Abstract
For decades, clinicians have developed medications and therapies to alleviate the symptoms of Parkinson’s disease, but no treatment currently can slow or even stop the progression of this localized neurodegeneration. Fortunately, sparked by the genetic revolution, stem cell reprogramming research and the advancing capabilities of personalization in medicine enable forward-thinking to unprecedented patient-specific modeling and cell therapies for Parkinson’s disease using induced pluripotent stem cells (iPSCs). In addition to modeling Parkinson’s disease more accurately than chemically-induced animal models, patient-specific stem cell lines can be created, elucidating the effects of genetic susceptibility and sub-populations’ differing responses to in vitro treatments. Sourcing cell therapy with iPSC lines provides ethical advantages because these stem cell lines do not require the sacrifice of human zygotes and genetically-specific drug trails can be tested in vitro without lasting damage to patients. In hopes of finally slowing the progression of Parkinson’s disease or re-establishing function, iPSC lines can ultimately be corrected with gene therapy and used as cell sources for neural transplantation for Parkinson’s disease. With relatively localized neural degeneration, similar to spinal column injury, Parkinson’s disease presents a better candidacy for cell therapy when compared to other diffuse degeneration found in Alzheimer’s or Huntington’s Disease. Neurosurgical implantation of pluripotent cells poses the risk of an innate immune response and tumorigenesis. Precautions, therefore, must be taken to ensure cell line quality before transplantation. While cell quality can be quantified using a number of assays, a yielding a high percentage of therapeutically relevant dopaminergic neurons, minimal de novo genetic mutations, and standard chromosomal structure is of the utmost importance. Current techniques focus on iPSCs because they can be matched with donors using human leukocyte antigens, thereby reducing the severity and risk of immune rejection. In August of 2018, researchers in Kyoto, Japan embarked on the first human clinical trial using iPSC cell therapy transplantation for patients with moderate Parkinson’s disease. Transplantation of many cell sources has already proven to reduce Parkinson’s disease symptoms in mouse and primate models. Here we discuss the history and implications for cell therapy for Parkinson’s disease, as well as the necessary safety standards needed for using iPSC transplantation to slow or halt the progression of Parkinson’s disease.
Keywords: alpha synuclein, animal model, cell therapy, dopaminergic neurons, induced pluripotent stem cells, neurodegeneration, Parksinson's disease, stem cells
Introduction
Of the intractable neurogenerative diseases plaguing humanity, Parkinson’s disease (PD) possesses unique characteristics that enable hope for successful future therapies. The disease promotes apoptotic pathways that result in the acute death of a relatively localized bundles of dopaminergic neurons (DAns) in the substantia nigra pars compacta (SNpc) (Hughes et al., 1992; Wakabayashi and Takahashi, 1997; Langston, 2006) and a cholinergic nucleus in the basalis of Meynert (Liu et al., 2015). These neural systems aid in influencing muscle coordination from the basal nuclei. After developmental pruning, normal individuals house around 50,000 of these DAns, but rigidity, tremors, bradykinesia, and other changes in gait-all hallmarks of PD can arise after the loss of even 7,800 SNpc DAns. Located deep within the midbrain, this neuronal death is currently impossible to confirm while the patient is alive and clinicians diagnose PD from symptom profiles. DAn death can only be confirmed through post-mortem examination. Given the difficulty to examine the mechanisms driving neurodegeneration in PD, a variety of theories of PDs causes abound to explain the characteristic presence of Lewy neurites and Lewy bodies. However, DAn survival within the SNpc is integral to retaining cognitive and motor function.
The most widely accepted postulation of PD progression focuses on the role of alpha-synuclein, thought to contribute to the molecular basis behind PD pathogenesis (Kahle et al., 2000). Alpha-synuclein, a small protein comprising 140 amino acids, exists in a dynamic equilibrium of conformational states that can quickly respond to changing conditions. One of these states is a membrane-bound, partially unfolded form that exposes a hydrophobic region. This conformation causes oligomerization, aggregation, and tell-tale Lewy bodies. In a healthy state, an accumulation of misfolded alpha-synuclein is quickly degraded using via ubiquitination. In pathological PD, however, mechanisms involved in alpha-synuclein degradation are disrupted at the level of alpha-synuclein expression, regulation, ubiquitination, or degradation. Alpha-synuclein also interacts with many proteins whose genetic mutations increase risk for PD. This may imply other down-stream effects of alpha-synuclein that could be critical to maintaining healthy neural tissue. Whether entanglement and aggregation are caused by innate or induced mechanisms of, alpha-synucleinopathy is present in almost all cases presenting symptoms of PD. In order to probe its effects and better understand the molecular and cellular basis of PD, researchers employ both cellular and animal models. Their utility hangs on their ability to mirror PD pathology as accurately as possible. I searched PubMed for reviews and primary articles on iPSC cell therapy for PD within the past 5 years. I searched from January 1, 2019 to April 15, 2019.
Induced Pluripotent Stem Cells Parkinson’s Disease Models in vitro
The unique qualities of induced pluripotent stem cells (iPSCs) provide many advantages when trying to model and study PD (Byers et al., 2012). One of their most profoundly influential characteristics is their specificity to the patient. Fibroblasts can be taken from an individual suffering from PD, reprogrammed to a pluripotent state using a handful of transcriptional factors, and then differentiated into the midbrain DAns directly affected by PD (Nashun et al., 2015; Phetfong et al., 2016). iPSC lines transformed scientific models and the study of neurodegeneration as well (Takahashi and Yamanaka, 2006). This model preserves the genetic susceptibility, native molecular machinery, and individual transcriptional pathways needed to accurately such a complex disease. The process of differentiating iPSC lines to the DA neural fate of the SNpc requires incredible complexity too. Since the first iPSC lines in 2006, it took 9 years to mimic this specific developmental pathway using small molecules over the course of 50 days. To start, mothers against decapentaplegic homolog inhibitors and fibroblast growth factor 8 are added in addition to neurotrophic factors that mimic patterns of the neural floor-plate are added to activate the sonic hedgehog signaling pathway (Ye et al., 1998; Fasano et al., 2010). To fine-tune differentiation requires activating additional WNT pathways using small CHIR molecules. CHIR produces a graded response of characteristic forebrain to hindbrain protein expression patterns, conditional on its concentration (Kirkeby et al., 2012). SNpc profiles from iPSC derivatives, with characteristic GIRK2 markers, result from the proper molarity of CHIR. To decrease the time needed for differentiation, mRNA transfection of transcription factors can be employed, but this method has yet to produce SNpc DAns. Additionally, spontaneous integration also limits its applicability for achieving clinical-grade differentiation. Though all current models can never fully mimic PD, iPSC models achieve robust and phenotypically-similar conditions to study PD (Playne and Connor, 2017). Retaining the genetic mutations of the PD patients themselves, the neurons produced from this “PD in a dish” maintain their natural apoptotic susceptibility and require less chemical induction to result in DAn death.
iPSC’s patient-specific capabilities open a window into assessing the genetic contributions to PD pathogenesis. Genome-wide association studies can identify polymorphism and mutation risk factors for PD, but iPSC models allow phenotypically-similar visualization of these effects. Similarly, their effects on cell death and susceptibility can be quantified and compared in controlled conditions. Through the differentiation process, changes in development may also be monitored, though direction via small-molecule induction in a dish hardly represents the embryological state perfectly. The first genetic link to PD discovered was a point mutation in the SNCA gene. In 2011, an iPSC line was established that contained this A53T transversion (Soldner et al., 2011). Similar lines were subsequently created with other PD genetic susceptibility including the LRRK2 gene (Nguyen et al., 2011; Reinhardt et al., 2013; Sanders et al., 2014), GBA mutations (Schondorf et al., 2014), and in PINK1 (Cooper et al., 2009). Employing the powerful capabilities of CRISPR/Cas9 system, the effects of single mutations can be isolated further by reducing clonal and genetic variations. With CRISPR, mutations can be fixed to allow co-culture and co-differentiation of both mutant and edited lines to neural dates that test positive for markers specific to DAns in the midbrain. The longevity of the resulting neurons can then be compared with controls to assess the hypersensitivity of the mutations, as well as the efficacy of gene editing. No longer deep in the midbrain, the cellular changes of mutation are physically seen in real-time. With the reduced cost of genetic sequencing, mutant and controlled lines will be able to limit the effects of background mutations. “PD in a dish” also presents other clinical applications. It may lend insight into diagnosing the severity of PD diagnosis with the ability to measure the effect of oxidative stress on the affected individual’s neurons in tandem with genetic screening.
With the addition of neurotoxins and oxidative stressors like 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine or 6-hydroxydopamine, iPSC models of DAns taken from PD patients currently demonstrate increased sensitivity to, an indication of a more accurate PD model (Byers et al., 2012). However, this added sensitivity must be viewed with healthy skepticism and within the larger context of PD research. Clearly, our models can and should be improved. During cellular reprogramming of fibroblasts, potentially critical aspects of the neural environment are lost. Reprogramming reverses some effects of the aging brain and similarly erases the patient’s epigenome, far from a perfect recreation of PD. Nevertheless, iPSC models have provided insight into the pathogenesis of PD, possibly implying that mitochondrial malfunction is at play. Further consensus is needed. iPSC models may also allow for quicker drug screening without human side-effects. Genetic differences, of both individuals and mutant sub-populations, facilitate the effects of drug uptake and response to PD treatment, contributing significantly to individual drug response (Jimenez-Jimenez et al., 2016; Kim and Jeon, 2016). Implementing personalized medicine based on genetic profile, factoring in PD risk mutations and variance in drug metabolism enzymes, offers many potential benefits for future PD patients. However, modalities of treatment will require constant fine-tuning and updates in response to the latest findings due to PD progression and dynamism. Drug therapies, in isolation, currently cannot slow or stop PD. By necessity, PD treatment may involve a combination of therapies, including drugs. Advances in iPSC technology now allow for an additional method to combat PD: cell therapy via patient-specific transplantation.
Cell Therapy in Model Organisms
Clinicians have sought to implement cell therapy for PD for decades. With PD’s relatively localized neurodegeneration, animal models are a great candidate for testing cell therapies’ efficacy. Model organisms (Drosophila, yeast, mice, and non-human primates) have also contributed significantly to our understanding of PD. When employing model organisms for study, researchers make careful tradeoffs between cost and anatomical, genetic, and behavior similarity of the specimen to humans. Animal models can be used to probe PD by overexpressing human genetic risk factors and monitoring the cellular or physiological response. Drosophila overexpressing the human gene SNCA, coding for the protein alpha-synuclein, exhibit a similar pattern to the age-dependent onset of DAn degeneration. Similarly, mice under the same conditions present with PD symptoms including decreased motor control, Lewy Body formation, and loss of DA terminals the basal nuclei. Not only have these contributions supported the causal role of alpha-synucleinopathy in PD progression, but quantifying phenotypic movement impairment in models has also assisted in assessing functional fitness in drug screening. Drug candidates are assessed in vivo to assess reduced impairment, however, none can restore or improve functionality long-term.
While other mammals produce dopamine within their central nervous system, PD primarily affects human beings. Thus, chemical induction of PD is achieved by injecting 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine into the model’s CNS. This causes severe oxidative stress and is necessary to mimic the loss of DAns in the SNpc in model organisms. A wide array of studies have examined the effects of cell therapy on chemically-induced PD in rats. Repeated reports have shown that rodents with neuro-progenitor cells injected into the midbrain after chemical lesion show signs of recovery. Using human embryonic stem cells (ESCs) as a cell source, transplantation led to both TH-positive donor cells and improvement in quantified motor skills tests (Rodriguez-Gomez et al., 2007; Peng et al., 2014). However, ESC sources have been shown to lead to tumorigenesis. Alternatively, human dopaminergic precursors can be derived from iPSC sources for transplantation in parkinsonian rats. Cell therapy using iPSCs did not result in a host immune response nor tumor formation (Doi et al., 2014). Additionally, brain biopsies in rats revealed more TH-positive cells and less serotonin-positive neurons when iPSC progenitors were cell-sorted prior to injection.
The logical bridge between rats and clinical trials is testing PD cell therapy in non-human primates. Supporting the findings in rodents, neural transplantation of human ESCs in non-human primates detected TH- and GABA-positive neurons at the site of injection. Clinical-grade human ESCs have also been transplanted with significant improvements in motor scores. Additionally, no significant tumor formation was observed when compared to control injections (Wang et al., 2018). iPSC transplantation has also shown promising results. In a longitudinal study following chemical induction of PD, non-human primates two years after transplantation of human iPSC cells still demonstrated significant improvement (Kikuchi et al., 2017). After post-mortem analysis, the neural environment surrounding the injection site revealed proliferation, robust growth, and neural integration into existing neural scaffolds and networks. (Hallett et al., 2015; Kikuchi et al., 2017). Staining also showed human iPSC transplanted cells functioned as TH+ midbrain DAns (Figure 1).
Figure 1.
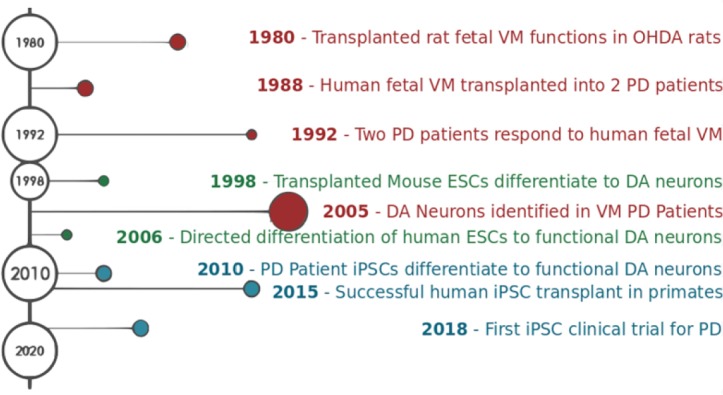
Advances in fetal ventromedial (VM) tissue, embryonic stem cell (ESC), and induced pluripotent stem cell (iPSC) therapy for Parkinson’s disease (PD).
In the 1980s, VM tissue was transplanted into the midbrain of two PD patients and their recovery was monitored over 20 years. Patients showed moderate recovery of symptoms, but low sample size limits the generalizability of the study. Advances in cell culture techniques allowed for successful ESC and iPSC differentiation to the dopaminergic neuronal fate of the midbrain in 2006 and 2010 respectively. After successful transplantation in primates, Takahashi (2019) launched the first clinical trial using iPSC therapy to treat PD. The clinical trial will follow seven patients with moderate PD. DA: Dopaminergic; OHDA: hydroxydopamine.
Studies in model organisms have aided in therapeutic selection, yet present many potential limitations. Artificial induction of PD, while critical for previous studies of blocked DA expression, fails to accurately mirror the intricate causes of PD alpha-synucleinopathy. Sensitive DAn neuropathology, Lewy neurites and Lewy bodies form naturally in endogenous PD. Transgenic animal and in vitro models give researchers a window into PD pathogenesis, yet chemically induced animal models of PD fail to portray the underlying mechanisms of disease and natural susceptibility to neurodegeneration in affected individuals. Animal cell therapy trials produced mixed results-clearly demonstrating the potential power of cellular intervention, yet they also highlight the limitations of these models. Due to cellular and gross anatomical differences, success in animal models may not predict the efficacy of cellular treatment for human neurodegeneration (Lemon, 2008). Though animal models may mimic human pathology in some cases, neurodegeneration involves a complex interplay of genetics, transcriptional feedback, and endogenous control by transcription factors. Thus, success in an animal model may not translate to success in the clinic.
Safety and Immune Response in Human Induced Pluripotent Stem Cell Transplantation
In the nearing future, research will examine the clinical efficacy of PD treatment using iPSC transplantation in humans. Successful trials of cell therapy in humans necessitates a number of precautions. The quality of potential cell sources must first be tested to assess the viability, state of differentiation, and integrity of genetic material. A variety of commercially available assays can be used to confirm the pluripotency of a cell line (Maherali and Hochedlinger, 2008). Cell quality can be quantified on a number of metrics including DNA methylation patterns, embryoid body formation, normal chromosome structure, expression of pluripotent markers, and teratoma formation. Similarly, cells can be screened to detect genetic changes induced by reprogramming and differentiation. Although stem cells accrue an impressively small amount of de novo mutations over time, even more impressive when compared to other immortalized cell lines, approximately 8 mutations are introduced with each passage and genetic abnormalities are introduced at a higher rate among high-passage lines (Spits et al., 2008). As such, proper lab techniques dictate the use of low-passage cells when possible. Finally, cell line quality can be determined by the expression of genes integral to the survival and function of mature, therapeutically relevant DA neurons, namely NURR1, PITX3, FOXA2, and TH (Cooper et al., 2012). However, patients of cell therapy will likely receive immature DA neurons that will complete differentiation post-implantation. Handling and delivery of cell lines must also be assessed if they are to be transported. Cells must be frozen in a partially differentiated state, ready for transplantation with minimal manipulation required once thawed. Additionally, precautions must be taken to limit immune rejection post-transplantation.
Human ESCs have been previously implanted in hopes of treating many neurological diseases ranging from spinal cord injury to macular degeneration. Though producing promising results in some instances, the main challenge for human ESC therapies is their allogeneic cell source, resulting in immune rejection by the recipient. To ensure acceptance of the cell graft, the recipient must endure strong immunosuppressant treatment in addition to the physical toll of the transplant. Technical and ethical roadblocks to cell therapies may ultimately lead to their infeasibility. But, this opportunity to find a treatment capable of restoring lasting function for PD patients is unprecedented. Human iPSC sources possess the advantage in that they are patient-specific, thereby limiting a possible immune response. However, a mounting pile of evidence suggests that some iPSC sources can be immunogenic. An effective strategy to produce foreign- or self-tolerance will be critical to transplantation success. The source of pluripotent cells, whether embryonic or induced, is not as central as quality when it comes to transplantation. Scaling the production of fully-functional DAns will be challenging and costly, especially to ensure a high percentage of functionality. The utmost precautions must be taken to ensure that the pluripotent cells can achieve a high-yield differentiation of DAns, functional stability, and limited proliferation once the culture has fully differentiated. Safe transplantation techniques for reprogrammed cells is also of importance. Infection, tumorigenesis, and graft-induced dyskinesia may all arise from transplantation in trials. Preparations must be taken to prevent these risks whenever possible. Cell therapies using iPSCs will always pose risks, but their advantages and previous animal studies present a compelling case for viable PD therapies in the future. One advantage to iPSCs is that patient lines are not as politically derisive. Lines are established without human embryo sacrifice (an ethical obstacle that ESC treatments face at scale). An additional advantage is that iPSCs lines can match patient and donors using human leukocyte antigen (HLA) profiles. Patient-specific iPSC grafts would limit patient variability seen in previous clinical trials using third-party cell sources. Researchers in Japan estimate that only 50 iPSC lines from donors homozygous at HLA loci can cover 73% of Japanese citizens when matched at 3 locations (A, DR, and B) (Okita et al., 2011). The cost of cell differentiation and reprogramming could pose added financial burdens on the patient, but reduced immune response and iPSC lines from generic donors will lower costs at scale. In the current renal transplant system, HLA matches result in lower rates of graft-loss, rejection, and death. Additionally, HLA matches required lower dosing of immunosuppressants post-operation. Primate studies of iPSC grafting have also shown to increase cell survival and reduce microglia and leukocyte responses when histocompatible (Morizane et al., 2017). The native immune response of the brain (and the attenuation through immunosuppressive drugs) may dictate the positive result of cell therapy in humans. Just as with the challenge of examining neurodegeneration within the brain, visualizing the neuroprotective effects of iPSCs post-operation cannot be achieved until post-mortem analysis.
Since 1987, researchers have probed the efficacy of cell therapy in treating PD using a variety of cell sources. Fetal tissue from the ventral midbrain has been shown to produce functionally viable DA neurons 20 years post-implantation, but treatment showed little behavioral improvement. ESCs were used in a 2017 human trial to treat PD, however, the Chinese team has yet to publish any findings (Cyranoski, 2017). Without data, speculation is currently unwarranted. Researchers in Japan began the first iPSC cell therapy for PD in 2018 (http://www.cira.kyoto-u.ac.jp/e/pressrelease/news/180730-170000.html). iPSCs have been used as cell sources in other clinical trials involving neural damage in spinal cord injury. However, the Japanese team has yet to publish data from their human trial for PD. The trial began in the late summer of last year, with the intent to transplant DAn progenitor cells derived from iPSCs into the SNpc (ID: UMIN000033564) (Takahashi and Sawamoto, 2018). This study, despite the history of success with cell therapy in animals, will be the first to treat clinical PD using iPSCs as opposed to a chemically-induced model. Lead by Takahashi at Kyoto University Hospital, seven patients with moderate PD were selected for early intervention and their progress will be monitored for 2 years (Takahashi and Sawamoto, 2018). Volunteers with moderate PD were selected, despite the increased risk of permanent damage, to reduce confounding variables and with the hopes of greater benefit. Cells were sourced from third-party HLA homozygous donors to reduce interference from patients’ genetic variability. Further testing of genetic integrity of the iPSC lines and additional tests in rat models were performed before implantation (Takahashi, 2019). Transplantation of 2.4 million cells was administered into 12 brain regions via two bored holes in the cranium (Takahashi and Sawamoto, 2018). Following the surgery, and due to the trial’s experimental nature, patients received drugs to modulate immunosuppression for extra precaution. The first patient to receive this treatment has had no adverse effects thus far. If the results hold, he will receive another round of 2.4 million cells. The other six patients are expected to be treated by 2020. Physicians will monitor his condition, including signs of excessive dopamine production that could result in involuntary muscle spasms, as well as other side-effects. Curing PD with cell treatments remains doubtful, and the forthcoming results must be examined in the context of other treatments. Many questions regarding the future of cell therapy for PD remain, but the future of treating neurodegeneration looks optimistic.
Conclusions
The patient-specific characteristics of iPSC sources offer unprecedented avenues for treating neurodegeneration. Neural degeneration does not affect PD patients alone. But with relatively localized neurodegeneration, in comparison to more diffuse forms of neural death like ALS or AD, PD remains one of the most viable targets for cell therapies in their early stages. An individual’s cells, taken from fibroblasts in the skin, may be harvested and subsequently de-differentiated into pluripotent stem cells. Using CRISPR technologies, genetic mutations predisposing the individual’s DAns can then be genetically corrected and then cultured into dopaminergic progenitor neurons. These progenitor cells can then be transplanted into the location of degeneration in the midbrain in hopes of restoring function. Losing a cluster of a few thousand neurons throws the fragility of the neural ecosystem into sharp relief. One begins to appreciate the interconnection of neural circuitry as the effects of this programmed death touch nearly all facets of life. This fragility adjures both clinician and researcher to spend all the more mental effort to ensure safety standards for research and treatments. As shown in model studies, cell therapy, in combination with future drug regiments, may offer a path forward to help mitigate the human suffering of PD.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: This work was supported by the Mallett Family.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the Mallett Family.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Byers B, Lee HL, Reijo Pera R. Modeling Parkinson’s disease using induced pluripotent stem cells. Curr Neurol Neurosci Rep. 2012;12:237–242. doi: 10.1007/s11910-012-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper O, Astradsson A, Hallett P, Robertson H, Mendez I, Isacson O. Lack of functional relevance of isolated cell damage in transplants of Parkinson’s disease patients. J Neurol. 2009;256(Suppl 3):310–316. doi: 10.1007/s00415-009-5242-z. [DOI] [PubMed] [Google Scholar]
- 3.Cooper O, Parmar M, Isacson O. Characterization and criteria of embryonic stem and induced pluripotent stem cells for a dopamine replacement therapy. Prog Brain Res. 2012;200:265–276. doi: 10.1016/B978-0-444-59575-1.00012-0. [DOI] [PubMed] [Google Scholar]
- 4.Cyranoski D. Trials of embryonic stem cells to launch in China. Nature. 2017;546:15–16. doi: 10.1038/546015a. [DOI] [PubMed] [Google Scholar]
- 5.Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, Sundberg M, Moore MA, Perez-Torres E, Brownell AL, Schumacher JM, Spealman RD, Isacson O. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell. 2015;16:269–274. doi: 10.1016/j.stem.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Agundez JA. Advances in understanding genomic markers and pharmacogenetics of Parkinson’s disease. Expert Opin Drug Metab Toxicol. 2016;12:433–448. doi: 10.1517/17425255.2016.1158250. [DOI] [PubMed] [Google Scholar]
- 10.Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, Yamamoto M, Okita K, Nakagawa M, Parmar M, Takahashi J. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017;548:592–596. doi: 10.1038/nature23664. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Jeon B. How close are we to individualized medicine for Parkinson’s disease. Expert Rev Neurother. 2016;16:815–830. doi: 10.1080/14737175.2016.1182021. [DOI] [PubMed] [Google Scholar]
- 13.Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 15.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 16.Liu AK, Chang RC, Pearce RK, Gentleman SM. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015;129:527–540. doi: 10.1007/s00401-015-1392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3:595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Morizane A, Kikuchi T, Hayashi T, Mizuma H, Takara S, Doi H, Mawatari A, Glasser MF, Shiina T, Ishigaki H, Itoh Y, Okita K, Yamasaki E, Doi D, Onoe H, Ogasawara K, Yamanaka S, Takahashi J. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 2017;8:385. doi: 10.1038/s41467-017-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nashun B, Hill PW, Hajkova P. Reprogramming of cell fate: epigenetic memory and the erasure of memories past. EMBO J. 2015;34:1296–1308. doi: 10.15252/embj.201490649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schüle B, Dolmetsch RE, Langston W, Palmer TD, Pera RR. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.OkitaOkita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 22.Peng J, Liu Q, Rao M, Zeng X. Survival and engraftment of dopaminergic neurons manufactured by a Good Manufacturing Practice-compatible process. Cytotherapy. 2014;16:1305–1312. doi: 10.1016/j.jcyt.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Phetfong J, Supokawej A, Wattanapanitch M, Kheolamai P, U-Pratya Y, Issaragrisil S. Cell type of origin influences iPSC generation and differentiation to cells of the hematoendothelial lineage. Cell Tissue Res. 2016;365:101–112. doi: 10.1007/s00441-016-2369-y. [DOI] [PubMed] [Google Scholar]
- 24.Playne R, Connor B. Understanding Parkinson’s disease through the use of cell reprogramming. Stem Cell Rev. 2017;13:151–169. doi: 10.1007/s12015-017-9717-5. [DOI] [PubMed] [Google Scholar]
- 25.Reinhardt P, Schmid B, Burbulla LF, Schöndorf DC, Wagner L, Glatza M, Höing S, Hargus G, Heck SA, Dhingra A, Wu G, Müller S, Brockmann K, Kluba T, Maisel M, Krüger R, Berg D, Tsytsyura Y, Thiel CS, Psathaki OE, et al. Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 2013;12:354–367. doi: 10.1016/j.stem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Gómez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, Green MV, Thanos PK, Ichise M, Pike VW, Innis RB, McKay RD. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25:918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders LH, Laganière J, Cooper O, Mak SK, Vu BJ, Huang YA, Paschon DE, Vangipuram M, Sundararajan R, Urnov FD, Langston JW, Gregory PD, Zhang HS, Greenamyre JT, Isacson O, Schüle B. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson’s disease patients: reversal by gene correction. Neurobiol Dis. 2014;62:381–386. doi: 10.1016/j.nbd.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schöndorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, Sardi SP, Valsecchi M, Hoffmann S, Schwarz LK, Hedrich U8, Berg D, Shihabuddin LS, Hu J, Pruszak J, Gygi SP, Sonnino S, Gasser T, Deleidi M. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- 29.Soldner F, Laganière J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S, Zhang L, Guschin D, Fong LK, Vu BJ, Meng X, Urnov FD, Rebar EJ, Gregory PD, Zhang HS, Jaenisch R. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K. Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol. 2008;26:1361–1363. doi: 10.1038/nbt.1510. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi J. Preparing for first human trial of iPSC-derived cells for Parkinson’s disease: an interview with Jun Takahashi. Regen Med. 2019;14:93–95. doi: 10.2217/rme-2018-0158. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi N, Sawamoto, N Kyoto . UMIN, Japan: 2018. Trial to Evaluate the Safety and Efficacy of iPSC-derived dopaminergic progenitors in the treatment of Parkinson’s Disease. [Google Scholar]
- 34.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 35.Wang YK, Zhu WW, Wu MH, Wu YH, Liu ZX, Liang LM, Sheng C, Hao J, Wang L, Li W, Zhou Q, Hu BY. Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Reports. 2018;11:171–182. doi: 10.1016/j.stemcr.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]


