotides making up the Iceman’s H. pylori strain were derived from AE1 (Figure 3C). This could suggest that this ancestral component was more widespread in Europe 5000 years ago compared to the present day, where extensive sampling throughout Europe and western Asia, only recovered four strains, each with an unusually high AE1 component (from the Netherlands, Estonia, Finland, and Kazakhstan, blue arrows Figure 3B and C).
Figure 3.
Population assignment of the ancient Helicobacter pylori strain using multilocus sequence typing. A: Comparison of the Iceman strain to a collection of strains representing the worldwide Helicobacter pylori (H. pylori) populations. The proportion of the Iceman strain is displayed on the right. B: Comparison of the Iceman strain to the H. pylori populations hpNEAfrica, hpEurope, hpAsia2. C: STRUCTURE linkage model analysis showing the proportion of Ancestral Europe (AE) 1 (from Central Asia) and AE2 (from northeast Africa).
Further high-resolution analysis of ancestral motifs using fineSTRUCTURE[43] showed that the ancient H. pylori genome shares high levels of ancestry with Indian hpAsia2 strains, but even higher co-ancestry with most European hpEurope strains. Furthermore, the fineSTRUCTURE analysis supports the MLST results by showing a low ancestry of the Iceman strain with the hpNEAfrica strain, a modern re-presentative of AE2. Comparative whole genome phylogeny based on 1121 core genes and 375 publicly available H. pylori genomes from Europe, Asia and Africa assigns the Iceman to Northern European genomes such as genomes from the United Kingdom and Sweden (Figure 4). Both these modern Northern European genomes and the Iceman share a high AE1 ancestry which let them group close to modern hpAsia2 genomes. Overall, we hypothesize that the Iceman’s strain belonged to a prehistoric European branch of hpAsia2 that is different from the modern hpAsia2 population from northern India.
Figure 4.
Helicobacter pylori core genome tree based on 1121 core genes with 375 publicly available Helicobacter pylori genomes from Europe, Asia and Africa including the Iceman (in bold). The core genome analysis was performed using the Roary pan-genome analysis tool[44] followed by phylogenomic analysis using the PhyML tool v 3.1[45].
Previously it was assumed that the admixture of the two hpEurope ancestors AE1 and AE2 has happened in the Middle East or Western Asia between 10000 and 52000 years ago and hpEurope was introduced into Europe with the first Early Neolithic farmers[4]. Our study, however, has shown that the Iceman’s H. pylori is a nearly pure representative of the bacterial population of Asian origin (AE1) that existed in Europe before hybridization. This suggests that the African ancestral H. pylori component (AE2) arrived in Europe within the past few thousand years, which is much more recent than previously hypothesized.
CONCLUSION
The power of modern molecular biology methods for studying ancient remains, coupled with the increasing repertoire of advanced data analysis approaches in computational biology, has enabled the reconstruction of ancient genomes of human pathogen including Helicobacter pylori. In contrast to the first PCR-based studies on ancient human remains open metagenomic studies with whole ancient pathogen genomes a completely new range of possible comparative sequence analysis that provide information to a yet unprecedented depth. Our metagenomic diagnostic approach and genome reconstruction revealed that the 5300-year-old H. pylori strain of the Iceman was a potentially virulent strain that is today strongly associated with gastric disease. Furthermore, comparative sequence analysis with modern H. pylori strains provided interesting insights into the ancestry and evolution of the pathogen and underlined the high complexity of ancient European population history. Encouraged by these first very promising results, we are currently collecting and analysing further ancient gut contents and coprolites. Overall, further ancient H. pylori genome sequences from all over the world will significantly contribute to our understanding of the basic biology and evolution of this important stomach pathogen and will add subsequently an additional chapter to the human demographic history from a bacterial point of view.
Footnotes
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: June 28, 2019
First decision: July 20, 2019
Article in press: November 1, 2019
P-Reviewer: Lee CL, Slomiany BL, Tarnawski AS, Zhu YL S-Editor: Tang JZ L-Editor: A E-Editor: Zhang YL
Contributor Information
Frank Maixner, Institute for Mummy Studies, EURAC Research, Bolzano 39100, Italy. frank.maixner@eurac.edu.
Kaisa Thorell, Department of Infectious Diseases, University of Gothenburg, Göteborg SE405 30, Sweden.
Lena Granehäll, Institute for Mummy Studies, EURAC Research, Bolzano 39100, Italy.
Bodo Linz, Department of Veterinary and Biomedical Sciences, Pennsylvania State University, University Park, PA 16802, United States.
Yoshan Moodley, Department of Zoology, University of Venda, Thohoyandou 0950, South Africa.
Thomas Rattei, Department of Microbiology and Ecosystem Science, University of Vienna, Vienna 1090, Austria.
Lars Engstrand, Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Stockholm 141 83, Sweden.
Albert Zink, Institute for Mummy Studies, EURAC Research, Bolzano 39100, Italy.
References
- 1.Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–1461. doi: 10.1016/S0140-6736(09)60938-7. [DOI] [PubMed] [Google Scholar]
- 2.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, Blaser MJ, Graham DY, Vacher S, Perez-Perez GI, Yamaoka Y, Mégraud F, Otto K, Reichard U, Katzowitsch E, Wang X, Achtman M, Suerbaum S. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 3.Moodley Y, Linz B, Yamaoka Y, Windsor HM, Breurec S, Wu JY, Maady A, Bernhöft S, Thiberge JM, Phuanukoonnon S, Jobb G, Siba P, Graham DY, Marshall BJ, Achtman M. The peopling of the Pacific from a bacterial perspective. Science. 2009;323:527–530. doi: 10.1126/science.1166083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moodley Y, Linz B, Bond RP, Nieuwoudt M, Soodyall H, Schlebusch CM, Bernhöft S, Hale J, Suerbaum S, Mugisha L, van der Merwe SW, Achtman M. Age of the association between Helicobacter pylori and man. PLoS Pathog. 2012;8:e1002693. doi: 10.1371/journal.ppat.1002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moodley Y, Linz B. Helicobacter pylori Sequences Reflect Past Human Migrations. Genome Dyn. 2009;6:62–74. doi: 10.1159/000235763. [DOI] [PubMed] [Google Scholar]
- 6.Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW, Yamaoka Y, Graham DY, Perez-Trallero E, Wadstrom T, Suerbaum S, Achtman M. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nell S, Eibach D, Montano V, Maady A, Nkwescheu A, Siri J, Elamin WF, Falush D, Linz B, Achtman M, Moodley Y, Suerbaum S. Recent acquisition of Helicobacter pylori by Baka pygmies. PLoS Genet. 2013;9:e1003775. doi: 10.1371/journal.pgen.1003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler C, Harding J, Hofmann D, Müller J. 2014. Movement of Plants, Animals, Ideas, and People in South-East Europe. Oxford University Press. [Google Scholar]
- 9.Gignoux CR, Henn BM, Mountain JL. Rapid, global demographic expansions after the origins of agriculture. Proc Natl Acad Sci USA. 2011;108:6044–6049. doi: 10.1073/pnas.0914274108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haak W, Lazaridis I, Patterson N, Rohland N, Mallick S, Llamas B, Brandt G, Nordenfelt S, Harney E, Stewardson K, Fu Q, Mittnik A, Bánffy E, Economou C, Francken M, Friederich S, Pena RG, Hallgren F, Khartanovich V, Khokhlov A, Kunst M, Kuznetsov P, Meller H, Mochalov O, Moiseyev V, Nicklisch N, Pichler SL, Risch R, Rojo Guerra MA, Roth C, Szécsényi-Nagy A, Wahl J, Meyer M, Krause J, Brown D, Anthony D, Cooper A, Alt KW, Reich D. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015;522:207–211. doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos KI, Harkins KM, Herbig A, Coscolla M, Weber N, Comas I, Forrest SA, Bryant JM, Harris SR, Schuenemann VJ, Campbell TJ, Majander K, Wilbur AK, Guichon RA, Wolfe Steadman DL, Cook DC, Niemann S, Behr MA, Zumarraga M, Bastida R, Huson D, Nieselt K, Young D, Parkhill J, Buikstra JE, Gagneux S, Stone AC, Krause J. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. Nature. 2014;514:494–497. doi: 10.1038/nature13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spyrou MA, Tukhbatova RI, Wang CC, Valtueña AA, Lankapalli AK, Kondrashin VV, Tsybin VA, Khokhlov A, Kühnert D, Herbig A, Bos KI, Krause J. Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague. Nat Commun. 2018;9:2234. doi: 10.1038/s41467-018-04550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correa P, Willis D, Allison MJ, Gerszten E. Helicobacter pylori in pre-Columbian mummies. Gastroenterology. 1998;114:A956. [Google Scholar]
- 14.Castillo-Rojas G, Cerbón MA, López-Vidal Y. Presence of Helicobacter pylori in a Mexican Pre-Columbian Mummy. BMC Microbiol. 2008;8:119. doi: 10.1186/1471-2180-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanston T, Haakensen M, Deneer H, Walker EG. The characterization of Helicobacter pylori DNA associated with ancient human remains recovered from a Canadian glacier. PLoS One. 2011;6:e16864. doi: 10.1371/journal.pone.0016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin DH, Oh CS, Hong JH, Lee H, Lee SD, Lee E. Helicobacter pylori DNA obtained from the stomach specimens of two 17th century Korean mummies. Anthropol Anz. 2018;75:75–87. doi: 10.1127/anthranz/2018/0780. [DOI] [PubMed] [Google Scholar]
- 17.Choe YH, Kim PS, Lee DH, Kim HK, Kim YS, Shin YW, Hwang TS, Kim HJ, Song SU, Choi MS. Diverse vacA allelic types of Helicobacter pylori in Korea and clinical correlation. Yonsei Med J. 2002;43:351–356. doi: 10.3349/ymj.2002.43.3.351. [DOI] [PubMed] [Google Scholar]
- 18.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatakeyama M. Helicobacter pylori CagA -- a bacterial intruder conspiring gastric carcinogenesis. Int J Cancer. 2006;119:1217–1223. doi: 10.1002/ijc.21831. [DOI] [PubMed] [Google Scholar]
- 20.Maixner F, Krause-Kyora B, Turaev D, Herbig A, Hoopmann MR, Hallows JL, Kusebauch U, Vigl EE, Malfertheiner P, Megraud F, O'Sullivan N, Cipollini G, Coia V, Samadelli M, Engstrand L, Linz B, Moritz RL, Grimm R, Krause J, Nebel A, Moodley Y, Rattei T, Zink A. The 5300-year-old Helicobacter pylori genome of the Iceman. Science. 2016;351:162–165. doi: 10.1126/science.aad2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaber O, Künzel KH. Man from the Hauslabjoch. Exp Gerontol. 1998;33:655–660. doi: 10.1016/s0531-5565(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 22.Spindler K. Munich: Goldman; 2000. Der Mann im eis. Neue sensationelle Erkenntnisse über die Mumie in den Ötztaler Alpen. [Google Scholar]
- 23.Lynnerup N. Mummies. Am J Phys Anthropol. 2007;45:162–190. doi: 10.1002/ajpa.20728. [DOI] [PubMed] [Google Scholar]
- 24.Keller A, Graefen A, Ball M, Matzas M, Boisguerin V, Maixner F, Leidinger P, Backes C, Khairat R, Forster M, Stade B, Franke A, Mayer J, Spangler J, McLaughlin S, Shah M, Lee C, Harkins TT, Sartori A, Moreno-Estrada A, Henn B, Sikora M, Semino O, Chiaroni J, Rootsi S, Myres NM, Cabrera VM, Underhill PA, Bustamante CD, Vigl EE, Samadelli M, Cipollini G, Haas J, Katus H, O'Connor BD, Carlson MR, Meder B, Blin N, Meese E, Pusch CM, Zink A. New insights into the Tyrolean Iceman's origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 2012;3:698. doi: 10.1038/ncomms1701. [DOI] [PubMed] [Google Scholar]
- 25.Maixner F, Overath T, Linke D, Janko M, Guerriero G, van den Berg BH, Stade B, Leidinger P, Backes C, Jaremek M, Kneissl B, Meder B, Franke A, Egarter-Vigl E, Meese E, Schwarz A, Tholey A, Zink A, Keller A. Paleoproteomic study of the Iceman's brain tissue. Cell Mol Life Sci. 2013;70:3709–3722. doi: 10.1007/s00018-013-1360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller W, Fricke H, Halliday AN, McCulloch MT, Wartho JA. Origin and migration of the Alpine Iceman. Science. 2003;302:862–866. doi: 10.1126/science.1089837. [DOI] [PubMed] [Google Scholar]
- 27.Murphy WA Jr, Nedden Dz Dz, Gostner P, Knapp R, Recheis W, Seidler H. The iceman: discovery and imaging. Radiology. 2003;226:614–629. doi: 10.1148/radiol.2263020338. [DOI] [PubMed] [Google Scholar]
- 28.Nerlich AG, Bachmeier B, Zink A, Thalhammer S, Egarter-Vigl E. Otzi had a wound on his right hand. Lancet. 2003;362:334. doi: 10.1016/S0140-6736(03)13992-X. [DOI] [PubMed] [Google Scholar]
- 29.Oeggl K, Kofler W, Schmidl A, Dickson JH, Egarter-Vigl E, Gaber O. The reconstruction of the last itinerary of “Ötzi”, the Neolithic Iceman, by pollen analyses from sequentially sampled gut extracts. Quaternary Sci Rev. 2007;26:853–861. [Google Scholar]
- 30.Gostner P, Pernter P, Bonattie G, Graefen A, Zink AR. New radiological insights into the life and death of the Tyrolean Iceman. J Archaeol Sci. 2011;38:3425–3431. [Google Scholar]
- 31.Maixner F, Turaev D, Cazenave-Gassiot A, Janko M, Krause-Kyora B, Hoopmann MR, Kusebauch U, Sartain M, Guerriero G, O'Sullivan N, Teasdale M, Cipollini G, Paladin A, Mattiangeli V, Samadelli M, Tecchiati U, Putzer A, Palazoglu M, Meissen J, Lösch S, Rausch P, Baines JF, Kim BJ, An HJ, Gostner P, Egarter-Vigl E, Malfertheiner P, Keller A, Stark RW, Wenk M, Bishop D, Bradley DG, Fiehn O, Engstrand L, Moritz RL, Doble P, Franke A, Nebel A, Oeggl K, Rattei T, Grimm R, Zink A. The Iceman's Last Meal Consisted of Fat, Wild Meat, and Cereals. Curr Biol. 2018;28:2348–2355.e9. doi: 10.1016/j.cub.2018.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spyrou MA, Bos KI, Herbig A, Krause J. Ancient pathogen genomics as an emerging tool for infectious disease research. Nat Rev Genet. 2019;20:323–340. doi: 10.1038/s41576-019-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. 2008;3:e2836. doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280–322. doi: 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlando L, Gilbert MT, Willerslev E. Reconstructing ancient genomes and epigenomes. Nat Rev Genet. 2015;16:395–408. doi: 10.1038/nrg3935. [DOI] [PubMed] [Google Scholar]
- 36.Kirsanow K, Burger J. Ancient human DNA. Ann Anat. 2012;194:121–132. doi: 10.1016/j.aanat.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Warinner C, Herbig A, Mann A, Fellows Yates JA, Weiß CL, Burbano HA, Orlando L, Krause J. A Robust Framework for Microbial Archaeology. Annu Rev Genomics Hum Genet. 2017;18:321–356. doi: 10.1146/annurev-genom-091416-035526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Garcerà M, Gigli E, Sanchez-Quinto F, Ramirez O, Calafell F, Civit S, Lalueza-Fox C. Fragmentation of contaminant and endogenous DNA in ancient samples determined by shotgun sequencing; prospects for human palaeogenomics. PLoS One. 2011;6:e24161. doi: 10.1371/journal.pone.0024161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briggs AW, Stenzel U, Johnson PL, Green RE, Kelso J, Prüfer K, Meyer M, Krause J, Ronan MT, Lachmann M, Pääbo S. Patterns of damage in genomic DNA sequences from a Neandertal. Proc Natl Acad Sci USA. 2007;104:14616–14621. doi: 10.1073/pnas.0704665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones KR, Whitmire JM, Merrell DS. A Tale of Two Toxins: Helicobacter Pylori CagA and VacA Modulate Host Pathways that Impact Disease. Front Microbiol. 2010;1:115. doi: 10.3389/fmicb.2010.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawson DJ, Hellenthal G, Myers S, Falush D. Inference of population structure using dense haplotype data. PLoS Genet. 2012;8:e1002453. doi: 10.1371/journal.pgen.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guindon S, Delsuc F, Dufayard JF, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol. 2009;537:113–137. doi: 10.1007/978-1-59745-251-9_6. [DOI] [PubMed] [Google Scholar]