Abstract
Environmental enrichment is known to be beneficial for cognitive improvement. In many animal models of neurological disorders and brain injury, EE has also demonstrated neuroprotective benefits in neurodegenerative diseases and in improving recovery after stroke or traumatic brain injury. The exact underlying mechanism for these phenomena has been unclear. Recent findings have now indicated that neuronal activity elicited by environmental enrichment induces Ca2+ influx in dorsal root ganglion neurons results in lasting enhancement of CREB-binding protein-mediated histone acetylation. This, in turn, increases the expression of pro-regeneration genes and promotes axonal regeneration. This mechanism associated with neuronal activity elicited by environmental enrichment-mediated pathway is one of several epigenetic mechanisms which modulate axon regeneration upon injury that has recently come to light. The other prominent mechanisms, albeit not yet directly associated with environmental enrichment, include DNA methylation/demethylation and N6-methyladenosine modification of transcripts. In this brief review, I highlight recent work that has shed light on the epigenetic basis of environmental enrichment-based axon regeneration, and discuss the mechanism and pathways involved. I further speculate on the implications of the findings, in conjunction with the other epigenetic mechanisms, that could be harness to promote axon regeneration upon injury.
Keywords: axon regeneration, CREB-binding protein, DNA methylation/demethylation, dorsal root ganglion, DRG neurons, environmental enrichment, epigenetics, histone acetylation, mechanistic target of rapamycin, mTOR, phosphatase and tensin homologue, PTEN
Introduction
Adult neurons, particularly those in the central nervous system (CNS), exhibit dismal levels of axon regeneration upon injury. Effective axon regeneration is blocked by both external inhibitory factors in the environment (Geoffroy and Zheng, 2014) and limited by neuronal intrinsic factors (He and Jin, 2016; Mahar and Cavalli, 2018), such as a robust response to injury with regards to new protein synthesis. In terms of cellular mechanisms that regulate axonal regeneration upon injury, a well-known signaling axis that underlie successful regeneration is that of the mechanistic target of rapamycin (mTOR) and phosphatase and tensin homologue (PTEN) (Park et al., 2010). mTOR activates the pro-survival phosphoinositide 3 kinase-AKT kinase pathway and enhances somatic and axonal protein synthesis, while PTEN antagonizes mTOR signaling. The latter is thus a key intrinsic inhibitor of axon regeneration, and PTEN deletion or silencing is invariably favorable for regeneration (Park et al., 2008; Christie et al., 2010; Liu et al., 2010; Zukor et al., 2013). It is well known that peripheral neurons are superior to CNS neurons in terms of axon regeneration, and a popular model to study intrinsic factors governing axon regeneration is the dorsal root ganglion (DRG) neurons (Nascimento et al., 2018). These pseudo-unipolar neurons have a stem axon with a peripheral and a central branch. Interestingly, the difficulty of axon regeneration by the central branch could be overcome by conditional injuries to the peripheral branch (Qiu et al., 2002), presumably via alteration of intrinsic regenerative capacity of the entire neuron.
Environmental enrichment (EE) is an experimental paradigm in which experimental animals are exposed to stimulatory physical and social surroundings. EE exposure has been shown, in general, to promote neuronal morphogenesis, synaptogenesis and increasing neuronal activity (Alwis and Rajan, 2014; Hannan, 2014). EE exposure has also been shown to be beneficial to a range of neurological disorder models. These include animal models of neurodegenerative diseases such as Alzheimer’s disease (Verret et al., 2013; Griñán-Ferré et al., 2018), Parkinson’s disease (Goldberg et al., 2012), amyotrophic lateral sclerosis (Stam et al., 2008) and Huntington’s disease (Spires et al., 2004), neurodevelopmental disorders such as Rett Syndrome (Kondo et al., 2008), as well as those of traumatic (de la Tremblaye et al., 2019) or ischemic (Gonçalves et al., 2018) brain injuries.
The mechanism underlying the neuronal protective and beneficial effects of EE has not been particularly clear, but could involve the production of pro-regenerative neurotrophins or cytokines (Ickes et al., 2000; Rossi et al., 2006; Zhang et al., 2016). Interestingly, epigenetic mechanisms involving histone acetylation has been shown to underlie the increase in the pro-survival and regenerative neurotrophin brain-derived growth factor (BDNF) in the hippocampus of aged rats (Neidl et al., 2016). Furthermore, enhanced synaptic plasticity after EE in adult male mice could be transgenerationally inherited via miRNAs (Benito et al., 2018). Pertaining to functional recovery after injury, EE-based factors likely act by promoting survival as well as the intrinsic regenerative capacity of adult neurons, including the capacity to regenerate severed or damaged axons. Recent findings made in DRG neurons have indeed implicate epigenetic changes in the form of enhanced histone acetylation in the damaged neuron as a major underlying driving processes for regeneration resulting from EE exposure. Here, I provide a short review of these findings, as well as other epigenetic mechanisms modulating axon regeneration that has come to light in the past 2–3 years. This narrative is informed by Medline database searches with the key word combinations of “environmental enrichment”, “epigenetics” and “axon regeneration”.
An Underlying Epigenetic Mechanism for Environmental Enrichment-Mediated Enhancement in Axon Regeneration of Proprioceptive Dorsal Root Ganglion Neurons
Hutson et al. (2019) have recently investigated the neuronal activity enhancement basis of EE on DRG neurons. The authors first observed that neurite outgrowth from DRG neurons from mouse placed in EE housing is significantly enhanced compared to those placed under standard housing. This enhancement was comparable to that induced by a conditioning injury, was gene transcription dependent, and was long lasting. Importantly, this enhancing effect of EE is larger than that induced by physical exercise alone. EE housing not only enhanced sciatic nerve axon regeneration, but also regeneration of sensory axons in a spinal cord injury (SCI) model, as traced by a retrograde marker. These regenerating sensory axons conferred a larger amplitude of compound action potentials recorded above the lesion site, which was selectively abolished by a chemo-genetics approach (using the Designer Receptors Exclusively Activated by Designer Drugs (DREADD) technology) targeting DRG neurons. The authors further noted that DRG neurons with axons regenerated across injury sites are positive for the proprioceptive neuron marker parvalbumin (PV), and that EE specifically enhanced neurite outgrowth from DRG neurons labeled by a PV promoter-driven fluorescent marker. Interestingly, EE-dependent (but not injury conditioning-dependent) DRG neurite outgrowth is largely abolished in Egr3–/– mice, which are defective in muscle spindle proprioceptive feedback but not PV-positive neuron population. These findings indicate that EE rather specifically enhances proprioceptive DRG axon regeneration, and that this enhancement is dependent on proprioceptive afferent feedback.
How did EE promote axonal regeneration? Interestingly, the authors observed no significant changes in either the DRG neurons or in the circulation in terms of neurotrophin and cytokine levels. RNAseq and proteomics analysis of whole DRGs (of mixed DRG neuronal cell types) or laser-captured DRG neurons with large diameter (which would include the proprioceptor and mechanoreceptor neuron populations) showed marked changes in the latter. In particular, EE induced an upregulation of genes and proteins associated with molecular pathways that are associated with regeneration, including those that regulate neuronal activity, calcium signaling, energy metabolism, neuronal projection and cytoskeleton dynamics. These changes in transcriptional and protein expression profiles are in line with the notion of an EE-induced pro-regenerative gene expression profile and activity in the DRG neuron subtypes examined. Indeed, Gi-coupled DREADD-mediated inhibition of adenylate cyclase and silencing of DRG neuronal activity attenuated axon regeneration of EE-exposed mice. Conversely, neuronal activity enhancement by Gq-coupled DREADD (which elicits inositol 1,4,5-trisphosphate-mediated Ca2+ release from intracellular stores) in mice on standard housing enhanced their DRG neurite outgrowth to a degree similar to those observed for the EE-exposed mice. Furthermore, the authors directly confirmed that EE exposure increases potassium stimulated Ca2+ release in the proprioceptive DRGs of transgenic mice carrying a calcium indicator GCaMP under the PV promoter (Hutson et al., 2019).
How did EE-induced neuronal activity elicit a global change in the proprioceptive DRG gene expression profile so as to enhance axon regeneration? The authors examined histone epigenetic marks and found that EE enhanced the acetylation of H3K27 and H4K8 in PV-positive DRG neurons. In line with the RNAseq finding of transcriptional upregulation, H4K8ac and H3K27ac are indeed markers of transcriptional activation. These histones could be acetylated by the CREB-binding protein (CBP), which harbors Ca2+-sensitive transactivation domains and is known to play important roles in activity-dependent neuroplasticity. In this regard, CBP activity is known to be controlled by Ca2+ and the calcium/calmodulin-dependent (CaM) protein kinases II and IV (CaMKII/IV) (Chawla et al., 1998; Hu et al., 1999). CBP has also been previously shown to be necessary for EE-induced neurogenesis and cognitive enhancement (Lopez-Atalaya et al., 2011). Indeed, the current work shows that EE exposure increased CBP phosphorylation and acetylated (active) CBP levels in PV-positive DRG neurons. Levels of acetylated H4K8, like the enhancement of neurite outgrowth capacity, persisted in PV-positive DRG neurons for a long time, even 5 weeks after EE exposure. Both the levels of acetylated H4K8 and acetylated CBP were reciprocally enhanced or inhibited by the DREADD-mediated manipulations of neuronal activity, thus linking calcium-dependent neuronal activity to both CREB activation and histone acetylation. Importantly, in mice with CBP conditionally knocked out in Ca2+-calmodulin-dependent protein kinase IIa (CaMKIIa)-positive neurons (including DRGs), loss of CBP abolished EE-exposure induced increase in neurite outgrowth. CBP-based acetylation is thus critical for mediating the persistent enhancement of neurite outgrowth and axon regeneration phenotype associated with neuronal activity resulting from EE exposure.
Could enhancement of CBP activity in the CNS therefore promote axon regeneration after injury? The authors demonstrated this point (Hutson et al., 2019) with a small molecule activator of CBP (TTK21) conjugated to glucose-derived carbon nanospheres (CSP) (Chatterjee et al., 2013), with the latter allowing effective delivery across the blood-brain barrier. CSP-TTK21 increased neurite outgrowth of DRG neurons in culture and promoted axonal regeneration as well as sensorimotor function in vivo after mid-thoracic dorsal hemisection in a mouse SCI model. In another SCI model of mid-thoracic spinal cord contusion in rats, CSP-TTK21’s enhancement of functional recovery appeared to correlate with the enhanced sprouting of both descending motor and ascending sensory axons, as well as an increase in the density of vGlut1-positive synaptic boutons from proprioceptive neurons found at the proximity of motor neurons. These findings, taken together, further supports the role of CBP activity in axon regeneration and attests to the translational value of this notion (Figure 1).
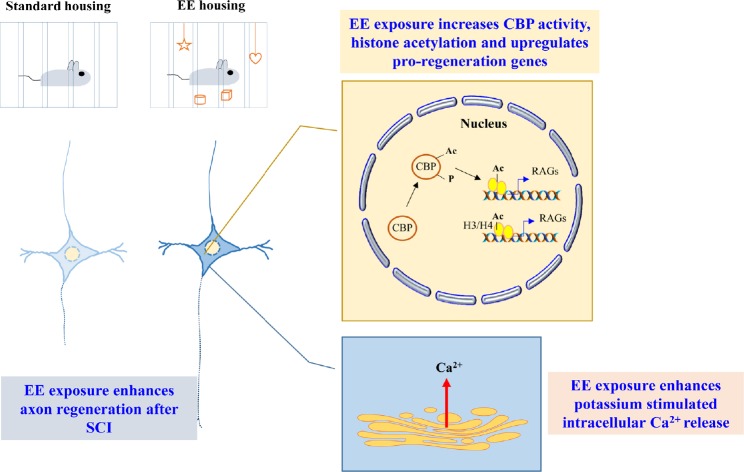
Figure 1.
Schematic diagram summarizing the findings of environmental enrichment (EE)-mediated enhancement in axon regeneration of proprioceptive dorsal root ganglion (DRG) neurons.
EE exposure enhanced axon regeneration through EE-elicited neuronal activity resulting in long lasting epigenetic markings in terms of CREB binding protein (CBP)-mediated histone acetylation, which promotes the expression of regeneration associated genes (RAGs). Among the latter are those that promote the ease of Ca2+ release with stimuli. Ac: Acetyl group; H3: histone protein 3; H4: histone protein 4; P: phosphorylation; SCI: spinal cord injury.
Epigenetics-Based Regulation of Axon Regeneration – Important Recent Findings
The findings of Hutson et al. (2019) illustrated an epigenetic mechanism underlying axon regeneration enhancement. In this regard, these findings add to other recent advances in our understanding of modulation of axon regeneration by several epigenetic mechanisms (Weng et al., 2016). There are major recent advances in our understanding of the role of DNA methylation in axon regeneration (Loh et al., 2017; Weng et al., 2017; Oh et al., 2018). Axotomy of mouse DRG neurons elevated the levels of the methylcytosine dioxygenase Ten-eleven translocation 3 (Tet3) (Loh et al., 2017; Weng et al., 2017). Tet3 mediates active DNA demethylation by catalyzing the conversion of 5-methylcytosine to 5-hydroxymethylcytosine and iteratively oxidizing it to 5-formylcytosine and 5-carboxycytosine. Genome-wide 5-hydroxymethylcytosine mapping showed that injury leads to distinct changes associated with regeneration-associated genes (RAGs), including the well-known ones such as Atf3, Bdnf, and Smad1 (Loh et al., 2017). Tet3 silencing significantly reduced the axonal outgrowth capacity of DRG neurons, and methylation at CpG dinucleotides was significantly reduced in the gene body and enhancer regions of Atf3 following injury (Weng et al., 2017). Thymine DNA glycosylase (Tdg) acts downstream of Tet3 by removing 5-formylcytosine and 5-carboxycytosine, thereby initiating base-excision repair to generate an unmodified cytosine. Weng et al. (2017) found that silencing or conditional knockout of thymine DNA glycosylase also attenuated axon regeneration and reduced RAGs expression. Interestingly, PTEN deletion-induced axon regeneration of mouse RGCs could be attenuated by the silencing of Tet1 instead of Tet3 (Weng et al., 2017).
On the other hand, another report by Oh et al. (2018) found that DNA methylation contributes to robust axon regeneration in the context of specific gene silencing. These authors showed that sciatic nerve injury of DRG neurons reduced the levels of the microRNA miR-9 (Jiang et al., 2017), which in turn resulted in the upregulation of the latter’s targets, including ubiquitin-like containing PHD ring finger 1 (UHRF1) and the RE1 silencing transcription factor (REST/NRSF), a master transcriptional regulator of neuron-specific genes (Hwang and Zukin, 2018). The DNA methytransferase inhibitor RG108 reduced the length of axons regenerating though the lesion site. Contrastingly, UHRF1 promoted axon regeneration of DRG neurons. UHRF1 silences gene expression by interacting with dimethylated and trimethylated H3K9, and by recruiting DNA methytransferase1 and DNA methytransferase3a to promote DNA methylation of gene promoters, which include that of PTEN. REST also appears to be important for axon regeneration as its inhibition or silencing both impaired the process in DRG (Oh et al., 2018). Presumably, REST’s suppression of neuron-specific genes promoted regeneration by a temporary loss of the terminal differentiation expression profile of adult neurons. However, the role of REST in this regard may be complex as it is itself transcriptionally repressed by UHRF1.
Epigenetic regulatory mechanisms are of course not limited to histone protein acetylation and DNA demethylation. A complex network of miRNAs regulates axonal growth and regeneration (Yoo, 2017), and the roles of a number of different miRNAs in axon regeneration have been extensively described in multiple species (Song et al., 2012; Liu et al., 2013; Gaudet et al., 2016; van Battum et al., 2018; Wang et al., 2018). More recently, Wenk et al. (2018) showed that sciatic nerve injury also elevates N6-methyladenosine (m6A)-tagged mRNA or transcripts of RAGs and those that encode components of the protein translation machinery in DRG neurons. m6A modification of mRNAs is mediated by a methyltransferase complex consisting of methyltransferase like 3 (Mettl3) and Mettl4 (Wang et al., 2016). Conditional knockout of Mettl14 or knockout of the m6A reader YTH domain-containing family protein 1 attenuated injury-induced translation of proteins in DRGs, reducing axon regeneration as well as associated functional recovery. PTEN deletion-induced axon regeneration of RGC neurons is likewise attenuated by Mettl14 silencing (Weng et al., 2018).
Environmental Enrichment-Elicited CREB-Binding Protein-Mediated Acetylation - Signaling Pathways and Connections
As EE has been previously shown to induce the expression of neurotrophins such as BDNF (Ickes et al., 2000; Rossi et al., 2006; Zhang et al., 2016), a lack of clear elevation of these factors in the Hutson et al (2019) study is mildly surprising. This is particularly so when the mechanism deciphered, CBP activation, is also known to induce BDNF expression (Chatterjee et al., 2013; Palomer et al., 2016). In particular, the use of CSP-TTK21 (Chatterjee et al., 2013) is likely to elevate BDNF expression in the SCI models, and this point would therefore need further confirmation.
CBP is a signal mediated transcription coactivator (Chawla et al., 1998) which has been previously shown to be necessary for EE-induced neurogenesis and cognitive enhancement (Lopez-Atalaya et al., 2011). If CBP activation resulting from neuronal activity elicited Ca2+ influx underlies the pro-regenerative phenotype, how is CBP activated by Ca2+? Although not specifically explored by the authors, fundamental mechamisms of neuronal calcium signaling are likely involved. Synaptic calcium influx of from excitatory neural transmission, for example activates synaptic CaMKII (Penny and Gold, 2018). CBP activity is known to be controlled by cAMP, CaMKII and CaMKIV (Hu et al., 1999). CaMKIV could shuttle from the cytoplasm to the nucleus and is a major nuclear CaM kinase (Lemrow et al., 2004). In the context of a pituitary cell line AtT20 (Chawla et al., 1998), nuclear calcium and CaMKIV are the major regulators of CBP/CREB-based transcription. Whether this is the case for DRG neurons remains to be ascertained.
Is the epigenetic mechanism of CBP activation in anyway related to the PTEN-mTOR pathway? Although the work of Hutson et al. emphasized on the role of CBP in histone acetylation, thus affecting axon regeneration via changes in gene expression, it should also be borne in mind that CBP also acetylates PTEN (Ikenoue et al., 2008), and PTEN acetylation is known to attenuate its activity (Okumura et al., 2006) and function (Ikenoue et al., 2008; Tang, 2019). Given that PTEN is a prominent inhibitor of axon regeneration in both PNS and CNS neurons (Park et al., 2010), CBP elevated during injury could conceivably also act through PTEN in terms of promoting DRG axon regeneration. On the other hand, the activity of mTOR, or one of its functional protein complex mTORC1, is essential for axon regeneration, and enhanced mTORC1 activity promotes the latter process (Miao et al., 2016; Carlin et al., 2019). In this regard, EE is known to improve learning and memory in young adult rats (Hullinger et al., 2015) as well as protect against photoreceptor neuron death in Retinitis Pigmentosa (Barone et al., 2012) through mTORC1 signaling. mTORC1 is conventionally consider to function in the cytoplasmic context on organellar membranes, but its non-canonical activity in the nucleus is gaining prominence (Audet-Walsh et al., 2017; Giguère, 2018). Interestingly, CBP has been recently shown to be a substrate of mTORC1, and the latter directly activates CBP by phosphorylation of several Ser residues at its C-terminal domain (Wan et al., 2017). The CBP-based epigenetic enhancement of axon regeneration via upregulation of pro-regeneration genes could therefore cross-talks extensively with the PTEN-mTOR pathway.
Some Caveats and Reservations
The recent findings on how epigenetic mechanisms modulate axon regeneration discussed above provided fresh insights and perspectives on the neuronal intrinsic aspects of axon regeneration upon injury. However, to fully comprehend the implications of some of these findings would require underlying mechanistic details to be better deciphered. Furthermore, some of the findings also appear, at least superficially, to contradict each other. Several caveats and unresolved issues remain. Firstly, whether DNA methylation is pro- or against axon regeneration appears unsettled, despite the use of similar models. The inferences drawn in different reports at the moment are therefore likely to be context-dependent. Secondly, the role of REST in axon regeneration appears to be complex, and both its levels and its impact on regenerative transcription profile may vary temporarily upon injury. Finally, mechanisms such as CBP-mediated histone acetylation is likely more prominent in some neuronal cell types (such as proprioceptive DRGs) than others. Taken together, the recent findings of epigenetic regulation of axon regeneration should be interpreted with some caution, and should prompt future work. Importantly, despite the above uncertainties, all these findings could have immense translation potential.
Could Environmental Enrichment-Mediated Enhancement in Axon Regeneration be Clinically Useful?
It is a conceivable future prospect that a cocktail of drugs eliciting multiple epigenetic pathways or mechanisms could be used to promote regeneration of injured axons in clinical settings. In terms of EE, clinical implementation has been discussed pertaining to enhancement of recovery from neuronal injuries, such as stroke (McDonald et al., 2018). A number of clinical trials with reasonably positive outcomes have also been reported (Janssen et al., 2014; Khan et al., 2016). However, a lack of qualitative understanding and quantitative gauge of which aspects of EE would be most useful or effective as well as the lack of a clear mechanistic understanding of how EE works, have not helped in its implementation in clinical settings beyond that of general neuro-habilitation. Now, along with the deciphering of the EE elicited, CBP-based histone acetylation mechanism and the somewhat promising efficacy of CSP-TTK21 (Chatterjee et al., 2013) as demonstrated in animal neuronal injury models (Hutson et al., 2019), the time may have come for further human subject-based investigations into EE’s use in neuronal injury recuperation settings, and for the development of EE mimetic drugs (Hannan, 2014).
Additional file: Open peer review report 1 (80.8KB, pdf) .
Footnotes
Conflicts of interest: The author declare no conflicts of interest.
Financial support: This work was supported by the National University of Singapore Graduate School for Integrative Sciences and Engineering (to BLT).
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Melissa Renee Andrews,University of Southampton, UK.
Funding: This work was supported by the National University of Singapore Graduate School for Integrative Sciences and Engineering (to BLT).
P-Reviewer: Andrews MR; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Alwis DS, Rajan R. Environmental enrichment and the sensory brain: the role of enrichment in remediating brain injury. Front Syst Neurosci. 2014;8:156. doi: 10.3389/fnsys.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Audet-Walsh É, Dufour CR, Yee T, Zouanat FZ, Yan M, Kalloghlian G, Vernier M, Caron M, Bourque G, Scarlata E, Hamel L, Brimo F, Aprikian AG, Lapointe J, Chevalier S, Giguère V. Nuclear mTOR acts as a transcriptional integrator of the androgen signaling pathway in prostate cancer. Genes Dev. 2017;31:1228–1242. doi: 10.1101/gad.299958.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barone I, Novelli E, Piano I, Gargini C, Strettoi E. Environmental enrichment extends photoreceptor survival and visual function in a mouse model of retinitis pigmentosa. PLoS One. 2012;7:e50726. doi: 10.1371/journal.pone.0050726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benito E, Kerimoglu C, Ramachandran B, Pena-Centeno T, Jain G, Stilling RM, Islam MR, Capece V, Zhou Q, Edbauer D, Dean C, Fischer A. RNA-dependent intergenerational inheritance of enhanced synaptic plasticity after environmental enrichment. Cell Rep. 2018;23:546–554. doi: 10.1016/j.celrep.2018.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlin D, Halevi AE, Ewan E, Moore AM, Cavalli V. Nociceptor deletion of Tsc2 enhances axon regeneration by inducing a conditioning injury response in dorsal root ganglia. eNeuro. 2019 doi: 10.1523/ENEURO.0168-19.2019. doi: 10.1523/ENEURO.0168-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, Cassel JC, Eswaramoorthy M, Kundu TK, Boutillier AL. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neurosci. 2013;33:10698–10712. doi: 10.1523/JNEUROSCI.5772-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- 8.Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci. 2010;30:9306–9315. doi: 10.1523/JNEUROSCI.6271-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Tremblaye PB, Cheng JP, Bondi CO, Kline AE. Environmental enrichment, alone or in combination with various pharmacotherapies, confers marked benefits after traumatic brain injury. Neuropharmacology. 2019;145:13–24. doi: 10.1016/j.neuropharm.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Gaudet AD, Mandrekar-Colucci S, Hall JCE, Sweet DR, Schmitt PJ, Xu X, Guan Z, Mo X, Guerau-de-Arellano M, Popovich PG. miR-155 deletion in mice overcomes neuron-intrinsic and neuron-extrinsic barriers to spinal cord repair. J Neurosci. 2016;36:8516–8532. doi: 10.1523/JNEUROSCI.0735-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geoffroy CG, Zheng B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol. 2014;27:31–38. doi: 10.1016/j.conb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giguère V. Canonical signaling and nuclear activity of mTOR-a teamwork effort to regulate metabolism and cell growth. FEBS J. 2018;285:1572–1588. doi: 10.1111/febs.14384. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg NRS, Fields V, Pflibsen L, Salvatore MF, Meshul CK. Social enrichment attenuates nigrostriatal lesioning and reverses motor impairment in a progressive 1-methyl-2-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol Dis. 2012;45:1051–1067. doi: 10.1016/j.nbd.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Gonçalves LV, Herlinger AL, Ferreira TAA, Coitinho JB, Pires RGW, Martins-Silva C. Environmental enrichment cognitive neuroprotection in an experimental model of cerebral ischemia: biochemical and molecular aspects. Behav Brain Res. 2018;348:171–183. doi: 10.1016/j.bbr.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Griñán-Ferré C, Izquierdo V, Otero E, Puigoriol-Illamola D, Corpas R, Sanfeliu C, Ortuño-Sahagún D, Pallàs M. Environmental enrichment improves cognitive deficits, AD hallmarks and epigenetic alterations presented in 5xFAD mouse model. Front Cell Neurosci. 2018;12:224. doi: 10.3389/fncel.2018.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannan AJ. Environmental enrichment and brain repair: harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol Appl Neurobiol. 2014;40:13–25. doi: 10.1111/nan.12102. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Jin Y. Intrinsic control of axon regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Hu SC, Chrivia J, Ghosh A. Regulation of CBP-mediated transcription by neuronal calcium signaling. Neuron. 1999;22:799–808. doi: 10.1016/s0896-6273(00)80738-2. [DOI] [PubMed] [Google Scholar]
- 19.Hullinger R, O’Riordan K, Burger C. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem. 2015;125:126–134. doi: 10.1016/j.nlm.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutson TH, Kathe C, Palmisano, Bartholdi K, Hervera A, De Virgiliis F, McLachlan E, Zhou L, Kong G, Barraud Q, Danzi MC, Medrano-Fernandez A, Lopez-Atalaya JP, Boutillier AL, Sinha SH, Singh AK, Chaturbedy P, Moon LDF, Kundu TK8, Bixby JL, Lemmon VP, et al. Cbp-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aaw2064. doi: 10.1126/scitranslmed.aaw2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang JY, Zukin RS. REST, a master transcriptional regulator in neurodegenerative disease. Curr Opin Neurobiol. 2018;48:193–200. doi: 10.1016/j.conb.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- 23.Ikenoue T, Inoki K, Zhao B, Guan KL. PTEN acetylation modulates its interaction with PDZ domain. Cancer Res. 2008;68:6908–6912. doi: 10.1158/0008-5472.CAN-08-1107. [DOI] [PubMed] [Google Scholar]
- 24.Janssen H, Ada L, Bernhardt J, McElduff P, Pollack M, Nilsson M, Spratt NJ. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: a pilot non-randomized controlled trial. Disabil Rehabil. 2014;36:255–262. doi: 10.3109/09638288.2013.788218. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Hu Y, Zhang B, Shi Y, Zhang J, Wu X, Yao P. MicroRNA-9 regulates mammalian axon regeneration in peripheral nerve injury. Mol Pain. 2017;13:1744806917711612. doi: 10.1177/1744806917711612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan F, Amatya B, Elmalik A, Lowe M, Ng L, Reid I, Galea MP. An enriched environmental programme during inpatient neuro-rehabilitation: A randomized controlled trial. J Rehabil Med. 2016;48:417–425. doi: 10.2340/16501977-2081. [DOI] [PubMed] [Google Scholar]
- 27.Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PPL, Hannan AJ. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome--Mecp2 gene dosage effects and BDNF expression. Eur J Neurosci. 2008;27:3342–3350. doi: 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- 28.Lemrow SM, Anderson KA, Joseph JD, Ribar TJ, Noeldner PK, Means AR. Catalytic activity is required for calcium/calmodulin-dependent protein kinase IV to enter the nucleus. J Biol Chem. 2004;279:11664–11671. doi: 10.1074/jbc.M312613200. [DOI] [PubMed] [Google Scholar]
- 29.Liu CM, Wang RY, Saijilafu, Jiao ZX, Zhang BY, Zhou FQ. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev. 2013;27:1473–1483. doi: 10.1101/gad.209619.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh YHE, Koemeter-Cox A, Finelli MJ, Shen L, Friedel RH, Zou H. Comprehensive mapping of 5-hydroxymethylcytosine epigenetic dynamics in axon regeneration. Epigenetics. 2017;12:77–92. doi: 10.1080/15592294.2016.1264560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Atalaya JP, Ciccarelli A, Viosca J, Valor LM, Jimenez-Minchan M, Canals S, Giustetto M, Barco A. CBP is required for environmental enrichment-induced neurogenesis and cognitive enhancement. EMBO J. 2011;30:4287–4298. doi: 10.1038/emboj.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19:323–337. doi: 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald MW, Hayward KS, Rosbergen ICM, Jeffers MS, Corbett D. Is environmental enrichment ready for clinical application in human post-stroke rehabilitation? Front Behav Neurosci. 2018;12:135. doi: 10.3389/fnbeh.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao L, Yang L, Huang H, Liang F, Ling C, Hu Y. mTORC1 is necessary but mTORC2 and GSK3β are inhibitory for AKT3-induced axon regeneration in the central nervous system. Elife. 2016;5:e14908. doi: 10.7554/eLife.14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nascimento AI, Mar FM, Sousa MM. The intriguing nature of dorsal root ganglion neurons: Linking structure with polarity and function. Prog Neurobiol. 2018;168:86–103. doi: 10.1016/j.pneurobio.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Neidl R, Schneider A, Bousiges O, Majchrzak M, Barbelivien A, de Vasconcelos AP, Dorgans K, Doussau F, Loeffler JP, Cassel JC, Boutillier AL. Late-life environmental enrichment induces acetylation events and Nuclear Factor κB-dependent regulations in the hippocampus of aged rats showing improved plasticity and learning. J Neurosci. 2016;36:4351–4361. doi: 10.1523/JNEUROSCI.3239-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh YM, Mahar M, Ewan EE, Leahy KM, Zhao G, Cavalli V. Epigenetic regulator UHRF1 inactivates REST and growth suppressor gene expression via DNA methylation to promote axon regeneration. Proc Natl Acad Sci USA. 2018;115:E12417–12426. doi: 10.1073/pnas.1812518115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okumura K, Mendoza M, Bachoo RM, DePinho RA, Cavenee WK, Furnari FB. PCAF modulates PTEN activity. J Biol Chem. 2006;281:26562–26568. doi: 10.1074/jbc.M605391200. [DOI] [PubMed] [Google Scholar]
- 40.Palomer E, Carretero J, Benvegnù S, Dotti CG, Martin MG. Neuronal activity controls Bdnf expression via Polycomb derepression and CREB/CBP/JMJD3 activation in mature neurons. Nat Commun. 2016;7:11081. doi: 10.1038/ncomms11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 42.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penny CJ, Gold MG. Mechanisms for localising calcineurin and CaMKII in dendritic spines. Cell Signal. 2018;49:46–58. doi: 10.1016/j.cellsig.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 45.Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 46.Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan LY, Jan YN. Regeneration of drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 2012;26:1612–1625. doi: 10.1101/gad.193243.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spires TL, Grote HE, Varshney NK, Cordery PM, van Dellen A, Blakemore C, Hannan AJ. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stam NC, Nithianantharajah J, Howard ML, Atkin JD, Cheema SS, Hannan AJ. Sex-specific behavioural effects of environmental enrichment in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2008;28:717–723. doi: 10.1111/j.1460-9568.2008.06374.x. [DOI] [PubMed] [Google Scholar]
- 49.Tang BL. Why is NMNAT Protective against neuronal cell death and axon degeneration, but inhibitory of axon regeneration? Cells. 2019 doi: 10.3390/cells8030267. doi: 10.3390/cells8030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Battum EY, Verhagen MG, Vangoor VR, Fujita Y, Derijck AAHA, O’Duibhir E, Giuliani G, de Gunst T, Adolfs Y, Lelieveld D, Egan D, Schaapveld RQJ, Yamashita T, Pasterkamp RJ. An image-based miRNA screen identifies miRNA-135s as regulators of CNS axon growth and regeneration by targeting Krüppel-like factor 4. J Neurosci. 2018;38:613–630. doi: 10.1523/JNEUROSCI.0662-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verret L, Krezymon A, Halley H, Trouche S, Zerwas M, Lazouret M, Lassalle JM, Rampon C. Transient enriched housing before amyloidosis onset sustains cognitive improvement in Tg2576 mice. Neurobiol Aging. 2013;34:211–225. doi: 10.1016/j.neurobiolaging.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Wan W, You Z, Xu Y, Zhou L, Guan Z, Peng C, Wong CCL, Su H, Zhou T, Xia H, Liu W. mTORC1 phosphorylates acetyltransferase p300 to regulate autophagy and lipogenesis. Mol Cell. 2017;68:323–335. doi: 10.1016/j.molcel.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang XW, Li Q, Liu CM, Hall PA, Jiang JJ, Katchis CD, Kang S, Dong BC, Li S, Zhou FQ. Lin28 signaling supports mammalian PNS and CNS axon regeneration. Cell Rep. 2018;24:2540–2552.e6. doi: 10.1016/j.celrep.2018.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weng YL, An R, Cassin J, Joseph J, Mi R, Wang C, Zhong C, Jin SG, Pfeifer GP, Bellacosa A, Dong X, Hoke A, He Z, Song H, Ming GL. An intrinsic epigenetic barrier for functional axon regeneration. Neuron. 2017;94:337–346.e6. doi: 10.1016/j.neuron.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weng YL, Joseph J, An R, Song H, Ming GL. Epigenetic regulation of axonal regenerative capacity. Epigenomics. 2016;8:1429–1442. doi: 10.2217/epi-2016-0058. [DOI] [PubMed] [Google Scholar]
- 57.Weng YL, Wang X, An R, Cassin J, Vissers C, Liu Y, Liu Y, Xu T, Wang X, Wong SZH, Joseph J, Dore LC, Dong Q, Zheng W, Jin P, Wu H, Shen B, Zhuang X, He C, Liu K, et al. Epitranscriptomic m6A regulation of axon regeneration in the adult mammalian nervous system. Neuron. 2018;97:313–325.e6. doi: 10.1016/j.neuron.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoo S. Micro in size but not in function-microRNAs in axonal survival and regeneration. FEBS Lett. 2017;591:2089–2090. doi: 10.1002/1873-3468.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang XQ, Mu JW, Wang HB, Jolkkonen J, Liu TT, Xiao T, Zhao M, Zhang CD, Zhao CS. Increased protein expression levels of pCREB, BDNF and SDF-1/CXCR4 in the hippocampus may be associated with enhanced neurogenesis induced by environmental enrichment. Mol Med Rep. 2016;14:2231–2237. doi: 10.3892/mmr.2016.5470. [DOI] [PubMed] [Google Scholar]
- 60.Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci. 2013;33:15350–15361. doi: 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.