Abstract
The peripheral nervous system has an astonishing ability to regenerate following a compression or crush injury; however, the potential for full repair following a transection injury is much less. Currently, the major clinical challenge for peripheral nerve repair come from long gaps between the proximal and distal nerve stumps, which prevent regenerating axons reaching the distal nerve. Precise axon targeting during nervous system development is controlled by families of axon guidance molecules including Netrins, Slits, Ephrins and Semaphorins. Several recent studies have indicated key roles of Netrin1, Slit3 and EphrinB2 signalling in controlling the formation of new nerve bridge tissue and precise axon regeneration after peripheral nerve transection injury. Inside the nerve bridge, nerve fibroblasts express EphrinB2 while migrating Schwann cells express the receptor EphB2. EphrinB2/EphB2 signalling between nerve fibroblasts and migrating Schwann cells is required for Sox2 upregulation in Schwann cells and the formation of Schwann cell cords within the nerve bridge to allow directional axon growth to the distal nerve stump. Macrophages in the outermost layer of the nerve bridge express Slit3 while migrating Schwann cells and regenerating axons express the receptor Robo1; within Schwann cells, Robo1 expression is also Sox2-dependent. Slit3/Robo1 signalling is required to keep migrating Schwann cells and regenerating axons inside the nerve bridge. In addition to the Slit3/Robo1 signalling system, migrating Schwann cells also express Netrin1 and regenerating axons express the DCC receptor. It appears that migrating Schwann cells could also use Netrin1 as a guidance cue to direct regenerating axons across the peripheral nerve gap. Engineered neural tissues have been suggested as promising alternatives for the repair of large peripheral nerve gaps. Therefore, understanding the function of classic axon guidance molecules in nerve bridge formation and their roles in axon regeneration could be highly beneficial in developing engineered neural tissue for more effective peripheral nerve repair.
Keywords: axonal guidance, EphrinB2, nerve bridge, Netrin1, peripheral nerve, regeneration, Slit3, Sox2, transection injury
Introduction
Injuries to peripheral nerves are common and often result in the complete transection of the nerve (Pfister et al., 2011). Such an injury may create a gap between the proximal and distal nerve stumps, requiring either an autologous nerve graft or the use of an artificial nerve guidance conduit for repair when the gap is greater than 5 mm (Moore et al., 2009). Studies using a sciatic nerve transection injury model in mice and rats have shown that new nerve bridge tissue can form between the proximal and the distal nerve ends and that regenerating axons are able to navigate across the nerve gap using the newly-formed bridge tissue as a substrate (Parrinello et al., 2010; Cattin et al., 2015; Dun and Parkinson, 2015; Dun et al., 2019). Engineered neural tissues have been suggested as promising alternatives for the repair of large peripheral nerve gaps to replace the current gold standard of using nerve autografts (Georgiou et al., 2013). Therefore, understanding the molecular mechanism of nerve bridge tissue formation is crucial not only to the understanding of basic principles governing cell biology and tissue regeneration, but also to the development of novel engineered neural tissue for effective nerve repair. Previous studies on roles for Netrin1/DCC signaling and EphrinB2/EphB2 signaling (Parrinello et al., 2010; Webber et al., 2011; Rosenberg et al., 2014), and more recently our study on roles for Slit3/Robo1 signaling within the nerve bridge, have shown that classic axon guidance molecules play key roles in regulating Schwann cell migration and precise axon regeneration in the peripheral nerve bridge (Dun et al., 2019). In this review, we will summarize the current understanding of how classic axon guidance molecules regulate cell migration, nerve bridge formation and precise axon regeneration in the peripheral nerve gap after transection injury (Figure 1).
Figure 1.
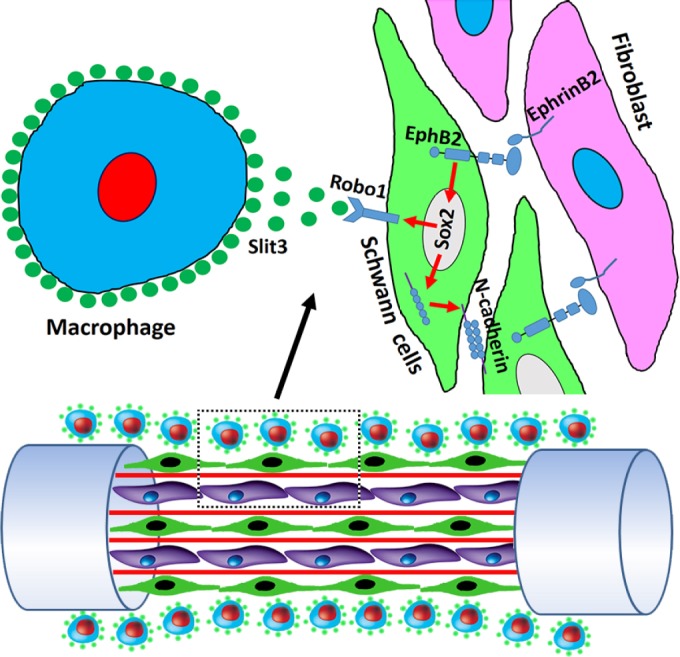
EphrinB2/EphB2, Slit3/Robo1 and Netrin1/DCC signalling control correct nerve bridge tissue formation and precise axon regeneration.
After transection of the sciatic nerve, the nerve bridge is formed between the proximal nerve end (left) and the distal nerve end (the right). EphrinB2/EphB2 signalling between migrating Schwann cells (in green) and nerve fibroblasts (in purple) controls Schwann cell sorting and Schwann cell cord formation in the nerve bridge. EphrinB2/EphB2 signalling increases Sox2 expression in Schwann cells and Sox2 promotes N-cadherin translocation to Schwann cell membrane. Sox2 also regulates Robo1 expression in Schwann cells. Macrophages (in blue) in the outermost layer of the nerve bridge secrete Slit3. Slit3 is required to interact with Robo1 on Schwann cells and keep migrating Schwann cells inside the nerve bridge. Schwann cells secrete Netrin1 and Netrin1 interacts with DCC on regenerating axons (in red) to guide regenerating axons across the nerve bridge.
The current scientific literature was searched using PubMed with the keywords Netrin, Slit, Ephrin or Semaphorin together with the key words peripheral, nerve and regeneration. The search for ‘Netrin’ retrieved 17 published papers; the search for ‘Slit’ retrieved 21 published papers; the search for ‘Ephrin’ retrieved 14 published papers and the search for ‘Semaphorin’ retrieved 28 published papers. Papers were then screened and assessed for inclusion according to their significance for the field of signalling in the formation of nerve bridge tissue following injury.
Netrin1/DCC Signaling between Migrating Schwann Cells and Regenerating Axons Directs Regenerating Axons Across the Nerve Bridge
Netrins are a family of extracellular, laminin-related proteins that are widely expressed in a range of embryonic tissues and play key roles in axonal guidance during nervous system development (Dun and Parkinson, 2017). In mammals, four secreted Netrins, Netrin1, 3, 4 and 5, have been identified, and so far Netrin1 is the best characterized member of the family (Lai Wing Sun et al., 2011; Yamagishi et al., 2015). Netrin1 was the first axon guidance molecule to be discovered in 1994, representing a true milestone in the research field of axonal guidance (Kennedy et al., 1994; Serafini et al., 1994). It can bind to a variety of receptors such as DCC, Neogenin, Unc5A-D and CD146 to transduce receptor specific biological functions but its attractive function for axon extension is largely transduced by the DCC receptor (Dominici et al., 2017; Dun and Parkinson, 2017). Netrin1 was shown to be up-regulated after peripheral nerve injury and the expression was primarily in Schwann cells (Madison et al., 2000; Petrausch et al., 2000; Webber et al., 2011; Jaminet et al., 2013; Rosenberg et al., 2014). Studies have shown that Netrin1 plays a vital role in adult peripheral nerve regeneration (Webber et al., 2011; Jaminet et al., 2013; Rosenberg et al., 2014; Ke et al., 2015). Netrin1 heterozygous mice showed a much slower functional recovery after peripheral nerve crush injury and full functional recovery was not observed even at 50 days following injury (Jaminet et al., 2013). Injection of Netrin1 overexpressing bone marrow mesenchymal stem cells into the crush site of rat sciatic nerve promoted more rapid axon regeneration and functional recovery (Ke et al., 2015). In the zebra fish mutant deleting DCC in motor neurons, regenerating motor axons in the nerve bridge strayed away from their original path onto ectopic trajectories, recapitulating the phenotype observed in zebra fish mutant lacking migrating Schwann cells in the nerve bridge (Rosenberg et al., 2014). Binding of Netrin1 to the DCC receptor typically transduces Netrin1 attractive signaling (Dominici et al., 2017; Dun and Parkinson, 2017). Thus, these findings indicated that Schwann cell could utilise Netrin1 as a guidance cue to direct axon regeneration in peripheral nerve bridge via the DCC receptor expressed on regenerating axons.
EphrinB2/EphB2 Signaling between Fibroblast and Migrating Schwann Cells Regulates Schwann Cell Cord Formation
Eph receptors (erythropoietin-producing human hepatocellular receptors) belong to the largest family of tyrosine kinase receptors and they are activated upon the binding of their ligands, the Eph receptor-interacting proteins, Ephrins (Lisabeth et al., 2013). Ephrin/Eph signaling not only has a critical function in embryonic nervous system development by regulating axonal guidance and cell migration but also plays an important role in tissue repair following injury (Coulthard et al., 2012). Previously, Parrinello et al. (2010) showed that EphrinB2/EphB2 signaling between fibroblasts and Schwann cells is critical for the formation of Schwann cell cords within the nerve bridge during regeneration. In the nerve bridge, migrating Schwann cells are surrounded and contacted by nerve fibroblasts but the two cell types do not appear to mix with each other. In co-culture experiments, Schwann cells and nerve fibroblasts sorted into mutually exclusive cell clusters. Examining nerve bridge tissue as well as cultured nerve fibroblasts and Schwann cells, Parrinello et al. (2010) demonstrated that Schwann cells express the EphB2 receptor while nerve fibroblasts express the EphrinB2 ligand, suggesting that EphrinB2/EphB2 signaling may be required for the coordination between nerve fibroblasts and Schwann cells in the nerve bridge. By manipulating the levels of EphrinB2 and EphB2 in cell co-cultures, Parrinello et al. (2010) demonstrated that EphrinB2/EphB2 signaling between nerve fibroblasts and Schwann cells is necessary and sufficient for Schwann cell sorting and cluster formation. This study also examined axon regeneration in the nerve bridge of EphB2 knockout mice or in wild-type rats in which an inhibitory EphB2-Fc fusion protein was delivered to the nerve bridge via mini osmotic pumps. In both cases, axonal regrowth appeared less organized in the nerve bridge compared to control experiments. As regenerating axons are guided by the Schwann cell cords in the nerve bridge, these findings demonstrated that EphrinB2/EphB2 signaling between nerve fibroblasts and Schwann cells in the nerve bridge is important for correct nerve bridge tissue formation.
Although Ephrin/Eph signaling is thought to primarily induce rapid cell responses by controlling actin dynamics, Parrinello et al. (2010) found that increased EphrinB2/EphB2 signaling increases the expression of the transcription factor Sox2 in Schwann cells. Sox2 expression in Schwann cells promotes tissue repair under several different circumstances including peripheral nerve bridge formation, skin wound healing and digit tip regeneration (Johnston et al., 2016; Carr and Johnston, 2017; Parfejevs et al., 2018). Parrinello et al. (2010) showed that EphrinB2/EphB2 signaling via Sox2 promotes the translocation of the cell surface adhesion molecule N-cadherin into the Schwann cell membrane. This increased level of N-cadherin promoted adhesion between Schwann cells. By knockdown of EphB2, Sox2 and N-cadherin levels in cultured Schwann cells, Parrinello et al. (2010) showed that N-cadherin is necessary and sufficient for cell sorting downstream of EphB2 and Sox2 in vitro. Sox2 overexpression rescued the Schwann cell sorting deficiency of cultured Schwann cells from EphB2 knockout mice, indicating that Sox2 acts downstream of EphB2 to regulate Schwann cell sorting. Thus, these findings uncovered a new signaling pathway downstream of EphB2 activation via Sox2 and N-cadherin that mediated the Schwann cell sorting process in the nerve bridge, ultimately leading to the formation of Schwann cell cords in the nerve bridge.
Macrophage-Derived Slit3 Controls Correct Nerve Bridge Tissue Formation
In another recent paper, we examined the pattern of axon regeneration and the trajectory of Schwann cell migration in the nerve bridge of mice with a Schwann cell-specific knockout of Sox2 (Dun et al., 2019). We showed a dramatic defect of both axon pathfinding and Schwann cell migration in the sciatic nerve bridge of Sox2 knockout mice following nerve injury. At both 10 and 14 days following sciatic nerve transection, large numbers of axons had left the nerve bridge and a completely abnormal nerve bridge formation was still clearly observed even at three months post-injury. To test whether the axon regeneration defects in Sox2 knockout mice were caused by ectopic Schwann cell migration, we used green fluorescent protein-labelled Schwann cells by crossing Sox2 knockout mice with a proteolipid protein promoter driving green fluorescent protein line (Mallon et al., 2002). Abnormal Schwann cell migration within the nerve bridge of Sox2 knockout animals was observed at 6 days following sciatic nerve transection with regenerating axons following the ectopic migrating Schwann cells. Ectopic migrating Schwann cells in Sox2 knockout nerves were still observed at 14 days post-injury and did not form correct Schwann cell cords connecting the proximal and the distal nerve stumps. To examine potential Sox2 targets in Schwann cells, we performed in vitro microarray analysis by overexpressing Sox2 in cultured primary rat Schwann cells and identified that Sox2 also regulates the expression of the axon guidance receptor Robo1. The regulation of Robo1 expression by Sox2 in Schwann cells was further confirmed in both Schwann cell-specific Sox2 knockout and Sox2 overexpressing mice after sciatic nerve transection injury (Dun et al., 2019).
The ligands for Robo1 are the secreted Slit glycoproteins. So far, three Slits (Slit1/2/3) have been identified in vertebrates with a spatio-temporal expression pattern in the central and peripheral nervous system during development and all of them bind to the Robo1 and Robo2 receptors with high affinity (Blockus and Chedotal, 2016). Interaction of Slit1–3 ligands with their Robo1–2 receptors form one of the most crucial ligand-receptor pairs among the classic axon guidance molecule family proteins and serves as a repulsive signaling pathway to control precise axon pathfinding and neuronal migration during nervous system development (Blockus and Chedotal, 2016). This is a function that is conserved in flies, worms, and vertebrates and which has been validated in numerous genetic studies (Blockus and Chedotal, 2016). After identifying that Sox2 regulates Robo1 expression in Schwann cells, we moved on to investigate the expression of Slit1–3 and Robo1–2 in the nerve bridge and found that Slit3 and Robo1 are highly expressed in the mouse sciatic nerve bridge during regeneration (Dun et al., 2019). Macrophages in the outermost layer of the nerve bridge express high levels of Slit3 while migrating Schwann cells inside the nerve bridge express high levels of its receptor Robo1. The expression of Slit3 by macrophages in the outermost layer of the nerve bridge and the expression of Robo1 in migrating Schwann cells inside the nerve bridge, led us to speculate that macrophage derived Slit3 could regulate the trajectory of Robo1 expressing Schwann cell migration within the nerve bridge.
To understand a primary role of Slit3/Robo1 signaling in Schwann cell migration, mice with reduced expression of both Slit3 and Robo1 (Slit3+/–/Robo1+/–) were generated and the effects upon Schwann cell migration and axonal pathfinding were tested. In control animals, at 14 days post-transection, Schwann cells migrated and stayed within the bridge and formed cell cords connecting the proximal and distal nerve ends. However, in Slit3+/–/Robo1+/– mice, a large population of Schwann cells left the nerve bridge from both the proximal and the distal nerve ends, confirming that Slit3 is required for controlling the trajectory of Schwann cell migration within the nerve bridge. We also co-cultured rat primary Schwann cells with control Slit3+/+ or Slit3–/– bone marrow macrophages and demonstrated an apparent Slit3/Robo1 repulsive signaling between Schwann cells and macrophages. A further examination of nerve bridge formation confirmed that the nerve bridge tissue was not correctly formed in Slit3+/–/Robo1+/– mice. Further studies have been carried out to examine the pattern of axon regeneration in the nerve bridge of Slit1–3 and Robo1–2 gene mutant mice at 14 days following sciatic nerve transection injury. In line with the observation of ectopic Schwann cell migration in Slit3 and Robo1 gene mutant mice, a remarkable axon regeneration defect was observed in the nerve bridge of Slit3+/–, Slit3–/–, Robo1+/– and Slit3+/–/Robo1+/– mice but not in Slit1–/–, Slit2+/– or Robo2+/– mice. Thus, our studies have demonstrated that the Slit3/Robo1 repulsive signaling between macrophages and Schwann cells in the nerve bridge plays an important role in regulating the trajectory of Schwann cell migration. Angiogenesis is a key event for correct nerve bridge formation and all classic axon guidance molecules have important functions in angiogenesis (Adams and Eichmann, 2010). However, we found that Slit3/Robo1 signaling does not appear to be required for angiogenesis in the nerve bridge following nerve transection (Dun et al., 2019). It will be interesting to study if other classic axonal guidance signaling pathways are involved in regulating angiogenesis in the nerve bridge.
Future Direction
Successful peripheral nerve bridge formation requires the coordination of multiple cell types and the activation of multiple signaling pathways in the nerve bridge. The nerve bridge consists of newly formed tissue connecting the proximal and distal nerve stumps and comprises macrophages, fibroblasts, endothelial cells and Schwann cells (Cattin et al., 2015). Previously, Parrinello et al. (2010) showed that the coordination between nerve fibroblasts and Schwann cells is controlled by EphrinB2/EphB2 signaling, and recently we showed that the coordination between macrophages and Schwann cells is controlled by the Slit3/Robo1 signaling. During development, classic axon guidance molecules Netrin, Slit, Ephrin and Semaphorin often cooperate with each other to regulate cell migration and axon pathfinding (Bashaw and Klein, 2010). Cell coordination in the nerve bridge between perineurial cells and Schwann cells (Lewis and Kucenas, 2014), between macrophages and endothelial cells (Cattin et al., 2015), and between macrophages and fibroblasts (Dun et al., 2019) have all now been reported. It will be interesting to study how other axonal guidance signaling pathways are involved in controlling cell coordination in the nerve bridge, and how the synergistic function among classic axon guidance molecules controls correct nerve bridge tissue formation and precise axon targeting. We believe understanding these key cellular and molecular mechanisms could help to develop better engineered neural tissue for more effective peripheral nerve repair.
Additional file: Open peer review report 1 (98.8KB, pdf) .
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Diego Noé Rodríguez Sánchez, São Paulo State University, Brazil.
P-Reviewer: Rodríguez Sánchez DN; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blockus H, Chedotal A. Slit-Robo signaling. Development. 2016;143:3037–3044. doi: 10.1242/dev.132829. [DOI] [PubMed] [Google Scholar]
- 4.Carr MJ, Johnston AP. Schwann cells as drivers of tissue repair and regeneration. Curr Opin Neurobiol. 2017;47:52–57. doi: 10.1016/j.conb.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulthard MG, Morgan M, Woodruff TM, Arumugam TV, Taylor SM, Carpenter TC, Lackmann M, Boyd AW. Eph/Ephrin signaling in injury and inflammation. Am J Pathol. 2012;181:1493–1503. doi: 10.1016/j.ajpath.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, Bernet A, Mehlen P, Chedotal A. Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature. 2017;545:350–354. doi: 10.1038/nature22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dun XP, Parkinson DB. Visualizing peripheral nerve regeneration by whole mount staining. PLoS One. 2015;10:e0119168. doi: 10.1371/journal.pone.0119168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dun XP, Parkinson DB. Role of Netrin-1 signaling in nerve regeneration. Int J Mol Sci. 2017 doi: 10.3390/ijms18030491. doi: 10.3390/ijms18030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dun XP, Carr L, Woodley PK, Barry RW, Drake LK, Mindos T, Roberts SL, Lloyd AC, Parkinson DB. Macrophage-derived Slit3 controls cell migration and axon pathfinding in the peripheral nerve bridge. Cell Rep. 2019;26:1458–1472 e1454. doi: 10.1016/j.celrep.2018.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Georgiou M, Bunting SC, Davies HA, Loughlin AJ, Golding JP, Phillips JB. Engineered neural tissue for peripheral nerve repair. Biomaterials. 2013;34:7335–7343. doi: 10.1016/j.biomaterials.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Jaminet P, Kohler D, Schaufele M, Rahmanian-Schwarz A, Lotter O, Fornaro M, Ronchi G, Geuna S, Rosenberger P, Schaller HE. Evaluating the role of Netrin-1 during the early phase of peripheral nerve regeneration using the mouse median nerve model. Restor Neurol Neurosci. 2013;31:337–345. doi: 10.3233/RNN-120277. [DOI] [PubMed] [Google Scholar]
- 13.Johnston AP, Yuzwa SA, Carr MJ, Mahmud N, Storer MA, Krause MP, Jones K, Paul S, Kaplan DR, Miller FD. Dedifferentiated Schwann cell precursors secreting paracrine factors are required for regeneration of the mammalian digit tip. Cell Stem Cell. 2016;19:433–448. doi: 10.1016/j.stem.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Ke X, Li Q, Xu L, Zhang Y, Li D, Ma J, Mao X. Netrin-1 overexpression in bone marrow mesenchymal stem cells promotes functional recovery in a rat model of peripheral nerve injury. J Biomed Res. 2015;29:380–389. doi: 10.7555/JBR.29.20140076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 17.Lewis GM, Kucenas S. Perineurial glia are essential for motor axon regrowth following nerve injury. J Neurosci. 2014;34:12762–12777. doi: 10.1523/JNEUROSCI.1906-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol. 2013 doi: 10.1101/cshperspect.a009159. doi: 10.1101/cshperspect.a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madison RD, Zomorodi A, Robinson GA. Netrin-1 and peripheral nerve regeneration in the adult rat. Exp Neurol. 2000;161:563–570. doi: 10.1006/exnr.1999.7292. [DOI] [PubMed] [Google Scholar]
- 20.Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore AM, Kasukurthi R, Magill CK, Farhadi HF, Borschel GH, Mackinnon SE. Limitations of conduits in peripheral nerve repairs. Hand (N Y) 2009;4:180–186. doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parfejevs V, Debbache J, Shakhova O, Schaefer SM, Glausch M, Wegner M, Suter U, Riekstina U, Werner S, Sommer L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat Commun. 2018;9:236. doi: 10.1038/s41467-017-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrausch B, Jung M, Leppert CA, Stuermer CA. Lesion-induced regulation of netrin receptors and modification of netrin-1 expression in the retina of fish and grafted rats. Mol Cell Neurosci. 2000;16:350–364. doi: 10.1006/mcne.2000.0877. [DOI] [PubMed] [Google Scholar]
- 25.Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg AF, Isaacman-Beck J, Franzini-Armstrong C, Granato M. Schwann cells and deleted in colorectal carcinoma direct regenerating motor axons towards their original path. J Neurosci. 2014;34:14668–14681. doi: 10.1523/JNEUROSCI.2007-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 28.Webber CA, Christie KJ, Cheng C, Martinez JA, Singh B, Singh V, Thomas D, Zochodne DW. Schwann cells direct peripheral nerve regeneration through the Netrin-1 receptors, DCC and Unc5H2. Glia. 2011;59:1503–1517. doi: 10.1002/glia.21194. [DOI] [PubMed] [Google Scholar]
- 29.Yamagishi S, Yamada K, Sawada M, Nakano S, Mori N, Sawamoto K, Sato K. Netrin-5 is highly expressed in neurogenic regions of the adult brain. Front Cell Neurosci. 2015;9:146. doi: 10.3389/fncel.2015.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


