Abstract
The zinc transporter ZnT8 (SLC30A8) localises to insulin secretory granules of β-cells where it facilitates zinc uptake for insulin crystallisation. ZnT8 abundance has been linked to β-cell survival and functional phenotype. However, the consequences of ZnT8 haploinsufficiency for β-cell zinc trafficking and function remain unclear. Since investigations in human populations have shown SLC30A8 truncating polymorphisms to decrease the risk of developing Type 2 Diabetes, we hypothesised that ZnT8 haploinsufficiency would improve β-cell function and maintain the endocrine phenotype. We used CRISPR/Cas9 technology to generate ZnT8 haploinsufficient mouse MIN6 β-cells and showed that ZnT8 haploinsufficiency is associated with downregulation of mRNAs for Slc39a8 and Slc39a14, which encode for the zinc importers, Znt- and Irt-related proteins 8 (ZIP8) and 14 (ZIP14), and with lowered total cellular zinc content. ZnT8 haploinsufficiency disrupts expression of a distinct array of important β-cell markers, decreases cellular proliferation via mitogen-activated protein (MAP) kinase cascades and downregulates insulin gene expression. Thus, ZnT8 cooperates with zinc importers of the ZIP family to maintain β-cell zinc homeostasis. In contrast to the hypothesis, lowered ZnT8 expression reduces MIN6 cell survival by affecting zinc-dependent transcription factors that control the β-cell phenotype.
Keywords: ZnT8, ZIP, zinc, MIN6 cells, β-cells, Type 2 Diabetes
1. Introduction
In mammals, the 14 Znt- and Irt-related proteins (ZIP) transport zinc or other metal ions into the cytosol of cells, and cytosolic zinc efflux is mediated by proteins of the zinc transporter (ZnT) family, of which most mammals have 10 paralogues [1]. Approximately 50% of cellular zinc is distributed to the cytosol, where it is buffered in such a way that the “free” available zinc concentrations are about 0.4 nM in rat INS-1E β-cells [2]. Zinc can be involved in cellular signalling and is otherwise tightly bound to metalloproteins as a structural or catalytic cofactor [3]. The zinc transporter ZnT8 highly expressed in β-cells localises to the membrane of insulin secretory granules where it regulates granule zinc uptake and accumulation, important for proper insulin maturation, storage and secretion [4]. ZnT8 expression is associated with altered β-cell function and ZnT8 polymorphisms and is linked to Type 2 Diabetes risk [5,6,7,8].
The role of ZnT8 in β-cell function has been extensively explored [4], although the details how the common tryptophan/arginine (W/R) variants affect the Type 2 diabetes risk remain unresolved. Decreased ZnT8 expression impacts β-cell function and mass as observed in hypoxia [9], lipotoxicity [10] and cytokine stimulation [11]. ZnT8 overexpression, on the other hand, protects against palmitate- and zinc chelating agent (TPEN)-induced decreases in glucose-stimulated insulin secretion (GSIS) in human islets [12] and increases the cellular zinc content and protects against zinc depletion-induced apoptosis in INS-1E cells [13]. In another study, it was found that ZnT8 is upregulated in islets of diabetic and insulin-resistant patients. Knockdown in mouse MIN6 β-cells increases proliferation and protects against inflammation-induced cell death [8]. Consistent with an association between ZnT8 and Type 2 Diabetes risk, ZnT8 haploinsufficiency through any of twelve rare loss-of-function nonsense and missense mutations is protective against the disease [14]. However, the effect of an absolute loss of function in humans remains unknown. Islets from ZnT8+/+ mice containing knock-in mutations of the most common loss-of-function variant with a pre-mature stop codon following arginine 138 (R138X) show reduced ZnT8 activity coupled with increased insulin secretory function [15]. ZnT8 knockout in the mouse induces large changes in secretory granule morphologies and lowers β-cell zinc content. However, few differences in glycaemic parameters were noted [16,17,18,19].
The polymorphic variant rs13266634 (R325W) in the human ZnT8 gene SLC30A8 is strongly associated with Type 2 Diabetes risk [5,6,7]. Curiously, the R risk allele appears to be associated with a better ability to transport zinc dependent on the lipid composition of the membrane [20]. The C-terminal domain (CTD) of ZnT8-R, which harbours the polymorphism, exhibits a higher thermostability but lower dimerization affinity compared with ZnT8-W, suggesting the mutation impacts transporter stability or sensing [21]. There is no difference in zinc transport activity between the two variants, but the ZnT8-R risk variant is more active in zinc transport than ZnT8-W when the lipid composition is altered in artificial liposomes [20,22]. In MIN6 cells, overexpression of ZnT8-R promotes granule zinc accumulation [16]. Likewise, mouse islets transgenic for human ZnT8-W exhibit reduced zinc content compared to human ZnT8-R controls [23]. Human ZnT8-R variant carriers present with increased proinsulin:insulin ratios [24], lowered β-cell function [25] and impaired insulin secretion during intravenous glucose tolerance tests [7,26]. Because these results have not clarified the exact role of the mutation in developing diabetes, further investigations of the consequences of ZnT8 expression for β-cell zinc metabolism and function are warranted.
We have previously shown that prolonged stimulation of insulin secretion, mimicking the stress β-cells experience in pre-diabetes, decreases the total zinc content of MIN6 cells and changes the expression of genes involved in cellular zinc regulation [27]. Since ZnT8 abundance is linked to β-cell survival and function [8,9,11,12,13,14] and since increased abundance during cellular stimulation elevates cytosolic free Zn2+ [28], ZnT8 is likely central to β-cell zinc trafficking and homeostasis. Here, we postulated that ZnT8 functions cooperatively with ZIP transporters. To address this hypothesis and to investigate potential mechanisms of protection against Type 2 Diabetes in ZnT8 haploinsufficient human populations, we knocked down ZnT8 by siRNA and in addition generated and characterised ZnT8 haploinsufficient MIN6 cells. Contrary to what might be expected based on the effect observed in humans with reduced ZnT8 function, the haploinsufficient MIN6 cells exhibited a changed β-cell phenotype in terms of decreased cellular zinc, insulin, and proliferation and lowered survival.
2. Results
2.1. ZnT8 Expression Is Associated with Expression of ZIP8 and ZIP14
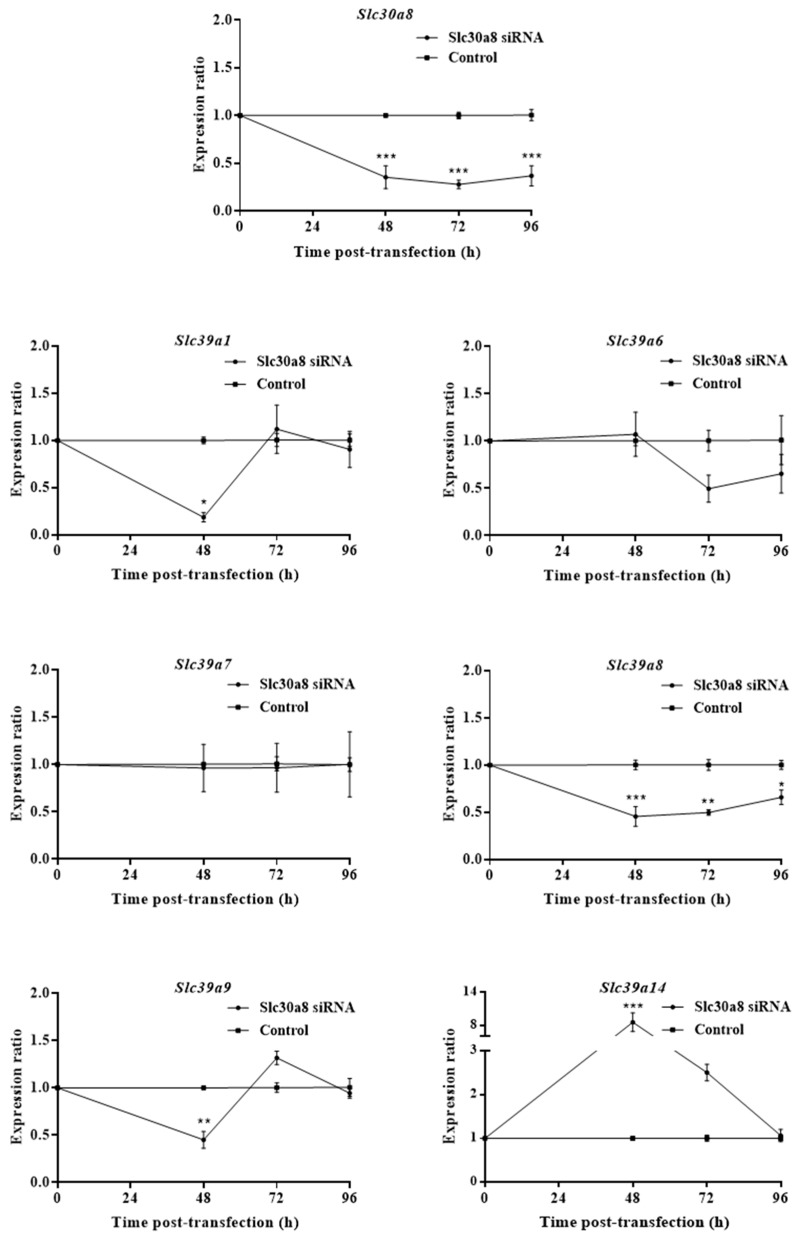
To explore the temporal ZIP response as cells adjust to ZnT8 deficiency, we knocked down Slc30a8 mRNA in MIN6 cells using siRNA and assayed expression of the Slc39a paralogues that we and others previously identified as important for β-cell function [28,29,30] at 48, 72 and 96 h (Figure 1). Slc30a8 showed >2-fold depletion at all three time-points. At 48 h, we observed upregulation of mRNA for Slc39a14 (8.56-fold), and downregulation for Slc39a1 (5.39-fold), Slc39a8 (2.20-fold) and Slc39a9 (2.22-fold). At both 72 and 96 h, only Slc39a8 remained differentially expressed compared to control cells (2.02-fold and 1.52-fold downregulation, respectively). These results show that ZnT8 expression affects the expression of ZIP transporters.
Figure 1.
Slc39a zinc transporter mRNA expression following transient Slc30a8 knockdown in MIN6 cells. Time-course for mRNA expression of Slc30a8, Slc39a1, Slc39a6, Slc39a7, Slc39a8, Slc39a9 and Slc39a14 following knockdown of Slc30a8 by siRNA. Expression was assayed at 48, 72 and 96 h post-transfection. Changes in mRNA abundances were calculated through qPCR and presented as expression ratios relative to MIN6 cells transfected with Silencer® Select negative control siRNA (control) at each time-point. Data were analysed by 2-way ANOVA followed by Sidak’s multiple comparison test. Error bars show ±SEM. n = 3. * p < 0.05, ** p < 0.005, *** p < 0.001.
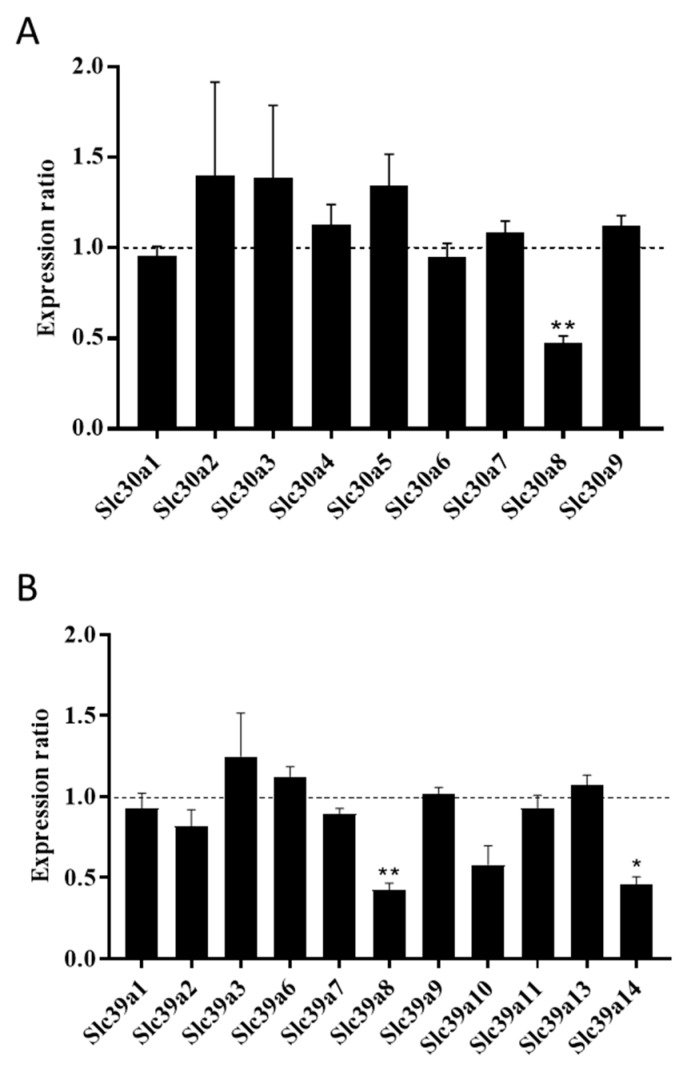
We next used CRISPR/Cas9 technology to knock out one of the Slc30a8 alleles in MIN6 cells (Figure S1) and examined the expression profiles for ZnT and ZIP paralogues in the gene-edited MIN6 cells. In silico sequence analysis predicted that our ZnT8 CRISPR MIN6 cells encode a 187 carboxy-terminal residue-truncated version of ZnT8 in addition to the 367 amino acid wild-type (Figure S2). We observed a 50% reduction of total Slc30a8 mRNA, suggesting that genome editing either prevented transcription of the truncated Slc30a8 copy variant or resulted in transcription of unstable, rapidly degraded mRNA, therefore confirming that the gene editing resulted in a ZnT8 haploinsufficient genotype (Figure 2A). We did not detect differences in mRNA abundances for any other ZnT paralogue (Figure 2A), indicating lack of transporter redundancy and/or a compensatory response. When we examined expression of the ZIP paralogues in ZnT8 haploinsufficient MIN6 cells, we recorded downregulation of mRNA for both Slc39a8 (2.37-fold) and Slc39a14 (2.32-fold) (Figure 2B).
Figure 2.
Zinc transporter expression in ZnT8 haploinsufficient MIN6 cells. The graphs show mRNA expression for (A) Slc30a and (B) Slc39a paralogues in ZnT8 haploinsufficient MIN6 cells generated by CRISPR/Cas9 technology. We were unable to detect quantifiable expression for Slc30a10, Slc39a4, Slc39a5 or Slc39a12. Changes in mRNA expression were measured by qPCR and are presented as the ratio relative to the expression level in sham-CRISPR MIN6 cells (control), which is indicated by the dotted line. Experiments were carried out at 48 h. Data were analysed through t-tests and corrected for multiple comparisons. Error bars show ±SEM. n = 3. * p < 0.05, ** p < 0.005.
2.2. ZnT8 Haploinsufficiency Decreases Zinc Uptake into MIN6 Cells
ZnT8 knockout in the mouse decreases the zinc content of islets [16,17,18,19,31,32], indicating ZnT8 affects normal zinc uptake and accumulation. Since ZIP8 and ZIP14 transport iron [33,34] and manganese [35] in addition to zinc, altered ZnT8 expression may also impact the cellular levels of these metal ions. Therefore, we next characterised the impact of ZnT8 haploinsufficiency on the zinc, iron and manganese contents of MIN6 cells. We incubated ZnT8 haploinsufficient MIN6 cells in media containing a normal concentration of zinc (8 μM) for 48 h and found that ZnT8 haploinsufficiency lowered total cellular zinc by 1.43-fold compared with sham-CRISPR MIN6 cells (Figure 3). We did not observe any statistically significant differences in the total cellular contents of iron (ZnT8 haploinsufficient cells: 0.55 nmol/mg; control cells: 0.61 nmol/mg; p = 0.95 (n = 4)) or manganese (ZnT8 haploinsufficient cells: 0.04 nmol/mg; control cells: 0.06 nmol/mg; p = 0.24 (n = 4)) at 48 h.
Figure 3.
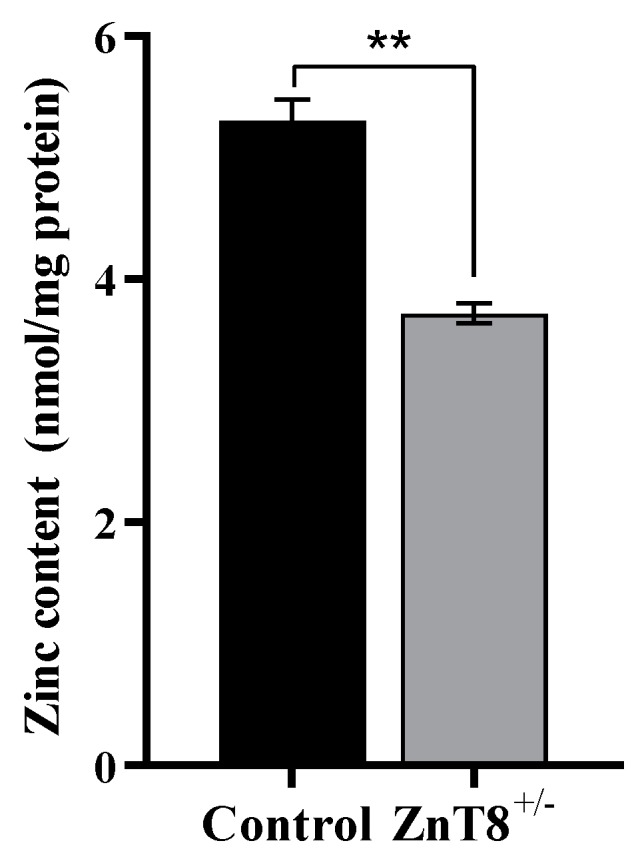
Zinc content of ZnT8 haploinsufficient MIN6 cells. Total cellular zinc content was determined through ICP-MS and normalised to protein. Experiments were carried out at 48 h. Data were analysed by 2-way ANOVA followed by Tukey’s multiple comparison test. n = 4. ** p < 0.005.
2.3. ZnT8 Haploinsufficiency Alters Expression of Important β-Cell Markers
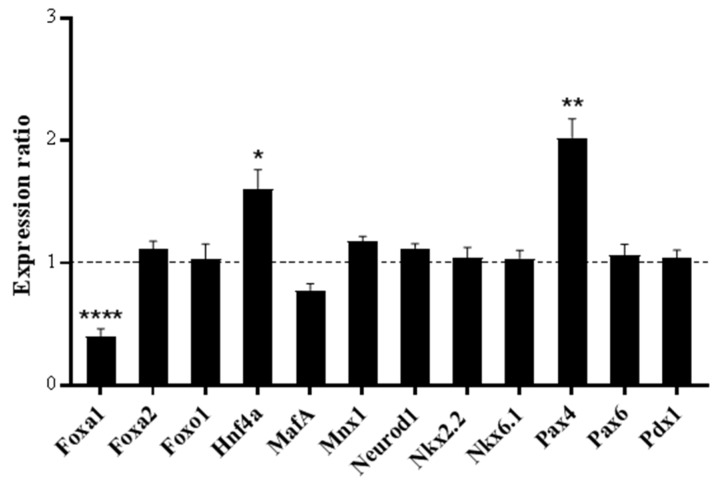
We have previously shown that multiple markers for β-cell identity and function are zinc-responsive (Hnf1b, Hnf4a, MafA, Mnx-1, Nkx2.2, Nkx6.1, Pax4, Pax6 and Pdx-1) [27]. Since lowered ZnT8 expression alters β-cell zinc trafficking and reduces total cellular zinc, it was deemed likely that ZnT8 haploinsufficiency impacts expression of these transcription factors. We examined mRNA abundances for the Zn2+-responsive transcription factors in ZnT8 haploinsufficient MIN6 cells, in addition to Foxa1, Foxa2, Foxo1 and Neurod1. We observed altered expression for three β-cell markers in ZnT8 haploinsufficient MIN6 cells (Figure 4), namely, upregulation of mRNA for Pax4 (2.03-fold) and Hnf4a (1.61-fold) and downregulation for Foxa1 (2.48-fold).
Figure 4.
mRNA expression for transcription factors in ZnT8 haploinsufficient MIN6 cells. Expression of each gene was assayed through qPCR and is presented as a ratio compared to the expression level in sham-CRISPR MIN6 cells (control), as indicated by the dotted line. Experiments were carried out at 48 h. Data were analysed through t-tests and corrected for multiple comparisons. Error bars show ±SEM. n = 3. * p < 0.05, ** p < 0.005, **** p < 0.0001.
2.4. ZnT8 Haploinsufficiency Reduces MIN6 Cell Proliferation and Ins1 Expression and Secretion
To investigate whether the affected markers for β-cell identity alter the β-cell functional phenotype, we examined the survival parameters and insulin secretory response of ZnT8 haploinsufficient MIN6 cells following culture with normal (8 μM) zinc concentration.
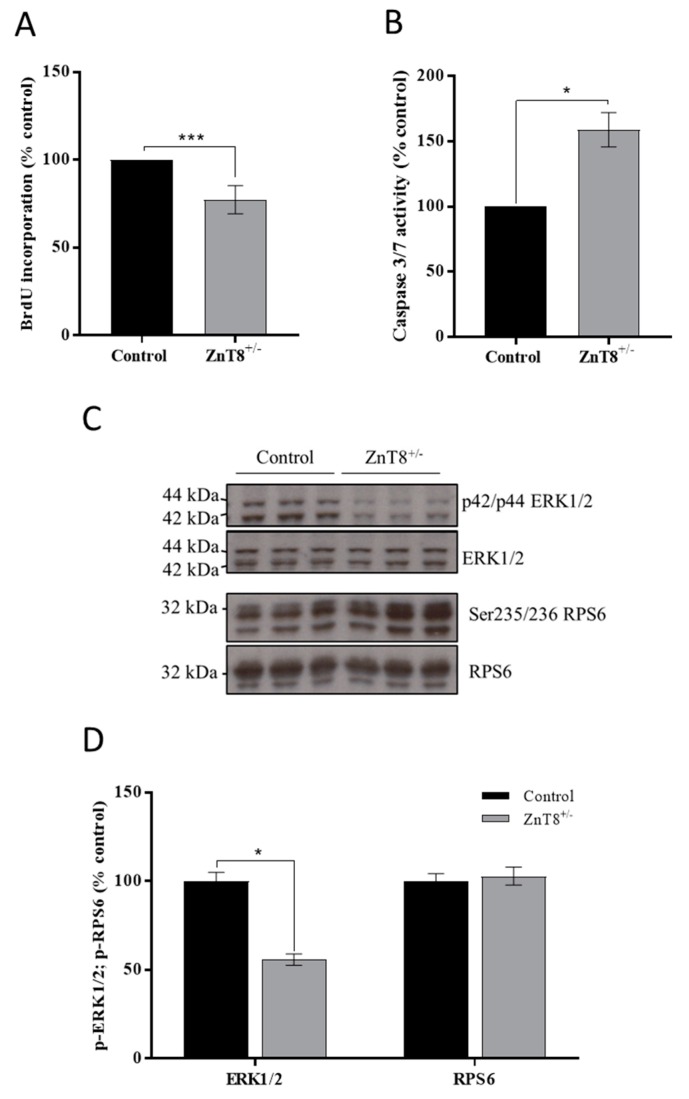
ZnT8 haploinsufficient MIN6 cells proliferate less (1.30-fold) compared with sham-CRISPR controls at 48 h. We additionally observed a 1.59-fold increase in apoptosis (Figure 5A,B). Mitogen-activated protein kinase (MAPK) cascades mediate proliferation through cell cycle regulation [36] and mechanistic target of rapamycin (mTOR) cascades promote proliferation through mediating mRNA translation [37]; both MAPK and mTOR pathways can be affected by zinc [38]. We therefore examined MAPK or mTOR signalling cascades by measuring phosphorylation of the downstream effectors ERK1/2 (Mapk1/3) and RPS6 (mTOR), which are both activated by phosphorylation. We observed decreased phosphorylation of ERK1/2 at p42/p44 compared to sham-CRISPR MIN6 cells (1.79-fold) but no effect on RPS6 (Figure 5C–D).
Figure 5.
Survival of ZnT8 haploinsufficient MIN6 cells in response to zinc depletion. (A) MIN6 cell proliferation, determined by bromodeoxyuridine (BrdU) incorporation. (B) MIN6 cell apoptosis, determined by Caspase 3/7 assays. (C,D) Phosphorylation of ERK1/2 and ribosomal protein S6 (RPS6) shown as (C) immunoblots of p42/p44 ERK1/2, ERK1/2, Ser235/236 RPS6 and RPS6 and (D) the quantified densities of immunoblot bands for p42/p44 ERK1/2 vs. ERK1/2, and Ser235/236 RPS6 vs. RPS6. Immunoblot quantification was carried out using ImageJ software. All experiments were carried out at 48 h. (A,B,D) Results are relative to sham-CRISPR MIN6 cells (control). Data were analysed by 2-way ANOVA followed by Sidak’s multiple comparison test. ZnT8 haploinsufficient MIN6 cells: ZnT8+/−. Error bars show ±SEM. n = 3. * p < 0.05, *** p < 0.001.
Finally, we explored the impact of ZnT8 haploinsufficiency on insulin expression and secretion, the downregulation of which is a hallmark of β-cell dysfunction and failure. Unlike in humans, rodents encode two insulin genes, Ins1 and Ins2 [39]. We observed 1.65-fold downregulation of Ins1 mRNA in ZnT8 haploinsufficient MIN6 (p < 0.05, n = 3) compared with control cells when cultured for 48 h with 8 µM zinc. However, ZnT8 haploinsufficient cells showed no change in abundance of Ins2 mRNA at either zinc concentration compared with the control (p > 0.05, n = 3).
3. Discussion
Complete loss of ZnT8 has not been reported in humans. ZnT8 haploinsufficient cells therefore represent a model to examine haploid loss-of-function ZnT8 genotypes [14] and/or ZnT8 downregulation in response to stresses that negatively impact β-cell function and mass [9,11,12].
3.1. Characteristics of Zinc Metabolism in ZnT8 Haploinsufficient MIN6 Cells
Upon GSIS, β-cells increase their cytosolic free Zn2+ to approximately 850 pM, presumably to increase zinc trafficking to insulin granules [28]; the activity of granule-localised ZnT8 may therefore be coordinated with ZIP-facilitated cytosolic zinc influx at the plasma membrane and/or membranes of intracellular zinc stores [40]. Concurrently, temporal loss of ZnT8 expression induced during β-cell dysfunction is coupled with decreased cytosolic free Zn2+ and a reduced insulin secretory capacity [9,11,12]. The lowered cytosolic Zn2+ is probably mediated by rapid changes to ZIP function at the plasma membrane and/or other activities coordinated with ZnT8. Following ZnT8 depletion with siRNA, we observed initial transcriptional upregulation of Slc39a14 and downregulation of Slc39a1, Slc39a8 and Slc39a9. Expression of ZIP1, ZIP8 and ZIP9 may be suppressed to prevent excess cytosolic free Zn2+ accumulation, which can be cytotoxic, in response to decreased zinc granular uptake. Of potential importance, ZIP9 functions as an androgen receptor as well as a zinc transporter, and downregulation may further impact β-cell function via associated G-protein signal transduction pathways [41].
Inconsistent information is available about ZnT8 and Type 2 Diabetes risk: downregulation is associated with cellular stress and disease development [9,11,12], whereas certain loss-of-function mutations are protective [14]. Lowered ZnT8 expression decreases β-cell/islet zinc content [16,17,18,19]. Consistent with this observation, we demonstrate that ZnT8 haploinsufficient MIN6 cells have reduced total zinc content. ZnT8 haploinsufficiency was not associated with disrupted mRNA expression for any other ZnT paralogues, indicating MIN6 cells do not induce transcription of any Slc30a gene to compensate for partial loss of ZnT8, consistent with observations recorded for ZnT8 null mouse islets [19]. ZnT8 haploinsufficiency was associated with lowered mRNA expression for Slc39a8 and Slc39a14, suggesting ZIP8 and ZIP14 are important for coordinating cytosolic zinc influx when ZnT8 transports zinc into granules. Loss of ZIP14 expression in ZnT8 haploinsufficient cells contrasts with our data for transient ZnT8 knockdown, possibly due to differences in zinc requirement following initial disruption. MIN6 cells may downregulate ZIP8 and ZIP14 to prevent destructive effects that could result from cytosolic free Zn2+ overload [42] when efflux into secretory granules is reduced. ZIP8 and ZIP14 are phylogenetically closely related [43] and both localise to the plasma membrane and membranes of intracellular zinc-storing organelles and vesicles [33,44]. ZIP8 and ZIP14 transport other metal ions in addition to zinc including non-transferrin bound iron [33,34], cadmium [45] and manganese [35]. Although we did not observe any differences in total cellular iron or manganese levels in ZnT8 haploinsufficient cells, altered transporter expression may induce off-target effects due to redistribution of these metal ions.
3.2. Phenotypes of ZnT8 Haploinsufficient MIN6 Cells
An array of endocrine-specific markers function to tightly maintain β-cell phenotype [46]. We have previously demonstrated that multiple transcription factors important for β-cell identity and endocrine function show Zn2+-responsive expression (HNF1B, HNF4A, MAFA, MNX-1, NKX2.2, NKX6.1, PAX4, PAX6 and PDX-1) [27]. Since ZnT8 haploinsufficient MIN6 cells exhibit lowered zinc content compared to controls, it is expected that β-cell transcription factor expression and identity is impacted. Accordingly, we observed that ZnT8 haploinsufficiency induced upregulation of Pax4 and Hnf4a, and downregulation of Foxa1. Although PAX4 and HNF4A are Zn2+-responsive [27,47], their expression is tightly maintained by multiple factors. PAX4 is a major transcriptional regulator of β-cell development, phenotype and function [48], promotes cellular survival by protecting against stress-induced apoptosis [48,49] and prevents β- to α-cell transdifferentiation through inhibiting glucagon expression [50], whereas HNF4A functions to regulate insulin gene expression [51]. Downregulation of FOXA1 additionally has the potential to disrupt β-cell function since FOXA1 acts in combination with FOXA2 to regulate insulin secretion and carbohydrate metabolism [52].
Tightly maintained zinc homeostasis is required for cellular signalling, differentiation and proliferation [53]. The changes to transcription factor expression observed in ZnT8 haploinsufficient cells suggest that ZnT8 downregulation impacts β-cell survival and phenotype. We demonstrated that ZnT8 haploinsufficiency lowers proliferation of MIN6 cells and increases apoptosis, possibly due to toxicity resulting from a reduced zinc storage capacity in granules. The lowered proliferation may be promoted by reduced MAPK signalling. MAPK cascades are activated by physiological concentrations of Zn2+ [54,55] to regulate gene expression, mitosis, differentiation, metabolism and programmed cell death [56]. The protective effects of reduced ZnT8 expression in vivo may be due to the ability to protect against cytokine cytotoxicity [57], which was not investigated in this study. Interestingly, the two zinc importers that showed changed expression in ZnT8 haploinsufficient cells were ZIP8 and ZIP14, which both have functions in the immune response [58,59]. ZIP14 expression has also been linked to insulin content, processing, and secretion in INS-1E β-cells [60]. Further, since ZnT8 expression is not restricted to β-cells [61,62], polymorphisms may induce effects systemically. The ZnT8 genotype has been associated with differential zinc homeostasis regulation and enhanced inflammatory response upon lipopolysaccharide stimulation in peripheral blood mononuclear cells from patients with Type 2 Diabetes [63].
It should be noted that our experiments were carried out in a single population of clonal MIN6 cells and, therefore, the observations may be clone-specific. To further establish the role of ZnT8 haploinsufficiency in β-cell zinc homeostasis and cellular function, further experiments should seek to replicate these findings in a range of genetically diverse clonal populations. Further, rescue experiments should be carried out to confirm the causative role of lowered ZnT8 expression in maintaining cellular function.
4. Materials and Methods
4.1. Cell Line and Culture
MIN6 cells (Mus musculus) [64] were cultured in Dulbecco’s Modified Eagle’s Medium containing 25 mM glucose (DMEM, Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 15% (v/v) fetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, MA, USA), 4 mM L-glutamine, 50 μM β-mercaptoethanol, 100 μg/mL streptomycin and 100 units/mL penicillin (all from Sigma Aldrich, St Louis, MI, USA), at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Cells were used from passages 24–38. For characterization of ZnT8 haploinsufficient cells, cells were cultured with growth medium containing either 1 µM (zinc deplete) or 8 μM (normal zinc) of total zinc. To achieve these specified concentrations, FBS was depleted of metal ions through incubation with 5% (w/v) Chelex-100 (Sigma Aldrich, St Louis, MI, USA) for 1 h, and sterile-filtered through a 0.22 μm syringe filter. Chelex-100- treated FBS was diluted 1:100 in 0.5% (v/v) nitric acid (HNO3) and metal analysis performed by inductively coupled plasma mass spectrometry (ICP-MS; PerkinElmer NexION 350D, Perkin Elmer Ltd, Beaconsfield, UK). Chelex-100–treated FBS was re-constituted with Ca2+, K+ and Na+ to the original concentrations. Chelex-100–treated FBS was added at 15% to DMEM containing 25 mM glucose, 4 mM/L-glutamine, 50 μM β-mercaptoethanol, 100 μg/mL streptomycin and 100 units/mL penicillin, and supplemented with ZnCl2 to total Zn2+ concentrations of 1 or 8 μM.
4.2. Cell Transfection
MIN6 cells were transfected with siRNA targeting Slc30a8 mRNA (s108999; 5′-3′ sequence: CUUUAAGCCUGACUACAAAtt (Thermo Fisher Scientific, Waltham, MA, USA)) or with Silencer® Select negative control siRNA (4390844; Thermo Fisher Scientific, Waltham, MA, USA) using Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA) 24 h after seeding, as per manufacturer’s instructions.
4.3. CRISPR/Cas9 Gene Editing
ZnT8/Slc30a8 gene editing was carried out using clustered regularly interspaced short palindromic repeats/ CRISPR associated protein 9 (CRISPR/Cas9) gene technology [65]. Cells were transfected with SLC30A8 Double Nickase Plasmid (sc-433687-NIC) or control CRISPR/Cas9 plasmid (sc-418922) (both Santa Cruz Biotechnologies, Santa Cruz, CA, USA) using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) (Figure S1). At 72 h following transfection, cells were selected using 1.25 ng/μL puromycin dihydrochloride (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) for 6 days and imaged for GFP fluorescence. Cells were transferred into a 96-well plate through serial dilution and clonal cell populations amplified through passaging cells into wells of increasing size. DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Venio, Netherlands). DNA targeted by the CRISPR/Cas9 constructs was amplified by PCR [forward primer: TCATCTCCGTGCTCAAACCC, reverse primer: TCTGTCATCGTGGCACTCAA (Sigma Aldrich, St Louis, MI, USA)] and amplicons separated on 2% (w/v) agarose gels, supplemented with 0.1% (v/v) gel red (Biotum, Freemont, CA, USA), at 40 V. Amplicons showing bands shifted from those from control cells (Figure S1) were purified using the QIAquick Gel Extraction Kit (Qiagen, Venio, Netherlands), subjected to a second PCR amplification, treated with ExoSAP-IT PCR Product Clean-up Reagent (Affymetrix, Santa Clara, CA, USA), and Sanger-sequenced at Eurofins Genomics, London, UK. To explore predicted translation of mutated proteins, edited mRNA sequences were uploaded into ExPASy bioinformatics software [66] and amino acid sequences were aligned to native sequences using the Clustal Omega multiple sequence alignment tool [67].
4.4. Gene Expression Analysis
Total RNA was extracted from MIN6 cells using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA), and treated with the high capacity RNA-to-cDNA kit (Thermo Fisher Scientific, Waltham, MA, USA). Primer Blast was used to predict whether primers bind to all SLC39A isoforms. PCR plates were loaded using the Biomek FX liquid handling robot (Beckman Coulter, Pasadena, CA, USA) and reactions (0–40 ng cDNA, 0.1 µM UPL probe, 0.2 µM forward primer, 0.2 µM reverse primer and 1X TaqMan Fast Advanced Mastermix (Applied Biosystems, Waltham, MA, USA)) amplified using the Prism7900HT sequence detection system (Applied Biosystems, Waltham, MA, USA) and analysed using sequence detection systems v2.4 software. Assay designs are provided in Table S1. Primer efficiencies ranged from 79 to 120%. The housekeeping gene ubiquitin C (UBC) was used as a control. Differential and relative expressions were calculated using the ΔΔCT method, and statistical significance was calculated using unpaired t-tests. Data shown are an average of three biological repeats.
4.5. Determination of Cellular Zinc, Iron and Manganese Levels
Cells were lysed in hot 0.2% (w/v) sodium dodecyl sulphate (Sigma Aldrich, St Louis, MI, USA). Samples were diluted 1:100 with trace element grade 0.5% nitric acid (Sigma Aldrich, St Louis, MI, USA) and ICP-MS was used to establish zinc concentrations. The total zinc, iron and manganese contents were expressed relative to total protein, which was determined through the Bradford assay (Bio-Rad, Hercules, CA, USA). Unpaired t-tests were used to calculate the statistical significance of differences in cellular zinc, iron and manganese levels.
4.6. Cellular Proliferation and Apoptosis
The rate of cellular proliferation was assessed using the Cell Proliferation ELISA, BrdU (colorimetric) assay (Sigma Aldrich (Roche), St Louis, MI, USA). A 2 h incubation period with BrdU labelling solution was used. The rate of cellular apoptosis was assessed using Caspase-Glo® 3/7 assays (Thermo Fisher Scientific, Waltham, MA, USA), using 50 μL reagent per 100 μL sample.
4.7. Immunoblotting
Protein lysates (20 µM) were prepared in 2× loading dye (Thermo Fisher Scientific, Waltham, MA, USA), heated (10 min, 72 °C), and separated on 12% acrylamide SDS-PAGE gels (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 0.1% (w/v) SDS (200 V, 40 min), and transferred onto nitrocellulose membranes [GE Healthcare Life Sciences Amersham, UK] (1 h, 100 V). Membranes were blocked with 5% (w/v) BSA/Tris-buffered saline [TBS (50 mM Tris-HCl pH 7.5, 150 mM NaCl)] and 0.05% (v/v) TWEEN-20 [TBST (1 h, room temperature], and incubated with primary antibodies diluted in 1% BSA/TBST [ERK1/2 (#9102, Cell Signalling Technology, Danvers, MA, USA, 1:1000), p42/44 ERK1/2 (#4370, Cell Signalling Technology, Danvers, MA, USA, 1:2000), RPS6 (#2217, Cell Signalling Technologies, Danvers, MA, USA, 1:1000), and Ser235/236 RPS6 (#4858, Cell Signalling Technologies, Danvers, MA, USA, 1:2000)] (16 h, 4 °C), followed by HRP-linked secondary antibodies diluted in 1% BSA/TBST (NA934V, GE Healthcare Life Sciences Amersham, UK, 1:15,000) (1 h, room temperature). Membranes were visualised following treatment with ECL Western Blotting Detection Reagent (GE Healthcare Life Sciences, Amersham, UK). For detection of additional proteins, membranes were treated with stripping buffer (Thermo Fisher Scientific, Waltham, MA, USA), as per manufacturer’s instructions. Band intensities were analysed using ImageJ software and phosphorylation of ERK1/2 and RPS6 were expressed as a ratio to total ERK1/2 or RPS6.
4.8. Insulin Secretion Assays
MIN6 cells were starved in DMEM containing 3 mM glucose (24 h, 37 °C). Cells were washed with PBS, incubated in Krebs-Ringer bicarbonate buffer (KRBH, 10 mM Hepes, 2 mM NaHCO3, 70 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 10 mM NaCl, 0.1% BSA, adjusted to pH 7.4 with NaOH) (30 min, 37 °C), supplemented with 3 mM glucose (30 min, 37 °C), followed by KRBH supplemented with 3 mM glucose, or 3 mM glucose and 40 mM KCl (30 min, 37 °C). The supernatant containing secreted insulin was aspirated and diluted 1:20. The amounts of secreted insulin were determined using the colorimetric Rat/Mouse Insulin ELISA assay kit (EZRMI-13K, Merck Millipore, Burlington, MA, USA), following manufacturer’s instructions, and normalised to total protein, determined through Bradford assays (Bio-Rad, Hercules, CA, USA).
5. Conclusions
We explored the impact of lowered ZnT8 expression on the phenotype of MIN6 cells only during zinc depletion. Diminished ZnT8 abundance downregulated ZIP8 and ZIP14 expression, decreased zinc accumulation, altered expression of key β-cell transcription factors and reduced cellular survival via MAPK signalling cascades in MIN6 cells, consistent with the effects of stress-induced ZnT8 suppression [9,11,12]. Overall, we demonstrate coordinated ZIP-ZnT8 zinc trafficking is important for β-cell zinc metabolism and that ZnT8 haploinsufficiency impacts β-cell phenotype and reduces cellular survival. The results suggest that protective effects of ZnT8 loss-of-function mutations are either different between mouse and human or are manifested at the organismal level rather than in cell culture.
Acknowledgments
R.L. was supported by the King’s Bioscience Institute and the Guy’s and St Thomas’ Charity Prize PhD Programme in Biomedical and Translational Science. The MIN6 cell line was a kind gift from Jun-ichi Miyazaki, distributed to W.M. under the appropriate materials transfer agreement. Metal analysis was performed by the London Metallomics Facility funded by the Wellcome trust (grant reference 202902/Z/16/Z). The authors would like to thank Andy Cakebread for performing the ICP-MS analyses.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/21/5485/s1.
Author Contributions
R.L. performed all experiments, analysis and drafted the manuscript. C.H. and W.M. supervised the study. All the authors reviewed, approved and contributed to the final version of this manuscript.
Conflicts of Interest
The funding body was not involved in the design of the study, collection, analysis or interpretation of data, or in writing the manuscript. The authors have no conflicts to declare.
References
- 1.Kambe T., Tsuji T., Hashimoto A., Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 2.Vinkenborg J.L., Nicolson T.J., Bellomo E.A., Koay M.S., Rutter G.A., Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 4.Davidson H.W., Wenzlau J.M., O’Brien R.M. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol. Metab. 2014;25:415–424. doi: 10.1016/j.tem.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sladek R., Rocheleau G., Rung J., Dina C., Shen L., Serre D., Boutin P., Vincent D., Bélisle A., Hadjadj S., et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 6.Saxena R., Voight B.F., Lyssenko V., Burtt N.P., De Bakker P.I.W., Chen H., Roix J.J., Kathiresan S., Hirschhorn J.N., Daly M.J., et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 7.Boesgaard T.W., Zilinskaite J., Vanttinen M., Laakso M., Jansson P.-A., Hammarstedt A., Smith U., Stefan N., Fritsche A., Häring H., et al. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients—The EUGENE2 study. Diabetologia. 2008;51:816–820. doi: 10.1007/s00125-008-0955-6. [DOI] [PubMed] [Google Scholar]
- 8.Huang L., Kirschke C. Down-regulation of zinc transporter 8 (SLC30A8) in pancreatic beta-cells promotes cell survival. Austin J. Endocrinol. Diabetes. 2016;3:1037. [Google Scholar]
- 9.Gerber P.A., Bellomo E.A., Hodson D.J., Meur G., Solomou A., Mitchell R.K., Hollinshead M., Chimienti F., Bosco D., Hughes S.J., et al. Hypoxia lowers SLC30A8/ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells. Diabetologia. 2014;57:1635–1644. doi: 10.1007/s00125-014-3266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy A.B., Wijesekara N., Genkin I., Prentice K.J., Bhattacharjee A., Kong D., Chimienti F., Wheeler M.B. Effects of high-fat diet feeding on Znt8-null mice: Differences between β-cell and global knockout of Znt8. Am. J. Physiol. Metab. 2012;302:E1084–E1096. doi: 10.1152/ajpendo.00448.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egefjord L., Jensen J.L., Bang-Berthelsen C.H., Petersen A.B., Smidt K., Schmitz O., Karlsen A.E., Pociot F., Chimienti F., Rungby J., et al. Zinc transporter gene expression is regulated by pro-inflammatory cytokines: A potential role for zinc transporters in beta-cell apoptosis? BMC Endocr. Disord. 2009;9:7. doi: 10.1186/1472-6823-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefebvre B., Vandewalle B., Balavoine A.-S., Queniat G., Moerman E., Vantyghem M.-C., Le Bacquer O., Gmyr V., Pawlowski V., Kerr-Conte J., et al. Regulation and functional effects of ZNT8 in human pancreatic islets. J. Endocrinol. 2012;214:225–232. doi: 10.1530/JOE-12-0071. [DOI] [PubMed] [Google Scholar]
- 13.Chimienti F., Devergnas S., Pattou F., Schuit F., Garcia-Cuenca R., Vandewalle B., Kerr-Conte J., Van Lommel L., Grunwald D., Favier A., et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 14.Flannick J., Go-T2D Consortium. Thorleifsson G., Beer N.L., Jacobs S.B.R., Grarup N., Burtt N.P., Mahajan A., Fuchsberger C., Atzmon G., et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 2014;46:357–363. doi: 10.1038/ng.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiner S., Gomez D., Megra B., Na E., Bhavsar R., Cavino K., Xin Y., Rojas J., Dominguez-Gutierrez G., Zambrowicz B., et al. Mice harboring the human SLC30A8 R138X loss-of-function mutation have increased insulin secretory capacity. Proc. Natl. Acad. Sci. USA. 2018;115:E7642–E7649. doi: 10.1073/pnas.1721418115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolson T.J., Bellomo E.A., Wijesekara N., Loder M.K., Baldwin J.M., Gyulkhandanyan A.V., Koshkin V., Tarasov A.I., Carzaniga R., Kronenberger K., et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes–associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijesekara N., Dai F.F., Hardy A.B., Giglou P.R., Bhattacharjee A., Koshkin V., Chimienti F., Gaisano H.Y., Rutter G.A., Wheeler M.B. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pound L.D., Sarkar S.A., Benninger R.K.P., Wang Y., Suwanichkul A., Shadoan M.K., Printz R.L., Oeser J.K., Lee C.E., Piston D.W., et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem. J. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemaire K., Ravier M.A., Schraenen A., Creemers J.W., Van de Plas R., Granvik M., Van Lommel L., Waelkens E., Chimienti F., Rutter G.A., et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merriman C., Huang Q., Rutter G.A., Fu D. Lipid-tuned zinc transport activity of human ZnT8 protein correlates with risk for type-2 diabetes. J. Biol. Chem. 2016;291:26950–26957. doi: 10.1074/jbc.M116.764605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsons D.S., Hogstrand C., Maret W. The C-terminal cytosolic domain of the human zinc transporter ZnT8 and its diabetes risk variant. FEBS J. 2018;285:1237–1250. doi: 10.1111/febs.14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho S., Molina-López J., Parsons D., Corpe C., Maret W., Hogstrand C. Differential cytolocation and functional assays of the two major human SLC30A8 (ZnT8) isoforms. J. Trace Elem. Med. Biol. 2017;44:116–124. doi: 10.1016/j.jtemb.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Li L., Bai S., Sheline C.T. hZnT8 (Slc30a8) Transgenic mice that overexpress the R325W polymorph have reduced islet Zn2+ and proinsulin levels, increased glucose tolerance after a high-fat diet, and altered levels of pancreatic zinc binding proteins. Diabetes. 2017;66:551–559. doi: 10.2337/db16-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchhoff K., Machicao F., Haupt A., Schäfer S.A., Tschritter O., Staiger H., Stefan N., Häring H.-U., Fritsche A. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51:597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 25.Dimas A.S., Lagou V., Barker A., Knowles J.W., Mägi R., Hivert M.-F., Benazzo A., Rybin D., Jackson A.U., Stringham H.M., et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014;63:2158–2171. doi: 10.2337/db13-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staiger H., Machicao F., Stefan N., Tschritter O., Thamer C., Kantartzis K., Schäfer S.A., Kirchhoff K., Fritsche A., Häring H.-U. Polymorphisms within novel risk loci for type 2 diabetes determine β-cell function. PLoS ONE. 2007;2:e832. doi: 10.1371/journal.pone.0000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson R., Maret W., Hogstrand C. Prolonged stimulation of insulin release from MIN6 cells causes zinc depletion and loss of β-cell markers. J. Trace Elem. Med. Biol. 2018;49:51–59. doi: 10.1016/j.jtemb.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Bellomo E.A., Meur G., Rutter G.A. Glucose regulates free cytosolic Zn2⁺ concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet β-cells. J. Biol. Chem. 2011;286:25778–25789. doi: 10.1074/jbc.M111.246082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson R., Maret W., Hogstrand C. Expression of the ZIP/SLC39A transporters in β-cells: A systematic review and integration of multiple datasets. BMC Genom. 2017;18:719. doi: 10.1186/s12864-017-4119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., Batchuluun B., Ho L., Zhu D., Prentice K.J., Bhattacharjee A., Zhang M., Pourasgari F., Hardy A.B., Taylor K.M., et al. Characterization of zinc influx transporters (ZIPs) in pancreatic beta cells: Roles in Regulating Cytosolic Zinc Homeostasis and Insulin Secretion. J. Biol. Chem. 2015;290:18757–18769. doi: 10.1074/jbc.M115.640524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pound L.D., Sarkar S.A., Ustione A., Dadi P.K., Shadoan M.K., Lee C.E., Walters J.A., Shiota M., McGuinness O.P., Jacobson D.A., et al. The physiological effects of deleting the mouse Slc30a8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS ONE. 2012;7:e40972. doi: 10.1371/journal.pone.0040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamaki M., Fujitani Y., Hara A., Uchida T., Tamura Y., Takeno K., Kawaguchi M., Watanabe T., Ogihara T., Fukunaka A., et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J. Clin. Investig. 2013;123:4513–4524. doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C.-Y., Jenkitkasemwong S., Duarte S., Sparkman B.K., Shawki A., MacKenzie B., Knutson M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012;287:34032–34043. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liuzzi J.P., Aydemir F., Nam H., Knutson M.D., Cousins R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheiber I.F., Wu Y., Morgan S.E., Zhao N. The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 2019;294:9147–9160. doi: 10.1074/jbc.RA119.008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Wong E.W., Chang F., Lehmann B., Terrian D.M., Milella M., Tafuri A., et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elghazi L., Bernal-Mizrachi E. Akt and PTEN: Beta-cell mass and pancreas plasticity. Trends Endocrinol. Metab. 2009;20:243–251. doi: 10.1016/j.tem.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nimmanon T., Ziliotto S., Morris S., Flanagan L., Taylor K.M. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics. 2017;9:471–481. doi: 10.1039/C6MT00286B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandrasekera P.C. Of rodents and men: Species-specific glucose regulation and type 2 diabetes research. ALTEX. 2014;31:157–176. doi: 10.14573/1309231. [DOI] [PubMed] [Google Scholar]
- 40.Lu Q., Haragopal H., Slepchenko K.G., Stork C., Li Y.V. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 2016;8:35–43. [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas P., Pang Y., Dong J., Berg A.H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology. 2014;155:4250–4265. doi: 10.1210/en.2014-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plum L.M., Rink L., Haase H. The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt-Ulms G., Ehsani S., Watts J.C., Westaway D., Wille H. Evolutionary descent of prion genes from the ZIP family of metal ion transporters. PLoS ONE. 2009;4:e7208. doi: 10.1371/journal.pone.0007208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bafaro E., Liu Y., Xu Y., Dempski R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017;2:17029. doi: 10.1038/sigtrans.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Muayed M., Raja M.R., Zhang X., MacRenaris K.W., Bhatt S., Chen X., Urbanek M., O’Halloran T.V., Lowe W.L., Lowe J.W.L. Accumulation of cadmium in insulin-producing β cells. Islets. 2012;4:405–416. doi: 10.4161/isl.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim G., Shin K.H., Pae E.K. Zinc up-regulates insulin secretion from β cell-like cells derived from stem cells from human exfoliated deciduous tooth (SHED) Int. J. Mol. Sci. 2016;17:2092. doi: 10.3390/ijms17122092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brun T., Franklin I., St-Onge L., Biason-Lauber A., Schoenle E.J., Wollheim C.B., Gauthier B.R. The diabetes-linked transcription factor PAX4 promotes β-cell proliferation and survival in rat and human islets. J. Cell Biol. 2004;167:1123–1135. doi: 10.1083/jcb.200405148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J., Li G., Lan M.S., Zhang S., Fan W., Wang H., Lu D. Pax4 paired domain mediates direct protein transduction into mammalian cells. Endocrinology. 2007;148:5558–5565. doi: 10.1210/en.2007-0636. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Meulen T., Huising M.O. Role of transcription factors in the transdifferentiation of pancreatic islet cells. J. Mol. Endocrinol. 2015;54:R103–R117. doi: 10.1530/JME-14-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartoov-Shifman R., Hertz R., Wollheim C.B., Bar-Tana J., Walker M.D., Wang H. Activation of the insulin gene promoter through a direct effect of hepatocyte nuclear factor 4. J. Biol. Chem. 2002;277:25914–25919. doi: 10.1074/jbc.M201582200. [DOI] [PubMed] [Google Scholar]
- 52.Gao N., Le Lay J., Qin W., Doliba N., Schug J., Fox A.J., Smirnova O., Matschinsky F.M., Kaestner K.H. Foxa1 and Foxa2 maintain the metabolic and secretory features of the mature beta-cell. Mol. Endocrinol. 2010;24:1594–1604. doi: 10.1210/me.2009-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beyersmann D., Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. BioMetals. 2001;14:331–341. doi: 10.1023/A:1012905406548. [DOI] [PubMed] [Google Scholar]
- 54.Azriel-Tamir H., Sharir H., Schwartz B., Hershfinkel M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Biol. Chem. 2004;279:51804–51816. doi: 10.1074/jbc.M406581200. [DOI] [PubMed] [Google Scholar]
- 55.Samet J.M., Graves L.M., Quay J., Dailey L.A., Devlin R.B., Ghio A.J., Wu W., Bromberg P.A., Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. Content. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- 56.Mebratu Y., Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merriman C., Fu D. Down-regulation of the islet-specific zinc transporter-8 (ZnT8) protects human insulinoma cells against inflammatory stress. J. Biol. Chem. 2019:jbc.RA119.010937. doi: 10.1074/jbc.RA119.010937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aydemir T.B., Chang S.-M., Guthrie G.J., Maki A.B., Ryu M.-S., Karabiyik A., Cousins R.J. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (Endotoxemia) PLoS ONE. 2012;7:e48679. doi: 10.1371/journal.pone.0048679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu M.-J., Bao S., Gálvez-Peralta M., Pyle C.J., Rudawsky A.C., Pavlovicz R.E., Killilea D.W., Li C., Nebert D.W., Wewers M.D., et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maxel T., Smidt K., Petersen C.C., Honoré B., Christensen A.K., Jeppesen P.B., Brock B., Rungby J., Palmfeldt J., Larsen A. The zinc transporter Zip14 (SLC39a14) affects beta-cell function: proteomics, gene expression, and insulin secretion studies in INS-1E cells. Sci. Rep. 2019;9:8589. doi: 10.1038/s41598-019-44954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deniro M., Al-Mohanna F.A. Zinc transporter 8 (ZnT8) expression is reduced by ischemic insults: A potential therapeutic target to prevent ischemic retinopathy. PLoS ONE. 2012;7:e50360. doi: 10.1371/journal.pone.0050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solomou A., Meur G., Bellomo E., Hodson D.J., Tomas A., Li S.M., Philippe E., Herrera P.L., Magnan C., Rutter G.A. The zinc transporter Slc30a8/ZnT8 is required in a subpopulation of pancreatic α-cells for hypoglycemia-induced glucagon secretion. J. Biol. Chem. 2015;290:21432–21442. doi: 10.1074/jbc.M115.645291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giacconi R., Malavolta M., Chiodi L., Boccoli G., Costarelli L., Bonfigli A., Galeazzi R., Piacenza F., Basso A., Gasparini N., et al. ZnT8 Arg325Trp polymorphism influences zinc transporter expression and cytokine production in PBMCs from patients with diabetes. Diabetes Res. Clin. Pr. 2018;144:102–110. doi: 10.1016/j.diabres.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Miyazaki J.-I., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y., Oka Y., Yamamura K.I. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 65.Ran F.A., Hsu P.D., Wright J., Agarwala V., A Scott D., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gasteiger E. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.