Multiple sclerosis (MS): MS is a neurodegenerative disease affecting around 2.5 million people worldwide, representing the second cause of disabilities in the young adult population. MS is a demyelinating pathology which originates in the autoimmune attack of T and B lymphocytes against myelin. This lack of myelin leads, in turn, to axonal degeneration, neuronal death and the consequent neurological disabilities (Franklin and Ffrench-Constant, 2017). A main hallmark of MS is a preserved local neuroinflammatory environment. It is now acknowledged that this persistent inflammatory scenario is a central and common condition in almost all neurodegenerative pathologies (as in Parkinson’s and Alzheimer’s diseases, among others) controlling and modulating the regulatory responses of the system to the triggering insult. In the case of MS, this original insult corresponds to the loss of myelin (Chitnis and Weiner, 2017; Franklin and Ffrench-Constant, 2017). Of particular interest for the understanding of MS progression, is how and when surrounded pro- and anti-inflammatory cytokines and chemokines modulate cross-glial communication in demyelinated lesions. After a demyelinated insult there is an -unfortunately deficient or incomplete- spontaneous myelin repair process (i.e., remyelination), characterized by the highly interdependent function of microglia, astrocytes and oligodendroglia, the latest corresponding to cells responsible for the formation of myelin in the central nervous system (CNS) (Chitnis and Weiner, 2017; Franklin and Ffrench-Constant, 2017). For instance, it is known that signaling molecules released by microglia induce the activation of astrocytes and promote differentiation of oligodendrocytes in demyelinated areas (Franklin and Ffrench-Constant, 2017). Similarly, astrocyte activity and secretion can promote oligodendrocyte maturation (Franklin and Ffrench-Constant, 2017). In the complex cellular interaction observed in demyelinated lesions, connexin (Cx)-based channels and hemichannels has been pointed out as a major components underlying glial communication (Vejar et al., 2018). However, less attention has been paid to the putative role of pannexin (Panx)-based channels, a functional equivalent of Cxs, usually involved in inflammatory processes, particularly in the CNS. Here we discuss evidence supporting a role of pannexin-based channels on the progression of MS that, we believe, deserves further investigation.
Role of Panx 1 in glial cells: Panxs are a family protein conformed by three members: Panx1, Panx2 and Panx3. These proteins form a channel, or pannexon, in the plasmatic membrane which is permeable to ions and other molecules such as ATP (Avendaño et al., 2015; Ahmadian et al., 2019). They are found in several cell types participating in diverse physio- and pathological events, such as cellular proliferation, differentiation, migration, inflammatory responses, cytokines release, muscle contraction, glucose uptake and modulation of the nervous system (Ahmadian et al., 2019). Different open conformation of Panx1-based channels has been proposed, displaying different permeabilities where ATP release (widely validated) is associated with a large conductance conformation (~500 pS) (Suadicani et al., 2012).
In the CNS Panx1 channels are expressed in neurons, glial (astrocytes, microglia and oligodendrocytes) and endothelial cells (Ahmadian et al., 2019). In neurons, it has been shown that reactive oxygen and nitrogen species (especially NO) increases the activity of Panx1-based channels during oxygen/glucose deprivation in a hippocampal ischemia/reperfusion model, suggesting a close relationship between oxidative stress and Panx1 (Ahmadian et al., 2019).
Regarding glial cells, Panx1 have been pointed out as a potential factor in astrocyte survival under physio- and pathological conditions (Suadicani et al., 2012; Freitas-Andrade and Nauss, 2016). Indeed, some authors hypothesize that Panx1 serve as K+ sensors in astrocytes, playing a central role in K+ homeostasis, the major documented astrocyte function in the CNS (Suadicani et al., 2012). In cultured spinal astrocytes the activation of purinergic ionotropic 2X receptors (P2XRs) by ATP trigger the opening of Panx1-based channels, leading to ATP-induced ATP release and the resulting Ca2+ entry. Although this mechanism is not unique (for instance, Cx43 hemichannels can be also opened) (Freitas-Andrade and Nauss, 2016), Panx1-based channel has a key role maintaining the ATP signaling, since in cultured astrocytes from a Panx1-null mouse, the ATP-dependent calcium wave propagation is impaired (Suadicani et al., 2012).
Several studies link Panx1-based channels activity with neurodegenerative disorders and/or inflammation conditions (Avendaño et al., 2015; Freitas-Andrade and Nauss, 2016; Ahmadian et al., 2019). For example, a prenatal inflammatory condition (triggered by lipopolysaccharide exposure during pregnancy) increases the release of ATP from astrocytes in the offspring by opening Panx1 channels (along with Cx43 hemichannels) (Avendaño et al., 2015). In the same study authors reported that Panx1 and Cx43 surface levels were up-regulated, resulting in an increase of neuronal death mediated by the activation of neuronal Panx1-based channels and P2X7 receptors (Avendaño et al., 2015). This increase of astrocytic Panx1 channels activity was induced by an increment in the levels of tumor necrosis factor-α and interleukin-1β (IL-1β), that in turn increased ATP release through Panx1 (and Cx43)-based (hemi)channels in astrocytes of the offspring (Avendaño et al., 2015). Also, under inflammatory conditions nod-like-receptor pyrin domain-containing 3 inflammasome activation is inhibited by probenecid, a Panx1 channel blocker (Ahmadian et al., 2019). Since nod-like-receptor pyrin domain-containing 3 plays a key role in the pathogenesis of Parkinson’s disease it is likely that Panx1-based channels activity contributes importantly to the inflammatory cascade underlying this neurodegenerative disease (Ahmadian et al., 2019). In this line, the administration of probenecid reduces inflammation, cerebral edema and neuronal death, in mice subjected to transient focal ischemia, reinforcing (i) an ubiquitous role for Panx1 in neuroinflammatory/detrimental conditions and (ii) the notion that an increase of Panx1-based channels activity might be a harmful signal, since neuroprotection is observed when a Panx1 channel blocker is applied (from Freitas-Andrade & Nauss, 2016)
Pannexin 1 as a novel regulator of MS: Given the well supported role of Panx1-based channels in inflammation and important neurodegenerative disorders, a putative function of these channels in MS progression–characterized precisely by a neuroinflammatory context– is expected. Indeed, recent findings showed that probenecid reduced clinical symptoms (disease score) in the experimental autoimmune encephalomyelitis MS model, reducing inflammation, the number of T lymphocytes infiltrating the spinal cord, and the loss of oligodendroglia lineage cells (Hainz et al., 2016, 2017b). Importantly, the same authors also showed that probenecid improved remyelination of the optic nerve in a cuprizone-induced demyelinated model of MS (Hainz et al., 2017a). Even though the subcellular mechanisms are still missing, these studies suggest a specific improved outcome after probenecid treatment, namely: myelin regeneration.
An interesting observation is that Panx1 levels are upregulated by IL-1β in peripheral tissue (bladder mucose) of experimental autoimmune encephalomyelitis mice (Negoro et al., 2013), as was also report in a systemic inflammatory condition (triggered by lipopolysaccharide) (Avendaño et al., 2015), confirming that cytokines can modify Panx1 levels in a relevant clinical model of MS (Negoro et al., 2013), thus these authors suggest that Panx1 signaling provides positive feedback in a dysfunction associated with MS (Negoro et al., 2013). Supporting a putative role for Panx1-based channels in MS, IL-1β is one of the main cytokines released by the activated microglia recruited in demyelinated regions, being thus likely that this signaling molecule regulates Panx1 level in MS lesions.
But how could Panx1-based channels activity regulates (re)myelination? A first direct possibility might be by modulating the extracellular ATP levels. It has been reported that myelin synthesis depends, at least partially, on ATP signaling (Wake et al., 2011). Thus, under physiological conditions, since ATP is released by high conductance Panx1-based channels, their activity could greatly modify the local amounts of available ATP that in turn could signaling to oligodendroglia controlling its potential to synthesize new myelin (Wake et al., 2011). However, in an inflammatory scenario, an increase of Panx1 levels and/or Panx1-channel activity could lead to cell damage by a toxic entry of calcium through a P2XRs overactivation in oligodendroglia (Figure 1). Alternatively, indirect modifications of another cellular partners such as astrocytes, can modify the potential for myelin regeneration. Indeed, blocking Panx1 expression in astrocytes by siRNA transfection, inhibited cytokine (interleukin-6 and -8) and glutamate release (Wei et al, 2015), both molecules pointed out as key factors in the process of myelination and remyelination, raising thus another possible mechanism for Panx1 involvement on MS: the modulation of molecules and cells commonly present in demyelinated lesions.
Figure 1.
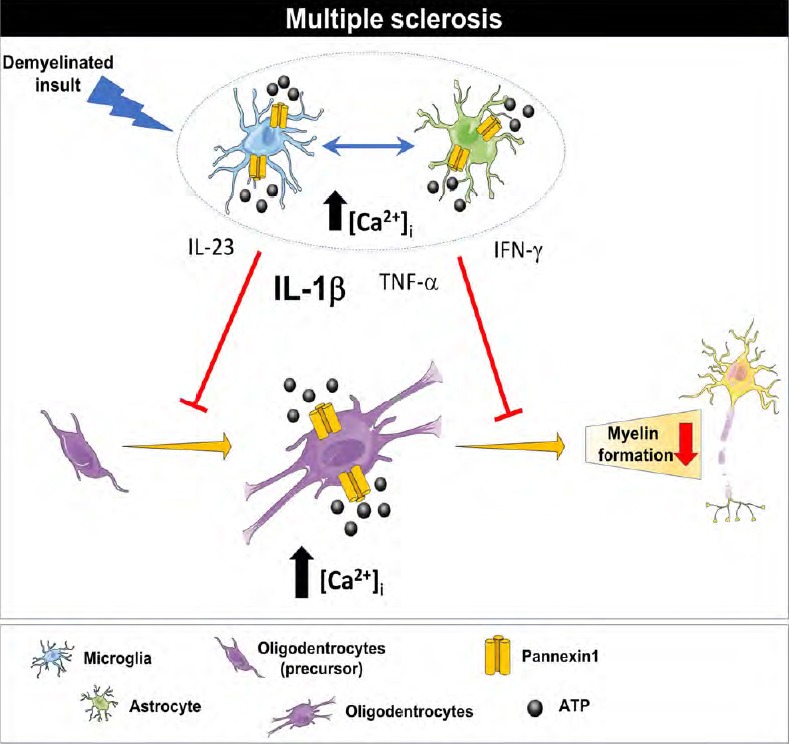
Summary of the proposed mechanism.
After a demyelinated insult, recruited microglia and astrocytes release several inflammatory molecules such as IL-1β, IL-23, IFN-γ, TNF-α, among others, that can regulate the potential for remyelination by modifying oligodendroglia proliferation and maturation. Under multiple sclerosis neuroinflammatory conditions, an increase in the IL-1β levels might increase the activity and levels of Panx-1 based channels which in turn might induce a toxic level of intracellular calcium and impaired ATP signaling in the glial cell population leading to an impaired remyelination. IFN-γ: Interferon-γ; IL: interleukin; TNF-α: tumor necrosis factor-α.
Concluding remarks: Currently there are no effective treatments for MS, therefore the study of relevant factors underlying the pathophysiological progression of the disease is a challenge for translational research. As other neurodegenerative disorders, MS is characterized by a chronic neuroinflammatory environment as well as the release of chemo- and cytokines as well as reactive oxygen and nitrogen species. Since (i) Panx1 is a common molecule interacting with these factors during the progression of neuroinflammation in many different scenarios, (ii) Panx1 levels and activity is modified in response to molecules commonly present in demyelinated lesions, (iii) Panx1 is expressed in glial cells participating in the myelin repair process, (iv) ATP, a molecule implicated in myelin synthesis, can be released by Panx1-based channels and (v) recent studies have shown that blocking Panx1 channels improve the symptomatology and promote myelin repair in MS animal models; we here proposed that Panx1 might play a central role on the progression of MS, and therefore deserves further investigation. To determine Panx1 involved pathways at cellular and subcellular levels becomes especially important when a clinically approved drug targeting Panx1-based channels (i.e., probenecid) is available. Next research lines might consider the putative mechamisms proposed herein (Figure 1)in order to shed light on novel mechanisms underlying MS progression and myelin repair.
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), No. 11160536 (to CP) and 11160616 (to FCO).
Additional file: Open peer review reports 1 (91.3KB, pdf) and 2 (92.6KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Hailong Song, University of Pennsylvania, USA; Marvin Soriano-Ursua, Escuela Superior de Medicina, Mexico.
P-Reviewers: Song H, Soriano-Ursua M; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Ahmadian E, Eftekhari A, Samiei M, Maleki Dizaj S, Vinken M. The role and therapeutic potential of connexins, pannexins and their channels in Parkinson’s disease. Cell Signal. 2019;58:111–118. doi: 10.1016/j.cellsig.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Avendaño BC, Montero TD, Chávez CE, von Bernhardi R, Orellana JA. Prenatal exposure to inflammatory conditions increases Cx43 and Panx1 unopposed channel opening and activation of astrocytes in the offspring effect on neuronal survival. Glia. 2015;63:2058–2072. doi: 10.1002/glia.22877. [DOI] [PubMed] [Google Scholar]
- 3.Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Invest. 2017;127:3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin RJ, Ffrench-Constant C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat Rev Neurosci. 2017;18:753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 5.Freitas-Andrade M, Naus CC. Astrocytes in neuroprotection and neurodegeneration: The role of connexin43 and pannexin1. Neuroscience. 2013;323:207–221. doi: 10.1016/j.neuroscience.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Hainz N, Becker P, Rapp D, Wagenpfeil S, Wonnenberg B, Beisswenger C, Tschernig T, Meier C. Probenecid-treatment reduces demyelination induced by cuprizone feeding. J Chem Neuroanat. 2017a;85:21–26. doi: 10.1016/j.jchemneu.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Hainz N, Wolf S, Beck A, Wagenpfeil S, Tschernig T, Meier C. Probenecid arrests the progression of pronounced clinical symptoms in a mouse model of multiple sclerosis. Sci Rep. 2017b;7:17214. doi: 10.1038/s41598-017-17517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hainz N, Wolf S, Tschernig T, Meier C. Probenecid application prevents clinical symptoms and inflammation in experimental autoimmune encephalomyelitis. Inflammation. 2016;39:123–128. doi: 10.1007/s10753-015-0230-1. [DOI] [PubMed] [Google Scholar]
- 9.Negoro H, Lutz SE, Liou LS, Kanematsu A, Ogawa O, Scemes E, Suadicani SO. Pannexin 1 involvement in bladder dysfunction in a multiple sclerosis model. Sci Rep. 2013;3:2152. doi: 10.1038/srep02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suadicani SO, Iglesias R, Wang J, Dahl G, Spray DC, Scemes E. ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia. 2012;60:1106–1116. doi: 10.1002/glia.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vejar S, Oyarzún JE, Retamal MA, Ortiz FC, Orellana JA. Connexin and pannexin-based channels in oligodendrocytes: implications in brain health and disease. Front Cell Neurosci. 2019;13:3. doi: 10.3389/fncel.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


