Abstract
Daxueteng, the liana stem of Sargentodoxa cuneata, is a widely used Traditional Chinese Medicine facing the overflow of its commercial adulterants. A method for discriminating adulterants and screening potential candidate alternatives of S. cuneata was thus established. Total phenols and flavonoids of S. cuneata and its six adulterants and their abilities to scavenge DPPH• and ABTS•+, to absorb peroxyl radicals (ORAC), and to inhibit AAPH-induced supercoiled plasmid DNA strand scission were comprehensively assessed. Polygonum cuspidatum and Bauhinia championii, two of the six adulterants of S. cuneate, shared considerably higher antioxidant activities as well as phenolic contents and, therefore, were considered as potential candidate alternatives. Phenolic compositions of the two potential candidate alternatives and S. cuneata itself were further determined by UPLC-QTOF-MS/MS. Totally 38 phenolics, including four hydroxybenzoic acids, two tyrosols, two caffeoylquinic acids, seven flavanol or its oligomers, two lignans, three hydroxycinnamic acids, six stilbenes, seven anthraquinones, and five flavanones were determined from three species. Furthermore, contents of different phenolic categories were semi-quantified and the major antioxidant contributors of S. cuneata and the two potential candidate alternatives were subsequently determined. It is concluded that tyrosols and caffeoylquinic acids were unique categories making great antioxidant contributions in S. cuneata and thus were considered as effective biomarkers in distinguishing its potential candidate alternatives.
Keywords: Sargentodoxa cuneata (Oliv.) Rehd. et Wils, medicinal linana, adulterants, potential candidate alternatives, phenolics, antioxidant activity, UPLC-DAD-QTOF-MS/MS analysis, HPLC fingerprint
1. Introduction
Sargentodoxa cuneata, a deciduous woody liana that belongs to Sargentodoxaceae (previously attributed to Lardizabalaceae), widely distributed over mountain forests from East Asia to central China [1] and sporadically occurred in Laos and Northern Vietnam [2]. According to the Pharmacopoeia of People’s Republic of China [3], the liana stem of S. cuneata has long been utilized as heat-clearing folk medicine named “Daxueteng” in China for its significant anti-inflammatory [4], anti-thrombotic, anti-tumor [5], anti-bacterial [6], anti-viral [7], and anti-allergic [8] effects. Furthermore, Li et al., [9] suggested that S. cuneata exhibited the highest total phenols and antioxidant capacities among 45 selected medicinal plants.
The World Health Organization (WHO) has estimated that over 80% of the earth’s inhabitants rely on the plant extracts and their active components of herbal medicines for their primary health care needs [10]. According to a report from Global Industry Analysts, the demand for herbal medicine increased with compound annual growth rate (CAGR) of 6–10% and this market is forecasted to reach $107 billion by the year 2017 (http://www.strategyr.com/MarketResearch). Data from the National Bureau of Statistics of China also indicated that the production of herbs used for Chinese Traditional Medicine reached 3.5 million tons in 2015 and over 3.7 million tons in 2016 (http://data.stats.gov.cn). With such a large production scale, pharmacovigilance is urgently required for those herbal medicinal products and drug safety should not be considered secondary to efficacy or be compromised by this increasing demand [10]. One of the major safety problems always faced by herbal drugs is the appearance of their commercial adulterants owing to the difficulty to identify herbs visually, especially for liana herbal medicine which occurred in markets as dry slices of their stems. Undeclared chemical and active ingredients of the adulterants could result in various degrees of side effects [11], and one of the most appalling examples is that the adulterants of herbal medicines have induced seriously anticholinergic poisoning in Hong Kong during 1989–2012 [11]. Specific to S. cuneata, the natural resources of S. cuneata have been declining dramatically due to long-term harvest, which particularly led to the overflow of its adulterants [12]. Therefore, it is essential to establish a rapid method for distinguishing S. cuneata from its adulterants and for screening its potential candidate alternatives from authentic herbs possessing similar medical effects.
In previous studies, many sound methods and tools were reported for the screening of adulterants for herbs or foods. Among which, liquid chromatography-diode array detector-quadrupole time-of-flight-mass spectrometer/mass spectrometer (LC-DAD-QTOF-MS/MS) [13,14,15] and DNA barcoding [16,17] were frequently applied. DNA barcoding is able to judge the relationship of various species at the genetic level. However, it requires elaborate sample preparation starting from fresh plants rather than processed herbal products like dry stems of S. cuneate occurring in the market. Comparing with DNA barcoding, LC-DAD-QTOF-MS/MS requires less sample condition and skill of operator, and thus contribute to the discovery of chemical constituents in crude herbal extracts and makes the overall comparison of chemical composition among authentic herbs and their adulterants possible.
Recently, studies have been focused on isolation and identification of bioactive components in S. cuneata, and several types of compounds including phenolics [18,19], lignans [19], triterpenes [20], anthraquinones [21], and phenylpropanoids [19] were characterized. Chang and Case [22] demonstrated that among 12 compounds isolated from the water-soluble constituents of S. cuneata stem, only two phenolic glycosides (cuneatasides A and B) possessed significant inhibitory activity against two gram-positive organisms, Staphylococcus aureus and Micrococcus epidermidis. Meanwhile, Zeng et al., [6] also suggested that hydroxytyrosol, a phenolic acid, possessed the highest antibacterial activity against S. aureus and Acinetobacter baumannii among 39 isolated compounds from the stem of S. cuneata. These researches indicated that phenolics in S. cuneata contribute not only to the well-known antioxidant ability but also to the antibacterial activity. Meanwhile, as inflammatory responses often include formation of tissue-damaging oxidation products, antioxidant ability could also be correlated to anti-inflammatory activity [23]. Therefore, phenolics could be selected as marker components for establishing methods for distinguishing adulterants and screening alternatives of S. cuneata.
In the present study, in order to screen adulterants and potential candidate alternatives of S. cuneata efficiently, total phenols and flavonoids and overall antioxidant activities of S. cuneata and its six adulterants were comprehensively assessed. Furthermore, an efficient HPLC fingerprint was developed for distinguishing S. cuneata from its adulterants, Polygonum cuspidatum and Bauhinia championii were determined as potential candidate alternatives for S. cuneata, and their antioxidant contributors were identified and semi-quantified with LC-DAD-QTOF-MS/MS. To the best of our knowledge, this is the first report of a method for screening adulterants and potential candidate alternatives of S. cuneata, and thus provide an efficient guideline for its industrial utilization.
2. Results and Discussion
2.1. Differences in Total Phenols and Flavonoids in Stems of S. cuneata and Its Six Adulterants
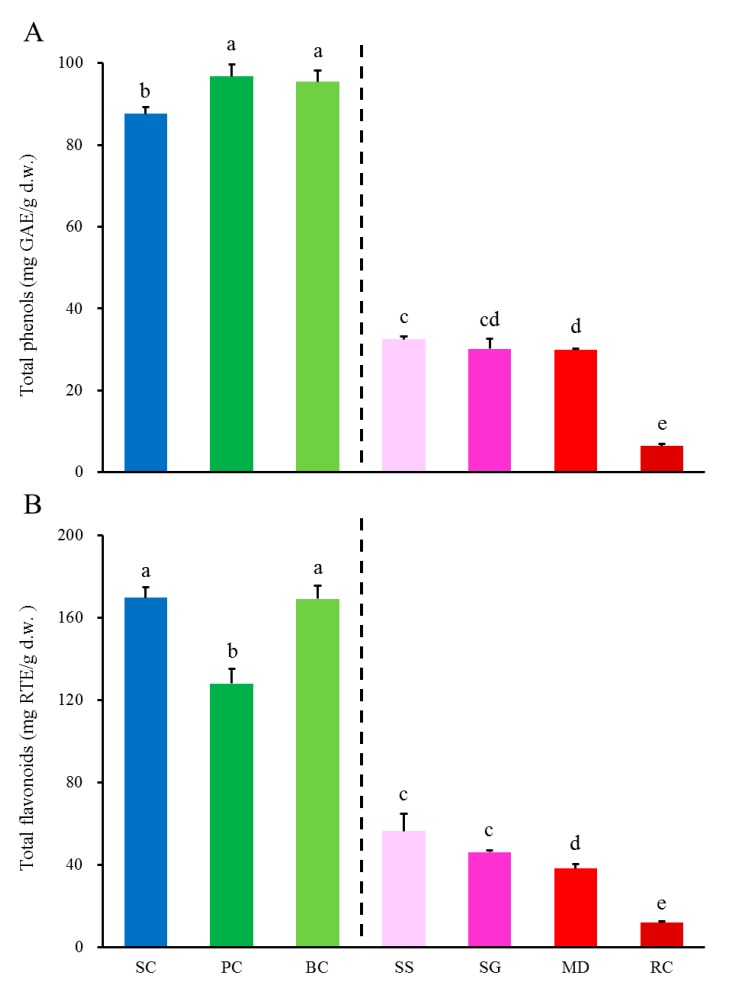
The Folin-Ciocalteu method was applied in determining total phenols in stems of S. cuneata and its six adulterants. As shown in Figure 1A, different samples exhibited diverse levels of total phenols with an order as Polygonum cuspidatum (PC) and Bauhinia championii (BC) > Sargentodoxa cuneate (SC) >> Schisandra sphenanthera (SS), Schisandra grandiflora (SG) and Millettia dielsiana (MD) > Rubia cordifolia (RC), namely, total phenols of SC were significantly lower than those of PC and BC, but far higher (5.03-fold in average) than all other four samples (SS, SG, MD, and RC) as hinted by the vertical dashed line. It should be noted that total phenols of SC determined in the present study (87.59 ± 1.56) were much higher compared with those determined by Li et al., [9] (52.35 ± 0.25), which was much likely due to a higher extraction efficiency caused by our ultrasonic method.
Figure 1.
Total phenols (A) and total flavonoids (B) of S. cuneata and its six adulterants. SC, PC, BC, SS, SG, MD, and RC were abbreviations of Sargentodoxa cuneata, Polygonum cuspidatum, Bauhinia championii, Schisandra sphenanthera, Schisandra grandiflora, Millettia dielsiana, and Rubia cordifolia in respective. Vertical lines provide hints that data on the left side are far greater than those on the right side. Different letters mean significant difference (p > 0.05), and data are mean ± SD (n = 3).
Figure 1B shows total flavonoids analyzed by aluminium chloride colorimetric assay with a similar order to that of total phenols as SC, BC > PC >> SS and SG > MD > RC. In other words, total flavonoids of SC, BC and PC were still far higher (5.44-fold in average) than those of SS, SG, MD and RC as hinted also by the vertical line.
Interestingly, in the group at the left side of the two vertical lines of Figure 1, PC exhibited the lowest total flavonoids (Figure 1B) but the highest total phenols (Figure 1A), and SC exhibited the highest total flavonoids (Figure 1B) but the lowest total phenols (Figure 1A), indicating that there existed divergent phenolic compositions in the three species (SC, PC, and BC) and total flavonoids were the major contributor to the total phenols in SC.
2.2. Differences in Antioxidant Activities in Stems of S. cuneata and Its Six Adulterants
2.2.1. DPPH• and ABTS•+ Scavenging Activities and ORAC
Radical scavenging abilities of S. cuneata and its six adulterants were determined with DPPH and ABTS assays. As shown in Figure 2A,B, DPPH• scavenging abilities ranked as SC, BC and PC >> SG > SS and RC > MD and SC, and ABTS•+ scavenging abilities ranked as SC and BC > PC >> MD, SG, and SS > RC. Most notably, the two-levels pattern found in both total phenols and flavonoids (Figure 1) also occurred in DPPH• and ABTS•+ scavenging abilities as hinted also by the dashed lines, with the left side group (i.e., SC, BC, and PC) were far higher (4.62-fold and 5.35-fold in averages in respective) than those of the right side group (i.e., SG, SS, RC and MD), indicating that total flavonoids were key constituents of total phenols, and total phenols were main contributors to radical scavenging abilities of S. cuneata and its six adulterants, especially those two (i.e., PC and BC) in the left side group. ABTS•+ as shown in Figure 2C, similar order to those of DPPH• and ABTS•+ scavenging abilities was not found when measuring ORAC which ranked as PC > SC > BC > SS and MD > SG > RC. Nevertheless, both PC, SC, and BC still showed higher values (3.74-fold on average) than those of the other four as arbitrarily divided by the fine vertical dotted line. This distinction could be interpreted by the mechanistic divergence between ORAC and DPPH or ABTS assays. To be more specific, radicals are mainly quenched via two chemical principles, namely, transfer of either a hydrogen atom or a single electron to convert radicals into a stable form [24]. DPPH and ABTS assays are based on the transfer of single electron and measures the electron-donating capacity of antioxidants to reduce DPPH• and ABTS•+ radicals. In the case of the ORAC assay, antioxidants slow the loss of fluorescence by quenching the peroxyl radicals produced by AAPH via transfer of hydrogen atom or radical addition. The difference in principles between these two types of mechanisms might be responsible for the variation between the rank order of S. cuneata and its six adulterants in the three assays for antioxidant abilities.
Figure 2.
Antioxidant activities of S. cuneata and its six adulterants determined with DPPH (A), ABTS (B), and ORAC (C) assays. SC, PC, BC, SS, SG, MD, and RC were abbreviations of Sargentodoxa cuneata, Polygonum cuspidatum, Bauhinia championii, Schisandra sphenanthera, Schisandra grandiflora, Millettia dielsiana, and Rubia cordifolia in respective. Vertical dashed (A,B)/dotted (C) lines provide hints that data at the left side are far greater/greater than those on the right side. Different letters mean significant difference (p > 0.05). Data are mean ± SD (n = 3).
2.2.2. Differences in Inhibition against Radicals-Induced Supercoiled Plasmid DNA Strand Scission
It is well known that overproduction of reactive oxygen species (ROS) [arising either from mitochondrial electron transport chain or excessive stimulation of NAD(P)H] leads to oxidative stress that results in deleterious damage to cell structures, including lipids and membranes, proteins, and DNA [25]. Under ROS stresses, the supercoiled plasmid DNA converts to open circular DNA followed by linear form via single or double-strand breaks, and this conversion could be used as an indicator of DNA damage [26]. In the present study, supercoiled plasmid DNA under AAPH-induced oxidative stress was treated with extracts from S. cuneata and its six adulterants. As shown in Figure 3A, DNA bands in supercoiled and open circular form were well separated, and different samples exhibited diverse inhibition abilities against supercoiled plasmid DNA strand scission. The calculated retention percentages of AAPH-induced supercoiled plasmid DNA strand scission were presented in Figure 3B. Results show that SC both at 0.075 and 0.150 mg/mL and PC at 0.150 mg/mL exhibited the highest protective effects. The inhibition ability of PC at 0.075 mg/mL was significantly lower than those of the above three but was still significantly far higher than all other treatments at two concentrations of the other five species. In contrast to the results of phenolic and flavonoids contents (Figure 1) and the three antioxidant abilities (Figure 2), the protective effect of BC was much lower comparing with the other two species (SC and PC) in the former higher-level group and was even lower than those of two in the lower-level group (SS and SG) as divided by the vertical lines.
Figure 3.
Agarose gel electrophoretograms of AAPH-induced supercoiled plasmid DNA strand scission (A) and their correspondingly calculated retention percentages (B). Lanes treated with PBS (lane 1), AAPH (lane 2), and AAPH + phenolic extracts of S. cuneata and its six adulterants (lanes 3-16) were presented. From lanes 3-16, lanes labeled with odd numbers were treated with extracts at a concentration of 0.075 mg/mL, and those labeled with even numbers were treated with those at a concentration of 0.150 mg/mL. SC, PC, BC, SS, SG, MD, and RC were abbreviations of Sargentodoxa cuneata, Polygonum cuspidatum, Bauhinia championii, Schisandra sphenanthera, Schisandra grandiflora, Millettia dielsiana, and Rubia cordifolia, respectively. Different letters in (B) mean significant difference (p > 0.05).
As phenols were demonstrated as key bioactive compounds and antioxidant ability could be correlated to anti-inflammatory activity in SC, PC and BC possessed the highest potential of being candidate alternatives of SC because of their similar higher total phenols, total flavonoids, and antioxidant abilities (Figure 1 and Figure 3). Among which, PC might be better than BC based further on the results of their ORAC values (Figure 2C) and inhibition abilities on radicals-induced supercoiled plasmid DNA strand scission.
2.3. Differences in HPLC Fingerprints of S. cuneata and Its Six Adulterants
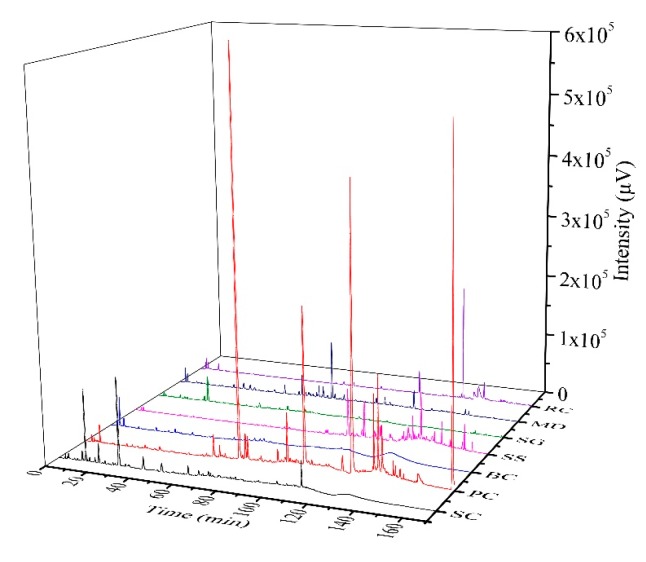
To further determine the detailed phenolic composition of S. cuneata and its adulterants, an HPLC fingerprint that suits for all samples was established. As shown in Figure 4, peaks were well separated in each sample, and all of them exhibited a diverse HPLC profile. Comparing with the profile of SC with those of its six adulterants by Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine software (Version 2004 A), none of the adulterants showed significant correlations with SC. Therefore, it could be concluded that the phenolic composition of all those adulterants, even the two possessing highest potential of being candidate alternatives (i.e., PC and BC) screened based on the above data, varied a lot from that of SC. Consequently, more detailed information on specific phenolic compounds in SC and its two potential candidate alternatives (PC and BC) needs to be further identified by the MS/MS technology, and antioxidant contributions of individual phenolics should be subsequently analyzed.
Figure 4.
HPLC fingerprints of S. cuneata and its six adulterants. SC, PC, BC, SS, SG, MD, and RC were abbreviations of Sargentodoxa cuneata, Polygonum cuspidatum, Bauhinia championii, Schisandra sphenanthera, Schisandra grandiflora, Millettia dielsiana, and Rubia cordifolia, respectively.
2.4. Characterization of Phenolic Compounds by UPLC-DAD-QTOF-MS/MS in S. cuneata and Its Two Potential Candidate Alternatives
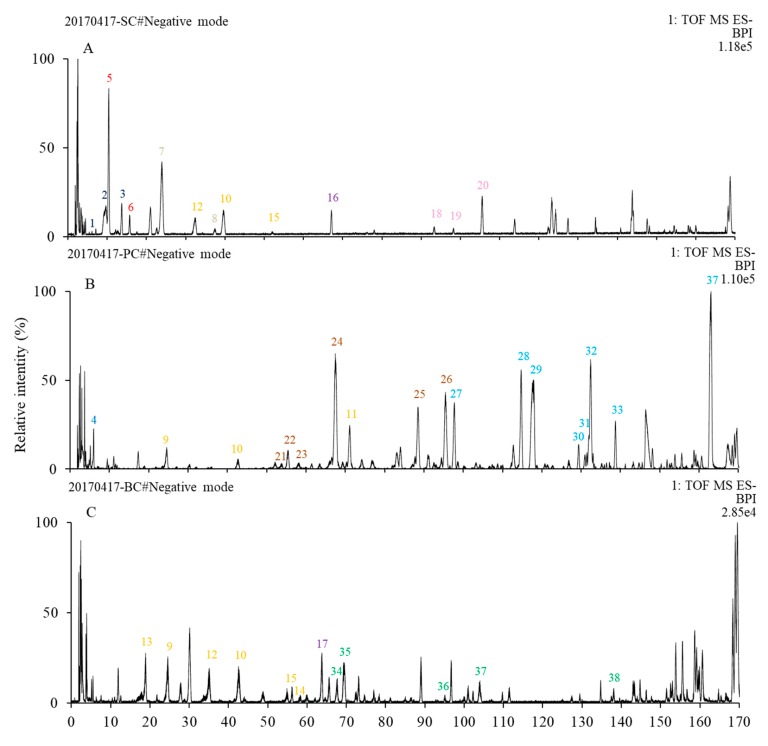
Although SC and its two potential candidate alternatives (i.e., PC and BC) exhibited similar DPPH, ABTS, and ORAC values as well as total phenols and flavonoids, varied phenolic profiles among them could still lead to diverse medical effects. Therefore, UPLC-DAD-QTOF-MS/MS was applied to further characterize their phenolic compositions, and to establish screening parameters for identifying their commercialized products. Both positive and negative ion modes were used aiming to obtain appropriate ionization. The results indicate that the negative ion mode possessed cleaner parent and fragment ion signals, better resolution, and lower background noise compared with the corresponding positive ion mode, which is likely due to the acidic nature of the detected compounds. Therefore, analyzes were conducted with the data obtained from the negative ion mode. The MS spectra of SC, PC, and BC were presented in Figure 5A–C, respective. It could be observed that the spectra obtained from different samples were quite complex, and 14, 17 and 12 phenolic compounds were tentatively identified from SC, PC, and BC, respectively.
Figure 5.
UPLC-QTOF-MS spectra of SC (A), PC (B) and BC (C). Numbers in (A–C) correspond to those peak numbers in Table 1. SC, PC, and BC were abbreviations of S. cuneata, P. cuspidatum, and B. championii, respectively.
Table 1 shows a list of totally 38 compounds tentatively identified through UPLC–DAD–QTOF-MS/MS along with their peak numbers, maximal UV absorptions, precursor ions, error values, predictive formula, MS/MS ions, compound names, compound group classified based on their structure, the samples where they were collected, and the references or MS database supporting the identification. Totally, among these 38 phenolics, four were identified as hydroxybenzoic acids, two are tyrosols, two are caffeoylquinic acids, seven are flavanol or its oligomers, two are lignans, three are hydroxycinnamic acid, six are stilbenes, seven are anthraquinones, and five are flavanones.
Table 1.
Phenolic compounds tentatively identified with UPLC-DAD-QTOF-MS/MS
| Peak No. | λmax | [M−H]− (m/z) | Error (ppm) | Formula | MS/MS Fragments (m/z) | Identified Compounds | Compound Group | Samples | References |
|---|---|---|---|---|---|---|---|---|---|
| (nm) | |||||||||
| 1 | 230, 250, 294 | 153 | −3.9 | C7H5O4 | 109 | Protocatechuic acid | Hydroxybenzoic acids | SC | [29] |
| 2 | 255, 286 | 167 | −2.4 | C8H7O4 | 108, 123, 152 | Hydroxybenzoic acids | SC | [27] | |
| 3 | 262 | 197 | −9.6 | C9H9O5 | 182, 166, 153 | Syringic acid | Hydroxybenzoic acids | SC | [30] |
| 4 | 228, 272 | 169 | 3.5 | C7H5O5 | 125 | Gallic acid | Hydroxybenzoic acids | PC | [31] * |
| 5 | 230, 280 | 315 | −1.6 | C14H19O8 | 153, 135 | Hydroxytyrosol-1-O-glucoside | Tyrosols | SC | [32] |
| 6 | 228, 276 | 299 | 2 | C14H19O7 | 137, 119, 101, 96 | Salidroside | Tyrosols | SC | [43] * |
| 7 | 242, 326 | 353 | 1.1 | C16H17O9 | 191 | 3-O-Caffeoylquinic acid | Caffeoylquinic acids | SC | [35] |
| 8 | 247, 316 | 353 | −0.8 | C16H17O9 | 191, 78, 96 | 5-O-Caffeoylquinic acid | Caffeoylquinic acids | SC | [35] * |
| 9 | 235, 280 | 289 | −0.3 | C15H13O6 | 137, 109 | Catechin | Flavanols | PC BC | [37] |
| 10 | 234, 278 | 289 | −0.7 | C15H13O6 | 137, 109 | Epicatechin | Flavanols | SC PC BC | [37] * |
| 11 | 239, 280 | 441 | −1.8 | C22H17O10 | 289, 169 | Catechin-gallate | Flavanols | PC | [38] |
| 12 | 236, 278 | 577 | 1.2 | C30H25O12 | 407, 289, 425 | B type proanthocyanidin dimer 1 | Flavanol oligomer | SC BC | [37] * |
| 13 | 237, 279 | 577 | 5.5 | C30H25O12 | 289, 407 | B type proanthocyanidin dimer 2 | Flavanol oligomers | BC | [43] |
| 14 | 243, 279 | 577 | −5 | C30H25O12 | 287, 289 | B type proanthocyanidin dimer 3 | Flavanol oligomers | BC | [43] * |
| 15 | 239, 278 | 865 | 2.1 | C45H37O18 | 577, 695, 407, 287 | B type proanthocyanidin trimer | Flavanol oligomers | SC BC | [38] |
| 16 | 238, 276 | 579 | −2.8 | C28H35O13 | 417, 181, 96 | Syringaresinol-4-O-glucoside | Lignans | SC | [22] |
| 17 | 242, 280 | 581 | 10.3 | C28H37O13 | 419 | Lyoniresinol glucoside | Lignans | BC | [43] |
| 18 | 245, 283, 330 | 359 | −4.2 | C18H15O8 | 359, 197, 161, 135 | Rosmarinate | Hydroxycinnamic acids | SC | [29] * |
| 19 | 245, 283 | 493 | −4.1 | C26H21O10 | 295 | Salvianolic acid A | Hydroxycinnamic acids | SC | [29] * |
| 20 | 244, 285 | 717 | −1.8 | C36H29O16 | 519, 321 | Salvianolic acid B | Hydroxycinnamic acids | SC | [29] * |
| 21 | 249, 300 | 469 | 8.3 | C20H21O11S | 227, 215 | Resveratrol-sulfoglucoside | Stilbenes | PC | [43] |
| 22 | 249, 304 | 389 | 3.1 | C20H21O8 | 227 | Resveratrol-4′-glucoside (Resveratroloside) | Stilbenes | PC | [38] |
| 23 | 245, 280 | 469 | 10 | C20H21O11S | 227, 407, 289 | Resveratrol-sulfoglucoside (isomer) | Stilbenes | PC | [43] |
| 24 | 236, 319 | 389 | 6.4 | C20H21O8 | 227 | Resveratrol-3-O-glucoside (Piceid) | Stilbenes | PC | [38] |
| 25 | 246, 300 | 541 | 3 | C27H25O12 | 313, 169, 227 | Resveratrol-galloylglucoside | Stilbenes | PC | [38] |
| 26 | 237, 306 | 227 | −3.5 | C7H15O8 | 185, 143 | Resveratrol | Stilbenes | PC | [41] * |
| 27 | 254, 282, 426 | 431 | −2.6 | C21H19O10 | 269 | Emodin-8-O-glucoside | Anthraquinones | PC | [38] * |
| 28 | 229, 281, 427 | 431 | 2.3 | C21H19O10 | 269 | Emodin-1-O-glucoside | Anthraquinones | PC | [38] * |
| 29 | 248, 280, 427 | 517 | −2.7 | C24H21O13 | 269, 473 | Emodin-8-O-(6′-O-malonyl)-glucoside | Anthraquinones | PC | [38] |
| 30 | 247, 272, 421 | 283 | 4.2 | C16H11O5 | 268, 240 | Physcion | Anthraquinones | PC | [38] |
| 31 | 250, 268, 441 | 285 | 4.6 | C15H9O6 | 267, 257, 255 | Citreorosein | Anthraquinones | PC | [43] |
| 32 | 249, 273, 441 | 299 | 0.7 | C15H7O7 | 255, 227 | Emodic acid | Anthraquinones | PC | [43] |
| 33 | 267, 288, 441 | 269 | 3 | C19H9O2 | 225, 241 | Emodin | Anthraquinones | PC | [38] * |
| 34 | 242, 279 | 417 | 10.1 | C21H21O9 | 255 | Liquiritin | Flavanones | BC | [37] |
| 35 | 242, 279 | 417 | 4.1 | C21H21O9 | 255 | Neoliquiritin | Flavanones | BC | [37] * |
| 36 | 244, 279 | 417 | −1.9 | C21H21O9 | 255 | Neoisoliquiritin | Flavanones | BC | [35] |
| 37 | 244, 279 | 255 | 5.9 | C15H11O4 | 119 | Liquiritigenin | Flavanones | BC | [37] * |
| 38 | 247, 279 | 255 | 4.7 | C15H11O4 | 119 | Isoliquiritigenin | Flavanones | BC | [37] * |
* Identification was verified via standards.
Four hydroxybenzoic acids: Compounds 1–4 were identified as hydroxybenzoic acid derivatives as they all exhibited a characteristic fragment ion with the loss of a carboxyl group (CO2) [27]. Compound 1 was assigned as protocatechuic acid with a [M-H]− at m/z 153 as it showed a fragment at m/z 109 with a loss of a CO2 (44 Da) [28]. Moreover, it exhibited similar maximal UV absorption with that reported by Liu et al., [29]. Compound 2 with a [M-H]− at m/z 167 was identified as vanillic acid as it showed fragment ions at m/z 152 due to the loss of a CH3 (15 Da), and at 123 with another loss of a CO2 (44 Da) [27]. Compound 3 with a [M-H]− at m/z 197 was identified as syringic acid because it exhibited product ions at m/z 182 due to the loss of a CH3 (15 Da), at m/z 166 with the loss of another CH3, and at m/z 153 with the loss of a CO2 (44 Da) [30]. Compound 4 was identified as gallic acid with a [M-H]− at m/z 169 as it showed a characteristic fragment at m/z 125 with the loss of a CO2 (44 Da) [31].
Two tyrosols: Compounds 5 and 6 both exhibited similar maximum UV absorbance at 228–230 and 276–280 nm. Therefore, it could be deduced that they shared similar structures. Compound 5 with a [M-H]− at m/z 315 was assigned as hydroxytyrosol-1-O-glucoside because it showed fragment ions at m/z 153, which was the fragment ion of hydroxytyrosol [32]. Compound 6 with a [M-H]− at m/z 299 was assigned as salidroside as its fragmentation patterns corresponded with those reported by Han et al., [33].
Two caffeoylquinic acids: Compounds 7 and 8 both showed a [M-H]− at m/z 353 and maximum UV absorbances at around 245 and 320 nm, thus they were identified as caffeoylquinic acid isomers. It is reported that compounds with similar chemical structures, like isomers or aglycone with different glycosides, always eluted in the same order in C18 column [34]. Based on their MS/MS fragments and elution order in the C18 column, compound 7 was identified as 3-O-caffeoylquinic acid, and compound 8 was identified as 5-O-caffeoylquinic acid [35].
Seven flavanols or its oligomers: Compounds 9-15 all showed two characteristic maximum UV absorbances at about 240 and 280 nm, indicating that they might be flavanols or its oligomers. Compounds 9 and 10 shared the same parent ion at m/z 289 and fragment ions at m/z 137 and 109. Due to their elution order in the C18 column, Compound 9 was identified as catechin and compound 10 was assigned as epicatechin [36,37]. Compound 11 with a [M-H]− at m/z 441 was assigned as catechin-gallate due to its fragmentation at m/z 289 (catechin moiety) and m/z 169 (gallic acid moiety) [38]. Compounds 12–14 exhibited the same parent ion at m/z 577 and characteristic product ions at m/z 289 and 287. As previously reported, proanthocyanidins yielded two different types of ions in the collision-induced MS analysis depending on whether they possessed the extension (287 Da) or terminal units (289 Da) of the corresponding proanthocyanidin molecule [4,39]. Therefore, compounds 12–14 were identified as B-type proanthocyanidin dimers 1–3. With the same fragmentation patterns, compound 15 with a [M-H]− at m/z 865 was identified as B-type proanthocyanidin trimer.
Two lignans: Compound 16, with a [M-H]− at m/z 579 and a characteristic fragment at m/z 417 (syringaresinol moiety), was identified as syringaresinol-4-O-glucoside [22,40]. Compound 17 with a [M-H]− at m/z 581 was assigned as lyoniresinol glucoside as it fragmented at m/z 419 (lyoniresinol moiety).
Three hydroxycinnamic acids: Compounds 18 with a [M-H]− at m/z 359 was identified as rosmarinate as it fragmented at m/z 197 (Danshensu moiety) [29]. Compound 19 with a [M-H]− at m/z 717 fragmented at m/z 519 with a loss of a Danshensu moiety (198 Da) and at m/z 321 with another loss of a Danshensu moiety (198 Da), thus was identified as salvianolic acid B. Following the same fragmentation patterns, compound 20 with a [M-H]− at m/z 493 was identified as salvianolic acid A.
Six stilbenes: Compound 26 with a precursor ion at m/z 227 was identified as resveratrol because it showed a product ion at m/z 185 representing a loss of a ketene molecule [41]. Meanwhile, compounds 21–25 all shared a resveratrol moiety (227 Da), indicating that they were resveratrol derivatives. Compounds 21 and 23 with a [M-H]− at m/z 469 were assigned as resveratrol-sulfoglucoside isomers, and compounds 22 and 24 with a [M-H]− at m/z 469 were identified as resveratrol-4′-glucoside (resveratroloside) and resveratrol-3-O-glucoside (piceid), respectively [38]. Compound 25 with a [M-H]− ion at m/z 541 was identified as resveratrol-galloylglucoside as it showed a fragment ion at m/z 169 (gallic acid moiety) [38].
Seven anthraquinones: Compound 32, with a precursor ion at m/z 299 and product ions at m/z 255 and 277 were identified as emodic acid, which corresponded with the data reported by Yao et al., [42]. Compound 33 with a [M-H]− at m/z 269 was identified as emodin as it showed a fragment ion at m/z 225 due to the loss of a CO2 (44 Da) [38]. With a characteristic ion at m/z 269, compounds 27-29 were assigned as emodin derivatives. Comparing their fragmentation patterns with those of literature compounds 27 and 28 with a [M-H]− at m/z 431 were identified as emodin-8-O-glucoside and emodin-1-O-glucoside in respective, and compound 29 with a [M-H]− at m/z 517 was assigned as emodin-8-O-(6′-O-malonyl)-glucoside [38]. Compound 30 with a [M-H]− at m/z 283 was identified as physcion since it exhibited a fragment at m/z 268 due to a loss of a CH3 (15 Da). Compound 31, with a [M-H]− at m/z 285, fragmented at m/z 267, 257 and 255, which corresponded with those recorded in the METLIN database (https://metlin.scripps.edu), was identified as citreorosein.
Five flavanones: Compounds 37 and 38, with the same parent ion at m/z 255 and daughter ion at m/z 119, were assigned as liquiritigenin isomers. Compounds 34–36 exhibited the same precursor ion at m/z 417 and product ion at m/z 255, thus were assigned as liquiritin isomers. Based on their elution order in the C18 column reported by Wang et al., [37], compounds 34–38 were identified as liquiritin, neoliquiritin, neoisoliquiritin, liquiritigenin and isoliquiritigenin in respective.
To validate the accuracy of our tentatively identification, 16 of the 38 phenolics (i.e., compounds 4, 6, 8, 10, 12, 14, 18, 19, 20, 26, 27, 28, 33, 35, 37, 38) were verified by comparing with their standards (Table 1 and Figure S1).
2.5. Antioxidant Contribution of Key Individual Phenolics in S. cuneata and Its Two Potential Candidate Alternatives
In order to unearth potential contributors to overall antioxidant activity in SC, PC and BC, and to screen markers for discriminating S. cuneata from its two potential candidate alternatives, antioxidant activities of nine representative phenolic compounds (or their aglycones) from the eight detected phenolic categories (Table 1) described above were evaluated with DPPH, ABTS, and ORAC assays. Furthermore, content for every single phenolic category measured by semi-quantification and its corresponding antioxidant contribution was subsequently calculated. For instance, gallic acid was selected as the representative phenolic compounds from hydroxybenzoic acids category, and its DPPH, ABTS, and ORAC values were determined as 3.98 ± 0.38, 6.08 ± 0.36, and 2.23 ± 0.03 (μmol Trolox equivalent/μmol) in respective (Table 2). Meanwhile, all hydroxybenzoic acids in SC were semi-quantified by comparing their peak areas with that of gallic acid, and the sum of all these hydroxybenzoic acids was defined as their semi-content. After that, antioxidant contribution of hydroxybenzoic acids in SC was obtained by multiplying their semi-content to DPPH, ABTS or ORAC values of gallic acid.
Table 2.
Antioxidant capacities of nine selected phenolic compounds assessed with DPPH, ABTS, and ORAC assays.
| Categories | Antioxidant Capacities * | Semi-Contents # | Contributions (%) to | |||||
|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | ORAC | DPPH | ABTS | ORAC | |||
| in SC | ||||||||
| gallic acid | Hydroxybenzoic acids | 3.98 ± 0.38 a | 6.08 ± 0.36 a | 2.23 ± 0.03 f | 9.19 ± 0.12 | 23.81 ± 3.49 | 31.88 ± 0.14 | 8.29 ± 0.01 |
| tyrosol | Tyrosols | 0.06 ± 0.02 f | 0.21 ± 0.04 e | 1.96 ± 0.03 g | 116.44 ± 0.44 | 5.60 ± 2.33 | 17.18 ± 0.00 | 113.63 ± 0.01 |
| chlorogenic acid | Caffeoylquinic acids | 0.69 ± 0.02 c | 0.41 ± 0.05 c | 5.01 ± 0.17 b | 112.04 ± 0.87 | 24.16 ± 2.24 | 12.58 ± 0.00 | 108.95 ± 0.01 |
| epicatechin | Flavanols | 1.37 ± 0.11 b | 0.41 ± 0.03 c | 4.45 ± 0.02 c | 21.47 ± 0.21 | 11.22 ± 2.36 | 2.94 ± 0.00 | 22.64 ± 0.00 |
| proanthocyanidin B2 | Flavanol oligomers | 0.10 ± 0.00 e | 0.16 ± 0.01 f | 6.93 ± 0.05 a | 22.24 ± 0.41 | 0.43 ± 0.00 | 0.60 ± 0.00 | 18.32 ± 0.00 |
| rosmarinic acid | Hydroxycinnamic acids | 0.65 ± 0.10 c | 0.53 ± 0.05 b | 4.80 ± 0.06 b | 26.32 ± 0.26 | 5.26 ± 2.63 | 3.76 ± 0.00 | 24.12 ± 0.00 |
| Sum of contributions | 70.48 ± 2.08 | 68.95 ± 0.02 | 295.94 ± 0.01 | |||||
| in PC | ||||||||
| gallic acid | Hydroxybenzoic acids | 3.98 ± 0.38 a | 6.08 ± 0.36 a | 2.23 ± 0.03 f | 6.8 ± 0.14 | 18.64 ± 2.58 | 29.91 ± 0.14 | 4.00 ± 0.01 |
| epicatechin | Flavanols | 1.37 ± 0.11 b | 0.41 ± 0.03 c | 4.45 ± 0.02 c | 62.62 ± 0.35 | 34.65 ± 6.89 | 10.89 ± 0.00 | 43.16 ± 0.00 |
| resveratrol | Stilbenes | 0.44 ± 0.01 d | 0.28 ± 0.11 d | 2.43 ± 0.03 e | 152.66 ± 1.18 | 34.51 ± 1.53 | 23.07 ± 0.00 | 73.07 ± 0.00 |
| emodin | Anthraquinones | ND h | 0.22 ± 0.04 e | 2.50 ± 0.10 e | 185.11 ± 0.62 | 0.00 ± 0.00 | 1.69 ± 0.00 | 76.98 ± 0.00 |
| Sum of contributions | 87.80 ± 2.75 | 65.56 ± 0.04 | 197.22 ± 0.01 | |||||
| in BC | ||||||||
| epicatechin | Flavanols | 1.37 ± 0.11 b | 0.41 ± 0.03 c | 4.45 ± 0.02 c | 112.39 ± 0.76 | 59.97 ± 12.36 | 15.49 ± 0.00 | 164.40 ± 0.00 |
| proanthocyanidin B2 | Flavanol oligomers | 0.10 ± 0.00 e | 0.16 ± 0.01 f | 6.93 ± 0.05 a | 212.98 ± 1.52 | 4.16 ± 0.00 | 5.75 ± 0.00 | 243.42 ± 0.00 |
| liquiritigenin | Flavanones | 0.02 ± 0.00 g | 0.01 ± 0.00 g | 2.89 ± 0.05 d | 21.01 ± 0.08 | 0.19 ± 0.00 | 0.08 ± 0.00 | 22.61 ± 0.00 |
| Sum of contributions | 64.32 ± 4.12 | 21.32 ± 0.00 | 430.42 ± 0.00 | |||||
* indicated that results were presented as μmol Trolox equivalent/μmol phenolic compound, different superscript letters in each row means significant difference. # presented the results as mg phenolic compound/g d.w., respectively.
As shown in Table 2, the nine representative phenolics exhibited diverse DPPH• scavenging abilities with a rank order as gallic acid > epicatechin > chlorogenic and rosmarinic acid > resveratrol > proanthocyanidin B2 > tyrosol > liquiritigenin > emodin. Gallic acid showed a significantly higher DPPH value comparing with other phenolics, while emodin exhibited no DPPH• scavenging ability comparing with others under the same concentrations. As for ABTS values, the nine phenolics exhibited a similar rank order to that of DPPH, and gallic acid still exhibited the highest ABTS•+ scavenging ability, indicating that hydroxybenzoic acids might possess higher electron-donating capacities comparing with other phenolic categories. In contrast, gallic acid only ranked the second to last among the nine phenolics in ORAC, and proanthocyanidin B2 possessed the highest ORAC value, followed by chlorogenic acid and rosmarinic acid. Tabart et al., [44] reported that gallic acid possesses a relatively similar activity in ABTS and ORAC assays while other phenolics including flavonols, flavan-3-ols, and flavanons exhibited a much higher ORAC value than ABTS value, supporting results of the present study.
SC possessed phenolics in six categories while PC and BC only have those in four and three categories, respectively. Caffeoylquinic acids, tyrosols, and hydroxycinnamic acids occurred only in SC while stilbenes and anthraquinones only existed in PC and flavanones were a unique category for BC (see dark red words in Table 2). In SC, hydroxybenzoic and caffeoylquinic acids and tyrosols made important contributions to overall antioxidant capacities as each of them made the largest contribution in one assay. As for PC, hydroxybenzoic acids, flavanols, and stilbenes played important roles in contributions to DPPH and ORAC, but anthraquinones ranked the first in ORAC assay. In BC, flavanols made the most contributions to the overall DPPH• and ABTS•+ scavenging abilities, and both flavanols and its oligomers contributed to the most of ORAC. Sum of the contributions made by all phenolic categories in an individual species ranged from 21.32% to 87.80% in DPPH and ABTS assays, but from 197.22% to 430.42% in ORAC assay. Those 1.97- and even 4.30-fold higher contributions comparing with overall ORAC values might partially result from the deviation caused by the semi-quantification, but more likely owing to an existing antagonistic effect. The reason for saying this is because the excessive levels of ORAC contributions occurred in all three species. Ciesla et al., [45] explored the antagonistic antioxidant interactions of common monoterpenes with DPPH assay, which verified that the antagonism effect existed in many combinations. Little information was gained concerning antagonism effect of phenolics for ORAC assays, thus more detailed experiments need to be conducted to testify the conjecture.
Considering tyrosols and caffeoylquinic and hydroxycinnamic acids occurred only in SC with the former two exhibiting relative higher antioxidant activity contributions in all the three assays, tyrosols and caffeoylquinic acids should be effective biomarkers for SC in distinguishing its alternatives.
3. Materials and Methods
3.1. Chemicals, Reagents and Plant Materials
All authentic standards were purchased from the National Institutes for Food and Drug Control (Beijing, China). Folin-Ciocalteu reagents, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2′-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH), and fluorescein were bought from Sigma Chemical (St. Louis, MO, USA). Supercoiled plasmid DNA (pBR 322 from E. coli) was purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Tris-acetate-EDTA (TAE) buffer and GelRedTM nucleic acid stain were obtained from Biotium Inc. (California, USA). HPLC-grade acetonitrile and formic acid were bought from Fisher Scientific (Pittsburgh, PA, USA). Other reagents (analytical grade) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Beijing, China).
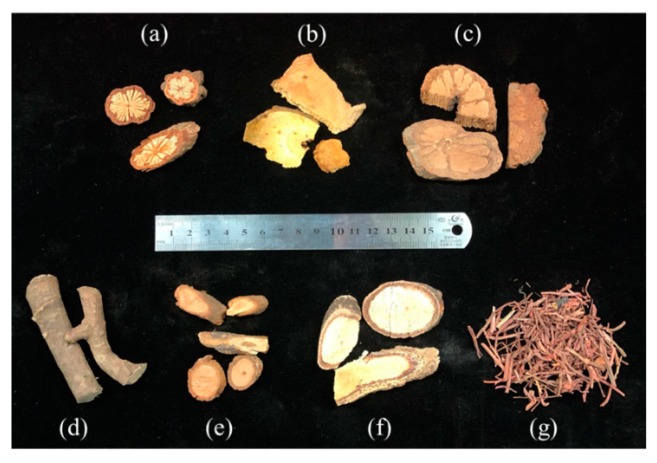
Dry stem slices of S. cuneata (Oliv.) Rehd. et Wils (namely, the authentic herbal medicine Daxueteng) were purchased from National Institutes for Food and Drug Control (Beijing, China). Seven herbs all being claimed as ‘Daxueteng’ were collected from different drugstores and tradesmen in Anguo herbal medicine market (Anguo, Hebei province, China), the biggest herbal medicine market in China. Experts from Capital Medical University identified these collected samples morphologically as seven species, i.e., S. cuneata (Oliv.) Rehd. et Wils., P. cuspidatum Sieb.et Zucc., B. championii (Benth.) Benth., S. sphenanthera Rehd. et Wils., S. grandiflora (Wall.) Hook. f. et Thoms, M. dielsiana Harms, and R. cordifolia L. (Table 3, Figure 6). After gentle cleaning, all collected samples were ground and passed a 250 × 250 μm2 sieve, then the powder was stored at −20 °C for subsequent extraction.
Table 3.
Collected herbs: S. cuneata and its six adulterants.
| Abbr. | Species | Collection Site | Collection Date | Material Conditions |
|---|---|---|---|---|
| SC | Sargentodoxa cuneata (Oliv.) Rehd. et Wils. | Beijing | 2015.7.26 | dry slices |
| PC | Polygonum cuspidatum Sieb.et Zucc. | Anguo, Heibei | 2015.8.30 | dry slices |
| BC | Bauhinia championii (Benth.) Benth. | Anguo, Heibei | 2015.8.30 | dry slices |
| SS | Schisandra sphenanthera Rehd. et Wils. | Anguo, Heibei | 2015.8.30 | dry slices |
| SG | Schisandra grandiflora (Wall.) Hook. f. et Thoms | Anguo, Heibei | 2015.8.30 | dry slices |
| MD | Millettia dielsiana Harms. | Anguo, Heibei | 2015.8.30 | dry slices |
| RC | Rubia cordifolia L. | Anguo, Heibei | 2015.8.30 | dry slices |
Figure 6.
Herb configurations: S. cuneata (a), P. cuspidatum (b), B. championii (c), S. sphenanthera (d), S. grandiflora (e), M. dielsiana (f), and R. cordifolia (g).
3.2. Extraction of Phenolics from Stems of S. cuneata and Its Six Adulterants
To prepare phenolic extracts, an ultrasonic-assisted extraction procedure was performed. To be specific, 15 mL of 80% methanol was added to 1.000 g dry powder of S. cuneata or each of its six adulterants, then the mixture was sonicated (KQ-300DE type, Kunshan Ultrasonic Instrument Co., Ltd., Kunshan, China) in a 300 W and 40 °C water bath for 30 min with occasional stirring. The sonicated mixture was filtrated by a 0.22 μm filter to obtain the supernatant. The extraction process was repeated twice more with the residue, and three supernatants were poured together and diluted to 45 mL with 80% methanol for further experiments.
3.3. Measurements of Phenolics and Assessments of Antioxidant Capacities
3.3.1. Total Phenols and Total Flavonoids
Total phenols were determined according to the Folin–Ciocalteu method [46], and total flavonoids were estimated by the aluminium chloride colorimetric assay [47], which were modified and detailed described in detail in one of our previous report [48]. Results were expressed as mg gallic acid equivalent (GAE)/g d.w. and mg rutin equivalent (RTE)/g d.w., respectively.
3.3.2. DPPH• and ABTS•+ Scavenging Activities and ORAC
DPPH• scavenging activity was determined as described by Brand-Williams et al., [49] and ABTS•+ scavenging capacity was evaluated by using the method reported by Re et al., [50], ORAC (oxygen radical absorbance capacity) assay was conducted according to a report by Sun et al., [51], and these three methods were modified and described in detail in one of our previous report [46]. Results were calculated by comparing the net AUC of each sample with that of the standard. All results were expressed as μmol Trolox equivalent (TE)/g d.w.
3.3.3. Inhibition of Radicals-Induced Supercoiled Plasmid DNA Strand Scission
Inhibition activity against peroxyl radicals-induced supercoiled DNA strand scission was evaluated according to reports by Chandrasekara and Shahidi [52] and Liyana-Pathirana and Shahidi [53] with some modifications. Supercoiled plasmid DNA (pBR 322 from E. coli RRI, 50 μg/mL), Trolox (5 and 10 μM), phenolic extracts (0.075 and 0.15 mg/mL), and AAPH (9 mM) were all dissolved in a 75-μM phosphate buffer solution (PBS, pH 7.4). In an Eppendorf tube (200 μL), PBS, supercoiled plasmid DNA, and phenolic extract or Trolox, 4 μL for each, and 8 μL AAPH were added in order to determine the inhibitory activity against peroxyl radicals-induced scission. A blank control with DNA alone and a negative control being devoid of extracts or Trolox were prepared with each set of phenolic extracts tested. The mixture was then incubated at 37 °C for 1 h in darkness [54]. The loading dye (4 μL), consisting of 0.25% bromophenol blue, 0.25% xylene cyanol and 50% glycerol in distilled water, was added to the reaction mixture at the end of the incubation.
The samples were then electrophoresed using GelRed™ (100 μL/L) as a gel stain and agarose gel (0.7%, w/v) prepared in a Tris-acetic acid-EDTA (TAE) buffer (40 mM Tris acetate, 1 mM EDTA, pH 8.5). Submarine gel electrophoresis was run in the TAE buffer for 1.5 h at room temperature and 60 V with a PowerPac™ Basic Power Supply (Bio-Rad Laboratories, Inc., California, USA). The bands were visualized under the transillumination of UV light using Azure Biosystems C600 (Azure Biosystems Inc, CA, USA). Images were analyzed using Azurespot software (Azure Biosystems Inc, CA, USA) to quantify DNA scission. Protective effects of phenolic extracts were calculated using retention percentage of the normalized supercoiled DNA as given below: DNA retention % = intensity of supercoiled DNA with AAPH and extract/intensity of supercoiled DNA in blank × 100.
3.4. Characterization and Quantification of Phenolic Compositions by UPLC-DAD-QTOF-MS/MS and HPLC-UV
The UPLC-DAD-QTOF-MS/MS system was comprised of an Acquity Ultra-Performance Liquid Chromatography (UPLC) system (Waters, Milford, MA, USA), a DAD detector and a QTOF-MS mass spectrometer (Xevo G2-XS, Waters). The chromatographic separation was performed on a reversed phase column (Diamonsil C18 5 μm 250 × 4.6 mm i.d., Dikma, China) with column temperature set at 30 °C. The mobile phase consisted of water with 0.4% formic acid (v:v) (A) and acetonitrile (B) under the following gradient program: 0–10 min, 5–7% B, 10–46 min, 7–10% B, 46–70 min, 10–16% B, 70–81 min, 16–17% B, 81–97 min, 17–21% B, 97–104 min, 21–23% B, 104–110 min, 23–25% B, 110–120 min, 25% B, 120–200 min, 25–80% B. The flow rate was set at 1 mL/min with an injection volume of 10 μL.
Mass spectra were recorded in the range of m/z 50–2000. MS experiments were performed both in positive and negative ionization modes under the following conditions: nitrogen drying gas flow, 10.0 L/min, nebulizer pressure, 45 psi, gas drying temperature, 370 °C, capillary and fragmentor voltage, 2.5 kV, MS/MS collision energies, 20 V. Peak identification was performed by comparing the UV and mass spectra and fragmentation ions to those reported by literatures and METLIN database (https://metlin.scripps.edu).
HPLC analyses were performed using a Shimadzu HPLC system (Shimadzu, Japan) equipped with two LC-10AT VP pumps, an SPDM20A UV-detector, and a SIL-20AC TH autosampler controlled by an analytical software (LC Solution-Release 1.23SP1). The reversed phase column, column temperature, solvent system, gradient program, as well as the flow rate were the same as those in the UPLC analysis described above. The flow rate was set at 1 mL/min with an injection volume of 10 μL. The detection wavelength was set at 280 nm to monitor more phenols simultaneously. At the end of each running, the column was flushed with 95% acetonitrile for 10 min to remove strongly retained constituents, and then equilibrated for 10 min under initial conditions.
Contents of gallic acid, tyrosol, chlorogenic acid, epicatechin, proanthocyanidin B2, rosmarinic acid, resveratrol, emodin, and liquiritigenin in each sample were quantified by comparing their peak areas with those of authentic standards. Furthermore, they were used as standards for semi-quantification of different phenolic categories (i.e., hydroxybenzoic acids, tyrosols, caffeoylquinic acids, flavanols, flavanol oligomers, hydroxycinnamic acids, stilbenes, anthraquinones, and flavanones), in respective.
3.5. Statistical Analyses
Each experiment was carried out in triplicate and data were expressed as mean ± standard deviation. The statistical significance (t-test: two-sample equal variance, using two-tailed distribution) was determined using SPSS software (Version 22.0, SPSS Inc., Chicago, IL, USA). p < 0.05 was set to be significant.
4. Conclusions
The present study reported for the first time a comprehensive method for screening the adulterants and alternatives for S. cuneata, which facilitates its industrial manufacture and should inspire other researchers in the process of screening herbal medicines with antioxidant capacities. Significantly, an efficient HPLC fingerprint was established for screening S. cuneata, and tyrosols and caffeoylquinic acids were considered as effective markers. P. cuspidatum and B. championii possessed similar phenolic contents and antioxidant activities but the diverse phenolic composition, indicating that they may be considered as potential candidate alternatives for S. cuneata. Furthermore, contents of different phenolic categories were semi-quantified and their antioxidant contributions were subsequently determined to clarify the differences between S. cuneata and its potential candidate alternatives, P. cuspidatum and B. championii. Nevertheless, to realize the substitution of P. cuspidatum and B. championii, cell and animal models should be further applied to validate the diversions of medical effects among S. cuneata, P. cuspidatum and B. championii.
Abbreviations
| DPPH | 2,2′-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azobis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| ORAC | oxygen radical absorbance capacity |
| AAPH | 2,2′-azobis (2-methylpropionamidine) dihydrochloride |
| UPLC-QTOF-MS/MS | ultra performance liquid chromatography-diode array detector-quadrupole time-of-flight-mass spectrometer/mass spectrometer |
| HPLC | high performance liquid chromatography |
| GAE | gallic acid equivalent |
| RTE | rutin equivalent |
| TE | Trolox equivalent |
| ROS | reactive oxygen species |
| SC | Sargentodoxa cuneata |
| PC | Polygonum cuspidatum |
| BC | Bauhinia championii |
| SS | Schisandra sphenanthera |
| SG | Schisandra grandiflora |
| MD | Millettia dielsiana |
| RC | Rubia cordifolia |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/21/5427/s1.
Author Contributions
L.Y., Y.L., and L.S. conceived and designed the experiments; L.Y., P.Y., and X.C. and L.Y. collected experiment samples; L.Y., P.Y., and X.C. performed the experiments; L.Y. and P.Y. analyzed all data; L.Y. wrote the paper; Y.L. and L.S. revised the manuscript; and all authors approved the final version of the manuscript.
Funding
This research was funded by the China Special Fund for Forestry Research in the Public Interest (Grant No. 201504606).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jiangsu New Medical College . Dictionary of Chinese Traditional Medicine. Shanghai Press of Science and Technology; Shanghai, China: 1986. pp. 268–270. [Google Scholar]
- 2.Chen D.Z., Shimizu T. Lardizabalaceae. In: Wu Z.Y., editor. Flora of China. Volume 6. Science Press; Beijing, China: Missouri Botanical Garden Press; St. Louis, MO, USA: 2001. pp. 440–454. [Google Scholar]
- 3.Chinese Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. Volume 1. China Medical Science and Technology Press; Beijing, China: 2015. p. 20. [Google Scholar]
- 4.Sakakibara I., Yoshida M., Hayashi K., Maruno M. Anti-inflammatory activities of glycosides from Sargentodoxa cuneata stems. Chem. Abstr. 1997;122 [Google Scholar]
- 5.Wan X.M., Zhou X.L., Chen H. Anti-thrombotic and anti-tumor effect of water extract of caulis of Sargentodoxa cuneata (Oliv) Rehd et Wils (Lardizabalaceae) in animal models. Trop. J. Pharm. Res. 2016;15:2391. [Google Scholar]
- 6.Zeng X., Wang H., Gong Z., Huang J., Pei W., Wang X., Zhang J., Tang X. Antimicrobial and cytotoxic phenolics and phenolic glycosides from Sargentodoxa cuneata. Fitoterapia. 2015;101:153–161. doi: 10.1016/j.fitote.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Guo J.P., Pang J., Wang X.W., Shen Z.Q., Jin M., Li J.W. In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J. Gastroenterol. 2006;12:4078–4081. doi: 10.3748/wjg.v12.i25.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 9.Li H.B., Wong C.C., Cheng K.W., Chen F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT. 2008;41:385–390. doi: 10.1016/j.lwt.2007.03.011. [DOI] [Google Scholar]
- 10.Barbour V., Clark J., Jones S., Norton M., Simpson P., Veitch E. Why drug safety should not take a back seat to efficacy. PLoS Med. 2011;8:e1001097. doi: 10.1371/journal.pmed.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan T.Y.K. Herbal Medicines Induced Anticholinergic Poisoning in Hong Kong. Toxins. 2016;8:80. doi: 10.3390/toxins8030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Z.X., Ye L.J., Zhang F., Hu W., Fan D.M., Zhang Z.Y. Development of microsatellite markers for Sargentodoxa cuneata (Lardizabalaceae) using next-generation sequencing technology1. Appl. Plant Sci. 2016;4:1600003. doi: 10.3732/apps.1600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng X., Zheng W., Zhou J., Gao X., Liu Z., Han N., Yin J. Study on the discrimination between Corydalis Rhizoma and its adulterants based on HPLC-DAD-Q-TOF-MS associated with chemometric analysis. J. Chromatogr. B. 2018;1090:110–121. doi: 10.1016/j.jchromb.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Heo S., Choi J.Y., Yoo G.J., Park S.K., Baek S.Y. Simultaneous analysis of 35 specific antihypertensive adulterants in dietary supplements using LC/MS/MS. Biomed. Chromatogr. 2017;31:e3856. doi: 10.1002/bmc.3856. [DOI] [PubMed] [Google Scholar]
- 15.Paíga P., Rodrigues M.J., Correia M., Amaral J.S., Oliveira M.B.P., Delerue-Matos C. Analysis of pharmaceutical adulterants in plant food supplements by UHPLC-MS/MS. Eur. J. Pharm. Sci. 2017;99:219–227. doi: 10.1016/j.ejps.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Gao Z., Liu Y., Wang X., Song J., Chen S., Ragupathy S., Han J., Newmaster S.G. Derivative Technology of DNA Barcoding (Nucleotide Signature and SNP Double Peak Methods) Detects Adulterants and Substitution in Chinese Patent Medicines. Sci. Rep. 2017;7:5858. doi: 10.1038/s41598-017-05892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J., Pang X., Liao B., Yao H., Song J., Chen S. An authenticity survey of herbal medicines from markets in China using DNA barcoding. Sci. Rep. 2016;6:18723. doi: 10.1038/srep18723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z.X., Liu D.L., Gao W.Y., Zhang T.J. A new macrolide and glycosides from the stem of Sargentodoxa cuneata. Chin. Chem. Lett. 2009;20:1339–1341. doi: 10.1016/j.cclet.2009.07.001. [DOI] [Google Scholar]
- 19.Tang J., Ma R.L., Ouyang Z., Chen H.S. Chemical constituents from the water-soluble fraction of wild Sargentodoxa cuneata. Chin. J. Nat. Med. 2012;10:115–118. doi: 10.3724/SP.J.1009.2012.00115. [DOI] [Google Scholar]
- 20.Miao K., Zhang J., Wang F., Qin Y. Studies on the chemical constituents of Sargentgloryvine (Sargentodoxa cuneata) Chin. Tradit. Herb. Drugs. 1995;26:171–173. [Google Scholar]
- 21.Zhang P., Yan S., Shao Y., Li Z. Effect of some water soluble substances of Sargentodoxa cuneata on myocardial ischemia. J. Shanghai Med. 1988;15:191–194. [Google Scholar]
- 22.Chang J., Case R. Phenolic glycosides and ionone glycoside from the stem of Sargentodoxa cuneata. Phytochemistry. 2005;66:2752–2758. doi: 10.1016/j.phytochem.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B., Deng Z., Tang Y., Chen P.X., Liu R., Ramdath D.D., Liu Q., Hernandez M., Tsao R. Reprint of “Bioaccessibility, in vitro antioxidant and anti-inflammatory activities of phenolics in cooked green lentil (Lens culinaris)”. J. Funct. Foods. 2017;38:698–705. doi: 10.1016/j.jff.2017.03.040. [DOI] [Google Scholar]
- 24.Sumczynski D., Kotásková E., Orsavová J., Valášek P. Contribution of individual phenolics to antioxidant activity and in vitro digestibility of wild rices (Zizania aquatica L.) Food Chem. 2017;218:107–115. doi: 10.1016/j.foodchem.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 25.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazúr M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Boil. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Wei Q.Y., Zhou B., Cai Y.J., Yang L., Liu Z.L. Synergistic effect of green tea polyphenols with trolox on free radical-induced oxidative DNA damage. Food Chem. 2006;96:90–95. doi: 10.1016/j.foodchem.2005.01.053. [DOI] [Google Scholar]
- 27.Heras R.M.L., Rada P.Q., Andrés A., Lamuela-Raventós R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS) J. Funct. Foods. 2016;23:370–377. doi: 10.1016/j.jff.2016.02.048. [DOI] [Google Scholar]
- 28.Hu X., Chen L., Shi S., Cai P., Liang X., Zhang S. Antioxidant capacity and phenolic compounds of Lonicerae macranthoides by HPLC–DAD–QTOF-MS/MS. J. Pharm. Biomed. Anal. 2016;124:254–260. doi: 10.1016/j.jpba.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Liu A.H., Lin Y.H., Yang M., Guo H., Guan S.H., Sun J.H., Guo D.A. Development of the fingerprints for the quality of the roots of Salvia miltiorrhiza and its related preparations by HPLC-DAD and LC-MS(n) J. Chromatogr. B. 2007;846:32–41. doi: 10.1016/j.jchromb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Cádiz-Gurrea M.D.L.L., Fernández-Arroyo S., Joven J., Carretero A.S. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013;50:197–204. doi: 10.1016/j.foodres.2012.09.038. [DOI] [Google Scholar]
- 31.Yang L., Yin P., Fan H., Xue Q., Li K., Li X., Sun L., Liu Y. Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer Truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity. Molecules. 2017;22:232. doi: 10.3390/molecules22020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos P.A.B., Santos S.A., Guerra Â.R., Guerreiro O., Felício L., Jerónimo E., Silvestre A.J., Neto C.P., Duarte M. Valorization of olive mill residues: Antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from olive oil by-products. Ind. Crops Prod. 2013;46:359–368. doi: 10.1016/j.indcrop.2013.02.020. [DOI] [Google Scholar]
- 33.Han F., Li Y., Ma L., Liu T., Wu Y., Xu R., Song A., Yin R. A rapid and sensitive UHPLC-FT-ICR MS/MS method for identification of chemical constituents in Rhodiola crenulata extract, rat plasma and rat brain after oral administration. Talanta. 2016;160:183–193. doi: 10.1016/j.talanta.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Lin L., Harnly J.M. LC-MS profiling and quantification of food phenolic components using a standard analytical approach for all plants. In: Greco L.V., Bruno M.N., editors. Food Science and Technology: New Research. Nova Publisher; New York, NY, USA: 2008. [Google Scholar]
- 35.Melguizo-Melguizo D., Díaz-De-Cerio E., Quirantes-Piné R., Švarc-Gajić J., Carretero A.S. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods. 2014;10:192–200. doi: 10.1016/j.jff.2014.05.019. [DOI] [Google Scholar]
- 36.Pollio A., Zarrelli A., Romanucci V., Di Mauro A., Barra F., Pinto G., Crescenzi E., Roscetto E., Palumbo G. Polyphenolic Profile and Targeted Bioactivity of Methanolic Extracts from Mediterranean Ethnomedicinal Plants on Human Cancer Cell Lines. Molecules. 2016;21:395. doi: 10.3390/molecules21040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang T., Ding L., Jin H., Shi R., Li Y., Wu J., Li Y., Zhu L., Ma Y. Simultaneous quantification of catechin, epicatechin, liquiritin, isoliquiritin, liquiritigenin, isoliquiritigenin, piperine, and glycyrrhetinic acid in rat plasma by HPLC-MS/MS: Application to a pharmacokinetic study of Longhu Rendan pills. Biomed. Chromatogr. 2016;30:1166–1174. doi: 10.1002/bmc.3662. [DOI] [PubMed] [Google Scholar]
- 38.Shan B., Cai Y.Z., Brooks J.D., Corke H. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem. 2008;109:530–537. doi: 10.1016/j.foodchem.2007.12.064. [DOI] [Google Scholar]
- 39.Furuuchi R., Yokoyama T., Watanabe Y., Hirayama M. Identification and Quantification of Short Oligomeric Proanthocyanidins and Other Polyphenols in Boysenberry Seeds and Juice. J. Agric. Food Chem. 2011;59:3738–3746. doi: 10.1021/jf104976n. [DOI] [PubMed] [Google Scholar]
- 40.Fiorentino A., DellaGreca M., D’Abrosca B., Oriano P., Golino A., Izzo A., Zarrelli A., Monaco P. Lignans, neolignans and sesquilignans from Cestrum parqui l’Her. Biochem. Syst. Ecol. 2007;35:392–396. doi: 10.1016/j.bse.2006.12.009. [DOI] [Google Scholar]
- 41.Huang X., Mazza G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 2011;1218:3890–3899. doi: 10.1016/j.chroma.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 42.Yao S., Zhang J., Wang D., Hou J., Yang W., Da J., Cai L., Yang M., Jiang B., Liu X., et al. Discriminatory components retracing strategy for monitoring the preparation procedure of Chinese patent medicines by fingerprint and chemometric analysis. PLoS ONE. 2015;10:e0121366. doi: 10.1371/journal.pone.0121366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metlin. [(accessed on 15 July 2019)]; Available online: https://metlin.scripps.edu/
- 44.Tabart J., Kevers C., Pincemail J., Defraigne J.O., Dommes J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009;113:1226–1233. doi: 10.1016/j.foodchem.2008.08.013. [DOI] [Google Scholar]
- 45.Ciesla L.M., Wojtunik-Kulesza K.A., Oniszczuk A., Waksmundzka-Hajnos M., Wojtunik-Kulesza K.A., Waksmundzka-Hajnos M. Antioxidant synergism and antagonism between selected monoterpenes using the 2,2-diphenyl-1-picrylhydrazyl method. Flavour Fragr. J. 2016;31:412–419. doi: 10.1002/ffj.3330. [DOI] [Google Scholar]
- 46.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Methods in Enzymology. Volume 1. Academic Press; New York, NY, USA: 1955. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent; p. 138. [Google Scholar]
- 47.Dewanto V., Wu X., Adom K.K., Liu R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 48.Yang L., Yin P., Li K., Fan H., Xue Q., Li X., Sun L., Liu Y. Seasonal dynamics of constitutive levels of phenolic components lead to alterations of antioxidant capacities in Acer truncatum leaves. Arab. J. Chem. 2018;11:14–25. doi: 10.1016/j.arabjc.2017.01.009. [DOI] [Google Scholar]
- 49.Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 50.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Boil. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 51.Sun L., Isaak C.K., Zhou Y., Petkau J.C., Karmin O., Liu Y., Siow Y.L. Salidroside and tyrosol from Rhodiola protect H9c2 cells from ischemia/reperfusion-induced apoptosis. Life Sci. 2012;91:151–158. doi: 10.1016/j.lfs.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 52.Chandrasekara A., Shahidi F. Antiproliferative potential and DNA scission inhibitory activity of phenolics from whole millet grains. J. Funct. Foods. 2011;3:159–170. doi: 10.1016/j.jff.2011.03.008. [DOI] [Google Scholar]
- 53.Liyana-Pathirana C.M., Shahidi F. Importance of Insoluble-Bound Phenolics to Antioxidant Properties of Wheat. J. Agric. Food Chem. 2006;54:1256–1264. doi: 10.1021/jf052556h. [DOI] [PubMed] [Google Scholar]
- 54.Hiramoto K., Ojima N., Sako K.I., Kikugawa K. Effect of Plant Phenolics on the Formation of the Spin-Adduct of Hydroxyl Radical and the DNA Strand Breaking by Hydroxyl Radical. Boil. Pharm. Bull. 1996;19:558–563. doi: 10.1248/bpb.19.558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.