Abstract
Diabetic retinopathy (DR) is one of the most severe microvascular complications of diabetes mellitus (DM). The (CA)n microsatellite variation of the aldose reductase (ALR) gene has been indicated to be associated with DR in previous studies; however, the results were inconclusive. To provide a more precise evaluation of the association between the (CA)n variations of ALR and the risk for DR, a meta-analysis was performed in the present study. Relevant articles were retrieved from the PubMed, Embase, Chinese National Knowledge Infrastructure and Cochrane Library databases. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the strength of the associations. The present meta-analysis included 17 studies comprising 1,575 DM patients with retinopathy and 1,741 DM patients without retinopathy. The results indicated that the Z-2 allele was a risk factor for DR in Asian (OR=1.82, 95% CI: 1.16–2.86, P=0.009) and Caucasian (OR=2.08, 95% CI: 1.14–3.79, P=0.02) populations, as well as in type 1 diabetes (T1D; OR=3.42, 95% CI: 1.46–8.04, P=0.005) and type 2 diabetes (T2D; OR=1.66, 95% CI: 1.05–2.63, P=0.03). Furthermore, the Z+2 allele was determined to be a protective factor for DR in Caucasian individuals (OR=0.50, 95% CI: 0.34–0.73, P=0.0004) and those with T1D (OR=0.39, 95% CI: 0.27–0.57, P<0.00001). Z+4 was also identified to be a protective factor, reducing the risk of DR in patients with T1D (OR=0.74, 95% CI: 0.57–0.96, P=0.02). Z-4 was revealed to be a risk factor for DR in Asian populations (OR=1.57, 95% CI: 1.22–2.03, P=0.0005) and in individuals with T1D (OR=1.62, 95% CI: 1.27–2.08, P=0.0001). However, no association was detected between the Z, Z+6 and Z-6 alleles and the risk of DR (P>0.05). In conclusion, the present results revealed the following: Z+2 may serve as a protective factor for DR in Caucasian individuals and those with T1D; Z+4 may be a protective factor for DR in patients with T2D; Z-2 may represent a risk factor for DR in all subgroups analyzed; and Z-4 may be a risk factor for DR in Asian populations and patients with T2D.
Keywords: aldose reductase, diabetic retinopathy, variation, meta-analysis
Introduction
Diabetic retinopathy (DR) is one of the major microvascular complications of diabetes mellitus (DM), including type 1 diabetes (T1D) and type 2 diabetes (T2D). It is one of the leading causes of blindness in patients with DM worldwide, particularly in industrialized countries (1,2). In addition to hypertension and the duration of diabetes, chronic hyperglycaemia has been considered to be the major risk factor for the development of microvascular complications in patients with diabetes (3). Pedigree studies have indicated the presence of genetic influences in the development of these complications (4,5). Recently, genome-wide linkage analyses have begun to identify possible genetic associations with DR. A number of candidate genes have been investigated, including vascular endothelial growth factor (6), monocyte chemoattractant protein-1 (7), vitamin D receptor (8), aldehyde dehydrogenease 2 (9) and aldose reductase [ALR; also known as aldoketo reductase family 1, member B1 (ALDR1)] (10,11).
ALR is the first and rate-limiting enzyme in the human polyol pathway, which occurs under hyperglycemic conditions (12). Excessive flux of glucose via the polyol pathway has been implicated in the pathogenesis of diabetic microvascular complications (13). The ALR gene, located on chromosome 7q35, extends over ~18 kb and consists of 10 exons that give rise to a 1,384-nucleotide mRNA sequence, which encodes a 316-amino-acid protein of 35,858 Da (14). It has been reported as a candidate locus for diabetic nephropathy (15,16). Previous candidate-gene studies and meta-analyses have examined the hypothesis that two common ALR gene variants, including 106C/T and (CA)n, are associated with the risk of DR (17–20). (CA)n is a microsatellite variant located 2.1 kb upstream of the ALR transcription initiation site that has been intensively studied. It has been demonstrated that (CA)n may cause increased enzyme levels or activity, thus contributing to diabetic nephropathy and DR (21–23). The Z-2 allele of the (CA)n ALR variant was determined to be associated with DR in Caucasian patients with T1D (24), in Chinese patients with T2D (25) and in Tunisian individuals (26). However, negative results were also reported in populations from Brazil (27) and Korea (28). For the Z+2 allele, significant associations were detected in Chinese (29), Tunisian (26) and Australian (24) populations, but not in Korean (28), Caucasian (30) and Brazilian (27) individuals.
Considering the relatively small sample size in individual studies and controversial results among others, the present study aimed to provide a more accurate evaluation of the association between (CA)n ALR variants and the risk of DR. A meta-analysis was performed to assess the association between ALR (CA)n variation (Z, Z-2/Z+2, Z-4/Z+4, Z-6/Z+6) and the susceptibility to DR.
Materials and methods
Search strategy
The meta-analysis of the present study followed the Cochrane collaboration definition (31) and PRISMA 2009 guidelines for meta-analyses and systematic reviews (32). A literature search was performed using the PubMed, Embase, Chinese National Knowledge Infrastructure (CNKI) and Cochrane Library databases. Studies investigating associations between ALR (CA)n variations and the risk for DR were identified using the following keywords: ‘aldose reductase’, ‘ALR’, ‘ALDR1’, ‘ADR’, ‘AR’, ‘aldoketo reductase family 1’, ‘AKR1B1’, ‘polymorphism’, ‘variant’, ‘diabetic retinopathy’ and ‘DR’. No language restriction was applied. The search included articles published until August 10, 2018. Additional eligible studies were retrieved manually.
Studies were included if the following applied: i) A case-control design that included diabetes with retinopathy and diabetes without retinopathy, separately; ii) adequate data to calculate odds ratios (ORs) and 95% confidence intervals (CIs); iii) evaluation of the association between ALR (CA)n variation and DR risk.
The following exclusion criteria were applied: Studies were excluded if they: i) Did not include the genetic association of ALR (CA)n variation and risk of DR; ii) were duplicates of studies, or letters, dissertations, abstracts or reviews; iii) lacked sufficient data for the determination of genotype frequencies.
Data extraction and quality assessment
The following terms were extracted from each eligible study: First author, year, age, percentage of males, disease duration, glycated hemoglobin, type of DM (T1D or T2D), number of patients with diabetes with retinopathy or without retinopathy. Two authors (YX and YB) independently extracted the above information. Any disagreement was resolved by discussion. The quality of each study was assessed by using the Newcastle-Ottawa Scale (NOS) (33). The NOS uses a ‘star’ rating system to judge the quality. The total score ranged from 0 (lowest quality) to 8 (highest quality). Studies with a score of ≥6 were considered as being of high quality.
Statistical analysis
Statistical analyses were performed using STATA 12.0 software (StataCorp) and Revman 5 (Cochrane Collaboration). ORs with 95% CIs were used to assess the strength of associations between the allelic model of (CA)n variation and the risk of DR. A Z-test was used to determine the pooled ORs. The heterogeneity among studies was evaluated by Cochrane's Q-statistic and I2 statistics. A random-effects model was used in case of significant heterogeneity (I2>50%, or P<0.05). Otherwise, the fixed-effects model was used. Sensitivity analyses were also performed using the fixed-effects model or random-effects model. A funnel plot in addition to an Egger's and Begg's test was used to assess potential publication bias. P<0.05 was considered to indicate statistically significant differences.
Results
Characteristics of the published studies
As presented in Fig. 1, 672 articles met the inclusion criteria and the full articles were retrieved from the PubMed, Embase, CNKI and Cochrane Library databases after the initial search. A total of 243 publications were excluded due to being duplicates. After screening the titles and abstracts, 175 publications were excluded for not documenting the association between ALR (CA)n and DR. In addition, 51 studies were excluded for being letters, dissertations, abstracts or reviews. Finally, 17 articles were included in the present meta-analysis (24–30,34–43). The characteristics of all studies included are summarized in Tables I and SI. The NOS scores of all eligible studies ranged from 6 to 8 stars, indicating high methodological quality of all of the studies. Among them, 12 studies were performed with Asian populations (25,26,28,29,34–38,40–42) and 5 studies were performed with Caucasian populations (24,27,30,39,43). Furthermore, 4 articles included patients with T1D (24,29,39,43) and 11 studies included patients with T2D (25,26,28,30,34,35,37,38,40–42). The type of DM of the subjects in 2 remaining articles was not specified (27,36).
Figure 1.
PRISMA flow chart of study inclusion and exclusion. DR, diabetic retinopathy; ALR, aldose reductase.
Table I.
Characteristics of eligible studies included in the meta-analysis.
| First author | Year | Nationality | Age, years (DR+/DR-) | M, % (DR+/DR-) | Duration, years (DR+/DR-)a | HbA1c, % (DR+/DR-) | Type of DM | DR+ | DR- | NOS | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kao | 1999 | Australian | 14.8±14.4/14.3±13.8 | 40.2/51.5 | 7.2±7.3/6.0±6.7 | 8.6±8.6/8.7±8.6 | T1D | 67 | 97 | 8 | (24) |
| Ko | 1995 | Chinese | 33.2±7.3/31.5±8.1 | 54.5/45.5 | 5.0±3.8/15.6±4.5 | 6.8±2.0/6.7±1.7 | T1D | 22 | 22 | 8 | (29) |
| Park | 2002 | Korean | 61.1±8.7/62.4±8.5 | 53.9/34.2 | 15.2±5.0/14.4±4.2 | 9.4±2.3/8.9±2.0 | T2D | 89 | 38 | 8 | (28) |
| Petrovic | 2005 | Caucasian | 65.6±9.7/71.3±7.0 | 49/49.4 | 18.7±9.1/16.8±6.8 | 8.7±1.8/8.4±1.7 | T2D | 124 | 81 | 8 | (30) |
| Richeti | 2007 | Brazilian | NA | NA | NA | NA | NA | 62 | 66 | 6 | (27) |
| Zghal-Mokni | 2005 | Tunisian | NA | NA | NA | NA | T2D | 47 | 28 | 6 | (26) |
| Li | 2001 | Chinese | 56.5±7.2/55.4±9.7 | NA | 12.5±10.3/12.5±11.4 | NA | T2D | 105 | 40 | 6 | (34) |
| Zou | 2000 | Chinese | 45.88±10.15/48.06±7.99 | 46/53 | 2.01±2.02/15.32±4.81 | 9.46±2.37/9.19±2.48 | T2D | 100 | 132 | 8 | (37) |
| Chen | 2013 | Chinese | 58.25±8.40/58.66±8.06 | NA | 16.13±4.23/16.29±3.09 | 8.63±2.4/8.62±1.95 | NA | 164 | 158 | 7 | (36) |
| Zhang | 2008 | Chinese | 59.6±11.3/58.7±13.1 | 58.9/54.2 | 3.4±1.3/14.4±3.1 | 8.00±1.41/7.65±2.04 | T2D | 39 | 35 | 8 | (35) |
| Liu | 1999 | Chinese | 60.7±12.2/57.8±12.5 | 46.1/59.7 | 8.9±7.8/3.9±4.9 | NA | T2D | 78 | 139 | 7 | (25) |
| Wang | 2003 | Chinese | 62.0±9.6/53.4±13.0 | 41.7/41.5 | 9.4±6.9/4.4±4.6 | 8.8±2.2/7.7±1.9 | T2D | 187 | 551 | 8 | (38) |
| Kumaramanickavel | 2003 | Indian | NA | 79/63 | 16/19.6 | NA | T2D | 105 | 109 | 7 | (42) |
| Isermann | 2000 | German | NA | NA | NA | NA | T1D | 170 | 84 | 5 | (43) |
| Demaine | 2000 | British | NA | 48.4/37.1 | 24.2±7.4/33.4±10.2 | NA | T1D | 159 | 70 | 7 | (39) |
| Ikegishi | 1999 | Japanese | 47.8±11.9/49.3±11.5 | 62.9/52.9 | 10.3±5.1/15.8±5.4 | 7.9±1.1/7.6±1.7 | T2D | 27 | 34 | 8 | (41) |
| Ichikawa | 1999 | Japanese | 54.4±15.7 | NA | 14.4±7.4/NA | 9.3±2.9/NA | T2D | 30 | 57 | 7 | (40) |
Duration (years) refers to the diabetic history or patients with DM. DR, diabetic retinopathy; M, males; DM, diabetes mellitus; NOS, Newcastle-Ottawa scale; NA, not available; T1D, type 1 diabetes; T2D, type 2 diabetes; DR+, diabetes with retinopathy; DR-, diabetes without retinopathy; HbA1c, glycated hemoglobin.
Results of the meta-analysis
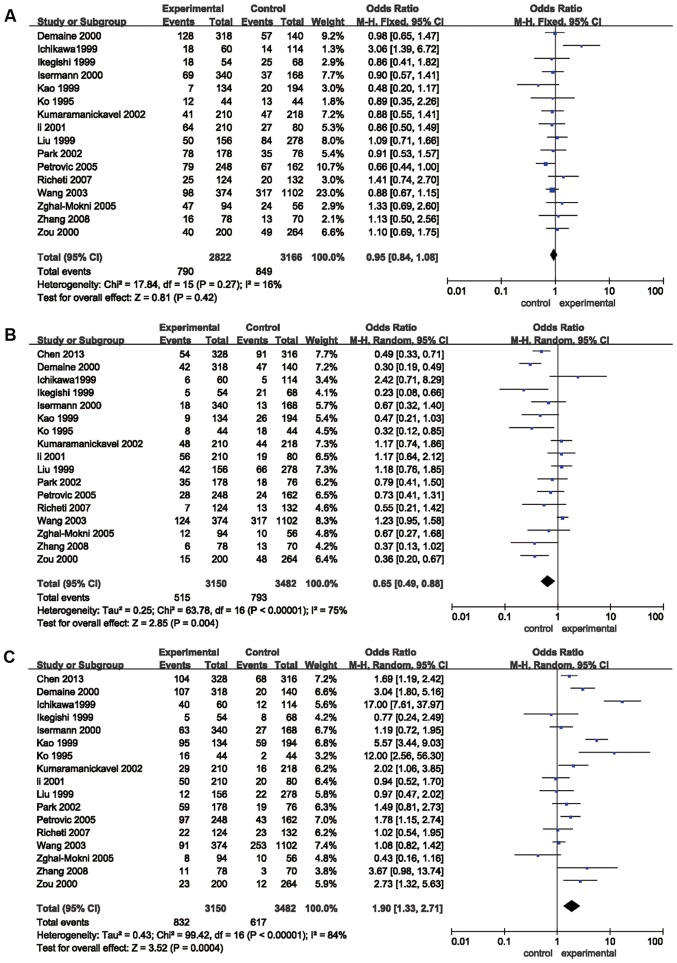
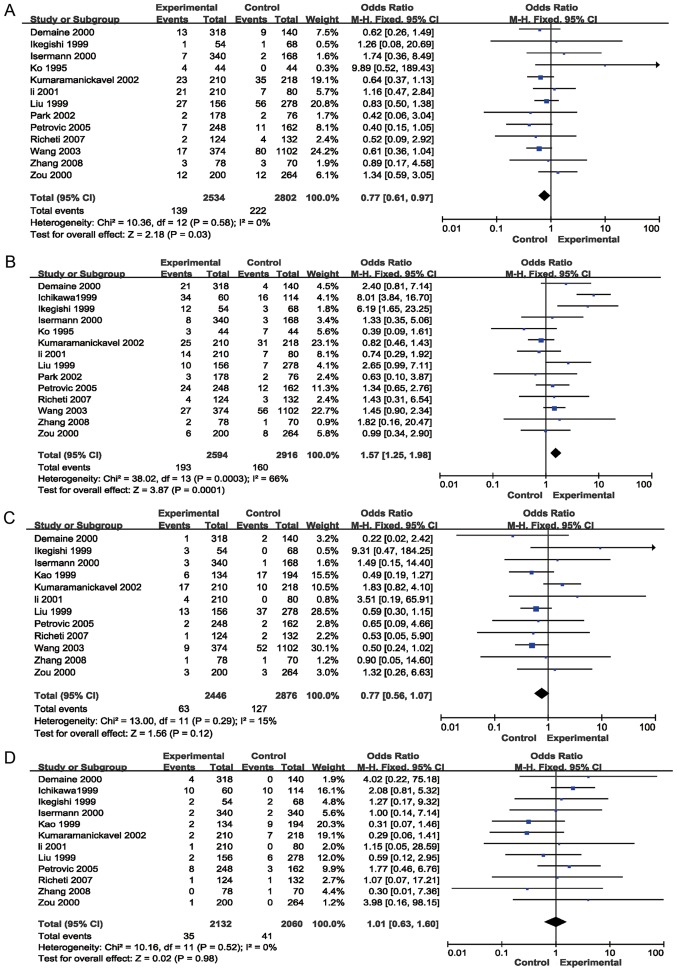
The results of the meta-analysis preformed in the present study are provided in Table II and Figs. 2 and 3. The pooled risk estimates indicated that the Z+2 allele was associated with a decreased risk of DR (OR=0.65, 95% CI: 0.49–0.88, P=0.004; Fig. 2B). However, the Z-2 allele was significantly associated with an increased risk of DR (OR=1.90, 95% CI: 1.33–2.71, P=0.0004; Table II, Fig. 2C). Significant associations were also detected between the Z+4 (OR=0.77; 95% CI, 0.61–0.97, P=0.03; Fig. 3A) and Z-4 (OR=1.57; 95% CI, 1.25–1.98; P=0.0001; Fig. 3B) alleles and the risk for DR. However, no association was detected between the Z, Z+6 or Z-6 alleles and the risk of DR (P>0.05; Table II; Figs. 2A, 3C and D, respectively). Stratification analyses based on ethnicity were then performed. The results indicated that the Z-2 allele was significantly associated with DR in Asian (OR=1.82, 95% CI: 1.16–2.86, P=0.009) and Caucasian (OR=2.08, 95% CI: 1.14–3.79, P=0.02) populations. Z+2 was significantly associated with DR in Caucasian individuals (OR=0.50, 95% CI: 0.34–0.73, P=0.0004), but not in Asian patients (P=0.07). Furthermore, Z-4 was significantly associated with DR in Asian populations (OR=1.57, 95% CI: 1.22–2.03, P=0.0005), but not in Caucasian individuals (P=0.09). In addition, the Z+4 allele was not significantly associated with DR in Asians or Caucasians (P>0.05). Stratification analyses based on the type of DM (T1D and T2D) were also performed. The results revealed that the Z-2 allele was associated with the risk of DR in patients with T1D (OR=3.42, 95% CI: 1.46–8.04, P=0.005) and T2D (OR=1.66, 95% CI: 1.05–2.63, P=0.03). The Z+2 allele was associated with the risk of DR in patients with T1D (OR=0.39, 95% CI: 0.27–0.57, P<0.00001), but not in patients with T2D (P>0.05). Furthermore, the Z+4 (OR=0.74; 95% CI, 0.57–0.96; P=0.02) and Z-4 (OR=1.62; 95% CI, 1.27–2.08; P=0.0001) alleles were associated with the risk of DR in patients with T1D, but not in patients with T2D (P>0.05; Table II).
Table II.
Pooled ORs and 95% CIs of the association between (CA)n of aldose reductase gene and diabetic retinopathy.
| Numbers | Test of association | Test of heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|
| Allele/subgroup | Number of studies | Cases | Controls | OR (95% CI) | P-value | Model | P-value | I2 (%) |
| Z | ||||||||
| Total | 16 | 2,822 | 3,166 | 0.95 (0.84, 1.08) | 0.42 | F | 0.27 | 16 |
| Asian | 11 | 1,658 | 2,370 | 1.00 (0.86, 1.16) | 0.98 | F | 0.38 | 6 |
| Caucasian | 5 | 1,164 | 796 | 0.86 (0.69, 1.06) | 0.16 | F | 0.21 | 32 |
| T1D | 4 | 836 | 546 | 0.88 (0.67, 1.15) | 0.34 | F | 0.56 | 0 |
| T2D | 11 | 1,862 | 2,488 | 0.95 (0.83, 1.10) | 0.51 | F | 0.71 | 29 |
| Z+2 | ||||||||
| Total | 17 | 3,150 | 3,482 | 0.65 (0.49, 0.88) | 0.004 | R | <0.00001 | 75 |
| Asian | 12 | 1,986 | 2,686 | 0.73 (0.52, 1.02) | 0.07 | R | <0.00001 | 75 |
| Caucasian | 5 | 1,164 | 796 | 0.50 (0.34, 0.73) | 0.0004 | F | 0.17 | 38 |
| T1D | 4 | 836 | 546 | 0.39 (0.27, 0.57) | <0.00001 | F | 0.32 | 14 |
| T2D | 11 | 1,862 | 2,488 | 0.82 (0.60, 1.12) | 0.22 | R | 0.0009 | 66 |
| Z-2 | ||||||||
| Total | 17 | 3,150 | 3,482 | 1.90 (1.33, 2.71) | 0.0004 | R | <0.00001 | 84 |
| Asian | 12 | 1,986 | 2,686 | 1.82 (1.16, 2.86) | 0.009 | R | <0.00001 | 83 |
| Caucasian | 5 | 1,164 | 796 | 2.08 (1.14, 3.79) | 0.02 | R | <0.0001 | 86 |
| T1D | 4 | 836 | 546 | 3.42 (1.46, 8.04) | 0.005 | R | <0.0001 | 87 |
| T2D | 11 | 1,862 | 2,488 | 1.66 (1.05, 2.63) | 0.03 | R | <0.00001 | 93 |
| Z+4 | ||||||||
| Total | 13 | 2,534 | 2,802 | 0.77 (0.61, 0.97) | 0.03 | F | 0.58 | 0 |
| Asian | 9 | 1,504 | 2,200 | 0.81 (0.62, 1.05) | 0.11 | F | 0.53 | 0 |
| Caucasian | 4 | 1,030 | 602 | 0.61 (0.35, 1.06) | 0.08 | F | 48 | 0 |
| T1D | 3 | 702 | 352 | 1.09 (0.54, 2.21) | 0.80 | R | 13 | 51 |
| T2D | 9 | 1,708 | 2,318 | 0.74 (0.57, 0.96) | 0.02 | F | 0.65 | 0 |
| Z-4 | ||||||||
| Total | 14 | 2,594 | 2,916 | 1.57 (1.25, 1.98) | 0.0001 | R | 0.0003 | 66 |
| Asian | 10 | 1,564 | 2,314 | 1.57 (1.22, 2.03) | 0.0005 | R | <0.0001 | 76 |
| Caucasian | 4 | 1,030 | 602 | 1.57 (0.94, 2.62) | 0.09 | F | 0.84 | 0 |
| T1D | 3 | 702 | 352 | 1.29 (0.66, 2.53) | 0.45 | R | 0.13 | 50 |
| T2D | 11 | 1,768 | 2,432 | 1.62 (1.27, 2.08) | 0.0001 | R | 0.0001 | 73 |
| Z+6 | ||||||||
| Total | 12 | 2,446 | 2,876 | 0.77 (0.56, 1.07) | 0.12 | F | 0.29 | 15 |
| Asian | 7 | 1,282 | 2,080 | 0.85 (0.59, 1.23) | 0.39 | F | 0.11 | 42 |
| Caucasian | 5 | 1,164 | 796 | 0.54 (0.26, 1.09) | 0.08 | F | 0.85 | 0 |
| T1D | 3 | 792 | 502 | 0.52 (0.23, 1.16) | 0.11 | F | 0.51 | 0 |
| T2D | 8 | 1,530 | 2,242 | 0.85 (0.59, 1.21) | 0.36 | F | 0.16 | 33 |
| Z-6 | ||||||||
| Total | 12 | 2,132 | 2,060 | 1.01 (0.63, 1.60) | 0.98 | F | 0.52 | 0 |
| Asian | 7 | 968 | 1,092 | 1.02 (0.56, 1.85) | 0.96 | F | 0.38 | 6 |
| Caucasian | 5 | 1,164 | 968 | 0.99 (0.48, 2.06) | 0.98 | F | 0.44 | 0 |
| T1D | 3 | 792 | 674 | 0.71 (0.27, 1.85) | 0.48 | F | 0.28 | 22 |
| T2D | 8 | 1,216 | 1,254 | 1.12 (0.65, 1.93) | 0.67 | F | 0.45 | 0 |
F, fixed-effects model; R, random-effects model; OR, odds ratio; CI, confidence interval; T1D, type 1 diabetes; T2D, type 2 diabetes.
Figure 2.
Forest plots of odds ratios for the association between aldose reductase (CA)n and the risk of diabetic retinopathy. (A) Z; (B) Z+2; (C) Z-2. M-H, Mantel-Haentzel; df, degrees of freedom.
Figure 3.
Forest plots of odds ratios for the association between aldose reductase (CA)n and the risk of diabetic retinopathy. (A) Z+4; (B) Z-4; (C) Z+6; (D) Z-6. M-H, Mantel-Haentzel; df, degrees of freedom.
Heterogeneity
Statistically significant heterogeneity across studies was observed when analyzing the Z+2 (I2=75%; P<0.00001), Z-2 (I2=84%; P<0.00001) and Z-4 (I2=66%; P=0.0003) alleles (Table II). Significant heterogeneity regarding Z+2 was primarily identified in the studies by Zou et al (37), Wang et al (38), Liu et al (25), Demaine et al (39), Li et al (34) and Ichikawa et al (40). Removal of these studies from the meta-analysis resulted in 40% (P=0.08) heterogeneity. Significant heterogeneity regarding Z-2 was primarily present in the studies by Kao et al (24), Ko et al (29), Demaine et al (39), Zghal-Mokni et al (26), and Ichikawa et al (40). Removal of these studies from the meta-analysis resulted in 36% (P=0.10) heterogeneity. Furthermore, the significant heterogeneity in Z-4 was mainly due to the study by Ichikawa et al (40); upon removal of this study from the meta-analysis, heterogeneity was reduced to 27% (P=0.17) (Table SII). In the subgroup analysis, significant heterogeneity across studies was identified regarding the Z+2, Z-2 and Z-4 alleles of Asian patients with T2D (Table II).
Sensitivity analysis and publication bias
In the sensitivity analysis, the influence of a single study on the overall risk estimate was determined by removing one article at a time. The results revealed that the ORs were not significantly altered in each genotype (Fig. 4). Begg's funnel plots and Egger's test were performed to assess publication bias. Some outliers were observed on the Begg's funnels plots, which may be due to the missed researches including articles with negative results and the small sample sizes of the studies. However, the results of Egger's test demonstrated that P>0.05, which indicated the missed researches and the small sample sizes studies had no effects on the results. Therefore, the results indicated that there was no publication bias for the comparison of Z, Z+2, Z-2, Z+4, Z-4, Z+6 and Z-6 (Table III and Fig. 5).
Figure 4.
Sensitive analysis was performed by omitting data one study at a time to assess the influence of each study on the stability of the results regarding aldose reductase (CA)n. (A) Z; (B) Z+2; (C) Z-2; (D) Z+4; (E) Z-4; (F) Z+6; (G) Z-6. M-H, Mantel-Haentzel; df, degrees of freedom. On the x-axis, the brackets represent mean the pooled odds ratios whereas the vertical lines represent the 95% confidence intervals.
Table III.
Egger's linear regression test for funnel plot asymmetries of (AC)n in the aldose reductase gene.
| Allele | P-value | 95% CI |
|---|---|---|
| Z | 0.281 | −0.8225458–2.62835 |
| Z+2 | 0.084 | −4.256574–0.3006727 |
| Z-2 | 0.319 | −1.63998–4.707237 |
| Z+4 | 0.267 | −0.5488476–1.791998 |
| Z-4 | 0.967 | −2.481549–2.578416 |
| Z+6 | 0.340 | −0.7528741–1.981472 |
| Z-6 | 0.658 | −2.021688–1.334716 |
Figure 5.
Publication bias of studies on aldose reductase (CA)n were tested by Begg's funnel plots. (A) Z; (B) Z+2; (C) Z-2; (D) Z+4; (E) Z-4; (F) Z+6; (G) Z-6. s.e., standard error; logor, log of odds ratio.
Discussion
ALR is an NADPH-dependent enzyme that exhibits glucose-reducing activity (44). Elevated glucose levels may enhance ALR activity in the case of a hyperglycaemic event, which then increases the flux of glucose through the polyol pathway (45). This pathway has been revealed to serve an important role in diabetic complications, including nephropathy and retinopathy. Furthermore, significantly elevated ALR mRNA and protein levels have been detected in the peripheral blood monocytes of patients with DR (46). Therefore, inhibition of ALR expression may offer a therapeutic strategy for patients suffering from DR.
Recently, two common variants in the ALR gene, including a (CA)n dinucleotide repeat microsatellite and a 106C/T polymorphism, have been examined for their genetic association with DR in several populations (36,38). The (CA)n variant, located 2.1 kb upstream of the transcription initiation site of ALR, may alter the secondary structure of DNA (47) or RNA (48), consequently affecting splicing and the generation of non-coding RNA. Multiple alleles have been identified for the (CA)n variation, including Z, Z+2, Z-2, Z+4, Z-4, Z+6, Z-6, Z+8, Z-8, Z+10 and Z-10. Z, Z+2 and Z-2 were the most common alleles identified as (CA)n variations. Furthermore, the Z-2 allele has been reported to be linked to increased expression of the ALR gene in the peripheral mononuclear cells of patients with diabetes or hyperglycaemia (49). In vitro gene reporter assays have revealed that the susceptibility polymorphism Z-2 increases transcriptional activity (49). In addition, the Z-2 allele has been associated with the susceptibility to DR (24). Other studies have also indicated that the Z+2 allele serves a protective role against DR (24,26,29). However, the results indicating the association between the Z-2 or Z+2 and DR were inconsistent and inconclusive. Among the 17 studies included in the present analysis, only 5 (29,36,37,39,41) reported significant associations between Z+2 and DR, and the 12 remaining studies indicated negative results. In addition, regarding Z-2, 7 studies reported a significant association with DR (24,29,30,36,37,39,40,42). However, the 10 remaining studies indicated no association between Z-2 and DR. The meta-analysis of the present study demonstrated that the Z-2 allele is likely to be a genetic risk factor (OR=1.90), while the Z+2 may exert a protective effect to reduce the susceptibility to DR (OR=0.65). However, a certain heterogeneity regarding Z+2 and Z-2 alleles was identified among the results of individual studies.
To determine the role of the genetic background and types of DM in DR, a subgroup analysis based on ethnicity and type of DM was performed in the present study. Subgroup analysis with stratification by ethnicity revealed that Z+2 exerted protective effects for Caucasian patients with DR only. Furthermore, Z-2 was demonstrated to be a risk factor for DR in Asian as well as Caucasian populations. These inconsistent results in different populations may indicate the important role of the genetic background in the pathogenesis of DR (50–53). In addition, subgroup analysis with stratification by type of DM revealed that Z-2 was associated with DR in T1D and T2D. Furthermore, Z+2 was associated with DR in T1D, but not in T2D. The prevalence of DR was higher in patients with T1D, with sight-threatening retinopathy reported to be up to 2.5× more common than in those with T2D (54). However, despite including a larger number of patients with T2D, the meta-analysis of the present study only demonstrated a slight association between Z-2 and DR in T2D. This may indicate the importance of a larger sample size to provide a more accurate evaluation of the association between gene polymorphisms and disease susceptibility.
A significant association between Z+4 or Z-4 and DR was determined in the present meta-analysis. Based on a larger number of patients, the Z+4 allele was determined to be a protective factor for DR, whereas the Z-4 allele was determined to be a risk factor for DR. However, none of the studies included detected any significant associations between Z+4 and DR. Furthermore, only one study reported a significant association between Z-4 and DR (41). These results may indicate the importance of larger sample sizes to provide a more accurate evaluation of the genetic association and increased calculation power in the combined analysis. Subgroup analysis based on ethnicity and type of DM revealed that Z-4 may be a risk factor for DR in Asian populations, but not in Caucasians. In addition, Z+4 and Z-4 were only determined to be associated with DR in patients with T2D. To the best of our knowledge, the present study is the first to identify a significant association between Z+4 or Z-4 and the susceptibility for DR by performing a meta-analysis. However, to fully confirm these genetic associations, larger numbers of case-control studies with more patients are necessary.
The present meta-analysis had various limitations. First, the sample size in the present meta-analysis was relatively small. Although 17 studies with 1,575 cases and 1,741 controls were included, the sample size in the subgroup analysis, particularly in the Caucasian and T1D subgroups, was insufficient. Furthermore, only the genetic association between alleles of the (CA)n variation and DR were assessed. No data on the genotype and haplotype were provided. In addition, multiple factors, including genetic and environmental factors, or the interaction between the two factors and other unknown risk factors, were not considered in the pathogenesis of DR. Furthermore, the studies included in the present meta-analysis had been performed in either Asian or Caucasian populations, but not in any subjects of other ethnicities. To more precisely assess the association between (CA)n polymorphisms and DR, analysis of multiple populations is necessary. Finally, the information on the cases was relatively limited, and the present analysis did not assess the approach (or medication) to control glucose due to lack of data in the individual studies.
In conclusion, the Z+2 allele may be a protective factor for DR in Caucasian patients with T1D. Furthermore, Z+4 may be a protective factor for DR in patients with T2D. However, Z-2 may be a risk factor for DR in all subgroups and Z-4 may be a risk factor for DR in Asian patients with T2D.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The current study was funded by the Youth Foundation of the Education Department of Hunan (grant no. 17B035) and the Foundation of the Education Department of Hunan (grant no. 18C1186).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WSM and YHB participated in data curation, methodology, software and validation. YX participated in project administration and the acquisition of funding. WSM wrote the original manuscript draft. YX was involved with the conception and design of the current study and revising the manuscript critically for important intellectual content. YX and YHB reviewed and edited the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complications. 2012;26:56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Chang YC, Wu WC. Dyslipidemia and diabetic retinopathy. Rev Diabet Stud. 2012;10:121–132. doi: 10.1900/RDS.2013.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuuminen R, Haukka J, Loukovaara S. Poor glycemic control associates with high intravitreal angiopoietin-2 levels in patients with diabetic retinopathy. Acta Ophthalmologica. 2015;93:e515–e516. doi: 10.1111/aos.12401. [DOI] [PubMed] [Google Scholar]
- 4.Grassi MA, Tikhomirov A, Ramalingam S, Below JE, Cox NJ, Nicolae DL. Genome-wide meta-analysis for severe diabetic retinopathy. Hum Mol Genet. 2011;20:2472–2481. doi: 10.1093/hmg/ddr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warpeha KM, Chakravarthy U. Molecular genetics of microvascular disease in diabetic retinopathy. Eye (Lond) 2003;17:305–311. doi: 10.1038/sj.eye.6700348. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Salinas R, Garcia-Gutierrez MC, Garcia-Aguirre G, Morales-Canton V, Velez-Montoya R, Soberon-Ventura VR, Gonzalez V, Lechuga R, Garcia-Solis P, Garcia-Gutierrez DG, et al. Evaluation of VEGF gene polymorphisms and proliferative diabetic retinopathy in Mexican population. Int J Ophthalmol. 2017;10:135–139. doi: 10.18240/ijo.2017.01.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, He M, Huang W. Association of monocyte chemoattractant protein-1 gene 2518A/G polymorphism with diabetic retinopathy in type 2 diabetes mellitus: A meta-analysis. Diabetes Res Clin Pract. 2016;120:40–46. doi: 10.1016/j.diabres.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Xia W, Lu P, Yuan HJ. The association between VDR gene polymorphisms and diabetic retinopathy susceptibility: A systematic review and meta-analysis. Biomed Res Int. 2016;2016:5305282. doi: 10.1155/2016/5305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li GY, Li ZB, Li F, Dong LP, Tang L, Xiang J, Li JM, Bao MH. Meta-analysis on the association of ALDH2 polymorphisms and type 2 diabetic mellitus, diabetic retinopathy. Int J Environ Res Public Health. 2017;14(pii):E165. doi: 10.3390/ijerph14020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujisawa T, Ikegami H, Kawaguchi Y, Shintani M, Kawabata Y, Ono M, Nishino M, Ogihara T, Okamoto N, Fukuda M. Genetic susceptibility to diabetic retinopathy: CA repeat polymorphism of the aldose reductase gene. Folia Jap Ophthalmol Clin. 2001;52:128–130. [Google Scholar]
- 11.Abhary S, Burdon KP, Laurie KJ, Thorpe S, Landers J, Goold L, Lake S, Petrovsky N, Craig JE. Aldose reductase gene polymorphisms and diabetic retinopathy susceptibility. Diabetes Care. 2010;33:1834–1836. doi: 10.2337/dc09-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crespo I, Giménez-Dejoz J, Porté S, Cousido-Siah A, Mitschler A, Podjarny A, Pratsinis H, Kletsas D, Parés X, Ruiz FX, et al. Design, synthesis, structure-activity relationships and X-ray structural studies of novel 1-oxopyrimido[4,5-c]quinoline-2-acetic acid derivatives as selective and potent inhibitors of human aldose reductase. Eur J Med Chem. 2018;152:160–174. doi: 10.1016/j.ejmech.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Radha V, Rema M, Mohan V. Genes and diabetic retinopathy. Indian J Ophthalmol. 2002;50:5–11. [PubMed] [Google Scholar]
- 14.Graham A, Brown L, Hedge PJ, Gammack AJ, Markham AF. Structure of the human aldose reductase gene. J Biol Chem. 1991;266:6872–6877. [PubMed] [Google Scholar]
- 15.Li YY, Wang H, Yang XX, Geng HY, Gong G, Lu XZ. AR C-106T gene polymorphism and diabetic nephropathy in the Eastern Asians with T2DM: A meta-analysis including 2120 subjects. Diabetes Res Clin Pract. 2017;130:244–251. doi: 10.1016/j.diabres.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Gupta B, Singh SK. Association of aldose reductase gene polymorphism (C-106T) in susceptibility of diabetic peripheral neuropathy among north Indian population. J Diabetes Complications. 2017;31:1085–1089. doi: 10.1016/j.jdiacomp.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Abhary S, Hewitt AW, Burdon KP, Craig JE. A systematic meta-analysis of genetic association studies for diabetic retinopathy. Diabetes. 2009;58:2137–2147. doi: 10.2337/db09-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olmos P, Bastías MJ, Vollrath V, Toro L, Trincado A, Salinas P, Claro JC, López JM, Acosta AM, Miquel JF, Castro J. C(−106)T polymorphism of the aldose reductase gene and the progression rate of diabetic retinopathy. Diabetes Res Clin Pract. 2006;74:175–182. doi: 10.1016/j.diabres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Zhou M, Zhang P, Xu X, Sun Z. The relationship between aldose reductase C106T polymorphism and diabetic retinopathy: An updated meta-analysis. Invest Ophthalmol Vis Sci. 2015;56:2279–2289. doi: 10.1167/iovs.14-16279. [DOI] [PubMed] [Google Scholar]
- 20.Song ZD, Tao Y, Han N, Wu YZ. Association of the aldose reductase-106TT genotype with increased risk for diabetic retinopathy in the Chinese han population: An updated meta-analysis. Curr Eye Res. 2015;41:1087–1091. doi: 10.3109/02713683.2015.1084642. [DOI] [PubMed] [Google Scholar]
- 21.Ng DP, Conn J, Chung SS, Larkins RG. Aldose reductase (AC)n microsatellite polymorphism and diabetic microvascular complications in Caucasian Type 1 diabtes mellitus. Diabetes Res Clin Pract. 2001;52:21–27. doi: 10.1016/S0168-8227(00)00239-4. [DOI] [PubMed] [Google Scholar]
- 22.dos Santos KG, Canani LH, Gross JL, Tschiedel B, Souto KE, Roisenberg I. The-106CC genotype of the aldose reductase gene is associated with an increased risk of proliferative diabetic retinopathy in Caucasian-Brazilians with type 2 diabetes. Mol Genet Metab. 2006;88:280–284. doi: 10.1016/j.ymgme.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Heesom AE, Hibberd ML, Millward A, Demaine AG. Polymorphism in the 5′-end of the aldose reductase gene is strongly associated with the development of diabetic nephropathy in type I diabetes. Diabetes. 1997;46:287–291. doi: 10.2337/diab.46.2.287. [DOI] [PubMed] [Google Scholar]
- 24.Kao YL, Donaghue K, Chan A, Knight J, Silink M. An aldose reductase intragenic polymorphism associated with diabetic retinopathy. Diabetes Res Clin Pract. 1999;46:155–160. doi: 10.1016/S0168-8227(99)00087-X. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Xiang K, Zheng T. A study of association between polymorphism of aldose reductase gene and diabetic microangiopathy. Chin J Endocrinol Metab. 1999;15:263–266. [Google Scholar]
- 26.Zghal-Mokni I, Arfa I, Elloumi-Zghal H, Abid A, Amrouche-Rached C, Kaabi B, Chakroun S, Blousa-Chabchoub S, Gaïgi S, Ayed S, et al. Association study between diabetic retinopathy and aldose reductase gene polymorphism in Tunisians. J Fr Ophtalmol. 2005;28:386–390. doi: 10.1016/S0181-5512(05)81069-1. (In French) [DOI] [PubMed] [Google Scholar]
- 27.Richeti F, Noronha RM, Waetge RT, de Vasconcellos JP, de Souza OF, Kneipp B, Assis N, Rocha MN, Calliari LE, Longui CA, et al. Evaluation of AC(n) and C(−106)T polymorphisms of the aldose reductase gene in Brazilian patients with DM1 and susceptibility to diabetic retinopathy. Mol Vis. 2007;13:740–745. [PMC free article] [PubMed] [Google Scholar]
- 28.Park HK, Ahn CW, Lee GT, Kim SJ, Song YD, Lim SK, Kim KR, Huh KB, Lee HC. (AC)(n) polymorphism of aldose reductase gene and diabetic microvascular complications in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2002;55:151–157. doi: 10.1016/S0168-8227(01)00299-6. [DOI] [PubMed] [Google Scholar]
- 29.Ko BC, Lam KS, Wat NM, Chung SS. An (A-C)n dinucleotide repeat polymorphic marker at the 5′ end of the aldose reductase gene is associated with early-onset diabetic retinopathy in NIDDM patients. Diabetes. 1995;44:727–732. doi: 10.2337/diabetes.44.7.727. [DOI] [PubMed] [Google Scholar]
- 30.Petrovic MG, Peterlin B, Hawlina M, Petrovic D. Aldose reductase (AC)n gene polymorphism and susceptibility to diabetic retinopathy in type 2 diabetes in Caucasians. J Diabetes Complications. 2005;19:70–73. doi: 10.1016/j.jdiacomp.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J, Green S, editors. The Cochrane Collaboration. 2011. Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated March 2011] [Google Scholar]
- 32.Boccia S. PRISMA: An attempt to improve standards for reporting systematic review and meta-analysis. Epidemiol Biostatist Public Health. 2009;6:E382. [Google Scholar]
- 33.Wells GA, Shea BJ, O'Connell D, Robertson J, Peterson J, Welch V, Losos M, Tugwell P. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Appl Eng Agric. 2014;18:727–734. [Google Scholar]
- 34.Li QJ, Xie P, Zeng WM, Song HP. Correlation between (AC)n polymorphism of the aldose reductase gene and retinopathy in type 2 diabetes mellitus. Chin J Endocrinol Metab. 2001;17:367–368. [Google Scholar]
- 35.Zhang JH, Wang HY, Li SY. Correlation between polymorphism of the aldose reductase gene and retinopathy in type 2 diabetes mellitus. J Shandong Univ. 2008;46:399–406. [Google Scholar]
- 36.Chen S, Liu CS, Wang XJ. The correlation study between the two gene polymorphism of aldose reductase gene 5′end and diabetic retinopathy. China Mod Med. 2013;20:13–15. [Google Scholar]
- 37.Zou X, Lu J. Study on the relationship between the polymorphism of (ac)n in the 5′-end of the ar gene and the susceptibility of microangopathy in type 2 diabetes mellitus. Med J Chin Peoples Liberat Army. 2000;25:353–356. [Google Scholar]
- 38.Wang Y, Ng MC, Lee SC, So WY, Tong PC, Cockram CS, Critchley JA, Chan JC. Phenotypic heterogeneity and associations of two aldose reductase gene polymorphisms with nephropathy and retinopathy in type 2 diabetes. Diabetes Care. 2003;26:2410–2415. doi: 10.2337/diacare.26.8.2410. [DOI] [PubMed] [Google Scholar]
- 39.Demaine A, Cross D, Millward A. Polymorphisms of the aldose reductase gene and susceptibility to retinopathy in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:4064–4068. [PubMed] [Google Scholar]
- 40.Ichikawa F, Yamada K, Ishiyama-Shigemoto S, Yuan X, Nonaka K. Association of an (A-C)n dinucleotide repeat polymorphic marker at the 5′-region of the aldose reductase gene with retinopathy but not with nephropathy or neuropathy in Japanese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:744–748. doi: 10.1046/j.1464-5491.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 41.Ikegishi Y, Tawata M, Aida K, Onaya T. Z-4 allele upstream of the aldose reductase gene is associated with proliferative retinopathy in Japanese patients with NIDDM, and elevated luciferase gene transcription in vitro. Life Sci. 1999;65:2061–2070. doi: 10.1016/S0024-3205(99)00329-X. [DOI] [PubMed] [Google Scholar]
- 42.Kumaramanickavel G, Sripriya S, Ramprasad VL, Upadyay NK, Paul PG, Tarun S. Z-aldose reductase allele and diabetic retinopathy in India. Ophthalmic Genet. 2003;24:41–48. doi: 10.1076/opge.24.1.41.13889. [DOI] [PubMed] [Google Scholar]
- 43.Isermann B, Susanne Schmidt, Bierhaus A, Schiekofer S, Borcea V, Ziegler R, Nawroth PP, Ritz E. (CA)(n) dinucleotide repeat polymorphism at the 5′-end of the aldose reductase gene is not associated with microangiopathy in Caucasians, with long-term diabetes mellitus 1. Nephrol Dial Transplant. 2000;15:918–920. doi: 10.1093/ndt/15.6.918. [DOI] [PubMed] [Google Scholar]
- 44.Kaur N, Vanita V. Association of aldose reductase gene (AKR1B1) polymorphism with diabetic retinopathy. Diabetes Res Clin Pract. 2016;121:41–48. doi: 10.1016/j.diabres.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Petrash JM, Flath M, Sens D, Bylander J. Effects of osmotic stress and hyperglycemia on aldose reductase gene expression in human renal proximal tubule cells. Biochem Biophys Res Commun. 1992;187:201–208. doi: 10.1016/S0006-291X(05)81479-2. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura C, Saito T, Ito T, Omori Y, Tanimoto T. High levels of erythrocyte aldose reductase and diabetic retinopathy in NIDDM patients. Diabetologia. 1994;37:328–330. doi: 10.1007/BF00398062. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, Risch E, Deng Q, Biglia N, Picardo E, Katsaros D, Yu H. An insulin-like growth factor-II intronic variant affects local DNA conformation and ovarian cancer survival. Carcinogenesis. 2013;34:2024–2030. doi: 10.1093/carcin/bgt168. [DOI] [PubMed] [Google Scholar]
- 48.Lu L, Katsaros D, Mayne ST, Risch HA, Benedetto C, Canuto EM, Yu H. Functional study of risk loci of stem cell-associated gene lin-28B and associations with disease survival outcomes in epithelial ovarian cance. Carcinogenesis. 2012;33:2119–2125. doi: 10.1093/carcin/bgs243. [DOI] [PubMed] [Google Scholar]
- 49.Shah VO, Scavini M, Nikolic J, Sun Y, Vai S, Griffith JK, Dorin RI, Stidley C, Yacoub M, Vander Jagt DL, et al. Z-2 microsatellite allele is linked to increased expression of the aldose reductase gene in diabetic nephropathy. J Clin Endocrinol Metab. 1998;83:2886–2891. doi: 10.1210/jcem.83.8.5028. [DOI] [PubMed] [Google Scholar]
- 50.Donaghue KC, Margan SH, Chan AK, Holloway B, Silink M, Rangel T, Bennetts B. The association of aldose reductase gene (AKR1B1) polymorphisms with diabetic neuropathy in adolescents. Diabet Med. 2005;22:1315–1320. doi: 10.1111/j.1464-5491.2005.01631.x. [DOI] [PubMed] [Google Scholar]
- 51.Liew G, Klein R, Wong TY. The role of genetics in susceptibility to diabetic retinopathy. Int Ophthalmol Clin. 2009;49:35–52. doi: 10.1097/IIO.0b013e31819fd5d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho H, Sobrin L. Genetics of diabetic retinopathy. Curr Diab Rep. 2014;14:515. doi: 10.1007/s11892-014-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ju Z, Daichao W, Yan L, Tan SJ. Association of luteinizing hormone/choriogonadotropin receptor gene polymorphisms with polycystic ovary syndrome risk: A meta-analysis. Gynecol Endocrinol. 2019;35:81–85. doi: 10.1080/09513590.2018.1498834. [DOI] [PubMed] [Google Scholar]
- 54.Roy MS, Klein R, O'Colmain BJ, Klein BE, Moss SE, Kempen JH. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol. 2004;122:546–551. doi: 10.1001/archopht.122.4.546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.