Abstract
BACKGROUND
There is a growing evidence regarding an increased risk of inflammatory bowel disease (IBD) among patients with airway diseases.
AIM
To investigate the influence of chronic obstructive pulmonary disease (COPD) on the risk of IBD.
METHODS
A nationwide, population-based study was conducted using data from the National Health Insurance Service database. A total of 1303021 patients with COPD and 6515105 non-COPD controls were identified. The COPD group was divided into the severe and the mild COPD group according to diagnostic criteria. The risk of IBD in patients with COPD compared to controls was analyzed by Cox proportional hazard regression models. The cumulative incidences of IBD were compared between the groups.
RESULTS
The COPD group had higher incidences of IBD compared to non-COPD controls (incidence rate, 9.98 vs 7.18 per 100000 person-years, P < 0.001). The risk of IBD in the COPD group was increased by 1.38 (adjusted hazard ratio (HR); 95%CI: 1.25-1.52). The incidence rate of IBD was higher in the severe COPD group than in the mild COPD group (12.39 vs 9.77 per 100000 person-year, P < 0.001). The severity of COPD was associated with an increased risk of IBD (adjusted HR 1.70 in severe COPD, 95%CI: 1.27-2.21 and adjusted HR 1.35 in mild COPD, 95%CI: 1.22-1.49)
CONCLUSION
The incidences of IBD were significantly increased in COPD patients in South Korea and the risk of developing IBD also increased as the severity of COPD increased.
Keywords: Claim data, Inflammatory bowel disease, Chronic obstructive pulmonary disease, Crohn’s disease, Ulcerative colitis
Core tip: In this nationwide population-based study, we showed that the incidence of inflammatory bowel disease (IBD) was higher in chronic obstructive pulmonary disease (COPD) patients compared to age-and sex-matched controls without IBD in South Korea. And the risk of developing IBD also increased as the severity of COPD increased. It is important to be aware of the gastrointestinal symptoms indicative of IBD in COPD patients. Accurate clinical assessment should be done, especially in patients with severe COPD in order to prevent complications and avoid excess medical expenses.
INTRODUCTION
Inflammatory bowel disease (IBD), which is divided into Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic idiopathic disorder causing inflammation of the gastrointestinal tract. However, IBD should be regarded as a systemic disorder not limited to the gastrointestinal tract because extraintestinal manifestations of IBD are frequent and may occur before or after IBD diagnosis[1]. Extraintestinal manifestation frequently affect joints, skin, hepatobiliary tract and eye. Although the lung is less affected than other organ, IBD is known to be associated with a variety of lung disease and airway disease is the most common respiratory manifestation in IBD patients[2-5].
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible airflow limitation, which is caused by chronic airway inflammation and lung parenchymal destruction. It is well-known that not only respiratory symptoms but also extra-pulmonary manifestations such as cardiovascular compromise, dysfunction of skeletal muscles, osteoporosis, and anemia can result in impaired functional capacity and increased mortality in COPD patients. The gastrointestinal manifestations are no exception to this, and a recent report showed that complaints of gastrointestinal symptoms may be higher in patients with COPD than in healthy individuals[6]. Furthermore, the associations of COPD with specific gastrointestinal disease were investigated. And there is a growing evidence regarding an increased risk of IBD incidence among patients with airway diseases including COPD[7-10].
South Korea has a considerably higher prevalence of COPD than other countries, with 15.5/1000 people diagnosed with COPD annually[11,12]. Meanwhile, the incidence of IBD in South Korea has increased approximately 10-fold over the last two decades, which has led to South Korea having one of the highest incidence of IBD among Asian countries[13-16]. These trends of incidence and prevalence may lead to considerable economic burdens and challenge for the healthcare system. Asian IBD is known to be different from that of the Western countries in s pathophysiology, clinical manifestation and response to treatment[17-19].
Thus, in the present study, we aimed to investigate the association between COPD and IBD represented by CD and UC using the large data in Asia. We also aimed to study the influence of COPD on the risk of IBD according to the severity of COPD. Consideration of this association may maximize the efficacy of prevention and treatment approaches to these chronic disease.
MATERIALS AND METHODS
Data source
This nationwide, population-based study was conducted using data from the National Health Insurance Service (NHIS) database. The South Korean government administers the NHIS as a mandatory health insurance system covering approximately 97% of the South Korean population; the remaining 3% represent the lower income population covered by the Medical Aid program. The NHIS database provides comprehensive information about demographics, medical treatments, procedures, outpatient and inpatient care, and disease diagnoses according to the International Classification of Disease, 10th revision (ICD-10). In addition, in 2007, the NHIS established a registration program for rare intractable disease (RID), which included IBD, to provide enhanced reimbursement for medical costs that were associated with rare diseases (affecting < 20000 people in Korea). To qualify for enrolment in the RID program, patients require a diagnosis from a certified physicians and approval by the NHIS.
Study population and patient identification
We identified COPD patients based on the following diagnostic criterion: Conditions for which an individual should visit the medical facility at least twice per year with both a COPD diagnostic code and a prescription for one or more COPD medications between January 2010 and December 2014. Similar to previous studies[20-22], the detailed diagnostic criteria of COPD are as follows: > 40 years of age; ICD-10 codes for COPD (J43-J44, except J430); and use of more than one drug for COPD such as a long-acting muscarinic antagonist (LAMA), long-acting beta-2 agonist (LABA), inhaled corticosteroid (ICS), ICS plus LABA, short-acting muscarinic antagonist (SAMA), short-acting beta-2 agonist (SABA), SAMA plus SABA, methylxanthines, systemic corticosteroids, and systemic beta agonists. Patients with COPD were divided into the mild COPD group and severe COPD group and severe COPD was defined according to the following severity criteria: (1) Tertiary hospital care patient who met the definition of COPD described above; and (2) Use of triple inhaler therapy at least once per year (ICS+LABA+LAMA). In addition, patients in the COPD group were subsequently 1:5 matched with individuals without COPD (non-COPD controls) for age and sex.
Incident cases of IBD were defined when the patients in the COPD group and non-COPD controls met the case definition for CD or UC during January 2010 and December 2014 and had been free of IBD diagnosis for at least 2 years prior to the beginning of the COPD case-defining period. We identified IBD patients using codes from the ICD-10 and the RID registration system (V code). Cases that involved CD were identified if they had both ICD-10 code K50 and V code V130, while cases that involved UC were identified if they had both ICD-10 code K51 and V code V131. Since IBD-unclassified is not registered in NHIS database and RID system as a definite diagnostic code, it was excluded from the analysis. We defined the time point at which this diagnosis was claimed using ICD-10 code and V code as “time 0” and identified IBD patients.
For inclusion in the present study, patients had to fulfill the diagnostic criteria for IBD, which were based on the clinical features, endoscopic findings, and histologic findings that are required for registration in the RID program. Previous studies have validated the accuracy of the RID database for both UC and CD diagnoses[14,23,24].
Statistical analysis
Statistical analyses were performed with the R program, version 3.4.3 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org) and SAS, version 9.2 (SAS Institute Inc., Cary, NC, United States) for Windows. Random selection of age- and sex-matched controls was performed using the SAS algorithm. Data for continuous variables are presented as the mean and standard deviation[25]. Data for categorical variables are presented as the numbers and percentages. Differences in baseline characteristics and comorbidities between the COPD group and non-COPD controls were analyzed with independent t-tests and χ2 tests, as appropriate. Incidence rates of IBD were calculated by dividing the number of events by 1000000 person-years of follow-up for each group. Cox proportional hazard regression models considering time-varying covariates were used to calculate the hazard ratio (HR) and 95% confidence interval (CI) for the risk of IBD in patients with COPD compared to controls[26]. The cumulative incidences of IBD were compared between the groups with the Kaplan-Meier method and the log-rank test. A P value < 0.05 was considered statistically significant.
Ethical consideration
All data were obtained without identifiable information, such as the registration number, patient’s name, or medical institution. This study’s protocol was approved by the institutional review board of Seoul National University Hospital (H-1703-107-840). All data base was encrypted and anonymous; we did not obtain informed consent from the study population.
RESULTS
Baseline characteristics of study population
A total of 1303021 patients with COPD and 6515105 non-COPD controls were included in this study. The mean age of the study population was 57.1 ± 10.7 years and the mean duration of follow-up was 3.9 ± 1.4 years. The number of patients with severe COPD was 111459, representing 8.6% of all COPD patients and 1191562 patients (91.4%) had mild COPD. (Table 1) Compared to non-COPD controls, the COPD group had lower proportions of urban. However, the COPD group had significantly higher proportions of individuals with the lower 20% incomes than non-COPD controls. In the case of comorbidity, the COPD group had a higher prevalence of diabetes mellitus, hypertension, and dyslipidemia.
Table 1.
Baseline characteristics of study population, n (%)
| Non-COPD controls (n = 6515105) | COPD group (n = 1303021) | P value | |
| Sex (Male %) | 2867470 (44.0) | 573494 (44.0) | 1 |
| Age (yr) | 57.1 ± 10.72 | 57.1 ± 10.72 | 1 |
| 40-64 | 5025580 (77.1) | 1005116 (77.1) | |
| 65- | 1489525 (22.9) | 297905 (22.9) | |
| COPD severity | |||
| Mild | 1191562 (91.4) | ||
| Severe | 111459 (8.6) | ||
| Income Low1 | 1597508 (24.5) | 357627 (27.5) | < 0.001 |
| Urban residents | 2965581 (45.8) | 567359 (44.1) | |
| Comorbidity | |||
| Diabetes mellitus | 640544 (9.8) | 164860 (12.7) | < 0.001 |
| Hypertension | 1703988 (26.2) | 418880 (32.2) | < 0.001 |
| Dyslipidemia | 1002279 (15.4) | 272449 (20.9) | < 0.001 |
| Follow up duration (yr) | 3.9 ± 1.4 | 3.9 ± 1.4 | 0.8803 |
Denotes subjects with annual income lower than 20% among total population. COPD: Chronic obstructive pulmonary disease.
Development of IBD in patients with chronic obstructive pulmonary disease
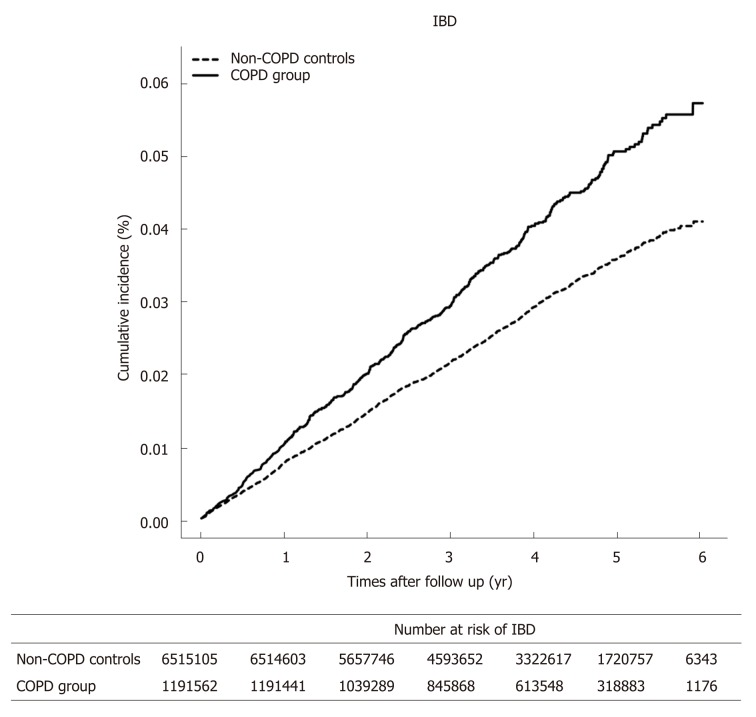
Comparison of the incidence rate and risk of IBD between patients with chronic obstructive pulmonary disease and con-COPD controls is presented in Table 2. In the COPD group, 513 patients (0.04%) developed IBD in the follow-up period, whereas 1,846 non-COPD controls (0.03%) developed IBD. The COPD group had higher incidences of total IBD compared to non-COPD controls (Figure 1). The risk of IBD in the COPD group was increased by 1.38 (adjusted HR; 95%CI: 1.25-1.52). Among the 513 patents with IBD in the COPD group, 406 were diagnosed with UC and 107 with CD. The incidence rate of UC was higher in the COPD group than in the non-COPD controls. CD also developed more frequently in the COPD group than in non-COPD controls. There was an increased risk of developing both UC and CD in the COPD group compared to in non-COPD controls.
Table 2.
Incidence rate and risk of inflammatory bowel disease in patients with chronic obstructive pulmonary disease
| Event | DURATION (Person-years) | Incidence rate of IBD | Model 11 HR (95%CI) | Model 22 HR (95%CI) | P value | ||
| IBD | |||||||
| COPD | No | 1846 | 25697723.08 | 7.18 | 1 (Ref.) | 1 (Ref.) | < 0.001 |
| Yes | 513 | 5139283.39 | 9.98 | 1.39 (1.26-1.53) | 1.379 (1.25-1.52) | ||
| UC | |||||||
| COPD | No | 1540 | 25697723.08 | 5.99 | 1 (Ref.) | 1 (Ref.) | < 0.001 |
| Yes | 406 | 5139283.39 | 7.9 | 1.32 (1.18-1.47) | 1.315 (1.18-1.47) | ||
| CD | |||||||
| COPD | No | 306 | 25697723.08 | 1.19 | 1 (Ref.) | 1 (Ref.) | < 0.001 |
| Yes | 107 | 5139283.39 | 2.08 | 1.75 (1.40-2.171) | 1.691 (1.35-2.10) | ||
Model 1: adjustment for age and sex;
Model 2: adjustment for model 1+ place of resident, income, diabetes mellitus, hypertension, dyslipidemia. CD: Crohn’s disease; CI: Confidence interval; COPD: Chronic obstructive pulmonary disease; HR: Hazard ratio; IBD: Inflammatory bowel disease; UC: Ulcerative colitis.
Figure 1.
The cumulative incidence of inflammatory bowel disease in chronic obstructive pulmonary disease group and non-chronic obstructive pulmonary disease controls. IBD: Inflammatory bowel disease; COPD: Chronic obstructive pulmonary disease.
Incidence rates and relative risks of IBD according to chronic obstructive pulmonary disease severity
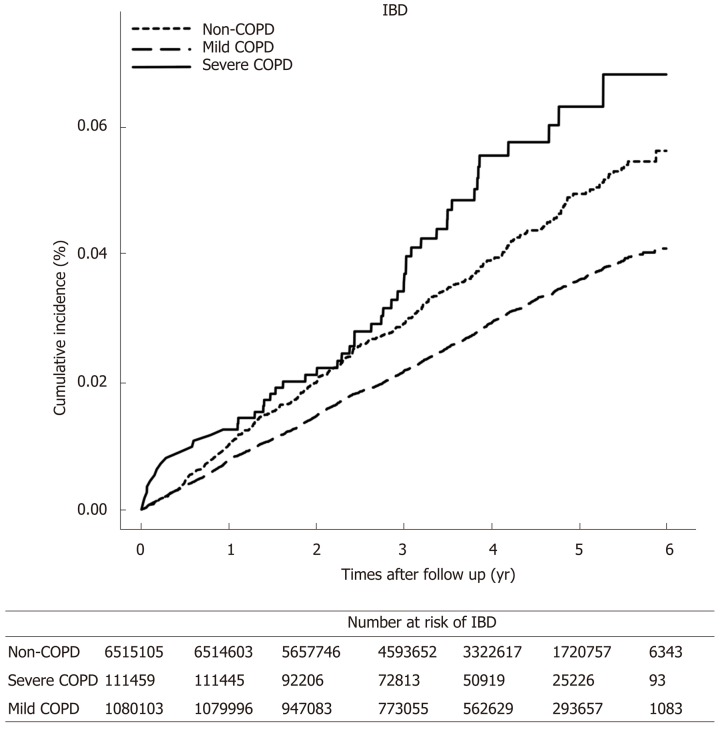
Table 3 reports the number of events, calculated incidence rates, and unadjusted and adjusted HRs for IBD in the COPD group according to COPD severity. The incidence rate of overall IBD was higher in the severe COPD group than in the mild COPD group) (Figure 2). Both UC and CD were developed more frequently in the severe COPD group than in the mild COPD group. The severity of COPD was associated with an increased risk of developing IBD. When IBD is classified as UC and CD separately, patients with severe COPD also had increased risk of developing UC and CD compared to not only non-COPD controls but also the mild COPD group. This tendency of increased risk of IBD according to the severity of COPD was more prominent in CD than in UC.
Table 3.
Incidence and risk of inflammatory bowel disease in patients with chronic obstructive pulmonary disease according to disease severity
| Event | DURATION (Person-years) | Incidence rate of IBD | Model 11 HR (95%CI) | Model 22 HR (95%CI) | P value | ||
| IBD | |||||||
| COPD Severity | Non | 1846 | 25697723.08 | 7.18 | 1 (Ref.) | 1 (Ref.) | < 0.001 |
| Mild | 461 | 4719752.21 | 9.77 | 1.36 (1.23-1.50) | 1.35 (1.22-1.49) | ||
| Sev-ere | 52 | 419531.18 | 12.39 | 1.717 (1.29-2.24) | 1.70 (1.27-2.21) | ||
| UC | |||||||
| COPD Severity | Non | 1540 | 25697723.08 | 5.99 | 1 (Ref.) | 1 (Ref.) | < 0.001 |
| Mild | 371 | 4719752.21 | 7.86 | 1.31 (1.17-1.47) | 1.31 (1.17-1.47) | ||
| Sev-ere | 35 | 419531.18 | 8.34 | 1.39 (0.97-1.91) | 1.38 (0.96-1.89) | ||
| CD | |||||||
| COPD Severity | Non | 306 | 25697723.08 | 1.19 | 1 (Ref.) | 1 (Ref.) | < 0.001 |
| Mild | 90 | 4719752.21 | 1.91 | 1.60 (1.26-2.02) | 1.548 (1.22-1.95) | ||
| Sev-ere | 17 | 419531.18 | 4.05 | 3.38 (2.00-5.33) | 3.29 (1.94-5.20) | ||
Model 1: adjustment for age and sex;
Model 2: adjustment for model 1+ place of resident, income, diabetes mellitus, hypertension, dyslipidemia. CD: Crohn’s disease; CI: Confidence interval; COPD: Chronic obstructive pulmonary disease; HR: Hazard ratio; IBD: Inflammatory bowel disease; UC: Ulcerative colitis.
Figure 2.
Comparison of cumulative incidence of inflammatory bowel disease in patients with chronic obstructive pulmonary disease according to the severity of chronic obstructive pulmonary disease. IBD: Inflammatory bowel disease; COPD: Chronic obstructive pulmonary disease.
Subgroup analysis according to sex and age
For the subgroup analysis, of the entire study population was dichotomized according to sex and age. The risk of IBD in the COPD group compared to the non-COPD controls was analyzed according to sex and age (Table 4). Male patients with COPD had increased risks of developing CD and UC compared to male patients without COPD. This result was same for female patients with COPD. When the risk of developing IBD was analyzed according to age, the risk of developing IBD in the COPD group was higher than that in the non-COPD controls at all ages. In addition, the risk of IBD increased with the severity of COPD irrespective of sex and age, and this tendency was more prominent in CD than in UC.
Table 4.
Risk of inflammatory bowel disease in patients with chronic obstructive pulmonary disease according to sex and age
| COPD | IBD | UC | CD | |
| Male | No | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 1.47 (1.23-1.60) | 1.35 (1.16-1.55) | 1.75 (1.26-2.39) | |
| Female | No | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 1.35 (1.16-1.55) | 1.27 (1.07-1.5) | 1.64 (1.20-2.21) | |
| Age (40-64) | No | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 1.36 (1.21-1.52) | 1.31 (1.15-1.48) | 1.62 (1.25-2.08) | |
| Age (65-) | No | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 1.46 (1.19-1.79) | 1.36 (1.07-1.71) | 1.93 (1.23-2.96) |
CD: Crohn’s disease; COPD: Chronic obstructive pulmonary disease; IBD: Inflammatory bowel disease; UC: Ulcerative colitis.
DISCUSSION
This is a large-scale and nationwide study to assess the risk IBD in COPD patients compared to non-COPD controls. Several studies of regions with high incidence of IBD such as Europe and Canada have reported similar findings[7-9]; however, this is the first study conducted in Asia with a previously low prevalence of IBD. In addition, to our best knowledge, this is the first study that revealed a close association between the severity of COPD and the risk of developing IBD.
We found that the incidence of IBD (UC and CD) in COPD patients was higher compared to that in non-COPD controls. The higher risk of IBD in COPD patients might be explained by the genetics, pathological background, and environmental factors shared by these two disease. There are several reports investigating genes that contribute to the development of both IBD and COPD. NOD2 is a cellular protein that recognizes the bacterial muramyl dipeptide as a major component of the bacterial cell wall. NOD2 mutations were significantly identified in patients with CD, and recent studies have also reported this mutation in COPD patients[27].
The Hedgehog-interacting protein gene that is shown to be a potential sus-ceptibility locus for COPD is also important in the development of the intestinal crypt axis and further studies are required to identify whether this gene contributes to the disease overlap between COPD and IBD[28]. Dysregulation of protease activity also has a role in both COPD and IBD. Increased levels of epithelial and leukocyte matrix metalloproteinases, which have a role in the digestion of key components in mucosal structural integrity have been associated with the pathogenesis of COPD and IBD[29-33].
Development of IBD in patients with COPD can also be explained by the hypothesis that systemic inflammation is caused by overspill of multiple inflammatory mediators including C-reactive protein, IL-6, fibrinogen and activated leucocytes resulting from lung inflammation[34]. Especially, several studies revealed that plasma tumor necrosis factor (TNF-α) and its soluble receptor are increased in patients with COPD than in healthy controls[35-37]. TNF-α is a pivotal cytokine in IBD pathogenesis and IBD can be assumed to be one of the systemic inflammation caused by COPD. However, despite this evidence and hypothesis, treatment with TNF-α inhibitors have not shown significant benefit in patients with COPD[38]. This may suggest that COPD is a highly complex inflammatory disease in which many other cytokines and mediators are involved, and blocking a single cytokine does not necessarily lead to a clinically significant effect[39].
In addition, microbiomes common to the lung and gastrointestinal tract, as well as autoimmune components of both diseases, can support the link between the two diseases. We also demonstrated that the risk of developing UC and CD in COPD patients also increased with the severity of COPD and this tendency was more pronounced in CD than in UC. This can be explained by understanding the effect of hypoxia on IBD pathogenesis. The intestinal mucosal barrier is made up of epithelial apical junction complexes, consisting of tight junctions and adherence junctions, which are sensitive to hypoxia. Severe hypoxemia caused by COPD is thought to evoke diminished splanchnic perfusion and result in inadequate oxygen delivery to the intestinal mucosal, causing tissue hypoxia, which is associated with increased enterocyte damage and integrity loss[40,41]. In addition, mucosal barrier loss can be accelerated by inflammatory mediators, which are known to circulate during COPD aggravation as above mentioned[42]. For instance, cytokines can lead to alterations in the structure of tight junctions, thereby resulting in enhanced para-cellular permeability and barrier loss[43]. The effects of hypoxia are expected to be more severe as the severity of COPD increases, and a recent study showed an increased intestinal permeability in patients undergoing acute exacerbations of COPD compared to the same patients in a stable condition of COPD[44].
In this study, the number of IBD patients with UC was higher than those with CD in the COPD group. This can be assumed to be due to the higher incidence of UC in the entire IBD population in Korea compared to that of CD[16]. In addition, it may be because of the nature of the disease that older patients are more likely to be present in the COPD group. Approximately 10%-15% of IBD is diagnosed after the age of 60 and older-onset UC is more common than CD[45-47].
Smoking is the most important risk factor for COPD, also affecting the pathogenesis of IBD, protecting against UC, and promoting the development of CD[48,49]. Nevertheless, our study could not make adjustment for the smoking status due to lack of information, and this is a weakness of this study. However, an analysis of the database including the smoking status of the Korean population from the national health screening program provided by the NHIS revealed that the proportion of ex- and current smokers was significantly higher in the COPD group than in non-COPD controls. (33.3% vs 31.1%, P < 0.001). And the proportion of IBD patients was also higher in the COPD group than in non-COPD controls (0.04% vs 0.03%, P < 0.001) (Supplementary material).
Our study could not reflect the actual clinical situation, and this is one of the weaknesses using administrative data. This limitation is associated with the possibility of overlooking the risk variables that are important for disease development. A well-designed prospective observational cohort study that combine administrative data and actual clinical data including medications and other clinical covariates is needed to reveal more precisely the association between COPD and IBD.
In conclusion, the incidences of both CD and UC were significantly increased in COPD patients in South Korea and the risk of developing IBD also increased as the severity of COPD increased. It is important to be aware of the gastrointestinal symptoms indicative of IBD in COPD patients. Accurate clinical assessment should be done, especially in patients with severe COPD in order to prevent complications and avoid excess medical expenses.
ARTICLE HIGHLIGHTS
Research background
Inflammatory bowel disease (IBD) is known to be associated with airway disease and there is a growing evidence of increased risk of IBD among patients with chronic obstructive pulmonary disease (COPD).
Research motivation
South Korea has a considerably high prevalence of COPD and is one of the highest incidence of IBD among Asian countries. Previous western studies have reported the risk of IBD in COPD patients, however, no research based on Asian data has been reported.
Research objectives
To estimate the incidence of IBD in patients with COPD compared to non-COPD controls and the risk of IBD development according to COPD severity.
Research methods
From January 2010 and December 2014, patients with COPD were identified using International Classification of Disease, 10th revision (ICD-10 code) and prescription records from the National Health Insurance (NHI) database. The COPD patients were divided into the severe and the mild COPD group. And these patients were subsequently 1:5 matches with individuals without COPD. Newly diagnosed IBD patients with Crohn’s disease and ulcerative colitis were identified using ICD-10 code and the rare intractable disease registration program codes from NHI database. The risk of IBD in COPD patients compared to controls was analyzed by Cox proportional hazard regression models. The cumulative incidence of IBD were compared between the groups.
Research results
The COPD group had higher incidences of IBD compared to non-COPD controls and the risk of IBD in the COPD group was increased. The incidence rate of IBD was higher in the severe COPD group than in the mild COPD group.
Research conclusions
The incidences of IBD were significantly increased in COPD patients in South Korea and the risk of developing IBD also increased as the severity of COPD increased.
Research perspectives
Careful monitoring the gastrointestinal symptoms indicative of IBD in COPD patients is important. Accurate clinical assessment should be done, especially in patients with severe COPD in order to determine the best strategies to prevent complications.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study protocol was approved by the Seoul National University Hospital Institutional Review Board (H-1703-107-840).
Informed consent statement: All personal information was encrypted and all data were anonymous. And informed consent was waived by the Seoul National University Hospital Institutional Review Board because of retrospective study design.
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Data sharing statement: No additional data are available.
Peer-review started: July 2, 2019
First decision: August 2, 2019
Article in press: October 17, 2019
P-Reviewer: Cheng DY, Can G, Jamali R, Madnani MA, Matowicka-Karna J, Serban ED, Tarnawski AS, Zhang ZH S-Editor: Wang J L-Editor: A E-Editor: Zhang YL
Contributor Information
Jooyoung Lee, Department of Internal Medicine and Healthcare Research Institute, Healthcare System Gangnam Center, Seoul National University Hospital, Seoul 06236, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul 03080, South Korea.
Jong Pil Im, Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul 03080, South Korea. jpim0911@snu.ac.kr.
Kyungdo Han, Department of Biostatics, College of Medicine, Catholic University of Korea, Seoul 06591, South Korea.
Seona Park, Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul 03080, South Korea.
Hosim Soh, Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul 03080, South Korea.
Kukhwan Choi, Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul 03080, South Korea.
Jihye Kim, Department of Internal Medicine, CHA Gangnam Medical Center, CHA University, Seoul 06135, South Korea.
Jaeyoung Chun, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul 06273, South Korea.
Joo Sung Kim, Department of Internal Medicine and Healthcare Research Institute, Healthcare System Gangnam Center, Seoul National University Hospital, Seoul 06236, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul 03080, South Korea.
References
- 1.Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982–1992. doi: 10.1097/MIB.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–532. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz A, Yilmaz Demirci N, Hoşgün D, Uner E, Erdoğan Y, Gökçek A, Cağlar A. Pulmonary involvement in inflammatory bowel disease. World J Gastroenterol. 2010;16:4952–4957. doi: 10.3748/wjg.v16.i39.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betancourt SL, Palacio D, Jimenez CA, Martinez S, Marom EM. Thoracic manifestations of inflammatory bowel disease. AJR Am J Roentgenol. 2011;197:W452–W456. doi: 10.2214/AJR.10.5353. [DOI] [PubMed] [Google Scholar]
- 6.Tielemans MM, Jaspers Focks J, van Rossum LG, Eikendal T, Jansen JB, Laheij RJ, van Oijen MG. Gastrointestinal symptoms are still prevalent and negatively impact health-related quality of life: a large cross-sectional population based study in The Netherlands. PLoS One. 2013;8:e69876. doi: 10.1371/journal.pone.0069876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raj AA, Birring SS, Green R, Grant A, de Caestecker J, Pavord ID. Prevalence of inflammatory bowel disease in patients with airways disease. Respir Med. 2008;102:780–785. doi: 10.1016/j.rmed.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Ekbom A, Brandt L, Granath F, Löfdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn's disease in a population suffering from COPD. Lung. 2008;186:167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 9.Brassard P, Vutcovici M, Ernst P, Patenaude V, Sewitch M, Suissa S, Bitton A. Increased incidence of inflammatory bowel disease in Québec residents with airway diseases. Eur Respir J. 2015;45:962–968. doi: 10.1183/09031936.00079414. [DOI] [PubMed] [Google Scholar]
- 10.Vutcovici M, Brassard P, Bitton A. Inflammatory bowel disease and airway diseases. World J Gastroenterol. 2016;22:7735–7741. doi: 10.3748/wjg.v22.i34.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee CK. High prevalence of chronic obstructive pulmonary disease in Korea. Korean J Intern Med. 2016;31:651–652. doi: 10.3904/kjim.2016.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leem AY, Park B, Kim YS, Jung JY, Won S. Incidence and risk of chronic obstructive pulmonary disease in a Korean community-based cohort. Int J Chron Obstruct Pulmon Dis. 2018;13:509–517. doi: 10.2147/COPD.S148618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB, Park ER, Kim KJ, Moon G, Yang SH. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Hann HJ, Hong SN, Kim KH, Ahn IM, Song JY, Lee SH, Ahn HS. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015;21:623–630. doi: 10.1097/MIB.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 15.Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111–119. doi: 10.5217/ir.2016.14.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung YS, Han M, Kim WH, Park S, Cheon JH. Incidence and Clinical Outcomes of Inflammatory Bowel Disease in South Korea, 2011-2014: A Nationwide Population-Based Study. Dig Dis Sci. 2017;62:2102–2112. doi: 10.1007/s10620-017-4640-9. [DOI] [PubMed] [Google Scholar]
- 17.Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Jr, Tysk C, O'Morain C, Moum B, Colombel JF Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD) Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 18.Ng SC. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014;28:363–372. doi: 10.1016/j.bpg.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Ye BD. Could early anti-tumor necrosis factor therapy change the prognosis of Crohn's disease? Intest Res. 2014;12:263–265. doi: 10.5217/ir.2014.12.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Lee JH, Kim Y, Kim K, Oh YM, Yoo KH, Rhee CK, Yoon HK, Kim YS, Park YB, Lee SW, Lee SD. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med. 2013;13:51. doi: 10.1186/1471-2466-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Rhee CK, Yoo KH, Kim YS, Lee SW, Park YB, Lee JH, Oh Y, Lee SD, Kim Y, Kim K, Yoon H. The health care burden of high grade chronic obstructive pulmonary disease in Korea: analysis of the Korean Health Insurance Review and Assessment Service data. Int J Chron Obstruct Pulmon Dis. 2013;8:561–568. doi: 10.2147/COPD.S48577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SC, Kim YS, Kang YA, Park EC, Shin CS, Kim DW, Rhee CK. Hemoglobin and mortality in patients with COPD: a nationwide population-based cohort study. Int J Chron Obstruct Pulmon Dis. 2018;13:1599–1605. doi: 10.2147/COPD.S159249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong SN, Kim HJ, Kim KH, Han SJ, Ahn IM, Ahn HS. Risk of incident Mycobacterium tuberculosis infection in patients with inflammatory bowel disease: a nationwide population-based study in South Korea. Aliment Pharmacol Ther. 2017;45:253–263. doi: 10.1111/apt.13851. [DOI] [PubMed] [Google Scholar]
- 24.Park S, Chun J, Han KD, Soh H, Choi K, Kim JH, Lee J, Lee C, Im JP, Kim JS. Increased end-stage renal disease risk in patients with inflammatory bowel disease: A nationwide population-based study. World J Gastroenterol. 2018;24:4798–4808. doi: 10.3748/wjg.v24.i42.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4:91. doi: 10.21037/atm.2016.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6:121. doi: 10.21037/atm.2018.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinose D, Ogawa E, Hirota T, Ito I, Kudo M, Haruna A, Marumo S, Hoshino Y, Muro S, Hirai T, Sakai H, Date H, Tamari M, Mishima M. A NOD2 gene polymorphism is associated with the prevalence and severity of chronic obstructive pulmonary disease in a Japanese population. Respirology. 2012;17:164–171. doi: 10.1111/j.1440-1843.2011.02069.x. [DOI] [PubMed] [Google Scholar]
- 28.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 29.Vernooy JH, Lindeman JH, Jacobs JA, Hanemaaijer R, Wouters EF. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest. 2004;126:1802–1810. doi: 10.1378/chest.126.6.1802. [DOI] [PubMed] [Google Scholar]
- 30.Pender SL, Li CK, Di Sabatino A, MacDonald TT, Buckley MG. Role of macrophage metalloelastase in gut inflammation. Ann N Y Acad Sci. 2006;1072:386–388. doi: 10.1196/annals.1326.019. [DOI] [PubMed] [Google Scholar]
- 31.Vlahos R, Bozinovski S, Jones JE, Powell J, Gras J, Lilja A, Hansen MJ, Gualano RC, Irving L, Anderson GP. Differential protease, innate immunity, and NF-kappaB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L931–L945. doi: 10.1152/ajplung.00201.2005. [DOI] [PubMed] [Google Scholar]
- 32.Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax. 2007;62:706–713. doi: 10.1136/thx.2006.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5:7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of 'overspill' of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- 35.Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- 36.Broekhuizen R, Grimble RF, Howell WM, Shale DJ, Creutzberg EC, Wouters EF, Schols AM. Pulmonary cachexia, systemic inflammatory profile, and the interleukin 1beta -511 single nucleotide polymorphism. Am J Clin Nutr. 2005;82:1059–1064. doi: 10.1093/ajcn/82.5.1059. [DOI] [PubMed] [Google Scholar]
- 37.Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1453–1455. doi: 10.1164/ajrccm.150.5.7952575. [DOI] [PubMed] [Google Scholar]
- 38.Matera MG, Calzetta L, Cazzola M. TNF-alpha inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm Pharmacol Ther. 2010;23:121–128. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Barnes PJ. Unexpected failure of anti-tumor necrosis factor therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:866–867. doi: 10.1164/rccm.200702-253ED. [DOI] [PubMed] [Google Scholar]
- 40.Takala J. Determinants of splanchnic blood flow. Br J Anaesth. 1996;77:50–58. doi: 10.1093/bja/77.1.50. [DOI] [PubMed] [Google Scholar]
- 41.Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, van Dam RM, Dejong CH, Buurman WA. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283–2291. doi: 10.2353/ajpath.2010.091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bathoorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:217–229. doi: 10.2147/copd.s1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 44.Sprooten RTM, Lenaerts K, Braeken DCW, Grimbergen I, Rutten EP, Wouters EFM, Rohde GGU. Increased Small Intestinal Permeability during Severe Acute Exacerbations of COPD. Respiration. 2018;95:334–342. doi: 10.1159/000485935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL Asia–Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e2. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Charpentier C, Salleron J, Savoye G, Fumery M, Merle V, Laberenne JE, Vasseur F, Dupas JL, Cortot A, Dauchet L, Peyrin-Biroulet L, Lerebours E, Colombel JF, Gower-Rousseau C. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423–432. doi: 10.1136/gutjnl-2012-303864. [DOI] [PubMed] [Google Scholar]
- 47.Taleban S, Colombel JF, Mohler MJ, Fain MJ. Inflammatory bowel disease and the elderly: a review. J Crohns Colitis. 2015;9:507–515. doi: 10.1093/ecco-jcc/jjv059. [DOI] [PubMed] [Google Scholar]
- 48.Birrenbach T, Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10:848–859. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. J Crohns Colitis. 2014;8:717–725. doi: 10.1016/j.crohns.2014.02.002. [DOI] [PubMed] [Google Scholar]