Keywords: catechol-O-methyltransferase, cognitive impairment, D1 dopamine receptors, fear memory, glycogen synthase kinase-3β, isoflurane, neurotoxicology, sevoflurane
Abstract
Isoflurane and sevoflurane are both inhalation anesthetics, but in clinical application, sevoflurane has been considered to be less suitable for long-term anesthesia because of its catabolic compounds and potential nephrotoxicity. Nevertheless, recent studies have shown that these two inhalation anesthetics are similar in hepatorenal toxicity, cost, and long-term anesthetic effect. Moreover, sevoflurane possibly has less cognitive impact on young mice. In this study, C57BL/6 mice aged 8–10 weeks were exposed to 1.2% isoflurane or 2.4% sevoflurane for 6 hours. Cognitive function and memory were examined in young mice using the novel object recognition, contextual fear conditioning, and cued-fear extinction tests. Western blot assay was performed to detect expression levels of D1 dopamine receptor, catechol-O-methyltransferase, phospho-glycogen synthase kinase-3β, and total glycogen synthase kinase-3β in the hippocampus. Our results show that impaired performance was not detected in mice exposed to sevoflurane during the novel object recognition test. Contextual memory impairment in the fear conditioning test was shorter in the sevoflurane group than the isoflurane group. Long-term sevoflurane exposure did not affect memory consolidation, while isoflurane led to memory consolidation and reduced retention. Downregulation of hippocampal D1 dopamine receptors and phosphorylated glycogen synthase kinase-3β/total glycogen synthase kinase-3β and upregulation of catechol-O-methyltransferase may be associated with differing memory performance after exposure to isoflurane or sevoflurane. These results confirm that sevoflurane has less effect on cognitive impairment than isoflurane, which may be related to expression of D1 dopamine receptors and catechol-O-methyltransferase and phosphorylation of glycogen synthase kinase-3β in the hippocampus. This study was approved by the Institutional Animal Care and Use Committee, Nanjing University, China on November 20, 2017 (approval No. 20171102).
Chinese Library Classification No. R453; R614.2; R741
Introduction
Neurotoxic effects of many commonly used volatile anesthetics have gained public attention. Animal studies consistently report brain injury and behavioral changes in animals exposed to anesthetics during critical periods of brain development (Zhang et al., 2015; Min et al., 2016; Wang et al., 2018).
Isoflurane and sevoflurane are the most widely used inhaled anesthetics worldwide because of their rapid induction and recovery coupled with low stimulation. However, commonly, isoflurane is more frequently applied as a long-term anesthetic than sevoflurane, most likely because of catabolic compound A production and its potential nephrotoxicity during sevoflurane anesthesia.
A previous study has compared the use of sevoflurane and isoflurane for long-term anesthesia and the effect of their metabolites on liver and kidney function. The results show no significant differences between the two groups following coronary artery bypass surgery (Jones et al., 2016). Moreover, a study compared the cost of isoflurane and sevoflurane use during surgery, and found sevoflurane to be no more expensive than isoflurane (Jones et al., 2016). These findings suggest that the use of sevoflurane for long-term anesthesia may not be inferior to the use of isoflurane. Furthermore, our previous work has shown that 1% isoflurane anesthesia for 6 hours can impair spatial memory in young mice (Xia et al., 2016; Tang et al., 2018). Compared with isoflurane, it is compelling that sevoflurane has no shortcomings in hepatotoxicity, nephrotoxicity, or cost (Dal Molin et al., 2014), while long-term isoflurane anesthesia can induce cognitive impairment in mice (Ni et al., 2015). In addition, although the effect of long-term sevoflurane anesthesia on cognitive function in young mice has rarely been reported, the notion that neonatal isoflurane exposure induces more necroptosis and impaired memory skills than sevoflurane is commonly acknowledged (Liang et al., 2010; Liu et al., 2017). Thus, it appears that sevoflurane could have advantages over isoflurane for long-term anesthesia.
In light of the fact that there is no direct comparison of the effects of long-term isoflurane and sevoflurane anesthesia on cognitive function in young adult mice, this experiment was designed to examine the hypothesis and provide a reference for clinical medicine.
Materials and Methods
Animals
All experiments were consistent with the applicable laws and guidelines on animal care, and were approved by the Institutional Animal Care and Use Committee, Nanjing University, China (approval No. 20171102) on November 20, 2017. All processing methods were conducted in accordance with the Directive 2010/63/EU of the European Parliament and Council. A total of 180 adult male C57BL/6 mice aged 8–10 weeks and weighing 20–25 g were obtained from the Model Animal Research Center of Nanjing University, China (license No. SYXK2014-0052). All mice were housed on controlled 12-hour light/dark cycles (lights turned on at 8:00). The room temperature and humidity conditions were maintained at 20–22°C and 50–60%, respectively. All mice were acclimatized with natural drink and food for at least 2 weeks before any treatments.
Anesthesia
Mice were randomly divided into isoflurane, sevoflurane, and control groups (n = 8), and were treated with isoflurane, sevoflurane, and air, respectively. The anesthesia box air inlet was connected to the anesthesia machine gas evaporation tank (Remain, Shanghai, China, consisting of an air connection and anesthesia monitor (Drager, Lubeck, Germany) for monitoring concentrations of isoflurane (license No. H20020267; Lunan Better Pharmaceutical Co., Ltd., Linyi, Shandong Province, China) and sevoflurane (license No. H20170172; Shanghai Hengrui Pharmaceutical Co., Ltd., Shanghai, China).
To ensure anesthetic potency of both anesthetics, minimum alveolar concentration was tested in young mice before the experiment using the general up-and-down method with a tail clamp (Callaway et al., 2012). A 1.0 minimum alveolar concentration multiple was chosen for our anesthetic procedure. Mice were settled in a chamber filled with a gas mixture that contained 1.2% isoflurane and 2.4% sevoflurane in pure oxygen flowing at 2 L/min for 6 hours. Lack of tail-clip response was a signal of anesthesia. A heated blanket was positioned under the chamber to maintain body temperature of the mouse, which was maintained at 37°C throughout anesthesia. During the procedure, an investigator monitored respiratory frequency and skin color. If signals of apnea or hypoxemia were detected, the mouse may have been exposed to air and was immediately removed from the experiment. Mice in the control group were kept in the container and exposed to air only for 6 hours.
Behavioral experiments
Novel object recognition test
The test box was composed of four acrylic cubes, and the test case was placed in a quiet, dimly lit room. The novel object recognition test included an adaptation phase, a familiar phase, and a testing phase. The adaptation phase lasted for 3 days, and during this stage, the experimenter handled each mouse for 1 minute every day. Mice were placed in an empty test box to acclimatize to the environment. In the familiar stage, mice were placed in the test box to become familiar with two identical objects, and were allowed to explore the area freely for 10 minutes. After 2 hours at the familiar stage, an object was removed from the test box and replaced with a new object of different shape and color. Subsequently, mice were free to explore for 10 minutes, and their behavior was recorded on video data. Mice were placed in the test room before the formal test for at least 1 hour to acclimatize to the environment in advance. To minimize adverse effects on the mice, the experimenter was asked to avoid making any noise or unnecessary movement. After testing each mouse, the test box was cleaned, and 75% alcohol was used to wipe the object and test box to reduce the impact of odor on the other mice during the experiment. Data were recorded as the time taken to explore familiar and new objects and the percentage of time spent exploring new objects. The discrimination index was used to evaluate learning and memory abilities of mice (Vigano et al., 2009). The discrimination index = (Tn–Tf)/(Tf+Tn), with Tn and Tf representing the time spent (during a 5-minute observation period) exploring new and familiar objects (or locations), respectively.
Contextual fear conditioning test
The contextual fear conditioning test was performed using a fear conditioning experimental system (Panlab, Barcelona, Spain). The experiment was divided into a training phase and a test phase. In the training phase, mice were freely allowed to explore a test chamber in silence and with no light for 5 minutes. Afterwards, the mice were administered a single high-frequency sound (4000 Hz, 80 dB) for 30 seconds of stimulation. During the final 2 seconds, the mice were given a current of 0.8 mA for an inescapable foot shock. After stimulation, the mice continued to explore the test chamber for 2 minutes. Following this, the mice were placed back in the cage. The test box was wiped with 75% alcohol at the end of each test to avoid the impact of odor on the experiment. After 24 hours of training, a context conditional fear memory test was performed. Mice were returned to the same test chamber as the previous day for 3 minutes without sound or shock. The percentage of time that mice froze over 3 minutes was calculated to estimate fear memory of the mice.
Cued-fear memory extinction test
Yizhar’s study was used as a reference for this protocol (Klavir et al., 2017). On the first day, mice were trained and tested for fear memory acquisition in a fear conditioning chamber (context A). Six pairings of the conditioned stimulus (CS) (5-Hz train of 100 ms, 5-kHz tone lasting for 30 seconds) and unconditioned stimulus (US) (continuous foot shock for 1 second, 0.8 mA) were performed to train the mice, with a 1-minute interval between each CS–US pairing. After 24 hours of training, fear memory consolidation was tested in a new chamber (context B), in which 20 repetitions of the CS were played for mice at 1-minute intervals. Context B was completely different than Context A in terms of lighting, smell, walls, and floor. A smooth aluminium sheet was used to cover on the original floor to change the tactile sense. The color and shape of walls were changed and light was turned on. Odor was removed with 75% ethanol to distinguish between context A and context B. On the third day, the mice were presented with 15 repetitions of the CS to quantify fear memory extinction in context B, which was the same as on day 2.
After the first day of training, even in the new environment, normal mice remember consequent shock stimulation and feel fearful. Hence, they do not move and exhibit a freezing-like state when the CS is presented in this context. Freezing time represents the percentage of duration that the mice remain motionless during total CS time to assess fear memory of all mice.
Western blot assay
Hippocampal protein extraction and western blots were performed as previously described (Xia et al., 2016). Samples were homogenized in sodium dodecyl sulphate buffer (Beyotime, Shanghai, China) with a mixture of protease and phosphatase inhibitor cocktail (Abcam, Cambridge, UK). Proteins from the hippocampus were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene difluoride membrane filters (Millipore, Burlington, MA, USA). Membrane blots were blocked with 5% bovine serum albumin for 2 hours at room temperature and incubated with primary antibodies overnight at 4°C (Wang et al., 2009). The primary antibodies were: anti-β-actin (1:1000; rabbit, Abcam, Cambridge, MA, USA), anti-D1 dopamine receptor (D1R) (1:1000; rabbit, Abcam), anti-catechol-O-methyltransferase (COMT) (1:1000; rabbit, Abcam), anti-phospho-glycogen synthase kinase-3β (p-GSK-3β)-serine 9 (1:1000; rabbit, Abcam), and anti-GSK-3β (1:1000; rabbit, Abcam). After washing the next day in Tris-buffered saline with Tween 20 four-times, membranes were incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000; Abcam) for 1 hour at room temperature. Membranes were visualized using a Western Chemiluminescent HRP Substrate kit (Millipore) with a Tanon 5200 chemiluminescent imager (Tanon Science & Technology, Shanghai, China). Relative protein expression levels were normalized to β-actin and quantified by ImageJ software (National Institute of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± SD. Statistical data were analyzed with a computerized statistical package (SPSS 22.0, IBM, Armonk, NY, USA). One-way or two-way analysis of variance followed by the least significant difference post hoc tests was used to evaluate quantitative differences in protein levels and behavioral data. A value of P < 0.05 was considered statistically significant.
Results
Only mice exposed to isoflurane exhibit impaired performance in the novel object recognition test
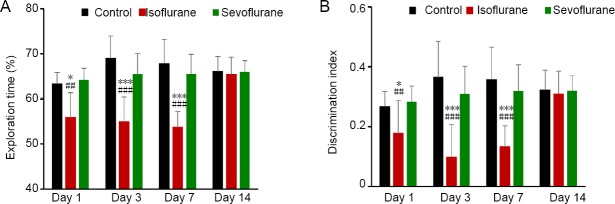
Results of the novel object recognition test showed that exploration time of a new object was decreased in the isoflurane group compared with the control and sevoflurane groups on days 1, 3, and 7 after anesthesia. This demonstrates that mice were not able to distinguish familiar and novel objects (P < 0.05, vs. control group and P < 0.01, vs. sevoflurane group on day 1; P ≤ 0.001, vs. control and sevoflurane groups on days 3 and 7; Figure 1A). Impaired memory was recovered on day 14 after anesthesia, and there was no significant difference among the three groups (P > 0.05; Figure 1A). Similarly, discrimination index values were higher in the control and sevoflurane groups than isoflurane group, as tested on days 1, 3, and 7 after anesthesia (P < 0.01; Figure 1B). There were no significant differences in discrimination index values among the three groups on day 14 (P > 0.05; Figure 1B).
Figure 1.
Effect of inhaled isoflurane and sevoflurane in the novel object recognition test.
(A) On days 1–7 after anesthesia, mice exposed to isoflurane failed in the object recognition test. (B) Discrimination index values in the control and sevoflurane groups (2.4%) were significantly greater compared with the isoflurane (1.2%) group. Data are expressed as the mean ± SD (n = 8). *P < 0.05, ***P < 0.001, vs. control group; ##P < 0.01, ###P < 0.001, vs. sevoflurane group (one-way analysis of variance followed by the least significant difference post hoc test).
Equivalent exposure to isoflurane induces prolonged contextual learning and memory impairment compared to sevoflurane in the fear conditioning test
In the contextual fear conditioning test, freezing time was significantly reduced in the isoflurane group compared with the control and sevoflurane groups on day 1 after anesthesia (P < 0.05; Figure 2A). On day 3, freezing times were shorter in the sevoflurane and isoflurane groups than the control group (P < 0.001; Figure 2B). Seven days after anesthesia, the results were the same as on day 1 (P < 0.05; Figure 2C). All groups exhibited similar freezing behaviors on day 14 (P > 0.05; Figure 2D).
Figure 2.
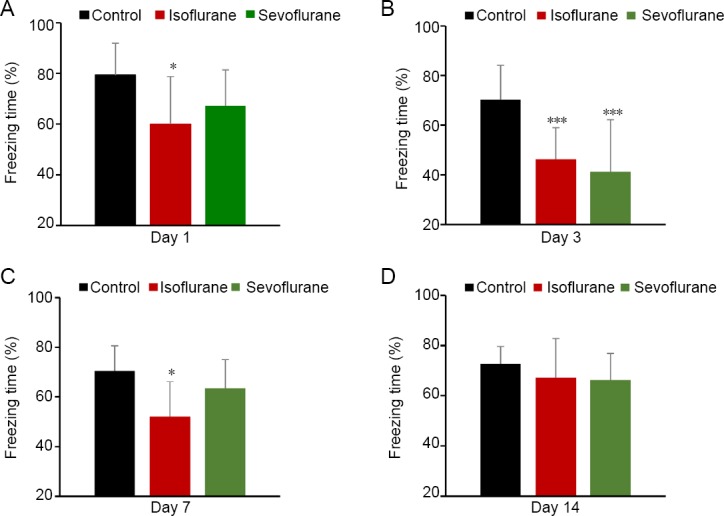
Effect of 6-hour isoflurane and sevoflurane exposure in the contextual fear conditioning test.
(A) Reduced freezing times after exposure to 1.2% isoflurane on day 1 after anesthesia. (B) Reductions in freezing time after exposure to 1.2% isoflurane and 2.4% sevoflurane on day 3 after anesthesia. There were no differences between the isoflurane and sevoflurane groups. (C) Reduced freezing time after exposure to 1.2% isoflurane on day 7 after anesthesia. (D) There were no significant differences in freezing time among the three groups on day 14. Data are expressed as the mean ± SD (n = 7). *P < 0.05, ***P < 0.001, vs. control group (one-way analysis of variance followed by the least significant difference post hoc test).
Long-term isoflurane exposure impairs memory consolidation and retention in young mice, while sevoflurane leads to impaired memory retention only
During fear memory acquisition, freezing responses in mice at the sound stage before the shock were similar among the three groups from the first to sixth sound (P > 0.05; Figure 3A). Compared with the control group, freezing time decreased in the isoflurane group on day 2 during both early (first 10 trials) and late (last 10 trials) memory consolidation (P < 0.05; Figure 3B). Meanwhile, no significant difference was observed in freezing time between the sevoflurane and control groups or isoflurane groups during this phase (P > 0.05; Figure 3B). The fear memory retention test was performed on day 3. Freezing behaviors were reduced in both isoflurane-treated and sevoflurane-treated mice during early (first 7 trials) and late (last 8 trials) extinction testing compared with control mice (P < 0.001, vs. isoflurane group and P < 0.05, vs. sevoflurane group in the first 7 trials; P < 0.001 in the last 8 trials; Figure 3C). There were no significant differences in freezing behavior between the sevoflurane and isoflurane groups during this phase (P > 0.05; Figure 3C).
Figure 3.
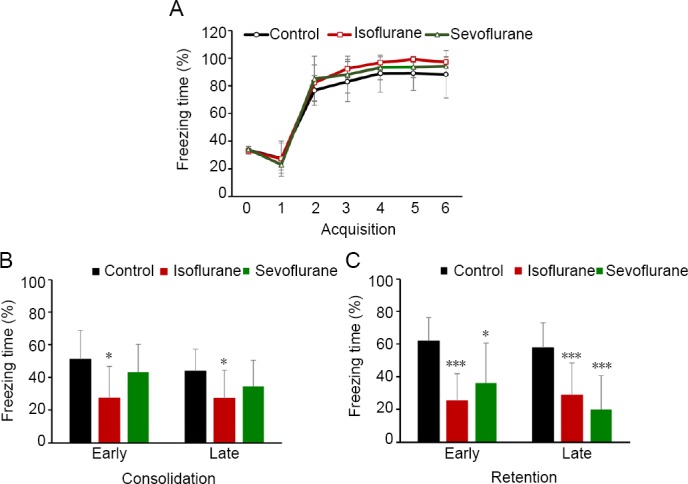
Effect of long-term isoflurane and sevoflurane exposure in the cued-fear memory extinction test.
(A) No significant differences in memory acquisition were observed among the three groups. (B) Memory consolidation was impaired in only mice exposed to 1.2% isoflurane for 6 hours. (C) Both 1.2% isoflurane-treated and 2.4% sevoflurane-treated mice exhibited memory retention deficits. Data are expressed as the mean ± SD (n = 7). *P < 0.05, ***P < 0.001, vs. control group. Percentage of freezing time was evaluated by two-way analysis of variance followed by the least significant difference post hoc test in the fear memory acquisition test, and by one-way analysis of variance followed by the least significant difference post hoc test in the memory consolidation and retention tests.
Expression changes of memory-related proteins in the mouse hippocampus after isoflurane or sevoflurane exposure
Hippocampal samples from different groups were homogenized, and expression levels of p-GSK-3β, t-GSK-3β, D1R, and COMT were determined after fear memory acquisition, consolidation, and retention testing (Figure 4). In the isoflurane group, levels of p-GSK-3β/t-GSK-3β were downregulated on the memory consolidation and retention testing days compared with the control group (P < 0.05). In the sevoflurane group, levels of p-GSK-3β and p-GSK-3β/t-GSK-3β were downregulated only after memory retention testing compared with the control group (P < 0.05). Furthermore, D1R levels were downregulated and COMT levels were upregulated in the isoflurane group on the memory consolidation and retention testing days compared with the control group (P < 0.05). In the sevoflurane group, these results only appeared after memory retention testing compared with the control group (P < 0.05). No significant differences were found between the isoflurane and sevoflurane groups (P > 0.05).
Figure 4.
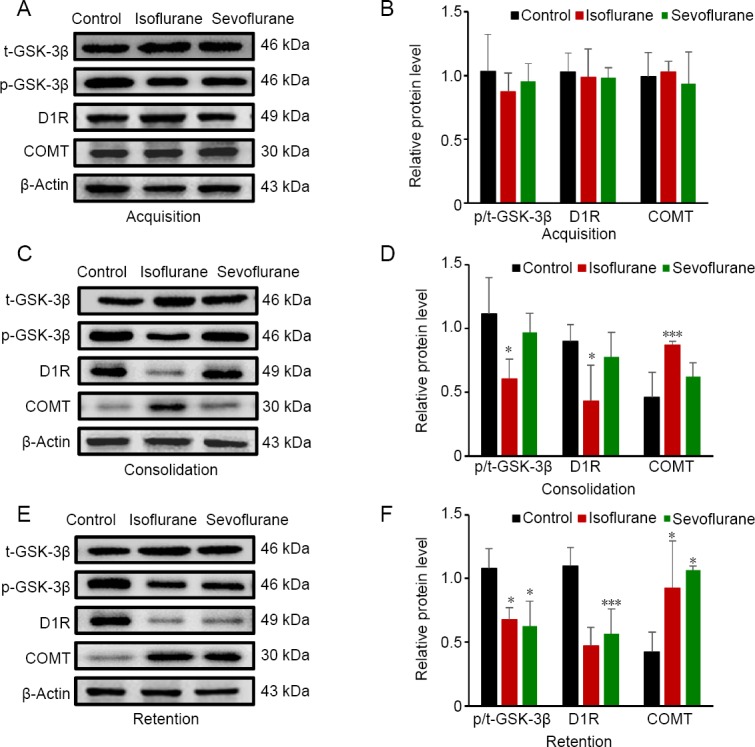
Changes in hippocampal p-GSK-3β, t-GSK-3β, D1R, and COMT protein expression in response to memory acquisition, memory consolidation, and memory retention in the cued-fear memory test in all groups.
(A, C, E) Representative protein blots. (B, D, F) Statistical analysis of relative protein expression. Data are expressed as the mean ± SD (n = 3). *P < 0.05, ***P < 0.001, vs. control group (one-way analysis of variance followed by the least significant difference post hoc test). COMT: Catechol-O-methyltransferase; D1R: D1 dopamine receptor; GSK: glycogen synthase kinase; p-GSK: phospho-GSK.
Discussion
In our study, we found that mice exposed to isoflurane exhibited impaired performance from day 1 to day 7 after anesthesia during the novel object recognition test and contextual fear conditioning test, while mice exposed to sevoflurane displayed only cognitive deficits in contextual fear conditioning on day 3 after anesthesia. Furthermore, long-term isoflurane exposure impaired memory consolidation and retention in young mice, while sevoflurane led to only impaired memory retention, which may be related to changes in hippocampal p-GSK-3β/t-GSK-3β, D1R, and COMT protein expression.
Isoflurane and sevoflurane are the most commonly used inhaled anesthetics, and have gained much attention due to their effects on the central nervous system. Although many previous studies have focused on this area, the results have been controversial most likely because of differences in the concentration or duration of anesthesia, age of animals, and methods of behavioral testing (Xia et al., 2007; Su et al., 2011; Jiang et al., 2017). Considering that minimum alveolar concentration represents the efficiency of anesthetics, we determined the minimum alveolar concentration in young adult mice to confirm that isoflurane or sevoflurane exposure in this experiment was at equivalent multiples, thereby enabling our results to be compared. Our data show that minimum alveolar concentration values were 1.2% for isoflurane and 2.38% for sevoflurane (Additional Figure 1 (327.3KB, tif) ). Under the same anesthesia regime, we found that during the novel object recognition test, isoflurane-treated mice exhibit cognitive impairment at 1, 3, 7, and 14 days after anesthesia, whereas no significant differences were detected between the sevoflurane-treated group and control group. These findings are consistent with a previous study showing that isoflurane, but not sevoflurane, may have adverse effects on cognitive function in young mice (Kilicaslan et al., 2013). However, in the contextual fear conditioning experiment, we found that sevoflurane-treated mice exhibit cognitive dysfunction on day 3 only, while isoflurane exposure for 6 hours elicited persistent cognitive dysfunction from day 1 to day 7. These results suggest that compared with the isoflurane group, sevoflurane treatment for 6 hours induced shorter times of cognitive impairment in young mice.
To investigate causes of differing results in both behavioral tests, we designed a cued-fear conditioning test to examine the effect of both anesthetics on the process of fear memory in mice (Kilicaslan et al., 2013). Our results suggest that only long-term sevoflurane anesthesia impaired retention of fear memory in young adult mice, while long-term isoflurane anesthesia impaired both memory consolidation and memory retention. This finding may also partly explain why the sevoflurane group did not display impaired cognitive function during the novel object recognition test. First contact with the two same objects can be interpreted as acquisition of memory. Placement of mice in contact with the old and another new object (mainly after 2 hours) represents memory recall and consolidation. It is reasonable that isoflurane-treated mice, whose memory consolidation was impaired, failed to recognize the new object, while memory consolidation-unaffected sevoflurane-treated mice could identify the new object. Nonetheless, different cognitive tests may involve multiple brain regions, thus the specific mechanism remains to be further studied.
Recent studies have shown a relationship between GSK-3β and memory retrieval and consolidation (Hong et al., 2012). It has also been recently reported that GSK-3β interacts with the dopamine D1 receptor to regulate dopamine receptor function, suggesting that D1R and GSK-3β may interact during inhaled anesthetic-induced fear memory impairment (Wang et al., 2017). Because dopamine plays a critical role in fear learning and extinction (Abraham et al., 2014), we also detected COMT, a S-adenosylmethionine-dependent methyltransferase enzyme, in the mouse hippocampus. The function of COMT is in metabolizing catecholamines, therefore it is essential for the regulation of dopamine levels and learning and memory function (Scheggia et al., 2017). In this study, D1R and p-GSK-3β/t-GSK-3β levels decreased in the isoflurane group during memory consolidation, whereas these changes were only observed during memory retention in the sevoflurane group. We also found that COMT levels were upregulated during fear memory consolidation and retention testing. Thus, lower basal catecholamine concentration combined with lower D1R levels in the hippocampus may contribute to the cognitive deficits. Considering that enhanced COMT expression facilitates fear memory extinction, the function of COMT itself cannot be ignored, albeit other catecholamines should be considered in future research.
Although our study suggests that long-term sevoflurane anesthesia has less effect on memory than isoflurane, and could provide clinical guidance for anesthesiologists, there are still a few limitations of our research. Only young mice were used in this study, and no results were obtained on the effects of long-term sevoflurane or isoflurane inhalation in infant or old mice. To investigate underlying mechanisms, this study only focused on the hippocampus, while protein changes in other brain regions associated with fear memory, such as the amygdala and prefrontal lobe, should be taken into consideration in the future.
In summary, compared with isoflurane, equivalent long-term exposure to sevoflurane resulted in less cognitive impairment owing to a limited effect on fear memory retention. To investigate the mechanism of these anesthetic effects on the memory process, we found that downregulation of D1R and p-GSK-3β/t-GSK-3β and upregulation of COMT may be implicated in the learning and memory process.
Additional file:
Additional Figure 1 (327.3KB, tif) : Scatter plot of the minimum alveolar concentration (MAC) of each mouse in the isoflurane and sevoflurane groups.
Scatter plot of the minimum alveolar concentration (MAC) of each mouse in the isoflurane and sevoflurane groups.
The MACs determined for isoflurane and sevoflurane were 1.2% (MAC = 1.2 ± 0.11%) and 2.38% (MAC = 2.38 ± 0.23%), respectively. Data are expressed as the mean ± SD (n = 10).
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81730033 (to XPG), No. 81701371 (to TJX). The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: This study was approved by the Institutional Animal Care and Use Committee, Nanjing University, China on November 20, 2017 (approval No. 20171102).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Ahmed E Abdel Moneim, Helwan University, Egypt.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81730033 (to XPG), No. 81701371 (to TJX).
P-Reviewer: Abdel Moneim AE; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: James R, Pack M, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiol Learn Mem. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaway JK, Jones NC, Royse AG, Royse CF. Sevoflurane anesthesia does not impair acquisition learning or memory in the Morris water maze in young adult and aged rats. Anesthesiology. 2012;117:1091–1101. doi: 10.1097/ALN.0b013e31826cb228. [DOI] [PubMed] [Google Scholar]
- 3.Dal Molin SZ, Kruel CR, de Fraga RS, Alboim C, de Oliveira JR, Alvares-da-Silva MR. Differential protective effects of anaesthesia with sevoflurane or isoflurane: an animal experimental model simulating liver transplantation. Eur J Anaesthesiol. 2014;31:695–700. doi: 10.1097/EJA.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 4.Hong JG, Kim DH, Lee CH, Park SJ, Kim JM, Cai M, Jang DS, Ryu JH. GSK-3beta activity in the hippocampus is required for memory retrieval. Neurobiol Learn Mem. 2012;98:122–129. doi: 10.1016/j.nlm.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Jiang S, Miao B, Chen Y. Prolonged duration of isoflurane anesthesia impairs spatial recognition memory through the activation of JNK1/2 in the hippocampus of mice. Neuroreport. 2017;28:386–390. doi: 10.1097/WNR.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 6.Jones PM, Bainbridge D, Chu MW, Fernandes PS, Fox SA, Iglesias I, Kiaii B, Lavi R, Murkin JM. Comparison of isoflurane and sevoflurane in cardiac surgery: a randomized non-inferiority comparative effectiveness trial. Can J Anaesth. 2016;63:1128–1139. doi: 10.1007/s12630-016-0706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilicaslan A, Belviranli M, Okudan N, Nurullahoglu Atalik E. Single and repeated sevoflurane or desflurane exposure does not impair spatial memory performance of young adult mice. Fundam Clin Pharmacol. 2013;27:641–649. doi: 10.1111/fcp.12027. [DOI] [PubMed] [Google Scholar]
- 8.Klavir O, Prigge M, Sarel A, Paz R, Yizhar O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci. 2017;20:836–844. doi: 10.1038/nn.4523. [DOI] [PubMed] [Google Scholar]
- 9.Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–1334. doi: 10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Zhao Y, Yang J, Zhang X, Zhang W, Wang P. Neonatal repeated exposure to isoflurane not sevoflurane in mice reversibly impaired spatial cognition at juvenile-age. Neurochem Res. 2017;42:595–605. doi: 10.1007/s11064-016-2114-7. [DOI] [PubMed] [Google Scholar]
- 11.Min N, Hu QF, Li XP, Nie XH, Yang LL. Isoflurane effects on the proliferation and differentiation of neural stem cells in the hippocampus of neonatal rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:118–122. [Google Scholar]
- 12.Ni C, Li Z, Qian M, Zhou Y, Wang J, Guo X. Isoflurane induced cognitive impairment in aged rats through hippocampal calcineurin/NFAT signaling. Biochem Biophys Res Commun. 2015;460:889–895. doi: 10.1016/j.bbrc.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 13.Scheggia D, Zamberletti E, Realini N, Mereu M, Contarini G, Ferretti V, Managò F, Margiani G, Brunoro R, Rubino T, De Luca MA, Piomelli D, Parolaro D, Papaleo F. Remote memories are enhanced by COMT activity through dysregulation of the endocannabinoid system in the prefrontal cortex. Mol Psychiatry. 2017;23:1040–1050. doi: 10.1038/mp.2017.126. [DOI] [PubMed] [Google Scholar]
- 14.Su D, Zhao Y, Wang B, Li W, Xiao J, Chen J, Wang X. Repeated but not single isoflurane exposure improved the spatial memory of young adult mice. Acta Anaesthesiol Scand. 2011;55:468–473. doi: 10.1111/j.1399-6576.2010.02385.x. [DOI] [PubMed] [Google Scholar]
- 15.Tang CL, Li J, Zhang ZT, Zhao B, Wang SD, Zhang HM, Shi S, Zhang Y, Xia ZY. Neuroprotective effect of bispectral index-guided fast-track anesthesia using sevoflurane combined with dexmedetomidine for intracranial aneurysm embolization. Neural Regen Res. 2018;13:280–288. doi: 10.4103/1673-5374.226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vigano D, Guidali C, Petrosino S, Realini N, Rubino T, Di Marzo V, Parolaro D. Involvement of the endocannabinoid system in phencyclidine-induced cognitive deficits modelling schizophrenia. Int J Neuropsychopharmacol. 2009;12:599–614. doi: 10.1017/S1461145708009371. [DOI] [PubMed] [Google Scholar]
- 17.Wang JR, Sun PH, Ren ZX, Meltzer HY, Zhen XC. GSK-3β interacts with dopamine D1 receptor to regulate receptor function: implication for prefrontal cortical D1 receptor dysfunction in schizophrenia. CNS Neurosci Ther. 2017;23:174–187. doi: 10.1111/cns.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Lim G, Mao J, Sung B, Mao J. Regulation of the trigeminal NR1 subunit expression induced by inflammation of the temporomandibular joint region in rats. Pain. 2009;141:97–103. doi: 10.1016/j.pain.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Yin SW, Zhang N, Zhao P. High-concentration sevoflurane exposure in mid-gestation induces apoptosis of neural stem cells in rat offspring. Neural Regen Res. 2018;13:1575–1584. doi: 10.4103/1673-5374.237121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia P, Wang ZP, Jiang S, Zeng YM. Activation of glutamate transporters during sevoflurane precondition against ischemic brain damage. Zhongguo Zuzhi Gongcheng Yanjiu. 2007;11:2261–2264. [Google Scholar]
- 21.Xia T, Cui Y, Chu S, Song J, Qian Y, Ma Z, Gu X. Melatonin pretreatment prevents isoflurane-induced cognitive dysfunction by modulating sleep-wake rhythm in mice. Brain Res. 2016;1634:12–20. doi: 10.1016/j.brainres.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Dong Y, Zhou C, Zhang Y, Xie Z. Anesthetic sevoflurane reduces levels of hippocalcin and postsynaptic density protein 95. Mol Neurobiol. 2015;51:853–863. doi: 10.1007/s12035-014-8746-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plot of the minimum alveolar concentration (MAC) of each mouse in the isoflurane and sevoflurane groups.
The MACs determined for isoflurane and sevoflurane were 1.2% (MAC = 1.2 ± 0.11%) and 2.38% (MAC = 2.38 ± 0.23%), respectively. Data are expressed as the mean ± SD (n = 10).