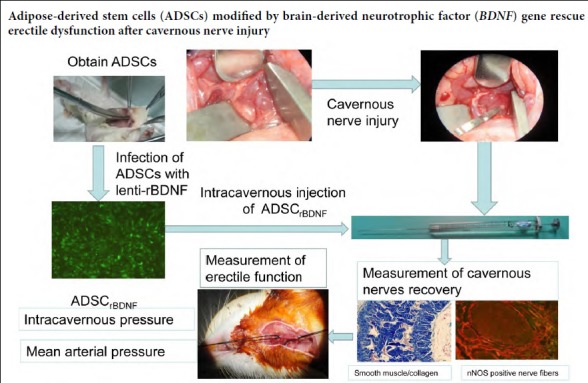
Keywords: adipose-derived stem cells, brain-derived neurotrophic factor, cavernous nerve injury, erectile dysfunction, infection, intracavernous injection, lentiviral vector, neuronal nitric oxide synthase, radical prostatectomy
Abstract
Cavernous nerve injury is the main cause of erectile dysfunction following radical prostatectomy. The recovery of erectile function following radical prostatectomy remains challenging. Our previous studies found that injecting adipose-derived stem cells (ADSCs) into the cavernosa could repair the damaged cavernous nerves, but the erectile function of the treated rats could not be restored to a normal level. We evaluated the efficacy of ADSCs infected with a lentiviral vector encoding rat brain-derived neurotrophic factor (lenti-rBDNF) in a rat model of cavernous nerve injury. The rats were equally and randomly divided into four groups. In the control group, bilateral cavernous nerves were isolated but not injured. In the bilateral cavernous nerve injury group, bilateral cavernous nerves were isolated and injured with a hemostat clamp for 2 minutes. In the ADSCGFP and ADSCrBDNF groups, after injury with a hemostat clamp for 2 minutes, rats were injected with ADSCs infected with lenti-GFP (1 × 106 in 20 μL) and lenti-rBDNF (1 × 106 in 20 μL), respectively. Erectile function was assessed 4 weeks after injury by measuring intracavernosal pressures. Then, penile tissues were collected for histological detection and western blot assay. Results demonstrated that compared with the bilateral cavernous nerve injury group, erectile function was significantly recovered in the ADSCGFP and ADSCrBDNF groups, and to a greater degree in the ADSCrBDNF group. Neuronal nitric oxide synthase content in the dorsal nerves and the ratio of smooth muscle/collagen were significantly higher in the ADSCrBDNF and ADSCGFP groups than in the bilateral cavernous nerve injury group. Neuronal nitric oxide synthase expression was obviously higher in the ADSCrBDNF group than in the ADSCGFP group. These findings confirm that intracavernous injection with ADSCs infected with lenti-rBDNF can effectively improve erectile dysfunction caused by cavernous nerve injury. This study was approved by the Medical Animal Care and Welfare Committee of Wuhan University, China (approval No. 2017-1638) on June 20, 2017.
Chinese Library Classification No. R459.9; R363; R364
Introduction
Recently, the detection of prostate-confined disease has been increasing because of earlier diagnosis by prostate-specific antigen screening, which has increased the number of radical prostatectomy procedures (Heidenreich et al., 2011; Martinez-Salamanca et al., 2015; Siegel et al., 2016). Radical prostatectomy is considered the gold standard for treatment of clinically localized prostate cancer (Heidenreich et al., 2011). Despite the use of nerve-sparing techniques, erectile dysfunction is a common complication of pelvic surgeries, such as radical prostatectomy, and it is associated with remarkably decreased quality of life (Burnett et al., 2008; Walz et al., 2010). It is very important to prevent this complication, because an increasing number of younger patients are undergoing radical prostatectomy procedures (Stephenson et al., 2005). To preserve potency in patients who undergo radical prostatectomy, research efforts have focused on pharmacological and gene- or stem cell-based therapies to improve neuroregeneration after cavernosal nerve injury (Bochiski et al., 2004; Albersen et al., 2008; Kato et al., 2009; Qiu et al., 2012; Hannan et al., 2013; May et al., 2013; Ying et al., 2013; You et al., 2013; Li et al., 2018). The primary etiology of erectile dysfunction following pelvic surgery is neurogenic and associated with iatrogenic trauma of the cavernous nerves, which provide autonomic regulation of penile erection. It is generally accepted that cavernous nerve injury is the main cause of erectile dysfunction (Sezen et al., 2009). Because the neuronal cell has poor ability to self-renew, it is difficult to return function to the damaged nerve. Thus, growth factors or stem cells represent a new approach in the treatment of erectile dysfunction (Harraz et al., 2010; Lin et al., 2016; Reed-Maldonado et al., 2016; Yiou et al., 2017; Wu et al., 2018; Zheng et al., 2018). Some studies have reported that growth factors and many neurotrophic factors, such as vascular endothelial growth factor and brain-derived neurotrophic factor (BDNF), play an important role in neural regeneration and recovery of erectile function after cavernous nerve injury (Lin et al., 2002; Hsieh et al., 2003). Studies have shown that BDNF, vascular endothelial growth factor, and the JAK/STAT signaling pathway are three important components in the regeneration of the cavernous nerve (Zhang et al., 2010; Luo et al., 2017; Wang et al., 2017). Among various neurotrophins, BDNF plays a specific and important role in penile nerve recovery after cavernous nerve injury (Bakircioglu et al., 2001). In addition, BDNF can promote neuronal survival during development and prevent neuronal death in experimental neuropathy models, and it is considered in the treatment of a variety of neurodegenerative diseases (Yan et al., 1997; Frostick et al., 1998; Apfel et al., 1999). The use of mesenchymal stem cells infected with recombinant adenoviruses expressing human BDNF (rAd/hBDNF) may have a better effect on erectile dysfunction caused by cavernous nerve injury compared with BDNF alone (Kim et al., 2012). However, the effects of combined therapies with BDNF and adipose-derived stem cells (ADSCs) have not yet been studied. Therefore, the aim of this study was to evaluate the effect of ADSCs infected with lentiviral vector expressing rat BDNF (lenti-rBDNF) on erectile function in a rat model of cavernous nerve injury.
Materials and Methods
Animals
Forty male specific-pathogen-free Sprague-Dawley rats weighing 250 to 300 g and aged 4 months with normal erectile function (verified by copulatory tests) were obtained from the Experimental Animal Center of Wuhan University, China (animal license No. SCXK (E) 2008-0004). This study was approved by the Medical Animal Care and Welfare Committee of Wuhan University, China (approval No. 2017-1638) on June 20, 2017 and treated in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
The rats were randomly divided into a control group (identification of the cavernous nerves bilaterally without further surgical manipulation; n = 10); a bilateral cavernous nerve injury (BCNI) group identification and injury of the cavernous nerves bilaterally by using a hemostat (clamp; n = 10); an ADSCGFP group (BCNI followed by injection with ADSCs via intracavernous injection with lenti-GFP; n = 10); and an ADSCrBDNF group (BCNI followed by intracavernous injection with ADSCs infected with lenti-rBDNF; n = 10).
Preparation of rat ADSCs
Isolation and culture of ADSCs were according to our previously published methods (Ying et al., 2012). Briefly, inguinal fat pads were collected from 1-month-old rats under anesthesia with intraperitoneal injection of 2% sodium pentobarbital 40 mg/kg. The tissues were washed in phosphate-buffered saline (PBS), cut into pieces, and digested with type I collagenase. Enzyme activity was neutralized with Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. The sections were centrifuged at 300 × g (1200 r/min) for 10 minutes to obtain high-density cellular particles. The stem cellular particles were collected following centrifugation and digested for 2 hours in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum in 5% humidified CO2 at 37°C. ADSCs were examined for surface markers (CD34, CD45, CD44) by flow cytometry (BD, Franklin, NJ, USA) as in our previous report (Ying et al., 2012).
Plasmid construction
pcDNA3.1 plasmid encoding rat BDNF (pcDNA3.1-rBDNF) was purchased from Shanghai Genechem Co. Ltd. (Shanghai, China). A third-generation self-inactivating lentiviral vector containing a CMV-driven enhanced green fluorescent protein (EGFP) reporter was purchased from Shanghai Genechem Co., Ltd. The lentiviral vector system had three parts before packaging: pGC-E1 vector, pHelper 1.0 (gag/pol element) vector, and pHelper 2.0 (VSVG element) vector. Primer pairs for amplifying rBDNF from pcDNA3.1-rBDNF and cloning into the AgeI site of pGC-E1vector are shown in Table 1.
Table 1.
Primer sequence
| ID | Sequence (5′–3′) | Product size (bp) |
|---|---|---|
| BDNF(5096-4)-P1 | GAG GAT CCC CGG GTA CCG GTC GCC ACC ATG ACC ATC CTT TTC CTT AC | 791 |
| BDNF(5096-4)-P2 | TCC TTG TAG TCC ATA CCT CTT CCC CTT TTA ATG GTC | 791 |
BDNF: Brain-derived neurotrophic factor.
The full-length cDNA of rBDNF was cloned into pGC-E1 by digesting with AgeI and ligating the resultant fragments into the AgeI site of the pGC-E1 vector (pGC-E1- rBDNF). PCR and DNA sequencing were used to confirm accurate insertion of rBDNF cDNA.
Production and titration of recombinant lentiviral vectors
To prepare pseudotyped lentiviral vectors, pHelper1.0 plasmid DNA (15 μg), pHelper 2.0 plasmid DNA (10 μg), and pGC-E1-rBDNF or pGC-E1-GFP plasmid DNA (20 μg) were co-transfected into subconfluent 293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Infectious lentiviruses were harvested at 48 hours post-transfection. The recombinant lenti-rBDNF and lenti-GFP viral vector titers were assessed by using fluorescence-activated cell sorting analysis of GFP+ 293T cells (Invitrogen). Virus titers were 109 transducing units/mL of medium.
Infection of ADSCs with lentiviral vectors
Third-passage cells were used for our study. On the day of infection, cells were plated at 4 × 103 cells/well in 96-well plates along with lenti-rBDNF at different multiplicities of infection (MOI) in serum-free growth medium containing 5 μg/mL Polybrene (Sigma-Aldrich, St. Louis, MO, USA) for 4 hours. Serum-containing growth medium was added for 48 hours and then replaced. At 4 and 5 days post-infection, reporter gene expression was examined using fluorescent microscopy. ADSCs were plated in T-75 flasks and infected at an MOI of 100 for transplantation. The ADSCGFP and ADSCrBDNF cells were passaged and prepared for cell transplantation.
Detection of BDNF expression
Total protein was extracted from either infected ADSCs or uninfected ADSCs. Lysate containing 20 μg of protein was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted. The membrane was sequentially incubated with monoclonal mouse anti-rat BDNF antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-GAPDH antibody (1:1000; Santa Cruz Biotechnology) at 37°C for 24 hours. Following goat anti-mouse secondary antibody (Santa Cruz Biotechnology) incubations at the ambient temperature for 1 hour, signals were visualized by enhanced chemiluminescence (Eastman Kodak, Rochester, NY, USA).
Rat models of cavernous nerve injury and cell transplantation
The rat cavernous nerve injury model was performed as described by Fandel et al. (2012). After abdominal anesthesia with 2% pentobarbital (40 mg/kg; Shanghai Bi-Yun-Tian Biotechnology, Shanghai, China), body temperature was maintained isothermically with a heating pad at 37°C. A midline incision was made on the abdominal skin to expose the bladder and prostate. The bilateral major pelvic ganglion was dissected from the lateral areas of the prostate with the aid of a surgical microscope (SXP-1C; Medical Optical Instruments Factory of Shanghai Medical Instruments Co., Ltd., Shanghai, China). From the bilateral major pelvic ganglion, cavernous nerves were identified toward the corpus cavernosum. The other three groups underwent bilateral cavernous nerve-crushing injury by using a hemostat clamp (Cheng-He Microsurgical Instruments Factory, Ningbo, Zhejiang Province, China) for 2 minutes. In the two treatment groups, ADSCGFP (1 × 106 in 20 μL), infected with lenti-GFP, or ADSCrBDNF (1 × 106 in 20 μL), infected with lenti-rBDNF, was injected into the penile cavernous tissue after BCNI.
Measurement of erectile function
Four weeks after model establishment, all rats were assessed for erectile function. In brief, after abdominal anesthesia with 2% pentobarbital (40 mg/kg; Shanghai Bi-Yun-Tian Biotechnology), the cavernous nerves and major pelvic ganglion were exposed bilaterally via midline laparotomy using a 23-G needle. After cavernous nerves were heparinized (250 U/mL), the 23-G needle end was connected to pressure transducers. The proximal parts of the cavernous nerves were activated using platinum electrodes connected to a stimulator. The stimulus parameters were 20 Hz, duration of 50 seconds, 0.2-ms pulse width, and 1.5 mA. After carotid artery cannulation, mean arterial pressure (MAP) and intracavernous pressure (ICP) were recorded using a data acquisition system (Chengdu TME Technology Co., Ltd., Chengdu, China). Finally, the maximal ICP/MAP ratio was calculated. The penis and cavernous nerves were then harvested for histology.
Immunofluorescence staining
Freshly dissected penis tissue was fixed for 4 hours in cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer, followed by overnight immersion in buffer solution containing 30% sucrose. Tissues were frozen in optimum cutting temperature compound (Sakura Finetek, Torrance, CA, USA) and stored at −80°C until use. Sections were cut at 5 mm, adhered to charged slides, air dried for 10 minutes, and rehydrated with PBS. Goat serum 3% in PBS was applied as blocking agent for 30 minutes. Sections were incubated overnight at 4°C with (1:400) rabbit anti-mouse monoclonal neuronal nitric oxide synthase (nNOS; Santa Cruz Biotechnology), followed by incubation with a 1:500 dilution of goat anti-rabbit secondary antibody (Santa Cruz Biotechnology) conjugated with Alexa Fluor 488 (Invitrogen) for 1 hour at 37°C. To analyze the content of nNOS, the total areas of the dorsal nerves and the areas of nNOS-positive fibers in the dorsal nerves were evaluated at 200× magnification using a fluorescence microscope (Adobe, San Jose, CA, USA).
Masson’s trichrome staining
Masson’s trichrome staining was conducted according to our previous method (Ying et al., 2013). Briefly, the middle part of penile tissue was fixed in formalin (10%) overnight, stored, and washed at 4°C with 70% alcohol. Then, the penile tissue was sliced into 5-μm paraffin-embedded sections for Masson’s trichrome staining. The color distribution of the muscle tissues was observed by using Adobe Photoshop CS 8.0 (Adobe). Using a light microscope (BX60; Olympus, Tokyo, Japan), we selected muscle tissues, which were stained red after all color distributions of the images were calculated.
Western blot assay
To conduct the western blot, the penis was excised, the urethra removed, and the corpus cavernosum homogenized in Tris-HCl buffer (pH 7.5). Cytosolic and membrane fractions were isolated to measure cytosolic nNOS protein content by western blot assay. Lysate containing 50 μg of protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted. The membranes were reacted with blocking buffer (5% skim milk in Tris-buffered saline-Tween) for 30 minutes at 4°C and incubated with rabbit anti-mouse nNOS monoclonal antibody (1:1000; Santa Cruz Biotechnology) and goat anti-rabbit GAPDH monoclonal antibody (1:1000; Santa Cruz Biotechnology) for 24 hours. Following secondary antibody incubations at 4°C for 1 hour, signals were visualized by enhanced chemiluminescence. Finally, the protein bands were visualized using an enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). GAPDH was used as an internal control for normalization to determine protein expression levels. The relative gray levels of nNOS to GAPDH were measured using an ultraviolet photometry imaging system (UVP LLC, Upland, CA, USA).
Statistical analysis
All data are reported as the mean ± SD. Statistical analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was used for the first analysis of our results. A Student-Newman-Keuls post hoc test was conducted if differences were significant. A value of P < 0.05 was considered statistically significant.
Results
Morphology and phenotype of cultured ADSCs
ADSCs expressed CD44 and were negative for CD34 and CD45 on the third passage (Figure 1A).
Figure 1.
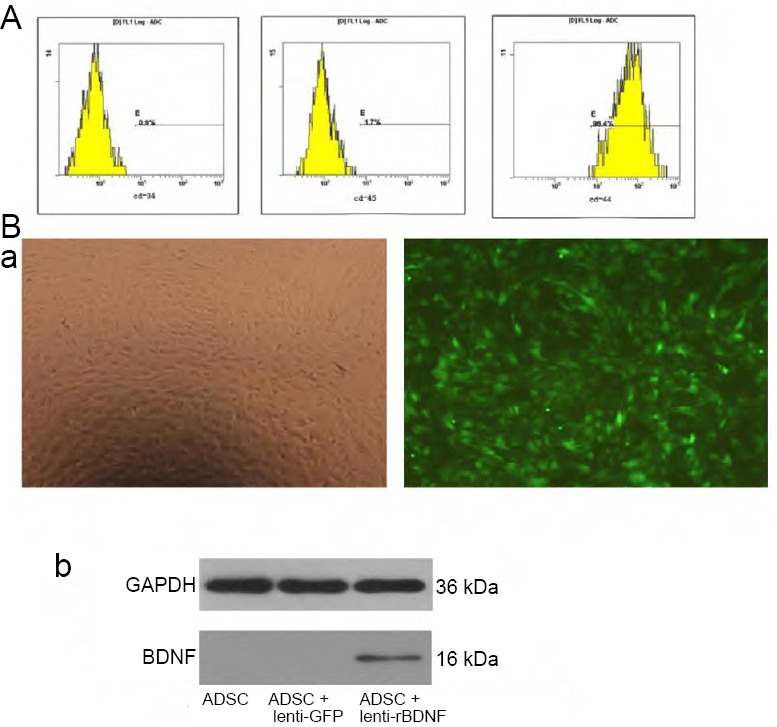
Identification and infection of ADSCs.
(A) Phenotypes of ADSCs at the third passage by flow cytometry; a: CD34 (0.9%), b: CD45 (1.7%), c: CD44 (96.4%). (B) Determination of lentiviral infection efficiency and rBDNF expression (arrows) in ADSCs. Panel (a) Infection efficiency 3 days after infection with lenti-rBDNF at an MOI of 100. GFP (green) was observed under light (left) or fluorescence (right) microscopy (original magnification, 100×). Panel (b) Representative immunoblot of rBDNF protein as measured by western blot assay. ADSCs were infected with control lentivirus (ADSC + lenti-GFP) or lenti-rBDNF (ADSC + lenti-rBDNF) at an MOI of 100. ADSCs: Adipose-derived stem cells; GFP: green fluorescent protein; MOI: multiplicity of infection; rBDNF: rat brain-derived neurotrophic factor.
Infection efficiency of lenti-rBDNF and rBDNF gene in ADSCs
The third-passage ADSCs were infected with lenti-rBDNF at different MOIs. Expression of GFP was examined 3 days after infection. The infection efficiency was –approximately 80% at an MOI of 100. (Figure 1B, panel a); therefore, an MOI of 100 was chosen for the study. To verify rBDNF gene expression in ADSCs, rBDNF protein levels were examined in infected ADSCs. The rBDNF protein was found in lenti-rBDNF-infected ADSCs, but not in ADSCs or lenti-GFP–infected ADSCs (Figure 1B, panel b).
Erectile function
The ICP and the ICP/MAP ratio of rats in the BCNI group were decreased compared with that of the control group (P < 0.05). The ICP of rats in the ADSCrBDNF group was much higher than that in rats in the ADSCGFP group (P < 0.05) but still lower than that of rats in the control group (P < 0.05). The ICP/MAP ratio was higher in the ADSCrBDNF group than in the BCNI group (P < 0.05) but lower than that in the control group (P < 0.05; Figure 2).
Figure 2.
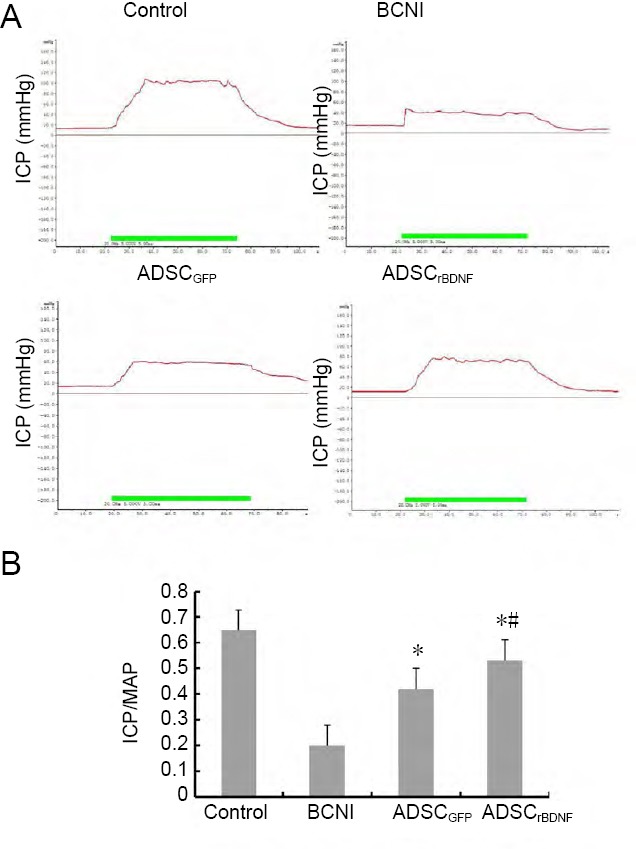
Effects of ADSCs infected with lenti-rBDNF on nerve conduction capacity.
(A) Representative recordings of ICP for each group in response to cavernous nerve stimulation. (B) ICP/MAP ratio was compared among each group of rats. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by the Student-Newman-Keuls post hoc test). *P < 0.05, vs. BCNI group; #P < 0.05, vs. ADSCGFP group. BCNI: Bilateral cavernous nerve injury; GFP: green fluorescent protein; ICP: intracavernosal pressure; MAP: mean arterial pressure; rBDNF: rat brain-derived neurotrophic factor.
ADSCs infected with lenti-rBDNF increase the number of nNOS-positive nerve fibers in the penile tissue of a rat model of cavernous nerve injury
nNOS-positive nerve fibers were diffusely distributed throughout the penis and were usually observed on the periphery of dorsal nerves of the penis. In the dorsal nerves, the number of the nNOS-positive nerve fibers was significantly decreased in the BCNI group compared with the control group (P < 0.05). The number of nNOS-positive nerve fibers was significantly higher in the ADSCrBDNF and ADSCGFP groups than in the BCNI group (P < 0.05) but less than in the control group (P < 0.05; Figure 3).
Figure 3.
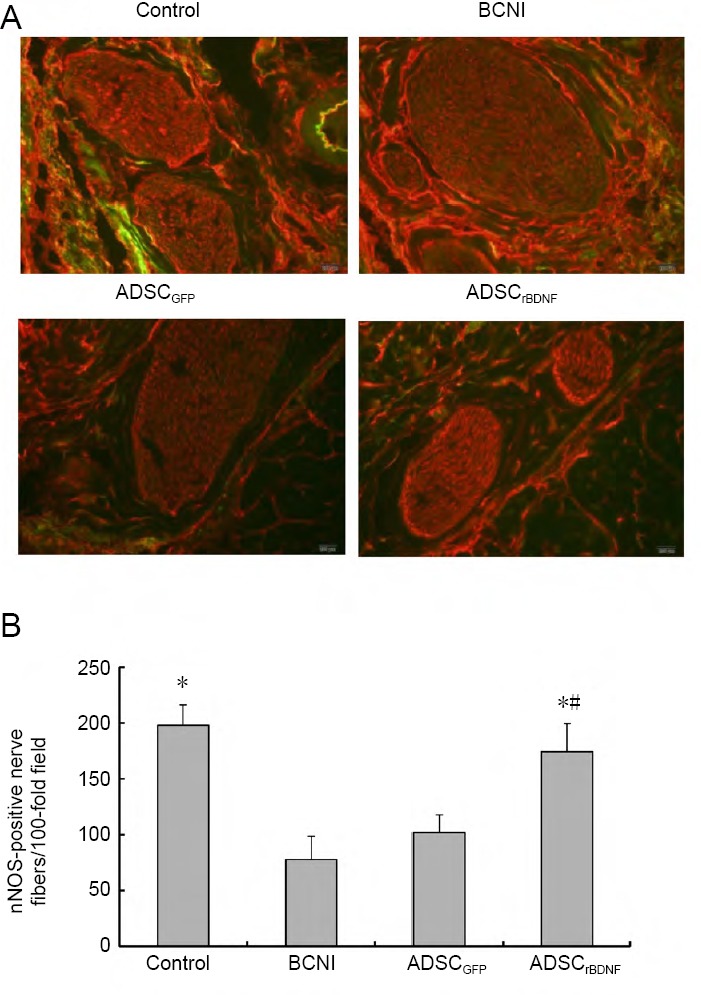
nNOS staining in a penile midshaft specimen.
(A) nNOS (red-stained fiber) in penile tissue (immunofluorescence microscope: 200×). Control group: Predominant red staining of nerve fibers; BCNI group: a paucity of red-stained fibers; ADSCrBDNF group: a significant increase in the number of red-stained nerve fibers compared with the ADSCGFP group. (B) nNOS-positive nerve fibers in penile midshaft sections. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by the Student-Newman-Keuls post hoc test). *P < 0.05, vs. BCNI group; #P < 0.05, vs. ADSCGFP group. ADSC: Adipose-derived stem cell; BCNI: bilateral cavernous nerve injury; GFP: green fluorescent protein; nNOS: neuronal nitric oxide synthase; rBDNF: rat brain-derived neurotrophic factor.
ADSCs infected with lenti-rBDNF increase smooth muscle in the penile tissue of a rat model of cavernous nerve injury
Computerized histomorphometric analysis showed that the ratio of smooth muscle/collagen was lower in the BCNI group than in the control group (P < 0.05). The ratio of smooth muscle/collagen was almost fully preserved in the ADSCrBDNF group compared with the control group (P > 0.05) and much higher than that in the ADSCGFP group (P < 0.05; Figure 4).
Figure 4.
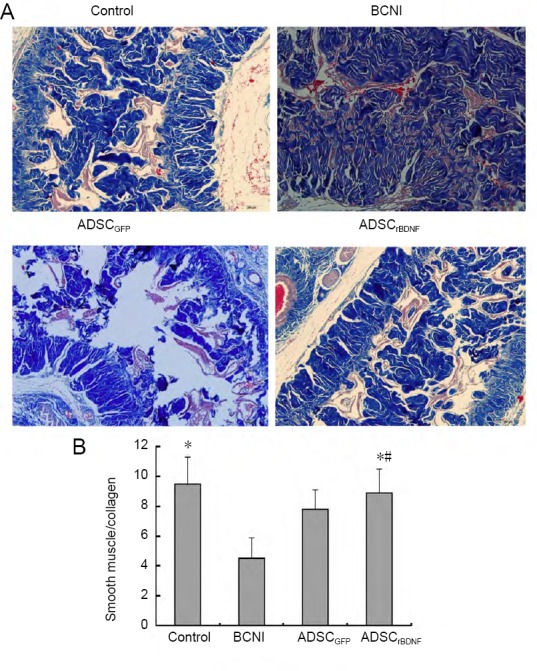
Effect of ADSCrBDNF on smooth muscle/collagen ratio in penile tissue of a rat model of cavernous nerve injury.
(A) Smooth muscle (red)/collagen (blue) changes in penile tissue with a light microscope (original magnification, 100×). (B) Ratio of smooth muscle/collagen. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by the Student-Newman-Keuls post hoc test). *P < 0.05, vs. BCNI group; #P < 0.05, vs. ADSCGFP group. ADSC: Adipose-derived stem cell; BCNI: bilateral cavernous nerve injury; GFP: green fluorescent protein; rBDNF: rat brain-derived neurotrophic factor.
Quantification of nNOS protein expression
Western blot assay results showed that nNOS expression was decreased in the BCNI group compared with the control group (P < 0.05). The expression of nNOS was significantly increased in the ADSCrBDNF and ADSCGFP groups compared with the BCNI group (P < 0.05; Figure 5).
Figure 5.
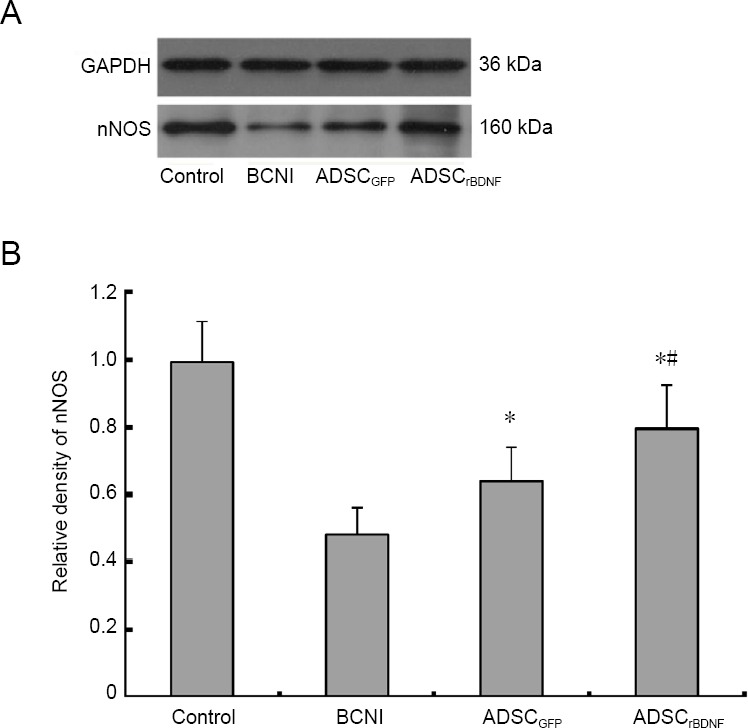
Effects of ADSCrBDNF on nNOS expression in the corpus cavernous tissue of rats after cavernous nerve crush injury.
(A) Western blot assay of nNOS expression in the corpus cavernosum at 4 weeks in the control group, BCNI group, ADSCGFP group, and ADSCrBDNF group. (B) Relative gray levels of nNOS to GAPDH. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by the Student-Newman-Keuls post hoc test). *P < 0.05, vs. BCNI group; #P < 0.05, vs. ADSCGFP group. ADSC: Adipose-derived stem cell; BCNI: bilateral cavernous nerve injury; GFP: green fluorescent protein; nNOS: neuronal nitric oxide synthase; rBDNF: rat brain-derived neurotrophic factor.
Discussion
Our studies showed a measurable neurotrophic effect of ADSCs and lenti-rBDNF on cavernous nerve regeneration following crush injury in a rat model. The restoration of erectile function was confirmed by both functional and morphological studies. The ICP changes and ICP/MAP indicated that the erectile response was better in the ADSCrBDNF and ADSCGFP groups than in the BCNI group at 4 weeks.
The ADSCrBDNF group showed a significant increase in nNOS content in the dorsal nerves. The nNOS content was significantly higher in the ADSCrBDNF and ADSCGFP groups than in the BCNI group, which indicated the recovery of cavernous nerves directly. In addition, the western blot assay showed that expression of nNOS proteins was increased in the ADSC-treated groups compared with the BCNI group.
Therapy using stem cells derived from adipose tissue has recently become an attractive treatment for tissue regeneration and engineering because of the differentiation potential of this cell type (An et al., 2010; Wu et al., 2010; Nagasaki et al., 2011; Ying et al., 2012; Jang et al., 2016; Li et al., 2017). ADSCs can be obtained from ubiquitously distributed adipose tissue with little harm to the patient and are expandable in vitro (Kim et al., 2008). In addition, ADSCs secrete neurotrophic factors that promote cavernous nerve regeneration (Albersen et al., 2010; Zhang et al., 2011). Therefore, many studies using ADSCs have reported restoration of erectile function following dysfunction caused by many factors, such as hypercholesterolemia, diabetes, and cavernous nerve injury (Wankhade et al., 2016; Zhu et al., 2017). In our study, intracavernous injection of ADSCGFP was expected to have a role in the restoration of erectile function. The ICP/MAP ratio was significantly higher in the ADSCGFP group than in the BCNI group. Furthermore, the preservation of cavernous smooth muscle was much better in the ADSCrBDNF group than in the BCNI group. These findings agree with those of other studies that evaluated the effect of various stem cells on neurogenic erectile dysfunction (Albersen et al., 2010; Harraz et al., 2010; Zhang et al., 2011). Considering these results, we propose that erectile function after cavernous nerve injury can be preserved by the regenerative effect of BDNF from ADSCrBDNF cells in the major pelvic ganglion.
Neurotrophins such as BDNF, growth differentiation factor-5, and neutrin act as neuromodulators in neuronal survival after cavernous nerve injury (Bella et al., 2009). BDNF belongs to the mammalian family of neurotrophins and can promote neuronal survival during development and prevent neuronal death in experimental neuropathy models (Yan et al., 1997; Frostick et al., 1998). BDNF provides neurotrophic support for a host of neuron types, such as peripheral sensory neurons and motor neurons (Sendtner et al., 1992). As we know, the JAK/STAT pathway modulates central and peripheral nerve regeneration by transducing cellular signals directly from the cell membrane to the nucleus. The mechanism by which BDNF promotes neurite growth in the major pelvic ganglion is through the JAK/STAT pathway (Bella et al., 2006; Lin et al., 2006).
Our results showed that intracavernous injection of ADSCrBDNF had a positive effect on cavernous nerve regeneration and functional recovery. Bakircioglu et al. (2001) found that intracavernous injection of adeno-associated virus (AAV)-BDNF may prevent the degeneration of nNOS-containing neurons in the major pelvic ganglion, facilitate the regeneration of nNOS-containing nerve fibers in penile tissue, and enhance the recovery of erectile function after BCNI. Piao et al. (2012) used ADSCs with BDNF-membrane on the cavernous nerve to improve erectile function in a rat model of erectile dysfunction post-prostatectomy. Their results showed that ADSC/BDNF-membrane treatment increased smooth muscle/collagen ratio, nNOS content, phospho-eNOS protein expression, and cyclic guanosine monophosphate level compared with BCNI and other treatment groups. Jeong et al. (2013) showed that udenafil combined with ADSC/BDNF-membrane protected the cavernous nerve and improved angiogenesis in the corpus cavernosum after cavernous nerve injury, which further maintained erectile function in a rat model of erectile dysfunction post-prostatectomy. In the current study, results of Masson’s trichrome staining showed that the muscle content was decreased and collagen content increased in the BCNI group. The smooth muscle/collagen ratio was higher in the ADSCrBDNF and ADSCGFP groups than in the BCNI group. The western blot assay and immunofluorescence showed that nNOS was observed in the corpus cavernosum of the ADSCrBDNF and ADSCGFP groups 4 weeks after injury. nNOS was remarkably elevated after intracavernous injection of ADSCs infected with lenti-rBDNF. These results are similar to those of previous studies (Bakircioglu et al., 2001; Piao et al., 2012; Jeong et al., 2013). Histologic evaluation of the dorsal nerves in the ADSCrBDNF group showed a dramatic increase in the number of myelinated axons and nNOS content, which could indicate regeneration of cavernous nerves.
Although our study demonstrated successful increase of erectile function following combined treatment with ADSCs and BDNF, further study is required. Our study had three important limitations. The first relates to clinical application: the safety of the lentivirus used as a vector for introduction of BDNF into ADSCs has not yet been established in humans. Second, our study lasted for 4 weeks, so we do not know whether the effect of ADSCrBDNF would persist up to 8 weeks or 3 months or longer. Third, the mechanism of preserving erectile function after cavernous nerve injury depends on whether ADSCs that differentiate into neural tissue have a regenerative effect in the major pelvic ganglion. Therefore, further investigations are needed.
In summary, functional and histological restoration of erectile dysfunction was better in the ADSCrBDNF group than in the ADSCGFP group after cavernous nerve injury. Injection of ADSCs infected with a lentiviral vector encoding rBDNF may be an effective method to treat erectile dysfunction following radical prostatectomy and deserves further clinical research.
Additional file: Open peer review report 1 (123KB, pdf) .
Acknowledgments:
We are very grateful to Rui Li from Experimental Animal Center of Wuhan University, China, for routine care and husbandry of rats used in these studies.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the Natural Science Foundation of Hubei Province of China, No. 2017CFB176 (to CCY); the Fundamental Research Funds for The Central Hospital of Wuhan of China, No. YB16A01 (to CCY). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The study was approved by the Medical Animal Care and Welfare Committee of Wuhan University, China (approval No. 2017-1638) on June 20, 2017. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Aldo Calliari, Veterinary School-UdelaR, Uruguay.
Funding: This study was supported by the Natural Science Foundation of Hubei Province of China, No. 2017CFB176 (to CCY); the Fundamental Research Funds for The Central Hospital of Wuhan of China, No. YB16A01 (to CCY).
P-Reviewer: Cook AD; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Adam L, Frenchman B, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albersen M, Joniau S, Claes H, Van H. Preclinical evidence for the benefits of penile rehabilitation therapy following nerve-sparing radical prostatectomy. Adv Urol. 2008;2008:594868. doi: 10.1155/2008/594868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An C, Cheng Y, Yuan Q, Li J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng. 2010;38:1647–1654. doi: 10.1007/s10439-009-9892-x. [DOI] [PubMed] [Google Scholar]
- 4.Apfel SC. Neurotrophic factors in peripheral neuropathies: therapeutic implications. Brain Pathol. 1999;9:393–413. doi: 10.1111/j.1750-3639.1999.tb00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakircioglu ME, Lin CS, Fan P, Sievert KD, Kan YW, Lue TF. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J Urol. 2001;165:2103–2109. doi: 10.1097/00005392-200106000-00078. [DOI] [PubMed] [Google Scholar]
- 6.Bella AJ, Lin G, Lin CS, Hickling DR, Morash C, Lue TF. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009;6(Suppl 3):347–352. doi: 10.1111/j.1743-6109.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bella AJ, Lin G, Tantiwongse K, Garcia M, Lin CS, Brant W. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part I. J Sex Med. 2006;3:815–820. doi: 10.1111/j.1743-6109.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 8.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, Lue TF. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–909. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AL, Teloken PE, Briganti A, Whitehurst T, Montorsi F. Intraoperative assessment of an implantable electrode array for cavernous nerve stimulation. J Sex Med. 2008;5:1949–1954. doi: 10.1111/j.1743-6109.2008.00865.x. [DOI] [PubMed] [Google Scholar]
- 10.Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61:201–210. doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Hannan J L, Albersen M, Kutlu O, Gratzke C, Stief CG, Burnett AL, Lysiak JJ, Hedlund P, Bivalacqua TJ. Inhibition of rho-kinase improves erectile function, increases nitric oxide signaling and decreases penile apoptosis in a rat model of cavernous nerve injury. J Urol. 2013;189:1155–1161. doi: 10.1016/j.juro.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harraz A, Shindel AW, Lue TF. Emerging gene and stem cell therapies for the treatment of erectile dysfunction. Nat Rev Urol. 2010;7:143–152. doi: 10.1038/nrurol.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidenreich A, Bellmunt J, Bolla M. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh PS, Bochinski DJ, Lin GT, Nunes L, Lin CS, Lue TF. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int. 2003;92:470–475. doi: 10.1046/j.1464-410x.2003.04373.x. [DOI] [PubMed] [Google Scholar]
- 16.Jang S, Cho HH, Kim SH, Lee KH, Cho YB, Park JS, Jeong HS. Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs. Neural Regen Res. 2016;11:994–1000. doi: 10.4103/1673-5374.184503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong HH, Piao S, Ha JN, Kim IG, Oh SH, Lee JH, Cho HJ, Hong SH, Kim SW, Lee JY. Combined therapeutic effect of udenafil and adipose-derived stem cell (ADSC)/Brain-derived Neurotrophic Factor (BDNF)-membrane system in a rat model of cavernous nerve injury. Urology. 2013;81:1108. doi: 10.1016/j.urology.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Kato R, Wolfe D, Coyle CH. Herpes simplex virus vector-mediated delivery of neurturin rescues erectile dysfunction of cavernous nerve injury. Gene Ther. 2009;16:26–33. doi: 10.1038/gt.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Lee MR, Kim JH, Jee MK, Kang SK. IFATS collection: selenium induces improvement of stem cell behaviors in human adipose tissue stromal cells via SAPK/JNK and stemness acting signals. Stem Cells. 2008;26:2724–2734. doi: 10.1634/stemcells.2008-0184. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Choi SW, Hur KJ. Synergistic effect of mesenchymal stem cells infected with recombinant adenovirus expressing human BDNF on erectile function in a rat model of cavernous nerve injury. Korean J Urol. 2012;53:726–732. doi: 10.4111/kju.2012.53.10.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Lei H, Xu Y, Li H, Yang B, Yu C, Yuan Y, Fang D, Xin Z, Guan R. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology. 2018;6:927–935. doi: 10.1111/andr.12519. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Xu W, Cheng LY. Adipose-derived mesenchymal stem cells accelerate nerve regeneration and functional recovery in a rat model of recurrent laryngeal nerve injury. Neural Regen Res. 2017;12:1544–1550. doi: 10.4103/1673-5374.215267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CS, Ho HC, Chen KC, Lin G, Nunes L, Lue TF. Intracavernosal injection of vascular endothelial growth factor induces nitric oxide synthase isoforms. BJU Int. 2002;89:955–960. doi: 10.1046/j.1464-410x.2002.02792.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin G, Bella AJ, Lue TF, Lin CS. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J Sex Med. 2006;3:821–827. doi: 10.1111/j.1743-6109.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin H, Dhanani N, Tseng H, Souza GR, Wang G. Nanoparticle improved stem cell therapy for erectile dysfunction in a rat model of cavernous nerve injury. J Urol. 2016;195:788–795. doi: 10.1016/j.juro.2015.10.129. [DOI] [PubMed] [Google Scholar]
- 26.Luo G, Xu B, Huang Y. Icariside II promotes the osteogenic differentiation of canine bone marrow mesenchymal stem cells via the PI3K/AKT/mTOR/S6K1 signaling pathways. Am J Transl Res. 2017;9:2077–2087. [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Salamanca JI, La Fuente JM, Fernandez A, Martinez-Salamanca E, Pepe-Cardoso AJ. Nitrergic function is lost but endothelial function is preserved in the corpus cavernosum and penile resistance arteries of men after radical prostatectomy. J Sex Med. 2015;12:590–599. doi: 10.1111/jsm.12801. [DOI] [PubMed] [Google Scholar]
- 28.May F, Buchner A, Schlenker B, Gratzke C, Arndt C, Stief C, Weidnerb N, Matiasek K. Schwann cell‐mediated delivery of glial cell line-derived neurotrophic factor restores erectile function after cavernous nerve injury. Int J Urol. 2013;20:344–348. doi: 10.1111/iju.12078. [DOI] [PubMed] [Google Scholar]
- 29.Nagasaki H, Shang Q, Suzuki T. Low-serum culture system improves the adipogenic ability of visceral adipose tissue-derived stromal cells. Cell Biol Int. 2011;35:559–568. doi: 10.1042/CBI20100406. [DOI] [PubMed] [Google Scholar]
- 30.Piao S, Kim IG, Lee JY, Hong SH, Kim SW, Hwang TK, Heang S, Lee JH, Ra JC, Lee JY. Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J Sex Med. 2012;9:1968–1979. doi: 10.1111/j.1743-6109.2012.02760.x. [DOI] [PubMed] [Google Scholar]
- 31.Qiu X, Fandel TM, Ferretti L, Albersen M M, Orabi H, Zhang HY, Lin GT, Lin CS, Schroeder T, Lue TF. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;62:720–727. doi: 10.1016/j.eururo.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed-Maldonado AB, Lue TF. The current status of stem-cell therapy in erectile dysfunction: a review. World J Mens Health. 2016;34:155–164. doi: 10.5534/wjmh.2016.34.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 34.Sezen SF, Lagoda G, Burnett AL. Role of immunophilins in recovery of erectile function after cavernous nerve injury. J Sex Med. 2009;6:340–346. doi: 10.1111/j.1743-6109.2008.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 36.Stephenson RA, Mori M, Hsieh YC, Beer TM, Stanford JL, Gilliland FD. Treatment of erectile dysfunction following therapy for clinically localized prostate cancer: patient reported use and outcomes from the surveillance, epidemiology, and end results prostate cancer outcomes study. J Urol. 2005;174:646–650. doi: 10.1097/01.ju.0000165342.85300.14. [DOI] [PubMed] [Google Scholar]
- 37.Walz J, Burnett AL, Costello AJ. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179. doi: 10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Chu Y, Yue B, Ma X, Zhang G, Xiang H, Liu Y, Wang T, Wu X, Chen B. Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget. 2017;8:23803–23816. doi: 10.18632/oncotarget.15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wankhade UD, Shen M, Kolhe R, Fulzele S. Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem Cells Int. 2016;2016:3206807. doi: 10.1155/2016/3206807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu G, Zheng X, Jiang Z. Induced differentiation of adipose-derived stromal cells into myoblasts. J Huazhong Univ Sci Technolog Med Sci. 2010;30:285–290. doi: 10.1007/s11596-010-0344-5. [DOI] [PubMed] [Google Scholar]
- 41.Wu H, Tang WH, Zhao LM, Liu DF, Yang YZ, Zhang HT, Zhang Z, Hong K, Lin HC, Jiang H. Nanotechnology-assisted adipose-derived stem cell (ADSC) therapy for erectile dysfunction of cavernous nerve injury: In vivo cell tracking, optimized injection dosage, and functional evaluation. Asian J Androl. 2018;20:442–447. doi: 10.4103/aja.aja_48_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT. Expression of brainderived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- 43.Ying C, Hu W, Cheng B, Zheng X, Li S. Neural differentiation of rat adipose-derived stem cells in vitro. Cell Mol Neurobiol. 2012;32:1255–1263. doi: 10.1007/s10571-012-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying C, Yang M, Zheng X, Hu W, Wang X. Effects of intracavernous injection of adipose-derived stem cells on cavernous nerve regeneration in a rat model. Cell Mol Neurobiol. 2013;33:233–240. doi: 10.1007/s10571-012-9890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yiou R, Hamidou L, Birebent B, Bitari D, Le Corvoisier P. Intracavernous injections of bone marrow mononucleated cells for postradical prostatectomy erectile dysfunction: final results of the INSTIN Clinical Trial. Eur Urol Focus. 2017;3:643–645. doi: 10.1016/j.euf.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 46.You D, Jang M J, Lee J, Jeong IG, Kim HS, Moon KH, Suh N, Kim CS. Periprostatic implantation of human bone marrow-derived mesenchymal stem cells potentiates recovery of erectile function by intracavernosal injection in a rat model of cavernous nerve injury. Urology. 2013;81:104–110. doi: 10.1016/j.urology.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 47.Zhang HY, Jin X B, Lue TF. Three important components in the regeneration of the cavernous nerve: brain-derived neurotrophic factor, vascular endothelial growth factor and the JAK/STAT signaling pathway. Asian J Androl. 2010;13:231–235. doi: 10.1038/aja.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin CS. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437–446. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng T, Zhang TB, Wang CL, Zhang WX, Jia DH, Yang F, Sun YY, Ding XJ, Wang R. Icariside II promotes the differentiation of adipose tissue-derived stem cells to Schwann cells to preserve erectile function after cavernous nerve injury. Mol Cells. 2018;41:553–561. doi: 10.14348/molcells.2018.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu LL, Zhang Z, Jiang HS, Chen H, Chen Y. Superparamagnetic iron oxide nanoparticle targeting of adipose tissue-derived stem cells in diabetes-associated erectile dysfunction. Asian J Androl. 2017;19:425–432. doi: 10.4103/1008-682X.179532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


