Abstract
Purpose of review
To review the impact of a new technology, 3D-bioprinting, in xenotransplantation research.
Recent findings
Genetically-engineered pigs, beginning with human (h) CD55-transgenic and Gal-knockout pigs, have improved the outcomes of xenotransplantation research. Today, there are >30 different genetically-engineered pigs either expressing human gene(s) or lacking pig gene(s). CRIPSR/cas9 technology has facilitated the production of multigene pigs (up to 9-genes in a single pig) which lack multiple pig xenoantigens, and express human transgenes, such as hCD46, hCD55, hThrombomodulin, hCD39, etc. Although recent studies in nonhuman primates (NHPs) have demonstrated prolonged survival after life-supporting pig kidney, heart, and islet xenotransplantation, researchers have difficulty determining the best genetic combination to test in NHPs due to a potential >100,000 genetic combinations. 3D-bioprinting of genetically-engineered pig cells (i) is superior to 2D in vitro testing, (ii) enables organ-specific testing, (iii) helps to understand differences in immunogenicity between organs, and (iv) is faster and cheaper than testing in NHPs. Moreover, 3D-bioprinted cells can be continuously perfused in a bioreactor, controlling for all variables, except the studied variable.
Summary
3D-bioprinting can help in the study of the impact of specific genes (human or pig) in xenotransplantation in a rapid, inexpensive, and reliable way.
Keywords: 3D-bioprinting, scaffold-free; bioreactor; genetic engineering; quick testing; xenotransplantation
Introduction
Outcomes of organ and cell transplantation continue to improve. However, the field still faces the problem of the shortage of transplantable organs despite the use of marginal organs and organs from living donors [1]. Xenotransplantation (i.e., cross-species transplantation between pig and humans) could offer an unlimited and readily- available supply of transplantable organs [2]. Recently, exciting developments have been reported in preclinical xenotransplantation models.
A life-supporting genetically-engineered pig kidney has functioned in a monkey for more than one year [3**], and genetically-engineered orthotopically-placed pig hearts have maintained the lives of baboons for 6 months [4**]. These achievements in preclinical trials (in nonhuman primates, NHPs) added to ongoing discussions of potential clinical trials of xenotransplantation [5–7].
The recent celebration of the 20th anniversary of the International Xenotransplantation Association [8], highlights the world-wide spread of xenotransplantation research beyond United States and Europe for acquiring techniques that could potentially offer unlimited supply of transplantable organs to patients on the waiting list across the world [9–12].
However, this success comes with its price because preclinical studies in NHPs take a long time (up to 3–4 years) and are expensive [13*]. Financial support, either state-wide [14] or through industry [15*], is essential for carrying out these research studies.
In this review, we highlighted the potential role of new technology, 3D-bioprinting, to partially overcome the burden of time and the lack of financial support in preclinical xenotransplantation research.
Mathematics of gene testing
There are currently >30 genetically-engineered pigs in xenotransplantation research [16] and the number increases each day with the identification of new targets and their genetic manipulation [17*-18]. Although multiple-gene pigs (currently up to 9-gene) exist and their organs are being used in preclinical trials, no researcher has tested 6, 7, or 8 gene pigs in order to compare outcomes in the presence or absence of a specific gene. In fact, the search of the ideal genetically-engineered pig for clinical xenotransplantation has been ongoing for decades [19,20,21*,22].
A simple mathematical formula (combination) can help to calculate the potential genetic combinations to be tested in order to scientifically understand the true role of each gene (and their combination) in regard to the human immune and coagulation systems. Figure 1 highlights the obstacles in preclinical pig-to-NHP xenotransplantation.
Figure 1: Summary of preclinical pig-to-nonhuman primate xenotransplantation testing.
Mathematical formula applied to calculate the number of potential genetic combinations (with the available genetic manipulations reported in the literature).
We calculated how many gene combinations are needed in order to create a 10-gene pig out of 20 available genes. Ten genes out of 30 could be eliminated on the basis of almost 40 years of experience in preclinical xenotransplantation research [23]. The calculation indicates that there would be >180,000 different genetic combinations to be tested. If we were to test only 1% of these genetic combinations for different organs (heart, lung, liver, kidney, etc), 30,000 to 50,000 transplants in NHPs might be required (Figure 1).
Active xenotransplantation research laboratories are aware of the magnitude of preclinical xenotransplantation experiments in NHPs. Oftentimes, one genetically-engineered pig donates one studied organ. An even better scenario, in which the pig donates two organs for different experiments, would require a total number of >5,000 genetically-engineered pigs to study 1% of the possible genetic combinations (Figure 1).
Currently, an active xenotransplantation research laboratory could transplant and manage 10–20 preclinical NHP transplants per year. Assuming that there would be 10 active xenotransplantation research laboratories for preclinical research, the time needed to test genetic combinations in NHPs would total 166–322 years per research laboratory (Figure 1). Therefore, not counting the financial burden, (which could rise to US$5–6 billion to test 1% of genetic combinations in NHPs), the time required to perform all experiments in a single laboratory is clearly prohibitive.
Other obstacles in preclinical models
Although the current NHP preclinical model is the best in vivo model [24] to test genetically-engineered pig organs, cells, and tissues before any clinical trials, it has several limitations. Table 1 highlights the uncontrolled variables in pig-to-NHP studies.
Table 1:
Uncontrolled variables of pig-to-nonhuman primate (NHP) xenotransplantation research experiments.
| - Different species of NHPs (baboon, Rhesus, Cynomolgus monkeys, Tibetan monkeys, and potentially New World [capuchin] monkeys) |
| - Different blood groups in NHPs (A, B, AB, O) |
| - Different pre-transplant immunological conditions in NHP recipients (e.g. preformed antibodies) |
| - Different ratio of recipient NHP / donor pig organs |
| - Different time-points in performing surgeries |
| - Different time-points in performing experimental assays |
| - Unavailability of testing certain gene(s) in most NHP species |
Experimental studies are being carried out in different NHP species, such as baboons, cynomolgus monkeys, and rhesus monkeys, as recipients. Rarely, different NHPs species, such as Tibetan monkeys were used as recipients. To date, there is no side-to-side comparison to determine whether one NHP species is preferable to another as a surrogate for humans. Each researcher has her/his own experience in one species (e.g. baboon or rhesus monkey) and persists with it, building a personal or single laboratory-based outcome.
Another important problem with the current NHP experiments in xenotransplantation is the inability to study the impact of N-glycolylneuraminic acid (Neu5Gc) which is a sialic acid molecule found in all Old World NHPs. Humans cannot synthesize Neu5Gc because the human gene CMAH (cytidine monophosphate-N-acetylneuraminic acid hydroxylase) is irreversibly mutated, and thus humans have a different sialic acid, N-acetylneuraminic acid (Neu5Ac). Therefore, Neu5Gc or CMAH-knockout pigs cannot be tested in most NHPs because they do not form anti-Neu5Gc antibodies. New World capuchin monkeys, which have anti-Neu5Gc antibodies, could serve as recipients to test Neu5Gc-knockout pig organs in preclinical trials [24-25*]. However, this would add another, possibly confounding, variable in the attempt to define the best NHP species for preclinical trials in xenotransplantation.
Heterogeneity and immunogenicity of pig organs and cells
Increased evidence showed that there is heterogeneity of organ-specific endothelial cells [26-27**]. Tissue-specific endothelial cells may originate from the same progenitor cells as tissue-specific cells, and they display distinct organ-specific barrier properties, angiogenic potential, and metabolic rate and support specific to the organ [27–28]. mRNA from several endothelial cell lines revealed heterogeneous signatures even in passaged cells, providing evidence that epigenetic modification mediates differential gene expression (transcriptome) profiles [27]. Moreover, another important study demonstrated that gene expression varies across cells and species, which shapes innate immunity [29**].
This heterogeneity and variable gene expression between cells reflects to two important phenomena in xenotransplantation research [30]; (i) genetically-engineered pigs produced by CRISPR/cas9 (clustered regularly interspaced short palindromic repeat/cas9) technology with knockout or knockin of genes (pig or human) may have different expression levels in different organs and tissues (D. Ayares personal communication), and (ii) immunogenicity of different organs and tissues (such as aorta, kidney, lungs) from the same genetically-engineered pig with similar gene transcription levels may respond differently to the same stimulant (recipient) (Ekser et al. unpublished data).
Potential solutions for rapid testing in xenotransplantation.
As mentioned above, when using the current approach, the time and resources required to test all of the potential pig genetic combinations in NHPs are prohibitive. The challenge will only increase when new, potentially-beneficial genetic manipulations are identified.
Therefore, researchers are exploring the possibility of testing xeno-responses in reliable in vitro or ex vivo studies in faster and cheaper ways. Unfortunately, although the initial xeno-response can be tested in 2D in vitro studies, due to the lack of cell population heterogeneity and niche environment, its reliability and immunogenic differences are questionable compared to 3D-bioprinted tissue constructs. Ex vivo studies perfusing pig organ(s) with human blood in a closed-circuit system could be an alternative to NHP studies, but there will still be the need to physically create genetically-engineered pigs for each genetic combination [31*].
Biomedical engineering, tissue engineering, 3D-bioprinting.
Advances in biomedical technology go in parallel with the development in genetic engineering. CRISPR/cas9 gene editing facilitated and shortened the creation of genetically-engineered pigs. Generating genetically-engineered pigs used to require 3 years using homologous recombination. Currently, with CRISPR/cas9 technology providing a faster and cheaper way of genetically-engineered pig production, pigs can be generated in 5 months [32-33*].
Although the techniques for decellularization and recellularization of organs offers promise for the future of transplantation, to date there is no single consensus for complete decellularization protocol. Oftentimes, these methods do not achieve complete decellularization, or if they do, they exhibit important deleterious side effects on the extracellular matrix [34-35**]. While some studies show no remaining immunogenicity to the decellularized scaffolds indicating some sort of tolerance [36], others show mixed results, which raises the question of whether complete decellularization has been achieved [37–38].
Most of these studies come from small animal models in phenotypically close species, such as mice-to-rat. Recently, Stahl et al studied the host immune response to completely decellularized lung scaffolds derived from wild-type and genetically-engineered (Gal-knockout) pigs in a NHP model by transplanting pieces of decellularized pig lung scaffolds underneath the skin [39*]. Interestingly, there was still host immune response to the decellularized genetically-engineered pig lung scaffold.
Chimeric animals and organs have been also studied. Wang et al. reported that replacing porcine CD31 with human CD31 in pig endothelial cells suppressed xenogeneic neutrophil-mediated cytotoxicity through the inhibition of NETosis [40]. However, the study was performed in vitro in a 2D culture environment. Oldani et al showed an interspecific graft remodeling in chimeric livers using triple knockout (Fah−/−Rag2−/−Il2rg−/−) mice in order to accommodate rat hepatocytes, and their differentiation (e.g. to bile duct cells) and remodeling [41*]. Although the study is exciting, it was performed in phenotypically close species, and its feasibility in pig-to-human model needs to be investigated [42].
Other developments included the formation of spheroids or organoids in 3D cell cultures which could be used to evaluate xeno-responses [43–44]. Although better than 2D in vitro experiments, these studies are limited to either single cell line in a 3D culture, or a one-time response, or inability to perfuse human blood though the matrix [43–44]. Recently, Sfriso et al. developed a 3D artificial micro-vessel to investigate endothelial cells under physiological flow conditions [45]. This technology could be used to continuously perfuse genetically-engineered pig endothelial cells. However, in order to study the true 3D pig microenvironment, other methodologies should be developed with multiple pig cell lines.
3D-bioprinting could also be a solution to create different organ models for rapid and cheaper testing. There are several different methods of 3D-bioprinting, such as laser-assisted bioprinting, inkjet bioprinting, and extrusion bioprinting [46]. However, the inclusion of scaffold biomaterials, such as bioink or exogenously-derived extracellular matrix, to cell-based complexes, and the inability to truly perfuse these constructs in a bioreactor, put some limitations on what can be achieved with bioprinted tissues [47–48].
Scaffold-free 3D-bioprinting of pig organ models
Scaffold-free 3D-bioprinting (SF3DBP) using the Kenzan (microneedle) (CyfuseBio K.K., Japan) method-based Regenova (CyfuseBio K.K, Japan) offers biomaterial free 3D-bioprinting that uses cell spheroids [49–50]. The Regenova bioprinter is a robotic platform capable of directly and precisely placing (bioprinting) spheroids on an array of microneedles into predesigned contiguous structures with micron-level precision [50]. The stainless-steel microneedles (“kenzans”) are used as a temporary support while cell spheroids fuse and make their own extracellular matrix within 5–21 days, depending on cell type and spheroid fusion conditions (e.g. 5–7 days for liver) [51]. After fusion, 3D-bioprinted constructs can be slid from the temporary support, achieving a free-standing 3D-bioprinted tissue or organ model [52**]. The continuous perfusion of 3D-bioprinted pig organ models with human blood is important, so that the pig-to-human model can be mimicked.
Smith et al. recently developed a bioprinter friendly 3D-printed bioreactor platform (i.e. FABRICA) (Indianapolis, IN), which was designed for 3D-bioprinted tissue construct culture, perfusion, observation, and analysis [52**]. Using the FABRICA bioreactor (Figure 2), the Indiana Group studied the flow profiles of 5 different media including pig and human blood. They also studied the impact of perfusion on the survival of 3D-bioprinted constructs. In preliminary studies, the Indiana Group created SF3DBP genetically-engineered pig liver model, and perfused it for one week (Figure 3) [52**]. The spheroid-based 3D-bioprinting was successful, and formed a scaffold-free free-standing tissue within a week (Figure 3). The application is unique and has several advantages (Table 2).
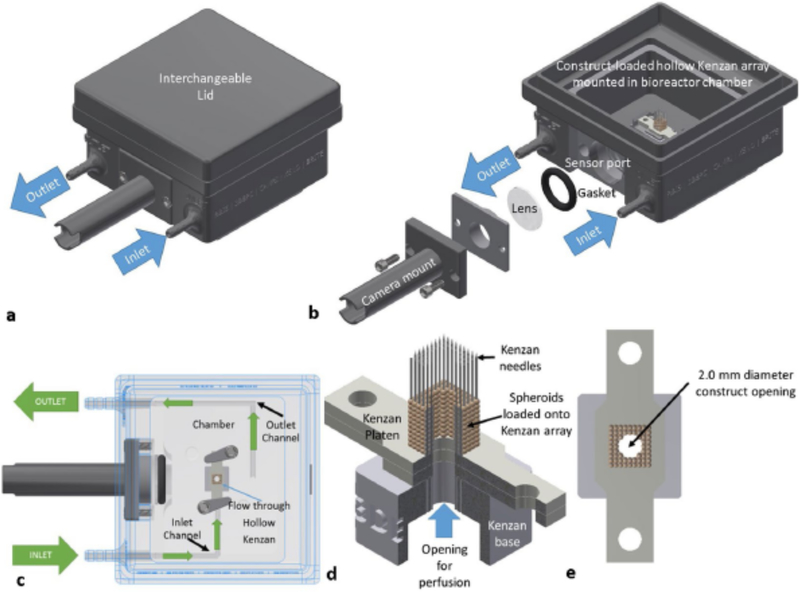
Figure 2: Fabrica bioreactor design.
(a) Fabrica bioreactor system with the interchangeable lid installed. (b) Fabrica bioreactor showing the inlet and outlet ports, how the camera mount is installed onto the camera port, and a tissue construct loaded on microneedles within the bioreactor chamber. (c) Top-down cutaway showing how constructs are perfused within the Fabrica. (d) Representative quarter section rendering of a hollow miconeedles with a perfusible tissue construct loaded onto the needles. (e) A top down rendering of the construct on the Kenzan showing the 2 mm diameter opening for perfusive flow. (Figure 2 is reproduced with permission from Smith et al [52**].)
Figure 3: Scaffold-free 3D-bioprinted genetically-engineered pig liver model.
(a) Microscope image of the 3D-bioprinted tissue construct immediately after printing on day 1. Individual spheroids can be appreciated. (b) Microscope image of the 3D-bioprinted tissue construct on the Kenzan microneedles after a week of continuous pulsatile perfusion. No gap between spheroids and no individual spheroids can be appreciated. This indicates the production of their own extracellular matrix. (c) Bright field image of the same construct after its removal from the Kenzan, as free-standing 3D-bioprinted tissue. (d) Photograph (naked-eye) of the perfused 3D-bioprinted construct after a week of continuous pulsatile perfusion. (e) Histology of the scaffold-free 3D-bioprinted liver tissue which was kept statically in the static culture media for one week. Solid bar indicates 100 μm. (f) Histology of the scaffold-free 3D-bioprinted liver tissue which was continuously perfused using the Fabrica bioreactor for one week, showing improved survival of cells in the tissue. Solid bar indicates 100 μm. (Figure 3 is reproduced with permission from Smith et al [52**].)
Table 2:
Uniqueness of scaffold-free 3D-bioprinting of genetically-engineered pig cells and their perfusion using a bioreactor.
| - No biomaterial other than pig cells (scaffold-free) |
| - Same human recipient conditions (same stimulants) for all genotypes being tested |
| - same PBMCs (from the same donor as serum/plasma) |
| - same serum/plasma (from the same donor as PBMCs) |
| - same pre-transplant preformed antibody levels |
| - Same pre-transplant conditions in both recipient and donor variables |
| - Same ratio, size-match of 3D-bioprinted tissues and recipient stimulants |
| - Same time points in performing surgeries and experimental assays |
| - Ability to study antigenicity of different organs (organ-specific) from the same genotype under the same conditions. |
| - No variability in NHP species (e.g., rhesus, cynomolgus monkeys, or baboons) |
| - Ability to study the impact of difference in recipient blood groups (O, A, B, AB) |
| - Ability to study the impact of low or high pre-formed antibody levels under the same conditions. |
PBMCs = peripheral blood mononuclear cells
The SF3DBP of genetically-engineered pig organ/tissue models and their continuous perfusion with human blood through the FABRICA bioreactor enabled the Indiana group to study (i) peripheral blood counts, including platelet count, (ii) T/B cell count, (iii) circulating cytokines, (iv) antibody binding and circulating antibody levels, (v) serum cytotoxicity, (vi) platelet aggregation, and (vii) histopathology of 3D constructs. By controlling other variables, the above-mentioned experiments helped the Indiana group to understand the true impact of only one variable (genotype) on human immune response (Table 2) (Ekser et al. unpublished).
In fact, using these experiments, organ-specific 3D models in xenotransplantation can be created and tested based on organ-specific immunogenicity in a quick and inexpensive manner, and thus help to identify the best genetic combination for clinical trials. As many preclinical xenotransplantation trials use an immunosuppressive regimen that might be applicable in the clinic [53], the system can be also used to test the impact of clinically-applicable immunosuppressive regimens.
Conclusion
After decades of research, xenotransplantation has reached an exciting era. While discussions on clinical xenotransplantation trials are ongoing, efforts continue to identify the best genetically-engineered pig either as a single organ donor or multiple organ donor. It is unclear whether a specific genetically-engineered pig will be ideal as a source of several different organs (kidneys, heart, liver, lungs), or whether it will be necessary to have organ-specific genetically-engineered pigs (because of organ-specific heterogeneity and immunogenicity). New technology, such as 3D-bioprinting, and even computational calculations [54*], may help us to obtain information we require (rapidly and less expensive), and thus help us to understand the remaining pathobiological and physiological barriers to that need to be overcome [55*-56].
KEY POINTS.
It is impossible to test all genetic combinations in genetically-engineered pig-to-nonhuman primate preclinical model due to amount of time and resources required.
Current nonhuman primates (baboons, cynomolgus and rhesus monkeys) are not able to test certain knockout gene(s) (e.g. Neu5Gc-knockout pigs).
Scaffold-free 3d-bioprinting of pig organ models using genetically-engineered pig cells, and their continuous perfusion with human blood through a bioreactor, can create organ-specific 3D models in a quick and inexpensive manner.
Organ-specific bioprinting could enhance our understanding of difference in immunogenicity from the same donor pig.
Acknowledgments
Work on xenotransplantation in the Xenotransplantation Research Laboratory at Indiana University has been supported by internal funds of the Department of Surgery, in part, with support by the Board of Directors of the Indiana University Health Values Fund for Research Award (VFR-457-Ekser), and the Indiana Clinical and Translational Sciences Institute funded, in part by Grant # UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The flow studies were supported by the IUPUI Research Support Funds Grant from the Office of the Vice Chancellor for Research. The 3D-bioprinter was supported by NIH Shared Instrumentation Grant (S10OD023595), and 3D-bioprinting in xenotransplantation was supported by the Sponsored Research Agreement between Indiana University and Lung Biotechnology LLC. Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959, and by a grant to UAB by United Therapeutics Corp., Silver Springs, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CMAH
cytidine monophosphate-N-acetylneuraminic acid hydroxylase
- Neu5Ac
N-acetylneuraminic acid
- Neu5Gc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- SF3DBP
scaffold-free 3D-bioprinting
Footnotes
Conflict of interest
D.A is the CEO of Revivicor, Inc. B.E is the owner of the patent for scaffold-free bioprinting of pig cells. L.J.S is the inventor of the patent-pending FABRICA bioreactor.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Ekser B, Cooper DK, Tector AJ. The need for xenotransplantation as a source of organs and cells for clinical transplantation. Int J Surg 2015. November;23(Pt B):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? Lancet 2012; 379: 672–683. [DOI] [PubMed] [Google Scholar]
- 3 **.Kim SC, Mathews DV, Breeden CP, et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am J Transplant. 2019. March 1. doi: 10.1111/ajt.15329. Reports the longest survival in renal xenotransplantation in a preclinical study
- 4 **.Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018; 564(7736): 430–433. Reports the longest survival in orthotopic heart xenotransplantation in a preclinical study.
- 5.Shaw BI, Kirk AD. Kidney Xenotransplantation: Steps toward Clinical Application. Clin J Am Soc Nephrol 2019; 14(4): 620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier RPH, Muller YD, Balaphas A, et al. Xenotransplantation: back to the future? Transpl Int 2018; 31(5): 465–477. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Ruiz I. Breakthrough in heart xenotransplantation. Nat Rev Cardiol 2019;16(2): 69. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DKC, Buhler LH. 20th Anniversary of IXA, 1998–2018. Xenotransplantation.2018; 25(3): e12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan D, Liu T, Lei T, Zhu H, Wang Y, Deng S. Progress in multiple genetically modified minipigs for xenotransplantation in China. Xenotransplantation. 2019; 26(1): e12492. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, Miyagawa S. Current activity of xenotransplantation in Japan.Xenotransplantation. 2019; 26(1): e12487. [DOI] [PubMed] [Google Scholar]
- 11.Park CG, Shin JS, Min BH, Kim H, Yeom SC, Ahn C. Current status of xenotransplantation in South Korea. Xenotransplantation. 2019; 26(1): e12488. [DOI] [PubMed] [Google Scholar]
- 12.Choi HJ, Hyon JY, Lee HK et al. Standardization of the proceedings for preparing clinical trials of corneal xenotransplantation in South Korea. Xenotransplantation. 2019; 26(1): e12448. [DOI] [PubMed] [Google Scholar]
- 13 *.Cooper DKC. Financial support for xenotransplantation research. Xenotransplantation. 2019. January 3:e12483. doi: 10.1111/xen.12483 Highlights the importance of the financial support in xenotransplantation.
- 14.Reichart B, Guethoff S, Brenner P, et al. Xenotransplantation of Cells, Tissues, Organs and the German Research Foundation Transregio Collaborative Research Centre 127. Adv Exp Med Biol 2015; 865: 143–55. [DOI] [PubMed] [Google Scholar]
- 15 *.Rothblatt M. A personal perspective on the science, ethics, and commercialization of xenotransplantation. Xenotransplantation. 2018; 25(3): e12414. Highlights the industry support from a dedicated parent and entrepreneur.
- 16.Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically engineered pigs in xenotransplantation research. J Pathol 2016; 238: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17 *.Ladowski JM, Reyes LM, Martens GR, et al. Swine Leukocyte Antigen Class II Is a Xenoantigen. Transplantation. 2018; 102(2): 249–254. First report to indicate that SLA class II is a xenoantigen.
- 18.Buermann A, Petkov S, Petersen B, et al. Pigs expressing the human inhibitory ligand PD-L1 (CD 274) provide a new source of xenogeneic cells and tissues with low immunogenic properties. Xenotransplantation. 2018; 25(5): e12387. [DOI] [PubMed] [Google Scholar]
- 19.Smood B, Hara H, Cleveland D, Cooper DKC. In Search of the Ideal Valve:Optimizing Genetic Modifications to Prevent Bioprosthetic Degeneration. Ann Thorac Surg 2019. March 2 pii: S0003–4975(19)30251–6. doi: 10.1016/j.athoracsur.2019.01.054. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladowski J, Martens G, Estrada J, Tector M, Tector J. The desirable donor pig to eliminate all xenoreactive antigens. Xenotransplantation. 2019. March 2:e12504. doi: 10.1111/xen.12504 [DOI] [PubMed] [Google Scholar]
- 21 *.Cooper DKC, Ezzelarab M, Iwase H, Hara H. Perspectives on the Optimal Genetically Engineered Pig in 2018 for Initial Clinical Trials of Kidney or Heart Xenotransplantation. Transplantation. 2018; 102(12): 1974–1982. Reports the ongoing search of optimal pigs for clinical trials.
- 22.Fischer K, Kind A, Schnieke A. Assembling multiple xenoprotective transgenes in pigs. Xenotransplantation. 2018; 25(6): e12431. [DOI] [PubMed] [Google Scholar]
- 23.Ekser B, Li P, Cooper DKC. Xenotransplantation: past, present, and future. Curr Opin Organ Transplant 2017; 22(6): 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porru M, Pompili L, Caruso C, Leonetti C. Xenograft as In Vivo Experimental Model. Methods Mol Biol 2018; 1692: 97–105. [DOI] [PubMed] [Google Scholar]
- 25 *.Li Q, Shaikh S, Iwase H, et al. Carbohydrate antigen expression and anti-pig antibodies in New World capuchin monkeys: Relevance to studies of xenotransplantation. Xenotransplantation. 2019. February 16:e12498. doi: 10.1111/xen.12498. The possibility to study Neu5Gc-knockout pig organs in a nonhuman primate model.
- 26.Altman MO, Gagneux P. Absence of Neu5Gc and Presence of Anti-Neu5Gc Antibodies in Humans—An Evolutionary Perspective. Front Immunol. 2019; 10: 789 10.3389/fimmu.2019.00789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27 **.Marcu R, Choi YJ, Xue J, et al. Human organ-specific endothelial cell heterogeneity. iScience. 2018; 4: 20–35. Very detailed report providing an evidence of heterogeneity in organ-specific endothelial cells
- 28.Boström KI, Yao J, Wu X, Yao Y. Endothelial Cells May Have Tissue-Specific Origins. J Cell Biol Histol 2018 Jun;1(1). pii: 104 2018. June 8. [PMC free article] [PubMed] [Google Scholar]
- 29 **.Hagai T, Chen X, Miragaia RJ, et al. Gene expression variability across cells and species shapes innate immunity. Nature 2018; 563: 197–202 Reports an evidence on the variability of gene expression across cells and species and its impact on innate immunity.
- 30.Song Z, Cooper DKC, Cai Z, Mou L. Expression and Regulation Profile of Mature MicroRNA in the Pig: Relevance to Xenotransplantation. Biomed Res Int 2018; 2018: 2983908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31 *.Abicht JM, Sfriso R, Reichart B, et al. Multiple genetically modified GTKO/hCD46/HLA-E/hβ2-mg porcine hearts are protected from complement activation and natural killer cell infiltration during ex vivo perfusion with human blood. Xenotransplantation. 2018; 25(5): e12390. Production of a multi-gene pig with overexpression of human HLA-E.
- 32.Cowan PJ, Hawthorne WJ, Nottle MB. Xenogeneic transplantation and tolerance in the era of CRISPR-Cas9. Curr Opin Organ Transplant. 2019; 24(1): 5–11 [DOI] [PubMed] [Google Scholar]
- 33 *.Naeimi Kararoudi M, Hejazi SS, Elmas E, et al. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Gene Editing Technique in Xenotransplantation . Front Immunol 2018; 9: 1711. doi: 10.3389/fimmu.2018.01711. Extensive review for the importance of CRISPR/cas9 in xenotransplantation.
- 34.Guruswamy Damodaran R, Vermette P. Tissue and organ decellularization in regenerative medicine. Biotechnol Prog 2018. November;34(6):1494–1505. [DOI] [PubMed] [Google Scholar]
- 35 **.Hillebrandt KH, Everwien H, Haep N, Keshi E, Pratschke J, Sauer IM. Strategies based on organ decellularization and recellularization. Transpl Int 2019. May 17. doi: 10.1111/tri.13462. Highlights decellularization and recellularization techniques of organs (heart, lung, kidney, liver) with promises and pitfalls with a close perspective in transplantation.
- 36.Dalgliesh AJ, Parvizi M, Lopera-Higuita M, Shklover J, Griffiths LG. Graft-specific immune tolerance is determined by residual antigenicity of xenogeneic extracellular matrix scaffolds. Acta Biomater 2018; 79: 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong C, Xia J, Xie B, et al. Immunogenicity analysis of decellularized cardiac scaffolds after transplantation into rats. Regen Med 2019. May 9. doi: 10.2217/rme-2018-0139. [DOI] [PubMed] [Google Scholar]
- 38.Heuschkel MA, Leitolis A, Roderjan JG, et al. In vitro evaluation of bovine pericardium after a soft decellularization approach for use in tissue engineering. Xenotransplantation. 2018. September 28:e12464. doi: 10.1111/xen.12464. [DOI] [PubMed] [Google Scholar]
- 39 *.Stahl EC, Bonvillain RW, Skillen CD, et al. Evaluation of the host immune response to decellularized lung scaffolds derived from α-Gal knockout pigs in a non-human primate model. Biomaterials. 2018; 187: 93–104. First report to test the immunogenicity of a genetically-engineered pig decellularized lung scaffold in nonhuman primates.
- 40.Wang HT, Maeda A, Sakai R, et al. Human CD31 on porcine cells suppress xenogeneic neutrophil-mediated cytotoxicity via the inhibition of NETosis. Xenotransplantation. 2018; 25(5): e12396. [DOI] [PubMed] [Google Scholar]
- 41 *.Oldani G, Peloso A, Vijgen S, et al. Chimeric liver transplantation reveals interspecific graft remodelling. J Hepatol 2018; 69(5): 1025–1036. Highlights the promise of chimeric liver grafts with intragraft remodeling.
- 42.Ekser B, Lagasse E. Interspecies chimeric livers: A step closer to solving the problem of transplantable organ shortage? J Hepatol 2018; 69(5): 999–1001. [DOI] [PubMed] [Google Scholar]
- 43.Jarockyte G, Dapkute D, Karabanovas V, Daugmaudis JV, Ivanauskas F, Rotomskis R. 3D cellular spheroids as tools for understanding carboxylated quantum dot behavior in tumors. Biochim Biophys Acta Gen Subj 2018; 1862(4): 914–923. [DOI] [PubMed] [Google Scholar]
- 44.Sfriso R, Bongoni A, Banz Y, Klymiuk N, Wolf E, Rieben R. Assessment of the Anticoagulant and Anti-inflammatory Properties of Endothelial Cells Using 3D Cell Culture and Non-anticoagulated Whole Blood. J Vis Exp 2017. September 5;(127). doi: 10.3791/56227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sfriso R, Zhang S, Bichsel CA, et al. 3D artificial round section micro-vessels to investigate endothelial cells under physiological flow conditions. Sci Rep 2018. April 12; 8(1): 5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali M, Kumar A, Lee SJ, Jackson JD. Three-dimensional bioprintining of organ bioengineering: promise and pitfalls. Curr Opin Organ Transplant 2018; 23: 649–656 [DOI] [PubMed] [Google Scholar]
- 47.Daly AC, Critchley SE, Rencsok EM, Kelly DJ. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication. 2016. October 7;8(4):045002. [DOI] [PubMed] [Google Scholar]
- 48.Citro A, Moser PT, Dugnani E, et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials. 2019; 199: 40–51. [DOI] [PubMed] [Google Scholar]
- 49.Moldovan NI, Hibino N, Nakayama K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng Part B Rev. 2017; 23(3): 237–244. [DOI] [PubMed] [Google Scholar]
- 50.Aguilar IN, Olivos DJ, Brinker A, et al. Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer: spheroid characterization and osteogenic differentiation. Bioprinting 2019; 15: e00050 10.1016/j.bprint.2019.e00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aguilar IN, Smith LJ, Olivos DJ, Chu TG, Kacena MA, Wagner DR. Scaffold-free bioprinting of mesenchymal stem cells with the regenova printer: optimization of printing parameters. Bioprinting 2019; 15: e00048 10.1016/j.bprint.2019.e000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52 **.Smith LJ, Li P, Holland MR, Ekser B. FABRICA: A Bioreactor Platform for Printing, Perfusing, Observing, & Stimulating 3D Tissues. Sci Rep 2018. May 15;8(1):7561. doi: 10.1038/s41598-018-25663-7. First report to mention scaffold-free 3D-bioprinted pig liver model using genetically-engineered cells and its continuous perfusion with in-house developed special bioreactor.
- 53.Samy KP, Butler JR, Li P, Cooper DKC, Ekser B. The Role of Costimulation Blockade in Solid Organ and Islet Xenotransplantation. J Immunol Res 2017; 2017: 8415205. doi: 10.1155/2017/8415205. 2017. October 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54 *.Sego TJ, Kasacheuski U, Hauersperger D, Tovar A, Moldavan NI. A heuristic computational model of basic cellular processes and oxygenation during spheroid-dependent biofabrication. Biofabrication. 2017; 9(2): 024104. doi: 10.1088/1758-5090/aa6ed4. Highlights the impact of computational science in biomedical engineering.
- 55 *.Smood B, Hara H, Schoel LJ, Cooper DKC. Genetically-engineered pigs as sources for clinical red blood cell transfusion: What pathobiological barriers need to be overcome? Blood Rev 2019. January 28 pii: S0268–960X(18)30095-X. doi: 10.1016/j.blre.2019.01.003. Extensive review on the remaining barriers before clinical xenotransplantation trials start.
- 56.Shah JA, Lanaspa MA, Tanabe T, Watanabe H, Johnson RJ, Yamada K. Remaining Physiological Barriers in Porcine Kidney Xenotransplantation: Potential Pathways behind Proteinuria as well as Factors Related to Growth Discrepancies following Pig-to-Kidney Xenotransplantation. J Immunol Res 2018; 2018: 6413012. [DOI] [PMC free article] [PubMed] [Google Scholar]