Keywords: Ca2+/calmodulin-dependent protein kinase II, cognitive impairment, contextual fear conditioning, cyclic adenosine monophosphate response element binding protein, GLYX-13, isoflurane, N-methyl-D-aspartate receptor, novel object recognition, rapastinel
Abstract
Accumulating evidence indicates that inhalation anesthetics induce or increase the risk of cognitive impairment. GLYX-13 (rapastinel) acts on the glycine site of N-methyl-D-aspartate receptors (NMDARs) and has been shown to enhance hippocampus-dependent learning and memory function. However, the mechanisms by which GLYX-13 affects learning and memory function are still unclear. In this study, we investigated these mechanisms in a mouse model of long-term anesthesia exposure. Mice were intravenously administered 1 mg/kg GLYX-13 at 2 hours before isoflurane exposure (1.5% for 6 hours). Cognitive function was assessed using the contextual fear conditioning test and the novel object recognition test. The mRNA expression and phosphorylated protein levels of NMDAR pathway components, N-methyl-D-aspartate receptor subunit 2B(NR2B)-Ca2+/calmodulin dependent protein kinase II (CaMKII)-cyclic adenosine monophosphate response element binding protein (CREB), in the hippocampus were evaluated by quantitative RT-PCR and western blot assay. Pretreatment with GLYX-13 ameliorated isoflurane exposure-induced cognitive impairment and restored NR2B, CaMKII and CREB mRNA and phosphorylated protein levels. Intracerebroventricular injection of KN93, a selective CaMKII inhibitor, significantly diminished the effect of GLYX-13 on cognitive function and NR2B, CaMKII and CREB levels in the hippocampus. Taken together, our findings suggest that GLYX-13 pretreatment alleviates isoflurane-induced cognitive dysfunction by protecting against perturbation of the NR2B/CaMKII/CREB signaling pathway in the hippocampus. Therefore, GLYX-13 may have therapeutic potential for the treatment of anesthesia-induced cognitive dysfunction. This study was approved by the Experimental Animal Ethics Committee of Drum Tower Hospital affiliated to the Medical College of Nanjing University, China (approval No. 20171102) on November 20, 2017.
Chinese Library Classification No. R453; R842.1; R741
Introduction
N-methyl-D-aspartate receptors (NMDARs) belong to the ionotropic glutamate receptor family. NMDARs allow calcium influx, which induces Ca2+/calmodulin-dependent protein kinase II (CaMKII) phosphorylation, which in turn triggers multiple downstream signaling cascades, including the cyclic adenosine monophosphate response element binding protein (CREB) pathway, which is closely related to cognitive processes (Greer and Greenberg, 2008; Lisman et al., 2012; Müller et al., 2013; Sanhueza and Lisman, 2013; Vedder et al., 2013; Ferreira et al., 2017). Increasing evidence indicates that NMDAR hypofunction is a pathogenic trigger for schizophrenia and plays a role in neurodegenerative diseases, such as Alzheimer’s disease (Hardingham and Do, 2016).
GLYX-13 (rapastinel) is a tetrapeptide (threonine-proline-proline-threonine) that is a partial agonist at the glycine site of NMDAR subunit 2B (NR2B), increasing NR2B currents as well as protein levels (Burgdorf et al., 2013; Burgdorf et al., 2015). Furthermore, GLYX-13 increases the amplitude of long-term potentiation (Zhang et al., 2008) and improves spatial memory function in both young and old rodents (Moskal et al., 2005; Burgdorf et al., 2011).
The inhalation anesthetic isoflurane is widely used clinically. However, accumulating evidence indicates that inhalation anesthetics can impair cognitive function (Sen and Sen, 2016; Kang et al., 2017; Su et al., 2017; Zhao et al., 2019), and isoflurane has been shown to perturb synaptic plasticity (Briner et al., 2010). Our previous study showed that long-term isoflurane exposure induces cognitive impairment and a downregulation of NR2B expression in mice (Xia et al., 2016). In the present study, we investigate the effect of GLYX-13 on long-term isoflurane-induced cognitive dysfunction, as well as the role of the NR2B/CaMKII/CREB signaling pathway.
Materials and Methods
Animals
A total of 300 specific-pathogen-free male C57/B6J mice (weight 22–25 g, 8–10 weeks of age) were provided by the Model Animal Research Center of Nanjing University, Nanjing, Jiangsu, China (license No. SYXK (Su) 2014-0052). Animals were housed in groups under a 12/12-hour light/dark cycle, with controlled room temperature (23 ± 1°C) and humidity (50–70%). Ad libitum feeding was used throughout the study. All protocols and procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, and approved by the Experimental Animal Ethics Committee of Drum Tower Hospital affiliated to the Medical College of Nanjing University, China (approval No. 20171102) on November 20, 2017.
Experimental design
To study the effects of GLYX-13 on cognitive function in mice exposed to long-term isoflurane, mice were randomly assigned to control (Con, n = 5), isoflurane anesthesia (Anes, n = 5), GLYX-13 injection (GLYX-13, n = 5) and isoflurane anesthesia + GLYX-13 injection (Anes + GLYX-13, n = 5) groups. Mice in the Anes and Anes + GLYX-13 groups were exposed to 1.5% isoflurane for 6 hours. Mice in the GLYX-13 and Anes + GLYX-13 groups were intravenously administered GLYX-13 (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.9% saline 2 hours before isoflurane anesthesia at a dose of 1 mg/kg. The other two groups of mice were administered an equal volume of saline. Both contextual fear conditioning (CFC) test and novel object recognition (NOR) test were used to assess the cognitive function of mice 1, 3 and 7 days after isoflurane exposure. The mRNA and phosphoprotein levels of NR2B, CaMKII and CREB in the hippocampus were assessed by quantitative real time-polymerase chain reaction (qRT-PCR) and western blot assay 1, 3 and 7 days after isoflurane exposure (Figure 1).
Figure 1.
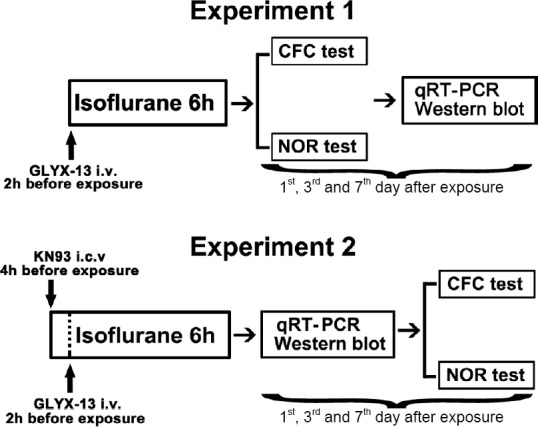
Schematic of the experimental design.
CFC: Contextual fear conditioning; GLYX-13: a tetrapeptide composed of threonine-proline-proline-threonine; i.c.v.: intracerebroventricular injection; i.v.: intravenous injection; KN93: a selective Ca2+/calmodulin-dependent protein kinase II inhibitor; NOR: novel object recognition; qRT-PCR: quantitative real time-polymerase chain reaction.
To clarify the mechanisms by which GLYX-13 affects cognitive function after long-term isoflurane exposure and to examine the role of the NR2B/CaMKII/CREB signaling pathway in this process, the CaMKII inhibitor KN93 was used. Mice were randomly assigned to isoflurane anesthesia (Anes, n = 5), isoflurane anesthesia + GLYX-13 injection (Anes + GLYX-13, n = 5), isoflurane anesthesia + KN93 injection (Anes + KN93, n = 5) and isoflurane anesthesia + GLYX-13 + KN93 injection (Anes + GLYX-13 + KN93, n = 5) groups. All mice were exposed to 1.5% isoflurane for 6 hours. KN93 (Tocris Bioscience, Bristol, UK) was dissolved in 0.9% saline containing 1% dimethyl sulfoxide and diluted to a concentration of 1 mM. Mice in the Anes + KN93 and Anes + GLYX-13 + KN93 groups were administered 1 μL of 1 mM KN93 by intracerebroventricular injection 4 hours before isoflurane exposure. Mice in the other two groups were injected with an equal volume of saline. Mice in the Anes + GLYX-13 and Anes + GLYX-13 + KN93 groups were intravenously injected 1 mg/kg GLYX-13 2 hours before isoflurane anesthesia. The mRNA and phosphoprotein levels of NR2B, CaMKII and CREB in the hippocampus were assessed by qRT-PCR and western blot assay 1, 3 and 7 days after isoflurane exposure. The CFC and NOR tests were used to evaluate cognitive function 1, 3 and 7 days after isoflurane exposure (Figure 1).
Isoflurane exposure
Mice were placed in a chamber with 4.2% isoflurane (license No. H20020267, Lunan Better Pharmaceutical Co., Ltd., Linyi, China) for induction and 1.5% isoflurane for maintenance for 6 hours. The other mice breathed air. During isoflurane exposure, an anesthesia monitor (Dragerwerk AG & Co. KGaA, Lübeck, Germany) was used to continuously monitor the concentration of isoflurane in the chamber, and respiration was observed to prevent respiratory depression. The chamber was placed on a heated sheet to maintain body temperature.
Intracerebroventricular injection
As described by Schaafsma et al. (2015), mice were anesthetized with isoflurane and placed in a stereotaxic apparatus (Shanghai Bio-will Co., Ltd., Shanghai, China). A microsyringe was used for injecting KN93 (1 μL/min) at the following stereotaxic coordinates: (from bregma) AP –0.5 mm; ML +1.0 mm; DV –2.0 mm (Paxinos and Franklin, 2001). The mice were returned to their home cages after recovery from anesthesia.
CFC test
The CFC test (Panlab, Barcelona, Spain) was performed in this study as previously described (Strekalova et al., 2003; Taniguchi et al., 2017). On day 1 (training stage), mice were placed in the chamber and allowed to explore freely for 5 minutes, and then exposed to a high frequency sound (4,000 Hz, 80 dB) for 30 seconds. During the final 2 seconds, an 0.8-mA foot shock was given. After the shock, mice were allowed to continue to explore the chamber for 2 minutes before returning to their home cages. Then, 24 hours later (testing stage), mice were placed into the same chamber for 5 minutes, and memory for the context was assessed by recording freezing behavior. After each test, 75% alcohol was used to clean the chamber to eliminate olfactory cues. Freezing time was automatically recorded and analyzed. Percent freezing time = freezing time/phase time × 100%.
NOR test
The NOR test was performed as previously described (Bevins and Besheer, 2006; Ferrante et al., 2018). Briefly, mice were allowed to habituate in an empty open field (25 cm × 25 cm × 40 cm) for 15 minutes for 3 consecutive days before the test. In the training stage, two identical objects (A and B) were placed 10 cm apart on the same side of the test box. Mice were placed in the test box and allowed to freely explore the two objects for 10 minutes. Then, 2 hours later (testing stage), the trial was repeated with object B removed from the test box and replaced with object C, which was of a similar size, but different in shape and color. The cumulative time spent (seconds) exploring each object in the training and testing stages were recorded. Exploratory behavior was considered as pointing the nose toward the object within 2 cm or sniffing the object, while standing, sitting or leaning on the object was not. After each test, the objects were cleaned to reduce the impact of olfactory cues. Percent novel object exploration time = novel object exploration time/total object exploration time × 100%. Discrimination index = (novel object exploration time-familiar object exploration time)/total object exploration time.
Tissue preparation
Mice were euthanized with a high concentration of isoflurane. The hippocampus was removed and stored at –80°C until use.
qRT-PCR analysis
An RNA extraction kit (Bioteke, Beijing, China) was used to extract total RNA, and the concentration was measured on a NanoDrop (Thermo, Waltham, MA, USA). PrimeScript RT master mix (RR036A; Takara Biotechnology Co., Ltd., Dalian, Liaoning Province, China) was used to reverse transcribe RNA into cDNA. SYBR Premix Ex Taq II master mix (Takara Biotechnology Co., Ltd.) was used for qRT-PCR on an ABI StepOne Plus Real-Time PCR system (Thermo). The primer sequences for qRT-PCR were as follows: β-actin: forward primer: 5′-CTG TCC CTG TAT GCC TCT G-3′; reverse primer: 5′-ATG TCA CGC ACG ATT TCC-3′; NR2B: forward primer: 5′-GGA TCT ACC AGT CTA ACA TG-3′; reverse primer: 5′-GAT AGT TAG TGA TCC CAC TG-3′; CaMKII: forward primer: 5′-TCT TAT TGA TCA GAT CCT TGG CTT C-3′; reverse primer: 5′-CTG GTG CCT ACG ATT TCC CAT-3′, CREB: forward primer: 5′-TTC TAC AGT ATG CAC AGA CCA CTG-3′; reverse primer: 5′-GGT ATG TTT GTA CAT CGC CTG A-3′. β-actin was used as a reference gene. The 2–ΔΔCt method was used to measure relative expression (Livak and Schmittgen, 2001).
Western blot assay
Hippocampi were sonicated in RIPA lysis buffer (Beyotime, Shanghai, China), and both protease and phosphatase inhibitors (Thermo) were used to prevent degradation. Protein concentrations were measured using the Bicinchoninic Acid Protein Assay Kit (Thermo). The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to Immobilon polyvinylidene fluoride membranes (Merck, Darmstadt, Germany). The blots were blocked with 5% bovine serum albumin for 2 hours and incubated with primary antibody (β-actin, 1:1,000, mouse monoclonal; p-NR2B, 1:1,000, rabbit monoclonal; phospho-CaMKII (p-CaMKII), 1:1,000, rabbit monoclonal; p-CREB, 1:1,000, rabbit monoclonal (all from Abcam, Cambridge, UK)) at 4°C overnight. After washing with Tris-buffered saline-Tween 20, membranes were incubated with the corresponding horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:20,000; Elabscience, Wuhan, China) for 2 hours. The blots were visualized using enhanced chemiluminescence solution (Thermo). The optical density values of the specific bands were measured using Image J (National Institutes of Health, Bethesda, MD, USA). β-Actin was used as control.
Statistical analyses
All values are shown as the mean ± standard error of the mean (SEM). One-way analysis of variance and least significant difference test were performed using SPSS 19.0 (IBM, Armonk, NY, USA). P < 0.05 was regarded as statistically significant.
Results
Pretreatment with GLYX-13 ameliorates cognitive impairment induced by long-term isoflurane anesthesia in mice
The cognitive function of mice in the Con, Anes, GLYX-13 and Anes + GLYX-13 groups was assessed using the CFC and NOR tests 1, 3 and 7 days after long-term isoflurane exposure. In the CFC test, compared with the Con group, the percent freezing time was significantly reduced in the Anes group 1 and 3 days after isoflurane exposure (P < 0.05). Furthermore, the percent freezing time in the Anes + GLYX-13 group was significantly increased compared with the Anes group (P < 0.05; Figure 2A). In the NOR test, no object preference was found in any group during the training stage (P > 0.05). In the testing stage, compared with the Con group, there were significant decreases in the percent novel object exploration time and discrimination index in the Anes group 1 and 3 days after isoflurane exposure (P < 0.05). Compared with the Anes group, the percent novel object exploration time and discrimination index were increased in the Anes + GLYX-13 group (P < 0.05). No significant difference was found among the four groups at 7 days after isoflurane anesthesia (P > 0.05; Figure 2B & C).
Figure 2.
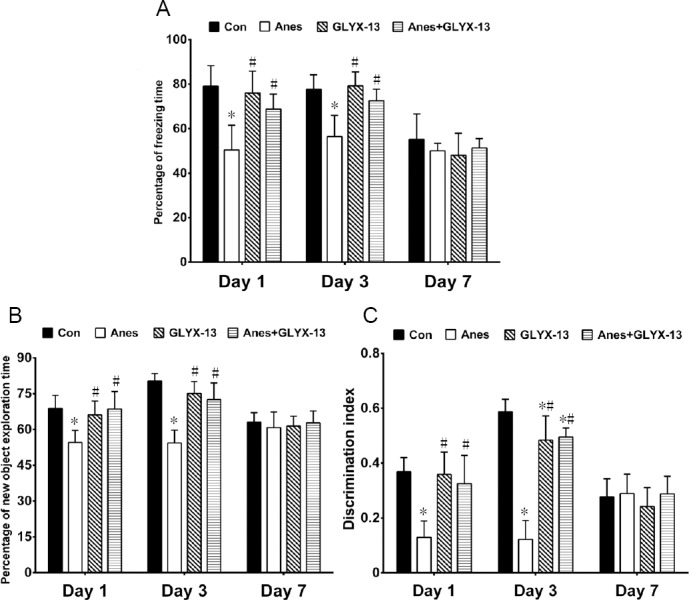
Effects of GLYX-13 pretreatment on cognitive function in mice 1, 3 and 7 days after isoflurane exposure.
(A) Percent freezing time in the CFC test. Percent freezing time (%) = freezing time/phase time × 100%. (B) Percent novel object exploration time in the NOR test. Percent novel object exploration time = novel object exploration time/total object exploration time × 100%. (C) Discrimination index in the NOR test. Discrimination index = (novel object exploration time-familiar object exploration time)/total object exploration time. Values are expressed as the mean ± SEM (n = 5). *P < 0.05, vs. Con group; #P < 0.05, vs. Anes group (one-way analysis of variance followed by the post-hoc least significant difference test). CFC: Contextual fear conditioning; NOR: novel object recognition. Con: Control group; Anes: isoflurane anesthesia group, in which mice were exposed to 1.5% isoflurane for 6 hours; GLYX-13: GLYX-13 injection group, in which mice were intravenously administered GLYX-13 (1 mg/kg); Anes + GLYX-13: isoflurane anesthesia + GLYX-13 injection group, in which mice were exposed to 1.5% isoflurane for 6 hours and intravenously pre-administered GLYX-13 (1 mg/kg) 2 hours earlier.
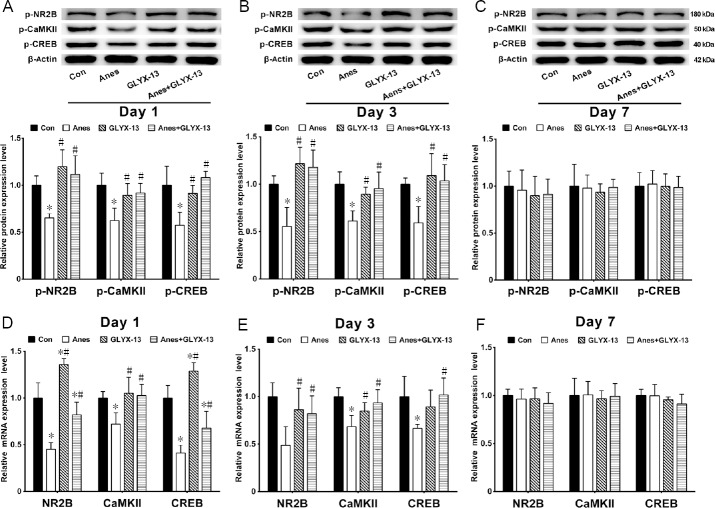
GLYX-13 suppresses the changes in the NR2B/CaMKII/CREB signaling pathway induced by isoflurane exposure
At 1 and 3 days after isoflurane anesthesia, the phosphoprotein levels of NR2B, CaMKII and CREB were significantly decreased in the Anes group compared with the Con group (P < 0.05). Furthermore, compared with the Anes group, the phosphoprotein levels of NR2B, CREB and CaMKII were significantly increased in the Anes + GLYX-13 group (P < 0.05; Figure 3A & B). No significant difference was found among the four groups 7 days after isoflurane exposure (P > 0.05; Figure 3C).
Figure 3.
Effects of GLYX-13 on the phosphorylation and gene transcription levels of NR2B, CaMKII and CREB in the hippocampus of mice after isoflurane exposure.
(A–C) The phosphorylation levels of NR2B, CaMKII and CREB at 1 (A), 3 (B) and 7 (C) days after isoflurane anesthesia, detected by western blot assay. Relative protein expression is expressed as the ratio of the target protein to β-actin. (D–F) NR2B, CaMKII and CREB mRNA levels at 1 (D), 3 (E) and 7 (F) days after isoflurane anesthesia detected by real time-polymerase chain reaction. Values are expressed as the mean ± SEM (n = 5). *P < 0.05, vs. Con group; #P < 0.05, vs. Anes group (one-way analysis of variance followed by post-hoc least significant difference test). CaMKII: Ca2+/calmodulin-dependent protein kinase II; CREB: cyclic adenosine monophosphate response element binding protein; NR2B: N-methyl-D-aspartate receptor subunit 2B. Con: Control group; Anes: isoflurane anesthesia group, in which mice were exposed to 1.5% isoflurane for 6 hours; GLYX-13: GLYX-13 injection group, in which mice were intravenously administered GLYX-13 (1 mg/kg); Anes + GLYX-13: isoflurane anesthesia + GLYX-13 injection group, in which mice were exposed to 1.5% isoflurane for 6 hours and intravenously pre-administered GLYX-13 (1 mg/kg) 2 hours earlier.
Compared with the Con group, NR2B, CaMKII and CREB gene expression levels were significantly reduced in the Anes group at 1 and 3 days after isoflurane exposure. NR2B, CaMKII and CREB gene expression levels were significantly increased in the Anes + GLYX-13 group compared with the Anes group (P < 0.05; Figure 3D & E). There was no significant difference in NR2B, CaMKII or CREB gene expression levels among the four groups 7 days after isoflurane exposure (P > 0.05; Figure 3F).
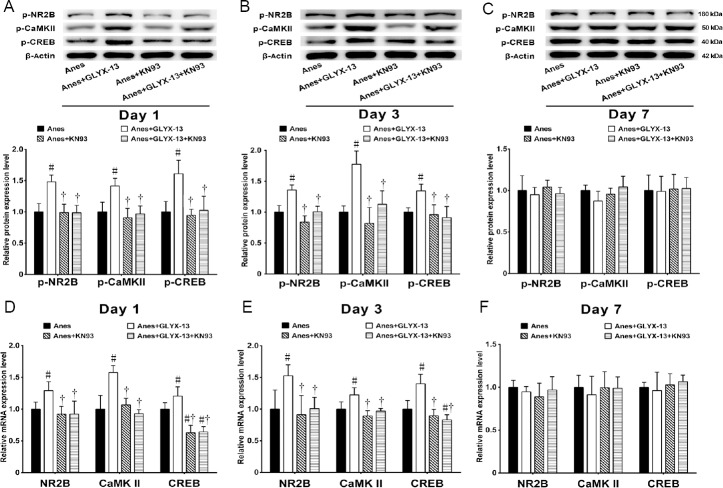
KN93 inhibits the NR2B/CaMKII/CREB signaling pathway
Western blot assay showed that the phosphoprotein levels of NR2B, CaMKII and CREB were significantly decreased in the Anes + GLYX-13 + KN93 group, 1 and 3 days after isoflurane anesthesia, compared with the Anes + GLYX-13 group (P < 0.05; Figure 4A & B). There was no significant difference in the phosphoprotein levels of NR2B, CaMKII or CREB between the Anes + KN93 and Anes groups (P > 0.05). No significant difference was found among the four groups 7 days after isoflurane anesthesia (P > 0.05; Figure 4C & F). The qRT-PCR results confirmed the western blot data, 1 and 3 days after isoflurane anesthesia (Figure 4D & E).
Figure 4.
Effects of combined administration of GLYX-13 and KN93 (a selective CaMKII inhibitor) on the phosphorylation and mRNA levels of NR2B, CaMKII and CREB in the hippocampus of mice after isoflurane anesthesia.
(A–C) The phosphorylation levels of NR2B, CaMKII and CREB at 1 (A), 3 (B) and 7 (C) days after isoflurane anesthesia, detected by western blot assay. Relative protein expression is expressed as the ratio of the target protein to β-actin. (D–F) NR2B, CaMKII and CREB mRNA levels at 1 (A), 3 (B) and 7 (C) days after isoflurane anesthesia, detected by real time-polymerase chain reaction analysis. Values are expressed as the mean ± SEM (n = 5). #P < 0.05, vs. Anes group; †P < 0.05, vs. Anes + GLYX-13 group (one-way analysis of variance followed by post-hoc least significant difference test). CaMKII: Ca2+/calmodulin-dependent protein kinase II; CREB: cyclic adenosine monophosphate response element binding protein; NR2B: N-methyl-D-aspartate receptor subunit 2B. Con: Control group; Anes: isoflurane anesthesia group, in which mice were exposed to 1.5% isoflurane for 6 hours; GLYX-13: GLYX-13 injection group, in which mice were intravenously administered GLYX-13 (1 mg/kg); Anes + GLYX-13: isoflurane anesthesia + GLYX-13 injection group, in which mice were exposed to 1.5% isoflurane for 6 hours and intravenously pre-administered GLYX-13 (1 mg/kg) 2 hours earlier.
GLYX-13 ameliorates cognitive impairment induced by long-term isoflurane exposure via the NR2B/CaMKII/CREB signaling pathway
In the CFC test, the percent freezing time was significantly reduced in the Anes + GLYX-13 + KN93 group, 1 and 3 days after isoflurane exposure, compared with the Anes + GLYX-13 group (P < 0.05; Figure 5A). In the NOR test, the percent novel object exploration time (Figure 5B) and discrimination index (Figure 5C) were decreased in the Anes + GLYX + KN93 group, 1 and 3 days after isoflurane exposure, compared with the Anes + GLYX-13 group (P < 0.05). No significant difference was found among the four groups 7 days after isoflurane anesthesia (P > 0.05; Figure 5).
Figure 5.
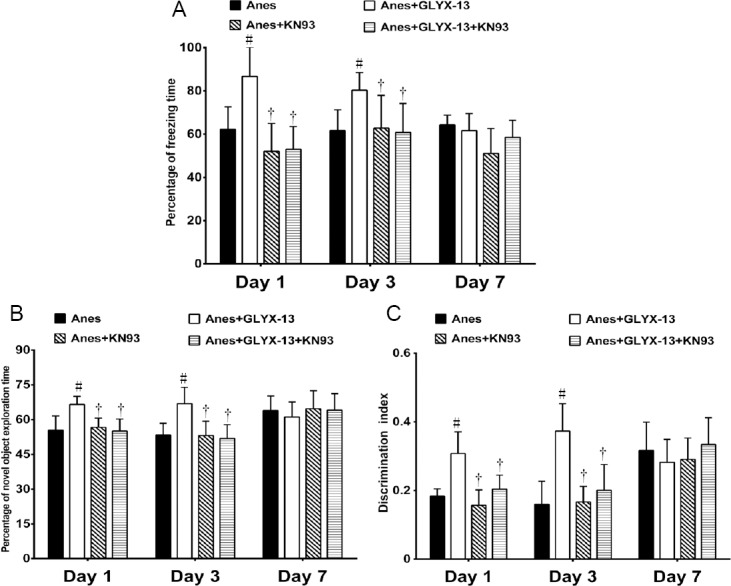
Effects of GLYX-13 on the cognitive impairment induced by long-term isoflurane exposure via the NR2B/CaMKII/CREB signaling pathway.
(A) Percent freezing time in the CFC test. Percentage freezing time (%) = freezing time/phase time × 100%. (B) Percent novel object exploration time in the NOR test. Percent novel object exploration time = novel object exploration time/total object exploration time × 100%. (C) Discrimination index in the NOR test. Discrimination index = (novel object exploration time-familiar object exploration time) /total object exploration time. Values are expressed as the mean ± SEM (n = 5). #P < 0.05, vs. Anes group; †P < 0.05, vs. Anes + GLYX-13 group (one-way analysis of variance followed by the post-hoc least significant difference test). CaMKII: Ca2+/calmodulin-dependent protein kinase II; CREB: cyclic adenosine monophosphate response element binding protein; KN93: a selective CaMKII inhibitor; NR2B: N-methyl-D-aspartate receptor subunit 2B. Con: Control group; Anes: isoflurane anesthesia group, in which mice were exposed to 1.5% isoflurane for 6 hours; GLYX-13: GLYX-13 injection group, in which mice were intravenously administered GLYX-13 (1 mg/kg); Anes + GLYX-13: isoflurane anesthesia + GLYX-13 injection group, in which mice were exposed to 1.5% isoflurane for 6 hours and intravenously pre-administered GLYX-13 (1 mg/kg) 2 hours earlier.
Discussion
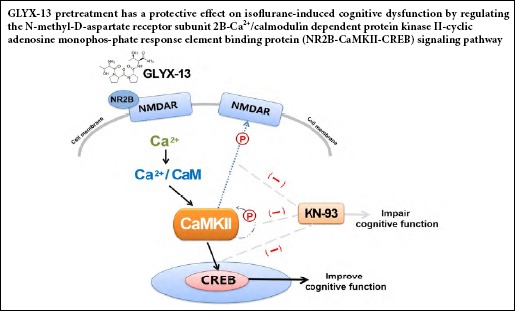
There is mounting evidence that inhalation anesthetics cause or increase the risk of cognitive dysfunction in both humans and rodents (Cheng et al., 2015; Min et al., 2016; Sen and Sen, 2016; Kang et al., 2017; Tang et al., 2018). However, the underlying mechanisms are still unclear and effective treatments are lacking. GLYX-13 acts as a partial agonist at the glycine site of NR2B-containing NMDARs and enhances hippocampus-related cognitive function by increasing NR2B protein expression levels and currents (Burgdorf et al., 2011, 2013). In the present study, GLYX-13 alleviated cognitive impairment induced by long-term isoflurane exposure and restored hippocampal memory-associated protein levels in mice. Furthermore, the CaMKII inhibitor KN93 blocked the neuroprotective effects of GLYX-13, which appear to involve the NR2B/CaMKII/CREB signaling pathway.
NMDARs are key targets of inhalation anesthetics. The antagonistic effects of inhalation anesthetics, such as isoflurane, on NMDARs may lead to neuronal degeneration and apoptosis, in part by blocking the neurotrophic support provided by glutamate (Dutton et al., 2002; Jevtovic-Todorovic et al., 2003). In this study, long-term isoflurane exposure induced cognitive impairment in mice, consistent with previous studies. There is considerable evidence for a central role of NR2B subunits in the regulation of synaptic plasticity (Murphy et al., 2014; Nakai et al., 2014). Accordingly, hippocampal NMDARs have become a key target in the development of cognitive function-modulating drugs (Ghersi et al., 2015). In the present study, the NMDAR agonist GLYX-13 protected against cognitive functional impairment by upregulating NR2B expression and by activating the NR2B/CaMKII/CREB signaling pathway.
The activity of CaMKII is largely regulated by calcium inflow controlled by NR2B, and the binding between CaMKII and calmodulin induces autophosphorylation (Molloy and Kennedy, 1991). In addition, there is evidence for a direct interaction between CaMKII and NR2B (Gambrill and Barria, 2011; Sanhueza et al., 2011), which maintains synaptic strength (Barcomb et al., 2016) and induces dendritogenesis in intermediate neurons in the hippocampus (Bustos et al., 2017). The NR2B/CaMKII complex promotes learning and memory either directly or indirectly through Ca2+. Because CaMKII is an important downstream target of NR2B, we speculated that GLYX-13 may improve cognitive function in mice through CaMKII. Therefore, we examined whether the CaMKII inhibitor KN93 could block the effects of GLYX-13. CREB mRNA expression and phosphorylation were significantly downregulated by the inhibitor, indicating that CREB is a key target of p-CaMKII.
One of the major hurdles to drug administration into the central nervous system is the blood-brain barrier. Therefore, it is noteworthy that GLYX-13 crosses this barrier effectively to achieve the desired concentration, as it is 80% as permeable as water (Moskal et al., 2005). Therefore, GLYX-13 is a potential drug for the treatment of cognitive impairment. While the plasma half-life of GLYX-13 is only several minutes (Moskal et al., 2014), it enhances hippocampal long-term potentiation 24 hours and 1 week following a single dose (Burgdorf et al., 2015). In the present study, we found no difference among the groups 7 days after isoflurane exposure. This suggests that cognitive function had recovered by this time. In the present study, GLYX-13 was administered 2 hours before isoflurane exposure; however, in the clinic, it can be administered again after anesthesia to bolster the neuroprotective effect of the drug.
GLYX-13 facilitates learning and memory in both young adult and learning-impaired aged rats in hippocampal-dependent trace eyeblink conditioning, alternating T-maze, and the MWM (Moskal et al., 2014). GLYX-13 also ameliorates the declarative memory impairment induced by phencyclidine and ketamine in the NOR test (Rajagopal et al., 2016). However, similar to our current findings, Lakshmi et al. found that GLYX-13 does not significantly enhance the discrimination index in mice with normal cognitive function in the NOR experiment (Ferreira et al., 2017). This finding was unexpected. We hypothesize that while GLYX-13 might hyperactivate NMDAR, adaptive regulation may prevent excessive activation of the NR2B/CAMKII/CREB signaling pathway.
Here, we found that pretreatment with GLYX-13 alleviated the cognitive impairment induced by long-term isoflurane exposure and restored the NR2B/CaMKII/CREB pathway in the hippocampus of mice. Furthermore, KN93, a selective CaMKII inhibitor, blocked these neuroprotective effects of GLYX-13. It is exciting that GLYX-13 has undergone phase II clinical pharmacological and toxicological trials in the United States. GLYX-13 was found to have satisfactory safety in humans.
In summary, our findings show that pretreatment with GLYX-13 attenuates long-term isoflurane exposure-induced cognitive impairment via the hippocampal NR2B/CaMKII/CREB signaling pathway. However, further clinical trials are needed to assess the therapeutic effectiveness of GLYX-13 against anesthesia-induced cognitive impairment in humans. There are some limitations to this study. For example, we did not examine the impact of surgical procedures, but will do so in a future study.
Additional file: Open peer review report 1 (111.9KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81730033 (to XPG), 81701371 (to TJX), 81801380 (to XZ) and Natural Science Foundation of Jiangsu Province of China, No. BK20170654 (to TJX), BK20170129 (to XZ). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Institutional review board statement: The experimental protocol was approved by the Experimental Animal Ethics Committee of Drum Tower Hospital affiliated to Medical College of Nanjing University (approval No. 20171102) on November 20, 2017.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Elizabeth D Kirby, Ohio State University, USA.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81730033 (to XPG), 81701371 (to TJX), 81801380 (to XZ) and Natural Science Foundation of Jiangsu Province of China, No. BK20170654 (to TJX), BK20170129 (to XZ).
P-Reviewer: Kirby ED; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel B, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Barcomb K, Hell JW, Benke TA, Bayer KU. The CaMKII/GluN2B Protein Interaction Maintains Synaptic Strength. J Biol Chem. 2016;291:16082–16089. doi: 10.1074/jbc.M116.734822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- 3.Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–556. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 4.Burgdorf J, Zhang XL, Weiss C, Matthews E, Disterhoft JF, Stanton PK, Moskal JR. The N-methyl-D-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol Aging. 2011;32:698–706. doi: 10.1016/j.neurobiolaging.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorf J, Zhang XL, Weiss C, Gross A, Boikess SR, Kroes RA, Khan MA, Burch RM, Rex CS, Disterhoft JF, Stanton PK, Moskal JR. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience. 2015;308:202–211. doi: 10.1016/j.neuroscience.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustos FJ, Jury N, Martinez P, Ampuero E, Campos M, Abarzua S, Jaramillo K, Ibing S, Mardones MD, Haensgen H, Kzhyshkowska J, Tevy MF, Neve R, Sanhueza M, Varela-Nallar L, Montecino M, van Zundert B. NMDA receptor subunit composition controls dendritogenesis of hippocampal neurons through CAMKII, CREB-P, and H3K27ac. J Cell Physiol. 2017;232:3677–3692. doi: 10.1002/jcp.25843. [DOI] [PubMed] [Google Scholar]
- 8.Cheng B, Zhang Y, Wang A, Dong Y, Xie Z. Vitamin C attenuates isoflurane-induced Caspase-3 activation and cognitive impairment. Mol Neurobiol. 2015;52:1580–1589. doi: 10.1007/s12035-014-8959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI., 2nd Isoflurane causes anterograde but not retrograde amnesia for pavlovian fear conditioning. Anesthesiology. 2002;96:1223–1229. doi: 10.1097/00000542-200205000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Ferrante A, Pezzola A, Matteucci A, Di Biase A, Attorri L, Armida M, Martire A, Chern Y, Popoli P. The adenosine A2A receptor agonist T1-11 ameliorates neurovisceral symptoms and extends the lifespan of a mouse model of Niemann-Pick type C disease. Neurobiol Dis. 2018;110:1–11. doi: 10.1016/j.nbd.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira DG, Temido-Ferreira M, Vicente Miranda H, Batalha VL, Coelho JE, Szegö ÉM, Marques-Morgado I, Vaz SH, Rhee JS, Schmitz M, Zerr I, Lopes LV, Outeiro TF. α-Synuclein interacts with PrPC to induce cognitive impairment through mGluR5 and NMDAR2B. Nat Neurosci. 2017;20:1569–1579. doi: 10.1038/nn.4648. [DOI] [PubMed] [Google Scholar]
- 12.Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A. 2011;108:5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghersi MS, Gabach LA, Buteler F, Vilcaes AA, Schioth HB, Perez MF, de Barioglio SR. Ghrelin increases memory consolidation through hippocampal mechanisms dependent on glutamate release and NR2B-subunits of the NMDA receptor. Psychopharmacology (Berl) 2015;232:1843–1857. doi: 10.1007/s00213-014-3817-6. [DOI] [PubMed] [Google Scholar]
- 14.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17:125–134. doi: 10.1038/nrn.2015.19. [DOI] [PubMed] [Google Scholar]
- 16.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang E, Jiang D, Ryu YK, Lim S, Kwak M, Gray CD, Xu M, Choi JH, Junn S, Kim J, Xu J, Schaefer M, Johns RA, Song H, Ming GL, Mintz CD. Early postnatal exposure to isoflurane causes cognitive deficits and disrupts development of newborn hippocampal neurons via activation of the mTOR pathway. PLoS Biol. 2017;15:e2001246. doi: 10.1371/journal.pbio.2001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Min N, Hu QF, Li XP, Nie XH, Yang LL. Isoflurane effects on the proliferation and differentiation of neural stem cells in the hippocampus of neonatal rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:118–122. [Google Scholar]
- 21.Müller L, Tokay T, Porath K, Köhling R, Kirschstein T. Enhanced NMDA receptor-dependent LTP in the epileptic CA1 area via upregulation of NR2B. Neurobiol Dis. 2013;54:183–193. doi: 10.1016/j.nbd.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Molloy SS, Kennedy MB. Autophosphorylation of type II Ca2+/calmodulin-dependent protein kinase in cultures of postnatal rat hippocampal slices. Proc Natl Acad Sci U S A. 1991;88:4756–4760. doi: 10.1073/pnas.88.11.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, Leander JD. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs. 2014;23:243–254. doi: 10.1517/13543784.2014.852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moskal JR, Kuo AG, Weiss C, Wood PL, O’Connor Hanson A, Kelso S, Harris RB, Disterhoft JF. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49:1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Murphy JA, Stein IS, Lau CG, Peixoto RT, Aman TK, Kaneko N, Aromolaran K, Saulnier JL, Popescu GK, Sabatini BL, Hell JW, Zukin RS. Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J Neurosci. 2014;34:869–879. doi: 10.1523/JNEUROSCI.4538-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakai T, Nagai T, Tanaka M, Itoh N, Asai N, Enomoto A, Asai M, Yamada S, Saifullah AB, Sokabe M, Takahashi M, Yamada K. Girdin phosphorylation is crucial for synaptic plasticity and memory: a potential role in the interaction of BDNF/TrkB/Akt signaling with NMDA receptor. J Neurosci. 2014;34:14995–15008. doi: 10.1523/JNEUROSCI.2228-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Franklin KB. New York: Academic Press; 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- 28.Rajagopal L, Burgdorf JS, Moskal JR, Meltzer HY. GLYX-13 (rapastinel) ameliorates subchronic phencyclidine- and ketamine-induced declarative memory deficits in mice. Behav Brain Res. 2016;299:105–110. doi: 10.1016/j.bbr.2015.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Mol Brain. 2013;6:10. doi: 10.1186/1756-6606-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaafsma W, Zhang X, van Zomeren KC, Jacobs S, Georgieva PB, Wolf SA, Kettenmann H, Janova H, Saiepour N, Hanisch UK, Meerlo P, van den Elsen PJ, Brouwer N, Boddeke HW, Eggen BJ. Long-lasting pro-inflammatory suppression of microglia by LPS-preconditioning is mediated by RelB-dependent epigenetic silencing. Brain Behav Immun. 2015;48:205–221. doi: 10.1016/j.bbi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Sen T, Sen N. Isoflurane-induced inactivation of CREB through histone deacetylase 4 is responsible for cognitive impairment in developing brain. Neurobiol Dis. 2016;96:12–21. doi: 10.1016/j.nbd.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su ZY, Ye Q, Liu XB, Chen YZ, Zhan H, Xu SY. Dexmedetomidine mitigates isoflurane-induced neurodegeneration in fetal rats during the second trimester of pregnancy. Neural Regen Res. 2017;12:1329–1337. doi: 10.4103/1673-5374.213554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strekalova T, Zorner B, Zacher C, Sadovska G, Herdegen T, Gass P. Memory retrieval after contextual fear conditioning induces c-Fos and JunB expression in CA1 hippocampus. Genes Brain Behav. 2003;2:3–10. doi: 10.1034/j.1601-183x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 35.Tang CL, Li J, Zhang ZT, Zhao B, Wang SD, Zhang HM, Shi S, Zhang Y, Xia ZY. Neuroprotective effect of bispectral index-guided fast-track anesthesia using sevoflurane combined with dexmedetomidine for intracranial aneurysm embolization. Neural Regen Res. 2018;13:280–288. doi: 10.4103/1673-5374.226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi M, Carreira MB, Cooper YA, Bobadilla AC, Heinsbroek JA, Koike N, Larson EB, Balmuth EA, Hughes BW, Penrod RD, Kumar J, Smith LN, Guzman D, Takahashi JS, Kim TK, Kalivas PW, Self DW, Lin Y, Cowan CW. HDAC5 and its target gene, Npas4, function in the nucleus accumbens to regulate cocaine-conditioned behaviors. Neuron. 2017;96:130–144.e6. doi: 10.1016/j.neuron.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vedder LC, Smith CC, Flannigan AE, McMahon LL. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus. 2013;23:108–115. doi: 10.1002/hipo.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia T, Cui Y, Chu S, Song J, Qian Y, Ma Z, Gu X. Melatonin pretreatment prevents isoflurane-induced cognitive dysfunction by modulating sleep-wake rhythm in mice. Brain Res. 2016;1634:12–20. doi: 10.1016/j.brainres.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55:1238–1250. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao ZF, Du L, Gao T, Bao L, Luo Y, Yin YQ, Wang YA. Inhibition of α5 GABAA receptors has preventive but not therapeutic effects on isoflurane-induced memory impairment in aged rats. Neural Regen Res. 2019;14:1029–1036. doi: 10.4103/1673-5374.250621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.