Abstract
Cancer is a worldwide epidemic and represents a major threat to human health and survival. Reactive oxygen species (ROS) play a dual role in cancer cells, which includes both promoting and inhibiting carcinogenesis. Tea remains one of the most prevalent beverages consumed due in part to its anti- or pro-oxidative properties. The active compounds in tea, particularly tea polyphenols, can directly or indirectly scavenge ROS to reduce oncogenesis and cancerometastasis. Interestingly, the excessive levels of ROS induced by consuming tea could induce programmed cell death (PCD) or non-PCD of cancer cells. On the basis of illustrating the relationship between ROS and cancer, the current review discusses the composition and efficacy of tea including the redox-relative (including anti-oxidative and pro-oxidative activity) mechanisms and their role along with other components in preventing and treating cancer. This information will highlight the basis for the clinical utilization of tea extracts in the prevention or treatment of cancer in the future.
Keywords: tea and its components, cancer, ROS homeostasis, anti-oxidative, pro-oxidative
1. Introduction
Tea (Camellia sinensis) is highly consumed making it one of the most prevalent beverages in the world [1]. There are diverse classifications of the methods used for tea production in different regions. According to the degree of fermentation tea is generally classified into three main types, including the unfermented green tea, the partially fermented oolong tea, and the fully fermented black or pu-erh tea [2]. The functional composition of tea consists of tea polyphenols, tea polysaccharides, L-theanine, tea pigments, caffeine, and tea saponin, some of which are secondary metabolites generated by tea. These active chemicals contribute to the many important properties of tea, such as anti-cancer, anti-aging, anti-microbial, anti-inflammatory, hypoglycemic, and hypotensive activities, along with maintaining health and controlling diseases in human [3,4,5,6,7]. As a major compound, polyphenols are rich in tea (about 30%) and possess anti- or pro-oxidative properties that have been studied widely for more than 30 years.
The redox balance is critical for the health of humans. Reactive oxygen species (ROS) are by-products of normal cellular metabolism which have been found to be associated with cancer. Generally, ROS accumulation can cause damage to DNA, proteins, and lipids, and eventually lead to carcinogenesis [8]. However, ROS-mediated oxidative stress may also induce the death of cancer cells [9]. Hence, modulating ROS production may be a potential strategy for cancer therapies. In this review, we will integrate the available information on the relationship between ROS and cancer, and the anti-cancer compounds found in tea, thereby summarizing the potential mechanisms of tea protecting against cancer.
2. Cancer
While cancer is a major cause of death little remains definitively known about it. Cancer, also known as malignant tumor or neoplasms, is a family of diseases that involve abnormal cell growth with the potential to invade or spread to other parts of the body [10,11]. However, cancer (particularly cervical cancer) barely produces obvious symptoms in the initial period leading to a late diagnosis and limited treatment options [12]. Cancer is a chronic disease requiring ongoing supports in four key areas, including prevention, surveillance, intervention for consequences of cancer and its treatment, and coordination between specialists and generalist providers [13]. Currently, the modern biomedical science community has accepted a theory that carcinogenesis is attributed to somatic mutation [14]. Somatic cells accumulate mutations in three stages, initially cell survival and proliferation are promoted, followed by the induction of mutations [15]. Thus, gene alterations, especially the activation of oncogenes and the inactivation of cancer suppressor genes, are the main causes of cancer [16,17,18,19,20].
3. ROS and Cancer
3.1. ROS Homeostasis and Regulation
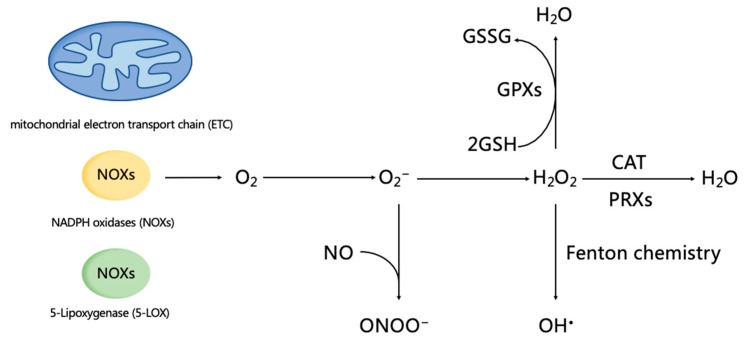
ROS are a group of highly reactive ions and molecules that are derived from molecular oxygen (O2) [21]. The endogenous generation of ROS is primarily derived from the mitochondrial electron transport chain (ETC), a family of membrane-bound NADPH oxidases (NOXs) and 5-Lipoxygenase (5-LOX) [22].
ROS homeostasis is required for normal cell survival and proper cell signaling. Under normal circumstances low levels of ROS can stimulate signaling pathways to regulate intracellular homeostasis and physiological functioning [21,23,24]. However, their fugitive properties and numerous cellular effects potentially make them indiscriminate and lethal oxidants [25]. Irregular ROS accumulation mainly stems from decreasing their elimination and increasing their production. Therefore, in order to guarantee ROS homeostasis, the antioxidant defense system must actively regulate ROS levels.
Enzymatic antioxidants and non-enzymatic antioxidants are the major components of the antioxidant defense system. Enzymatic antioxidants mainly consist of superoxide dismutases (SODs), peroxiredoxins (PRXs), glutathione peroxidases (GPXs), and catalase (CAT) [26,27]. ROS (i.e., O2−, H2O2, OH•, NO, and ONOO−) can be cleaned up and catalyzed to H2O by these antioxidant enzymes (Figure 1). The removal of ROS will then inhibit biomolecule damage, including DNA damage, lipid peroxidation, and protein denaturation [8,26,28,29]. Some non-enzymatic materials also play an important role for antioxidation. Glutathione (GSH) is the main non-enzymatic antioxidant in cells. Two molecules of GSH are oxidized by H2O2 which will produce glutathione disulfide (GSSG), and then GSSG is converted back to GSH by glutathione reductase (GR) and NADPH [30]. In addition, activation of nuclear factor erythroid 2–related factor 2 (Nrf2)-antioxidant response element signaling pathway can stimulate the cellular expression of antioxidant enzymes [31]. The tumor suppressor gene p53 is also implicated in the expression of some important antioxidant genes, and participates in the antioxidant defense system [32]. Thus, Nrf2 and p53 signaling may be considered as the important intracellular pathways for regulating antioxidant capacity.
Figure 1.
Reactive oxygen species (ROS) generation and homeostasis. The endogenous generation of ROS is primarily derived from the mitochondrial electron transport chain (ETC), including a family of membrane-bound NADPH oxidases (NOXs) and 5-Lipoxygenase (5-LOX). As a precursor of H2O2 and OH•, O2−can be converted into H2O2 by three isoforms of superoxide dismutases (SOD) (i.e., SOD1, 2, and 3) that locate in the cytoplasm, mitochondria matrix, and extracellular matrix. O2− reacts with nitric oxide (NO), and produces peroxynitrite (ONOO−), which will decrease antioxidant capacity. OH• is the production of H2O2 undergoing Fenton chemistry, and it owns the strongest chemistry activity, which can cause damage to biomolecules (such as DNA damage, lipid peroxidation, and protein denaturation). In addition, H2O2 can be catalyzed to H2O by peroxiredoxins (PRXs), glutathione peroxidases (GPXs), and catalase (CAT). Glutathione (GSH) is the main non-enzymatic antioxidant in cells, and plays an important role in the degradation of H2O2. Two molecules of GSH are oxidized by H2O2 through GPX, and are converted into glutathione disulfide (GSSG), and then are regenerated GSH via glutathione reductase (GR) and NADPH.
3.2. ROS and Carcinogenesis
Compared with normal cells cancer cells have higher levels of ROS and may maintain a persistent pro-oxidative state, impairing ROS homeostasis, and leading to an intrinsic oxidative stress [33]. Oxidative stress results from the massive accumulation of ROS and may induce carcinogenesis in normal cells [34]. Lipid peroxidation can be induced through ROS reacting with polyunsaturated fatty acids, and its end products includes malonaldehyde (MDA) and 4-hydroxynonenal (HNE) having mutagenicity and tumorigenicity on mammalian cells [35,36]. As a major hallmark lesion 8-oxo-7-hydrodeoxyguanosine (8-oxo-dG) is also generated by ROS reacting with both the DNA and nucleotide pool [37]. Moreover, ROS can specifically hyperactivate the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathways, stimulating some intracellular signaling cascades, and resulting in tumor development and metastasis through the regulation of cellular phenotypes including cancer cells survival, proliferation, and angiogenesis [38]. Riemann et al. showed that ROS prevented acidosis-induced MAPK phosphorylation, whereas the addition of H2O2 enhanced it in AT1 R-3327 prostate carcinoma cells [39]. Reuter et al. and Chetram et al. have reported that the ROS-mediated PTEN function further activating Akt is the hub of complex signaling networks that integrate a multitude of potentially oncogenic signals [40,41].
In addition, ROS act as inflammatory factors which can lead to carcinogenesis. First of all, ROS are involved in the respiratory burst of neutrophils, tumor-associated macrophages (TAM), and lymphocytes, as well as recruiting inflammatory cells into sites of inflammation [42]. Secondly, some studies have revealed that the development of cancer was associated with chronic inflammation [43,44]. The induction of chronic inflammation that predisposes cancers include microbial infections (such as Helicobacter pylori for gastric cancer and mucosal lymphoma), autoimmune diseases (such as inflammatory bowel disease for colon cancer), and inflammatory conditions of uncertain origin (such as prostatitis for prostate cancer) [45,46,47]. Zhang et al. also found that esophageal adenocarcinoma cells successfully achieved metastasis and progression through ROS-induced NF-κB signaling pathways, which increased the expression of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8 [48].
Besides inducing oxidative stress and inflammation, ROS-induced tumor progression and metastatic are also related to the neovascular response [49]. The increased levels of ROS and Akt phosphorylation promote angiogenesis and tube formation [50]. Vascular endothelial growth factor (VEGF) may be up-regulated by oxidative stress, which is known to play a crucial role in tumor angiogenesis [51]. Therefore, decreasing levels of ROS could prevent carcinogenesis and the proliferation of cancer cells.
3.3. ROS as a Cancer Therapy Agent
Interestingly, ROS are appreciated for having a dual role in cancer cells. If the generation of ROS exceeds the tolerance range of cancer cells, by using an oxidative stress inducer (such as STA-4783, ampelopsin), this will result in a threat to survival and even the death of cancer cells [9,52]. ROS promotes cancer cell death and autophagy through the activation of c-Jun N-terminal kinase (JNK) and p38 signaling pathways [53]. Recently, it has been shown that apoptotic cell death can occur through activating the endoplasmic reticulum (ER) stress/ROS/JNK axis and inhibiting Akt pro-survival signaling in colon cancer cells [54]. Therefore, cancer cells maintain ROS at a level without inducing cell death by promoting pro-tumorigenic signaling pathways and triggering their antioxidant capacity [27] along with controlling the production of mitochondrial ROS (mROS) via cytosolic isocitrate dehydrogenase-1 (IDH1)-dependent reductive carboxylation [55]. These evidences are in opposition to previously published studies. Then what is the true relationship between ROS and cancer? The role of ROS as an oncogenesis agent or a cancer therapy agent is determined by the concentrate and types of ROS, and its local antioxidant capacity [56]. Currently, it is unreliable to use ROS generators as a mono-approach to treat cancer in the clinic and the redox adaptation of cancer cells, which is largely in charge of therapeutic resistance and tumor relapse, needs to be taken into consideration when treating cancer.
4. Tea Resists Carcinogenesis
The major tea-producing countries are China, India, Japan, Sri Lanka, Indonesia, and Central African countries [57]. The argument that tea is a cancer preventive agent is no longer new. A pioneering study in the mid-1990s summarized the available epidemiologic information and found that tea consumption is likely to have beneficial effects on reducing the cancer risk in some people [1]. Recently, a meta-analysis found an inverse association between tea consumption and cancer risk [4,58,59,60,61]. However, some evidence does not support the hypothesis that tea can reduce the risk of cancer [62,63]. The above conflicting results could be due to variations in the types, dosage, and drinking manner of tea. In fact, the components and quality of tea are variable by the category, growth environment, storage time, and method of production, which will affect the original beneficial effects of tea.
4.1. Tea Polyphenols
Tea polyphenols are one of the most important ingredients in regulating the redox balance of tea. Our previous in vivo and in vitro studies reported that tea polyphenols have strong anti-oxidative capacities [64,65,66]. Tea polyphenols can reduce the incidence and development of tumors in the stomach, intestines, liver, lungs, skin and other parts of the whole body [67,68,69,70,71]. Catechins are the most abundant polyphenols in tea, mainly including epigallocatechin-3-gallate (EGCG), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epicatechin (EC) [34]. Among them, EGCG is the major catechin in tea, and may account for 50–80% of the total catechins [72].
Tea polyphenols could decrease the risk of skin cancer through inhibiting ultraviolet light B (UVB)-induced oxidative stress, such as the depletion of antioxidant enzymes, lipid oxidation, and the infiltration of inflammatory cells [68]. In a two-stage model of diethylnitrosamine (DEN)/phenobarbital (PB)-induced hepatocarcinogenesis of Sprague-Dawley rats, oral gavage of tea polyphenols five times weekly could significantly increase the total antioxidant capacity (T-AOC) and GPX activity in livers [67]. In the multistage mouse skin carcinogenesis model, peracetylated EGCG treatment could decrease the expression of oxidative enzymes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 [73]. In addition, tea polyphenols show a pro-oxidative activity. EGCG-induced oxidative stress and mitochondrial dysfunction, and played an anti-cancer role in oral cancer [74].
In addition to acting as preventive agents, tea polyphenols can also be used as adjuvant therapies for various cancers. When EGCG is combined with a conventional cancer therapy additive, synergistic effects have been proposed, which are mainly due to its anti-inflammatory and anti-oxidative activities that improve the side effects during cancer treatment [75]. Zhang et al. found that the administration of 400 mg EGCC three times daily potentiated the efficacy of radiotherapy in patients [3]. However, EGCG also has antagonistic interactions with PS-341, which will limit its clinical use. PS-341 is an anti-myeloma drug which activity would be blocked by EGCG through vicinal diols on polyphenols interacting with the boronic acid of PS-341 [76]. As a consequence, pre-clinical studies on tea polyphenols (particularly on the bioactive utilization, mechanism of action, and safety of EGCG) need to be carried out.
4.2. Others
In the cancer field studies involving tea polysaccharides (TPS), L-theanine, tea pigments, and caffeine are not adequate. TPS are a group of hetero-polysaccharides bonded with proteins [77]. Yang et al. reported that TPS (400–800 μg/mL) significantly improved the anti-oxidative capacity in a dose-dependent manner, and inhibited the cancerometastasis of gastric cancer in mice [78]. Selenium (Se)-containing TPS (IC50 of 140.1 μg/mL) induced ROS generation which made cells undergo G2/M phase arrest and apoptosis and exhibited effective inhibition of human breast cancer MCF-7 cell growth [79]. Moreover, compared with the utilization of doxorubicin (DOX) alone, a combination of TPS and DOX has a better suppression efficiency in lung cancer A549 cells [80].
L-theanine is a natural amino acid which is found specifically in tea plants and makes up 1–2% of the dry weight of tea leaves [81]. Liu et al. found that theanine and its derivates had no toxicity in mice [82]. Recent studies have shown that, in addition to relieving depression, memory improvement, and neuroprotection [83,84,85], L-theanine may also have anti-tumor activities. Adriamycin (ADR) was used to efficiently treat Ehrlich ascites carcinoma cells and its side effects, such as reducing antioxidant enzyme activity and increasing the level of lipid peroxidation, can be alleviated by the combined utilization of L-theanine [86].
Tea pigments are the oxidized products of polyphenols and their derivatives in tea leaves and mainly consist of theaflavins (TFs), thearubigins (TRs), and theabrownin [87]. The composition of tea pigments in black tea are similar to that of the tea polyphenols in green tea, but the former is chemically stable and may be an ideal chemopreventive agent [88]. In a rat liver precancerous lesion model the treatment with tea pigments suppressed cancer biomarkers such as glutathione S-transferase Pi (GST-Pi) mRNA and protein [89]. Furthermore, in an in vivo trial on 1,2-dimethylhydrazine (DMH)-induced rat colorectal carcinogenesis, treatment with 0.1% tea pigments reduced aberrant cryptic foci (ACF) and colonic tumor formation [90].
Caffeine, the most abundant alkaloid in tea, makes up 2–4% of the dry weight, and its structure is identified as 1,3,7-trimethylxanthine [91]. Caffeine has been shown to have both positive and negative health effects. The cancer preventative effects of caffeine in rodent hepatocellular carcinoma (HCC) models have also been demonstrated [92]. Chronic caffeine ingestion inhibited rat breast cancer, neither by interfering with the high prolactin levels that is a necessary step in murine tumor development, nor by causing hypocaloric intake [93]. However, an in vivo trial showed that the rats consuming caffeine and unsaturated fat had the earliest tumor development and the most multiple tumor occurrence [94].
4.3. Tea Types and Anti-Cancer
Along with studying the different components of tea, studies should also be undertaken to analyze their anti-cancer properties. Generally, tea is divided into three main types based on production, namely unfermented green tea, partially fermented oolong tea, and fully fermented black tea or pu-erh tea [2]. We have already summarized and discussed the anti-oxidant capacity of tea polyphenols derived from the differently produced teas but remain unable to draw a consistent conclusion [34]. The individual effects of green tea, black tea, and oolong tea on cancer are difficult to confirm using epidemiological research, mainly due to many consuming several tea types [95]. However, it appears that when comparing the anti-cancer effects between green tea and black tea, the former is more efficient [96,97]. This can be associated with the stronger antioxidant capacity and protective effects of green tea [95,98]. However, Record and Dreosti reported that treatment with black tea provided more protection than green tea in solar irradiation-induced skin cancer in hairless mice [99]. Until now, there has been no direct evidence that oolong tea, a semi-fermented tea, has the ability to fight cancer. Only one in vitro experiment showed that oolong tea has the worst inhibiting effect on the invasion and proliferation of AH109A compared with green and black teas [100].
5. Anti-Cancer Mechanisms of Tea through Regulating ROS Homeostasis
5.1. Anti-Oxidant Capacity of Tea
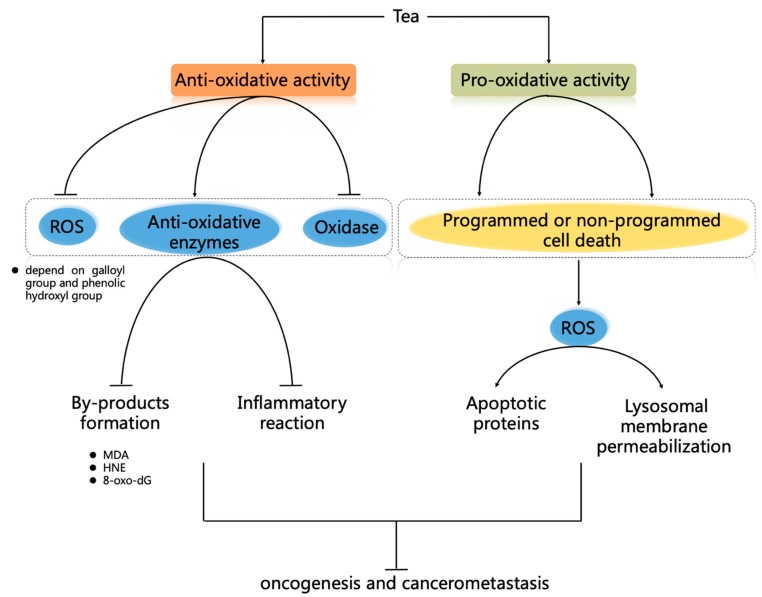
Even though a great amount of anti-cancer mechanisms of tea has been discovered the anti-oxidant capacity of tea is still considered as the most important mechanism (Figure 2). Tea polyphenols are the main anti-oxidant capacity component in tea. Tea polyphenols have higher antioxidant capacity than vitamins and offset vitamin’s disadvantages, including photosensitivity from natural vitamin intake and the side effects of synthetic vitamin [101].
Figure 2.
Anti-cancer mechanisms of tea by regulating ROS homeostasis. Anti-oxidative activity of tea. Tea polyphenols can directly scavenge ROS by depending on its B and D ring of the galloyl group, and the phenolic hydroxyl group. Tea can also indirectly eliminate ROS through improving anti-oxidative enzymes activities, decreasing the effects of the oxidases, decreasing by-products (including MDA, HNE, and 8-oxo-dG) formation, and decreasing the inflammatory reaction, which involves the regulation of Nrf2 and NF-κB signaling pathways. Moreover, tea can regulate the drug-metabolizing pathways or become a chelating agent to scavenge ROS. Pro-oxidative activity of tea. With pro-oxidative activity, tea can selectively induce programmed cell death (PCD) or non-PCD of cancer cells, and it is considered as a potential anti-cancer candidate. PCD of cancer cells includes ROS-induced apoptosis or autophagy by mitochondrial dysfunction, the reduction of Trx/TrxR, and ROS-induced hyperactivation of p38, JNK, and p53, which activate the expression of downstream apoptotic proteins (such as Bax, caspase-3, and caspase-9). In addition, tea can induce non-PCD of cancer cells through ROS-induced DNA degradation by endogenous copper and ROS-mediated lysosomal membrane permeabilization.
5.1.1. Tea as a Direct ROS Scavenger
In an in vitro oxidative hemolysis model of human red blood cells (RBC) green tea polyphenols, as natural antioxidants, efficiently suppressed the hemolysis in the sequence of EGCG > EGC > ECG ≈ EC [102]. Cancer induced by oxidative stress will produce abnormal levels of MDA, HNE, and 8-oxo-dG, which can be mitigated by tea polyphenols [34,103]. Oxidative DNA and protein damage was mitigated by the consumption of tea polyphenols in a mice model with prostate cancer cell subcutaneous xenografts [104]. The decrease of ROS-induced biomolecule damage could depend on EGCG, which is the most effective scavenger for O2−, OH•, and 1,1-diphenyl-2-picrylhydrazyl radicals among tea catechins [105]. EGCG scavenges ROS mainly through oxidizing the B and D ring of the galloyl group, and the oxidation of ECG occurs initially in the D ring [106]. After the phenolic hydroxyl groups of tea polyphenols combine with ROS, phenoxy radical is formed, and then the levels of ROS decrease [107].
Besides tea polyphenols, other components of tea may directly clean up ROS. Theabrownin exerts a ROS scavenging activity because it is the derivative of tea polyphenols [108]. In addition, TPS also can be a direct scavenger to eliminate excessive ROS, but the ROS scavenging activity of TPS depends on the preparation method, drying method, and its concentration [109,110,111].
5.1.2. Tea as an Indirect ROS Scavenger
EGCG may indirectly enhance the antioxidant capacity to suppress carcinogenesis, which is derived from the increasing activities of the antioxidant enzyme and the decreasing effects of the oxidases [103]. TPS can also improve the anti-oxidant function in gastric cancer mice via decreasing the levels of MDA and increasing the activity of antioxidant enzymes [78,112]. Tea extract could attenuate oxidative damage by improving the expression of SOD, CAT, and GPX, and downregulating the expression of iNOS and COX-2 in mice [113]. Further studies showed that EGCG inhibited iNOS gene expression, iNOS kinase and COX activities, which would reduce the generation of NO and prostacyclin, and decrease protein and DNA damage, and ultimately inhibit cancer [114,115].
Nrf2/the antioxidant response element (ARE), is the redox-sensitive signaling pathway that regulates antioxidant enzymes and xenobiotic detoxification of enzymes against oxidative stress to maintain the redox balance in normal cells [116]. In addition, multiple protein kinases such as MAPKs, extracellular regulated protein kinases (ERK), and protein kinase C (PKC), were involved in the regulation of Nrf2 transcriptional activity by inducing the phosphorylation of Nrf2 [117,118]. Tea polyphenols pretreatment could modulate the nuclear translocation of Nrf2 by stimulating the ERK1/2 phosphorylation and transcriptionally regulating the expression of antioxidant enzymes downstream (including heme oxygenase (HO)-1 and NQO-1) in HepG2 cells [117]. Gao et al. also reported that EGCG could upregulate the level of HO-1 to improve contrast-induced oxidative stress against carcinogenesis [119]. Interestingly, several studies have indicated that Nrf2 and HO-1 are frequently upregulated in cancer and correlate with a poor prognosis [120,121]. The dark side of the Nrf2/HO-1 axis could be inhibited by the combination of EGCG and metformin, reaching to a level suppressing lung cancer [122].
The ability of tea extracts to scavenge ROS involves the NF-κB signaling pathway [113]. NF-κB also modulates the expression of cancer-associated cytokines such as TNF-α and IL-8 [123]. Following combining with tumor necrosis factor receptor (TNFR), TNF-α stimulates the NOX complex-induced ROS via NF-κB activation and induces carcinogenesis [124,125,126]. IL-8 is a potent neutrophil chemoattractant which can promote the generation of ROS by activating NF-κB [127]. Fabiola et al. and Tomtitchong et al. found that treating gastric epithelial cells with different factors (such as IL-1β and Helicobacter pylori) can enhance IL-8 secretion, and tea polyphenols treatment decreased the risk of gastric cancer via inhibition of NF-κB [5,6]. In an analysis report, black tea consumption could mitigate ROS-induced DNA damage, and then prevent carcinogenesis by inhibiting NF-κB transcriptional expression in tobacco users [128].
The above-mentioned information implied that there is a potential cross relationship between Nrf2 and NF-κB that could be regulated by tea and its components during the inhibition of carcinogenesis [113,129]. A study found that the increasing susceptibility of Nrf2 deficient mice to dextran sulfate sodium (DSS)-induced colitis and colorectal cancer was associated with the decreased expression of antioxidant/phase II detoxifying enzymes, in parallel with the enhancement of pro-inflammatory cytokines through stimulating the NF-κB signaling pathways [130]. Furthermore, Li et al. found that tea polyphenols suppressed NF-κB-dependent inflammation, promoted Nrf2 nuclear translocation, and improved the antioxidant capacity during nonalcoholic steatohepatitis in mice fed a high-fat diet [113].
Some drug-metabolizing pathways also are targets of tea and its components. The aryl hydrocarbon receptor (AhR) and cytochrome P450 (CYP450) may convert procarcinogens into carcinogens, which induces cellular toxicity by regulating the generation of ROS [131]. Several studies reported that CYP450 took part in polycyclic aromatic hydrocarbons (PAHs) metabolism, and there is a carcinogenesis possibility causing oxidative DNA damage via ROS generation [132,133,134]. However, green tea and black tea decrease the risk of cancer through inhibiting AhR activation pathways and CYP450 activity in the rat liver [135]. Therefore, it is possible that tea and its components potentially are able to inhibit carcinogenesis through influencing drug-metabolizing pathways to impede ROS production.
Moreover, the antioxidant activity of EGCG reaching anti-carcinogenesis is also attributed to its chelation with metal ions [136]. Iron is required for neoplastic cells and will be prone to depletion [137]. Iron ion supplementation promotes tumor-associated macrophage (TAM) formation because of increased ROS production [138]. But the B ring of tea polyphenols may competitively bind metal ions (including iron), which will decrease the iron intake and ROS level in neoplastic cells [137,139]. Thus, tea polyphenols may reduce ROS generation via competing for metal ions.
5.2. Pro-Oxidative Activity of Tea
Pro-oxidative activity tea with its components are considered anti-cancer candidates. They selectively cause programmed cell death (PCD) of cancer cells, but do not damage normal cells (Figure 2). PCD of cancer cells involves hyperactivation of p38, JNK, and p53, which activates the expression of downstream apoptotic proteins (such as Bax, caspase-3, and caspase-9) [53,140,141,142]. In contrast to the anti-oxidative property of tea, EGCG-induced apoptosis or autophagy in cancer cells were due to the significant decrease in mitochondrial membrane potential, which results in mitochondrial dysfunction and increased intracellular ROS [143,144]. Then, as an important mitochondrial redox modulator, sirtuin 3 (SIRT3) and its downstream targets (including GSH and SOD) transcription could be inhibited through decreasing the nuclear localization of the estrogen-related receptor α (ERRα) by EGCG in oral cancer cells [145]. Thioredoxin (Trx) and thioredoxin reductase (TrxR), known as antioxidant agents and anti-apoptotic proteins, commonly are overexpressed in the human cancer cells [146]. EGCG treatment may reduce Trx/TrxR via the formation of EGCG-Trx1 (Cys(32)) and EGCG-TrxR (Cys/Sec) conjugates and promote cancer cell death [147]. Human telomerase reverse transcriptase (hTERT) is expressed in over 90% of cancers but not in normal cells [148]. Compared with normal cells, EGCG specifically induced ROS production (especially H2O2) in cancer cells; and ROS down-regulated hTERT expression to promote the apoptosis of cancer cells [149]. Interestingly, Nrf2 frequently overexpresses in human cancer cells and will improve the anti-oxidative activity of cancer cells [150]. However, a combination of EGCG (30 µM) with luteolin (10 µM) efficiently inhibits Nrf2 and synergistically increased apoptosis in the head and neck as well as lung cancer cell lines, including A549 [151].
Tea can also induce the non-programmed cell death of cancer cells. Tea and its components were also prone to have pro-oxidative activity with an excessive concentration of transition metal ions. For examples, tea polyphenols mediated the cellular DNA degradation of ROS-induced lymphoma through the involvement of endogenous copper [152]. Moreover, EGCG induces non-apoptotic death of human cancer cells (both HepG2 and HeLa) via ROS-mediated lysosomal membrane permeabilization [153].
6. Conclusions
ROS were appreciated for having a dual role in human cancer including promoting and inhibiting carcinogenesis. Generally, tea and its components act as efficient scavengers of ROS in direct and indirect manners. Interestingly, excessive tea-induced ROS can also cause the PCD or non-PCD of cancer cells. Therefore, tea could potentially be a cancer therapy agent. Moreover, we discussed tea and its bioactive components, especially tea polyphenols, on the basis of their anti- or pro-oxidative activity resisting oncogenesis and cancerometastasis through regulating human redox balance.
In addition, tea and its components may have synergistic effects with conventional anti-cancer measures in clinical practice. However, tea and its components still encounter lots of challenges for clinical application. Usage and dosage of tea and its components, and the various cancer types, may be the prerequisite regulating the bioavailability of tea and its components. Thus, further studies need to be done. For example, how to deliver tea and its components effectively to target sites and protect them from degradation. Furthermore, except for tea polyphenols, other components of tea need more studies in cancer treatment. Thus, we strongly recommend extensive clinical studies of the application of tea and its components with regards to its use as both a preventative and potential therapeutic for cancer.
Acknowledgments
Special thanks to Qing Yang from Oklahoma State University (USA), and Paul Dyce from Auburn University (USA), for editing the manuscript.
Author Contributions
Conceptualization, X.M. and D.C.; writing—original draft preparation, X.M. and X.X.; writing—review, D.C. and B.Y.; reference collection, X.X. and J.H.
Funding
This study was financially supported by the earmarked grant for China Agriculture Research System (CARS-35), the grant from the Science and Technology Support Project of Sichuan Province (2016NYZ0052), and the fund from the research program of “Sheng Yang” students’ association (B2016010).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yang C.S., Wang Z.Y. Tea and cancer. J. Natl. Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 2.Kuo K.L., Weng M.S., Chiang C.T., Tsai Y.J., Lin-Shiau S.Y., Lin J.K. Comparative studies on the hypolipidemic and growth suppressive effects of oolong, black, pu-erh, and green tea leaves in rats. J. Agric. Food Chem. 2005;53:480–489. doi: 10.1021/jf049375k. [DOI] [PubMed] [Google Scholar]
- 3.Zhang G., Wang Y., Zhang Y., Wan X., Li J., Liu K., Wang F., Liu K., Liu Q., Yang C., et al. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr. Mol. Med. 2012;12:163–176. doi: 10.2174/156652412798889063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan X., Wang J., Pan S., Lu C. Tea consumption and the risk of ovarian cancer: A meta-analysis of epidemiological studies. Oncotarget. 2017;8:37796–37806. doi: 10.18632/oncotarget.16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabiola G.O., Stephens B.R., Neilson A.P., Rodney G., Ferruzzi M.G., Bomser J.A. Green and black tea inhibit cytokine-induced IL-8 production and secretion in AGS gastric cancer cells via inhibition of NF-κB activity. Planta Med. 2010;76:1659–1665. doi: 10.1055/s-0030-1249975. [DOI] [PubMed] [Google Scholar]
- 6.Tomtitchong P., Robinson P.A., Crabtree J.E. The green tea catechin epigallocatechin-3-gallate inhibits H. pylori-Induced and TNF-α-induced IL-8 transcription and IL-8 secretion in gastric epithelial cells. Gastroenterology. 2009;136 doi: 10.1016/S0016-5085(09)61564-X. [DOI] [Google Scholar]
- 7.Fei T., Fei J., Huang F., Xie T., Yang P. The anti-aging and anti-oxidation effects of tea water extract in Caenorhabditis elegans. Exp. Gerontol. 2017;97:89–96. doi: 10.1016/j.exger.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Borra S.K., Mahendra J., Gurumurthy P., Jayamathi, Iqbal S.S., Mahendra L. Effect of curcumin against oxidation of biomolecules by hydroxyl radicals. J. Clin. Diagn. Res. 2014;8:CC01–CC05. doi: 10.7860/JCDR/2014/8517.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y., Shu F., Liang X., Chang H., Shi L., Peng X., Zhu J., Mi M. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS ONE. 2014;9:e89021. doi: 10.1371/journal.pone.0089021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoekstra H.J., Wobbes T., Heineman E., Haryono S., Aryandono T., Balch C.M. Fighting global disparities in cancer care: A surgical oncology view. Ann. Surg. Oncol. 2016;23:2131–2136. doi: 10.1245/s10434-016-5194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C.S., Lambert J.D., Ju J., Lu G., Sang S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J.F., Chen J.F., Canfell K., Feng X.X., Ma J.F., Zhang Y.Z., Zhao F.H., Li R., Ma L., Li Z.F., et al. Estimation of the costs of cervical cancer screening, diagnosis and treatment in rural Shanxi Province, China: A micro-costing study. BMC Health Serv. Res. 2012;12:123. doi: 10.1186/1472-6963-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips J.L., Currow D.C. Cancer as a chronic disease. Collegian. 2010;17:47–50. doi: 10.1016/j.colegn.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Duesberg P., Rasnick D. Aneuploidy, the somatic mutation that makes cancer a species of its own. Cell Motil. Cytoskelet. 2000;32:81–107. doi: 10.1002/1097-0169(200010)47:2<81::AID-CM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Martincorena I., Raine K.M., Gerstung M., Dawson K.J., Haase K., Van L.P., Davies H., Stratton M.R., Campbell P.J. Universal Patterns of Selection in Cancer and Somatic Tissues. Cell. 2017;171:1029–1041. doi: 10.1016/j.cell.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrello M.G., Degl’Innocenti D., Pierotti M.A. Inflammation and cancer: The oncogene-driven connection. Cancer Lett. 2008;267:262–270. doi: 10.1016/j.canlet.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 17.Uchino S., Noguchi M., Ochiai A., Saito T., Kobayashi M., Hirohashi S. p53 Mutation in gastric cancer: A genetic model for carcinogenesis is common to gastric and colorectal cancer. Int. J. Cancer. 2010;54:759–764. doi: 10.1002/ijc.2910540509. [DOI] [PubMed] [Google Scholar]
- 18.Spitschak A., Meier C., Kowtharapu B., Engelmann D., Pützer B.M. MiR-182 promotes cancer invasion by linking RET oncogene activated NF-κB to loss of the HES1/Notch1 regulatory circuit. Mol. Cancer. 2017;16:24. doi: 10.1186/s12943-016-0563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman S., Magnussen M., León T.E., Farah N., Mansour M.R. Activation of the LMO2 oncogene through a somatically acquired neomorphic promoter in T-cell acute lymphoblastic leukemia. Blood. 2017;129:3221–3226. doi: 10.1182/blood-2016-09-742148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamaspishvili T., Berman D.M., Ross A.E., Scher H.I., Marzo A.M.D., Squire J.A., Lotan T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018;15:222–234. doi: 10.1038/nrurol.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galadari S., Rahman A., Pallichankandy S., Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017;104:144–164. doi: 10.1016/j.freeradbiomed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Novo E., Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008;1:5. doi: 10.1186/1755-1536-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Autréaux B., Toledano M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 26.Fukai T., Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reczek C.R., Chandel N.S. The two faces of reactive oxygen species in cancer. Ann. Rev. Cancer Biol. 2017;1:79–98. doi: 10.1146/annurev-cancerbio-041916-065808. [DOI] [Google Scholar]
- 28.Peng T., Wong N.K., Chen X., Chan Y.K., Sun Z., Hu J.J., Shen J., Ei-Nezami H., Yang D. Molecular imaging of peroxynitrite with HKGreen-4 in live cells and tissues. J. Am. Chem. Soc. 2014;136:11728–11734. doi: 10.1021/ja504624q. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad P., Islam B.U., Allarakha S., Rabbani G., Dixit K., Moinuddin, Khan R.H., Siddiqui S.A., Ali A. Preferential recognition of peroxynitrite-modified human serum albumin by circulating autoantibodies in cancer. Int. J. Biol. Macromol. 2015;72:875–882. doi: 10.1016/j.ijbiomac.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Mailloux R.J., Mcbride S.L., Harper M.E. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem. Sci. 2013;38:592–602. doi: 10.1016/j.tibs.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Wu J., Sun B., Luo X., Zhao M., Huang M. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells: Via Nrf2 signaling. RSC Adv. 2018;8:10898–10906. doi: 10.1039/C8RA01162A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vurusaner B., Poli G., Basaga H. Tumor suppressor genes and ROS: Complex networks of interactions. Free Radic. Biol. Med. 2012;52:7–18. doi: 10.1016/j.freeradbiomed.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 33.Lara G., Marcello P., Milena N., Sara D.B., Erika R., Linda B., Andrea C. Interfering with ROS metabolism in cancer cells: The potential role of quercetin. Cancers. 2010;2:1288–1311. doi: 10.3390/cancers2021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao X., Gu C., Chen D., Yu B., He J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget. 2017;8:81649–81661. doi: 10.18632/oncotarget.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Chen S.Y., Hsu T., Santella R.M. Immunohistochemical detection of malondialdehyde–DNA adducts in human oral mucosa cells. Carcinogenesis. 2002;23:207–211. doi: 10.1093/carcin/23.1.207. [DOI] [PubMed] [Google Scholar]
- 36.Zhong H., Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolin C., Stedeford T., Cardozopelaez F. Single extraction protocol for the analysis of 8-hydroxy-2’-deoxyguanosine (oxo8dG) and the associated activity of 8-oxoguanine DNA glycosylase. J. Neurosci. Methods. 2004;136:69–76. doi: 10.1016/j.jneumeth.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Storz P. Reactive oxygen species in tumor progression. Front. Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 39.Riemann A., Schneider B., Ihling A., Nowak M., Sauvant C., Thews O., Gekle M. Acidic environment leads to ROS-induced MAPK signaling in cancer cells. PLoS ONE. 2011;6:e22445. doi: 10.1371/journal.pone.0022445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chetram M.A., Don-Salu-Hewage A.S., Hinton C.V. ROS enhances CXCR4-mediated functions through inactivation of PTEN in prostate cancer cells. Biochem. Biophys. Res. Commun. 2011;410:195–200. doi: 10.1016/j.bbrc.2011.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Athanasios V., Thomais V., Konstantinos F., Spyridon L. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health. 2013;10:3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basu S., Rossary A., Vasson M.P. Role of inflammation and eicosanoids in breast cancer. Lipid Technol. 2016;28:60–64. doi: 10.1002/lite.201600017. [DOI] [Google Scholar]
- 44.Zamora-Ros R., Shivappa N., Steck S.E., Canzian F., Landi S., Alonso M.H., Hébert J.R., Moreno V. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case–control study. Genes Nutr. 2015;10:447. doi: 10.1007/s12263-014-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 46.Hardbower D.M., De S.T., Chaturvedi R., Wilson K.T. Chronic inflammation and oxidative stress: The smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes. 2013;4:475–481. doi: 10.4161/gmic.25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahat M.A., Shakya J. Parallel aspects of the microenvironment in cancer and autoimmune disease. Mediat. Inflamm. 2016;2016:1–17. doi: 10.1155/2016/4375120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang R., Yin X., Shi H., Wu J., Shakya P., Liu D., Zhang J. Adiponectin modulates DCA-induced inflammation via the ROS/NF-κB signaling pathway in Esophageal Adenocarcinoma Cells. Dig. Dis. Sci. 2014;59:89–97. doi: 10.1007/s10620-013-2877-5. [DOI] [PubMed] [Google Scholar]
- 49.Cao Z., Shang B., Zhang G., Miele L., Sarkar F.H., Wang Z., Zhou Q. Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim. Biophys. Acta (BBA) Rev. Cancer. 2013;1836:273–286. doi: 10.1016/j.bbcan.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Bae J., Park D., Lee Y.S., Jeoung D. Interleukin-2 promotes angiogenesis by activation of Akt and increase of ROS. J. Microbiol. Biotechnol. 2008;18:377–382. [PubMed] [Google Scholar]
- 51.Katsabeki-Katsafli A., Kerenidi T., Kostikas K., Dalaveris E., Kiropoulos T.S., Gogou E., Papaioannou A.I., Gourgoulianis K.I. Serum vascular endothelial growth factor is related to systemic oxidative stress in patients with lung cancer. Lung Cancer. 2008;60:271–276. doi: 10.1016/j.lungcan.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Foley K., Bertin J., Chan K., Hutchings A., Inoue T., Kirshner J., Korbut T., Li L., Mihalek R., Rao P., et al. The oxidative stress inducer STA-4783 enhances the in vivo efficacy of multiple anti-cancer therapies in mouse tumor models. Mol. Cancer Ther. 2007:3430–3431. [Google Scholar]
- 53.Manohar M., Fatima I., Saxena R., Chandra V., Sankhwar P.L., Dwivedi A. (−)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J. Nutr. Biochem. 2013;24:940–947. doi: 10.1016/j.jnutbio.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L., Kim S.B., Luitel K., Shay J.W. Cholesterol depletion by TASIN-1 induces apoptotic cell death through the ER Stress/ROS/JNK signaling in colon cancer cells. Mol. Cancer Ther. 2018;17:945–951. doi: 10.1158/1535-7163.MCT-17-0887. [DOI] [PubMed] [Google Scholar]
- 55.Lei J., Shestov A.A., Swain P., Yang C., Parker S.J., Wang Q.A., Terada L.S., Adams N.D., Mccabe M.T., Pietrak B. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016;532:255–258. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandel N.S., Tuveson D.A. The promise and perils of antioxidants for cancer patients. N. Engl. J. Med. 2014;371:177–178. doi: 10.1056/NEJMcibr1405701. [DOI] [PubMed] [Google Scholar]
- 57.Sang S. Tea: Chemistry and Processing. Encycl. Food Health. 2016 doi: 10.1016/B978-0-12-384947-2.00685-1. [DOI] [Google Scholar]
- 58.Zhu G., Hua J., Wang Z., She F., Chen Y. Tea consumption and risk of gallbladder cancer: A meta-analysis of epidemiological studies. Mol. Clin. Oncol. 2015;3:613–618. doi: 10.3892/mco.2015.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riza E., Linos A., Petralias A., De Martinis L., Duntas L., Linos D. The effect of Greek herbal tea consumption on thyroid cancer: A case-control study. Eur. J. Public Health. 2015;25:1001–1005. doi: 10.1093/eurpub/ckv063. [DOI] [PubMed] [Google Scholar]
- 60.Sing M.F., Yang W., Gao S., Gao J., Xiang Y. Epidemiological studies of the association between tea drinking and primary liver cancer: A meta-analysis. Eur. J. Cancer Prev. 2011;20:157–165. doi: 10.1097/CEJ.0b013e3283447497. [DOI] [PubMed] [Google Scholar]
- 61.Sang L.X., Chang B., Li X.H., Jiang M. Green tea consumption and risk of esophageal cancer: A meta-analysis of published epidemiological studies. Nutr. Cancer. 2013;65:802–812. doi: 10.1080/01635581.2013.805423. [DOI] [PubMed] [Google Scholar]
- 62.Miura K., Hughes M.C.B., Arovah N.I., Pols J.C.V.D., Green A.L.C. Black tea consumption and risk of skin cancer: An 11-year prospective study. Nutr. Cancer. 2015;67:1049–1055. doi: 10.1080/01635581.2015.1073759. [DOI] [PubMed] [Google Scholar]
- 63.Braem M.G.M., Onland-Moret N.C., Schouten L.J., Tjønneland A., Hansen L., Dahm C.C., Overvad K., Lukanova A., Dossus L., Floegel A., et al. Coffee and tea consumption and the risk of ovarian cancer: A prospective cohort study and updated meta-analysis. Am. J. Clin. Nutr. 2012;95:1172–1181. doi: 10.3945/ajcn.111.026393. [DOI] [PubMed] [Google Scholar]
- 64.Li Y.Y. Ph.D. Thesis. Sichuan Agricultural University; Ya’an, China: 2011. Protective effects of tea polyphenols for weaned pigs challenged with oxidative stress. [Google Scholar]
- 65.Li Y.Y., Duan X.D., Zhao J., Yu B., Mao X.B., Mao Q., Chen H., Chen D.W. Effects of tea polyphenolon growth performance and immune function of weaned piglets chalenged with oxidative stress. Chin. J. Anim. Sci. 2011;47:53–57. [Google Scholar]
- 66.Chao Y.M., Chen D.W., Yu B., He J., Yu J., Mao X.B., Luo Y.H., Huang Z.Q., Luo J.Q., Wang S.H. Effects of tea polyphenol on growth performance, antioxidant capacity, carcass performance and meat quality of finishing pigs. Chin. J. Anim. Nutr. 2016;28:3996–4005. [Google Scholar]
- 67.Zhou F., Shen T., Duan T., Xu Y.Y., Khor S.C., Li J., Ge J., Zheng Y.F., Hsu S., De S.J. Antioxidant effects of lipophilic tea polyphenols on diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis in rats. Vivo. 2014;28:495–503. [PubMed] [Google Scholar]
- 68.Li H., Jiang N., Liu Q., Gao A., Zhou X., Liang B., Li R., Li Z., Zhu H. Topical treatment of green tea polyphenols emulsified in carboxymethyl cellulose protects against acute ultraviolet light B-induced photodamage in hairless mice. Photochem. Photobiol. Sci. 2016;15:1264–1271. doi: 10.1039/C6PP00073H. [DOI] [PubMed] [Google Scholar]
- 69.Park J.S., Khoi P.N., Joo Y.-E., Lee Y.H. EGCG inhibits recepteur d’origine nantais expression by suppressing Egr-1 in gastric cancer cells. Int. J. Oncol. 2013;42:1120–1126. doi: 10.3892/ijo.2013.1775. [DOI] [PubMed] [Google Scholar]
- 70.Morris J., Moseley V.R., Cabang A.B., Coleman K., Wei W., Garrettmayer E., Wargovich M.J. Reduction in promotor methylation utilizing EGCG (Epigallocatechin-3-gallate) restores RXRα expression in human colon cancer cells. Oncotarget. 2016;7:35313–35326. doi: 10.18632/oncotarget.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang P., Wu X., Wang X., Huang W., Feng Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget. 2016;7:43337–43351. doi: 10.18632/oncotarget.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sang S.M., Lambert J.D., Ho C.T., Yang C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Chiou Y.S., Sang S., Cheng K.H., Ho C.T., Wang Y.J., Pan M.H. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+ skin stem cells and skin tumors. Carcinogenesis. 2013;34:1315–1322. doi: 10.1093/carcin/bgt042. [DOI] [PubMed] [Google Scholar]
- 74.Ling T., Forester S.C., Lambert J.D. The role of reactive oxygen species in (−)-epigallocatechin-3-gallate (EGCG)-induced cell growth inhibition and apoptosis in oral cancer cells. Cancer Res. 2012;72:5436. [Google Scholar]
- 75.Lecumberri E., Dupertuis Y.M., Miralbell R., Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013;32:894–903. doi: 10.1016/j.clnu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Kim T.Y., Park J., Oh B., Min H.J., Jeong T.S., Lee J.H., Park S.B., Lee D.S. (−)-epigallocatechin-3-gallate (EGCG), green tea component, antagonize the anti-myeloma activity of proteasome inhibitor PS-341 by direct chemical interaction. Blood. 2007;110:4850. doi: 10.1182/blood.V110.11.4850.4850. [DOI] [Google Scholar]
- 77.Du L.L., Fu Q.Y., Xiang L.P., Zheng X.Q., Lu J.L., Ye J.H., Li Q.S., Polito C.A., Liang Y.R. Tea Polysaccharides and Their Bioactivities. Molecules. 2016;21:1449. doi: 10.3390/molecules21111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang J.J., Chen B., Gu Y. Pharmacological evaluation of tea polysaccharides with antioxidant activity in gastric cancer mice. Carbohydr. Polym. 2012;90:943–947. doi: 10.1016/j.carbpol.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 79.He N., Shi X., Zhao Y., Tian L., Wang D., Yang X. Inhibitory effects and molecular mechanisms of selenium-containing tea polysaccharides on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2013;61:579–588. doi: 10.1021/jf3036929. [DOI] [PubMed] [Google Scholar]
- 80.Wei N., Zhu Q., Chen J., Tong L.I., Li Y.F., Huang Y., Xiao M.A., Wang X., Sheng J. Tea polysaccharide increased Doxorubicin inhibition of lung cancer A549 cells. J. Tea Sci. 2016;36:477–483. [Google Scholar]
- 81.Li L., Wang X., Xiong Y., Ren W., Huang M., Zhao G., Ding Y., Wu X., Su D. L-theanine: A promising substance in tumor research. J. Food Agric. Environ. 2013;11:25–27. [Google Scholar]
- 82.Liu J., Sun Y., Zhang H., Ji D., Fei W., Tian H., Liu K., Ying Z., Wu B., Zhang G. Theanine from tea and its semi-synthetic derivative TBrC suppress human cervical cancer growth and migration by inhibiting EGFR/Met-Akt/NF-κB signaling. Eur. J. Pharmacol. 2016;791:297–307. doi: 10.1016/j.ejphar.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 83.Kakuda T. Neuroprotective effects of the green tea components theanine and catechins. Biol. Pharm. Bull. 2002;25:1513–1518. doi: 10.1248/bpb.25.1513. [DOI] [PubMed] [Google Scholar]
- 84.Nathan P.J., Lu K., Gray M., Oliver C. The neuropharmacology of L-theanine(N-ethyl-L-glutamine): A possible neuroprotective and cognitive enhancing agent. J. Herb. Pharmacother. 2006;6:21–30. doi: 10.1080/J157v06n02_02. [DOI] [PubMed] [Google Scholar]
- 85.Li G., Kang J., Yao X., Xin Y., Wang Q., Ye Y., Luo L., Yin Z. The component of green tea, L-theanine protects human hepatic L02 cells from hydrogen peroxide-induced apoptosis. Eur. Food Res. Technol. 2011;233:427–435. doi: 10.1007/s00217-011-1534-5. [DOI] [Google Scholar]
- 86.Sadzuka Y., Sugiyama T., Miyagishima A., Nozawa Y., Hirota S. The effects of theanine, as a novel biochemical modulator, on the antitumor activity of adriamycin. Cancer Lett. 1996;105:203–209. doi: 10.1016/0304-3835(96)04282-6. [DOI] [PubMed] [Google Scholar]
- 87.Tomita M., Irwin K.I., Xie Z.J., Santoro T.J. Tea pigments inhibit the production of type 1 (TH1) and type 2 (TH2) helper T cell cytokines in CD4+ T cells. Phytother. Res. 2002;16:36–42. doi: 10.1002/ptr.834. [DOI] [PubMed] [Google Scholar]
- 88.Han C., Gong Y. Experimental studies on the cancer chemoprevention of tea pigments. J. Hyg. Res. 1999;28:343. [PubMed] [Google Scholar]
- 89.Gong Y., Han C., Chen J. Effect of tea polyphenols and tea pigments on the inhibition of precancerous liver lesions in rats. Nutr. Cancer. 2000;38:81–86. doi: 10.1207/S15327914NC381_12. [DOI] [PubMed] [Google Scholar]
- 90.Jia X.D., Han C. Chemoprevention of tea on colorectal cancer induced by dimethylhydrazine in Wistar rats. World J. Gastroenterol. 2000;6:699–703. doi: 10.3748/wjg.v6.i5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graham D.M. Caffeine—Its Identity, Dietary Sources, Intake and Biological Effects. Nutr. Rev. 2010;36:97–102. doi: 10.1111/j.1753-4887.1978.tb03717.x. [DOI] [PubMed] [Google Scholar]
- 92.Okano J., Nagahara T., Matsumoto K., Murawaki Y. Caffeine inhibits the proliferation of liver cancer cells and activates the MEK/ERK/EGFR signalling pathway. Basic Clin. Pharmacol. Toxicol. 2010;102:543–551. doi: 10.1111/j.1742-7843.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 93.Petrek J.A., Sandberg W.A., Cole M.N., Silberman M.S., Collins D.C. The inhibitory effect of caffeine on hormone-induced rat breast cancer. Cancer. 1985;56:1977–1981. doi: 10.1002/1097-0142(19851015)56:8<1977::AID-CNCR2820560815>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 94.Minton J.P., Abou-Issa H., Foecking M.K., Sriram M.G. Caffeine and unsaturated fat diet significantly promotes DMBA-induced breast cancer in rats. Cancer. 1983;51:1249–1253. doi: 10.1002/1097-0142(19830401)51:7<1249::AID-CNCR2820510713>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 95.Lee A.H., Su D., Pasalich M., Binns C.W. Tea consumption reduces ovarian cancer risk. Cancer Epidemiol. 2013;37:54–59. doi: 10.1016/j.canep.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 96.Tang N., Wu Y., Zhou B., Wang B., Yu R. Green tea, black tea consumption and risk of lung cancer: A meta-analysis. Lung Cancer. 2009;65:274–283. doi: 10.1016/j.lungcan.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 97.Zheng J., Yang B., Huang T., Yu Y., Yang J., Li D. Green Tea and Black Tea Consumption and Prostate Cancer Risk: An Exploratory Meta-Analysis of Observational Studies. Nutr. Cancer. 2011;63:663–672. doi: 10.1080/01635581.2011.570895. [DOI] [PubMed] [Google Scholar]
- 98.Ye J.H., Jin J., Liang Y.R. Antioxidant Properties of Green Tea, Oolong Tea, Black Tea and Pu-erh Tea. J. Korea Tea. 2015;1:221–228. [Google Scholar]
- 99.Record I.R., Dreosti I.E. Protection by black tea and green tea against UVB and UVA + B induced skin cancer in hairless mice. Mutat. Res. 1998;422:191–199. doi: 10.1016/S0027-5107(98)00192-4. [DOI] [PubMed] [Google Scholar]
- 100.Zhang G., Miura Y., Yagasaki K. Effects of green, oolong and black teas and related components on the proliferation and invasion of hepatoma cells in culture. Cytotechnology. 1999;31:37–44. doi: 10.1023/A:1008076306672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dai D.Z., Chen S.H. Tea Polyphenols and Quercetin Preventing the Heart, Brain and Liver from the Injury by Free Radicals in Comparison with Ascorbic Acid. Chin. J. Nat. Med. 2004;2:223–231. [Google Scholar]
- 102.Ma L., Liu Z., Zhou B., Yang L., Liu Z. Inhibition of free radical induced oxidative hemolysis of red blood cells by green tea polyphenols. Chin. Sci. Bull. 2000;45:2052–2056. doi: 10.1007/BF03183525. [DOI] [Google Scholar]
- 103.Hussain S. Comparative efficacy of epigallocatechin-3-gallate against H2O2-induced ROS in cervical cancer biopsies and HeLa cell lines. Contemp. Oncol. 2017;21:209–212. doi: 10.5114/wo.2017.70110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henning S.M., Wang P., Said J., Magyar C., Castor B., Doan N., Tosity C., Moro A., Gao K., Li L., et al. Polyphenols in brewed green tea inhibit prostate tumor xenograft growth by localizing to the tumor and decreasing oxidative stress and angiogenesis. J. Nutr. Biochem. 2012;23:1537–1542. doi: 10.1016/j.jnutbio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y.L., Chen S.J., Zhang C. Capacity of hesperidin, limonene and tea polyphenols on sequestering free radical. Oral Sci. Res. 2015;31:11–14. [Google Scholar]
- 106.Kondo K., Kurihara M., Miyata N. Scavenging mechanisms of (−)-epigallocatechin gallate and (−)-epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action. Free Radical Biol. Med. 1999;27:855–863. doi: 10.1016/S0891-5849(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 107.Kuhn D., Lam W.H., Kazi A., Daniel K.G., Song S., Chow L.M., Chan T.H., Dou Q.P. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front. Biosci. 2005;10:1010–1023. doi: 10.2741/1595. [DOI] [PubMed] [Google Scholar]
- 108.Zhou X.J., Gao Y.X., Yuan Y.J., Yang H.J., Zhang J. Study on extraction technology and antioxidant activity of theabrownine from oolong tea. Chin. J. Exp. Tradit. Med. Formulae. 2011;17:36–40. [Google Scholar]
- 109.Zhao Z.Y., Huangfu L.T., Dong L.L., Liu S.L. Functional groups and antioxidant activities of polysaccharides from five categories of tea. Ind. Crops Prod. 2014;58:31–35. doi: 10.1016/j.indcrop.2014.04.004. [DOI] [Google Scholar]
- 110.Wang Y., Liu Y., Huo J., Zhao T., Ren J., Wei X. Effect of different drying methods on chemical composition and bioactivity of tea polysaccharides. Int. J. Biol. Macromol. 2013;62:714–719. doi: 10.1016/j.ijbiomac.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Xiao J., Huo J., Jiang H., Yang F. Chemical compositions and bioactivities of crude polysaccharides from tea leaves beyond their useful date. Int. J. Biol. Macromol. 2011;49:1143–1151. doi: 10.1016/j.ijbiomac.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 112.Nie S.P., Xie M.Y., Cao S.W. The antioxidative activity and anti-proliferation activity of purified tea polysaccharide against two colon cancer cell lines. Acta Nutr. Sin. 2007;29:46–50. [Google Scholar]
- 113.Liao Y., Zou X., Wang C., Xin Z. Insect tea extract attenuates CCl4-induced hepatic damage through its antioxidant capacities in ICR mice. Food Sci. Biotechnol. 2016;25:581–587. doi: 10.1007/s10068-016-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paquay J.B., Haenen G.R., Stender G., Wiseman S.A., Tijburg L.B., Bast A. Protection against nitric oxide toxicity by tea. J. Agric. Food Chem. 2000;48:5768–5772. doi: 10.1021/jf981316h. [DOI] [PubMed] [Google Scholar]
- 115.Zhong Y., Chiou Y.S., Pan M.H., Ho C.T., Shahidi F. Protective effects of epigallocatechin gallate (EGCG) derivatives on azoxymethane-induced colonic carcinogenesis in mice. J. Funct. Foods. 2012;4:323–330. doi: 10.1016/j.jff.2011.12.011. [DOI] [Google Scholar]
- 116.Sinha D., Biswas J., Bishayee A. Nrf2-mediated redox signaling in arsenic carcinogenesis: A review. Arch. Toxicol. 2013;87:383–396. doi: 10.1007/s00204-012-0920-5. [DOI] [PubMed] [Google Scholar]
- 117.Qi G., Mi Y., Rong F., Li R., Wang Y., Li X., Huang S., Liu X. Tea polyphenols ameliorate hydrogen peroxide- and constant darkness-triggered oxidative stress via modulating the Keap1/Nrf2 transcriptional signaling pathway in HepG2 cells and mice liver. RSC Adv. 2017;7:32198–32208. doi: 10.1039/C7RA05000C. [DOI] [Google Scholar]
- 118.Patel R., Maru G. Polymeric black tea polyphenols induce phase II enzymes via Nrf2 in mouse liver and lungs. Free Radic. Biol. Med. 2008;44:1897–1911. doi: 10.1016/j.freeradbiomed.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Gao Z., Han Y., Hu Y., Wu X., Wang Y., Zhang X., Fu J., Zou X., Zhang J., Chen X. Targeting HO-1 by epigallocatechin-3-gallate reduces contrast-induced renal injury via anti-oxidative stress and anti-inflammation pathways. PLoS ONE. 2015;11:e0149032. doi: 10.1371/journal.pone.0149032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Furfaro A.L., Traverso N., Domenicotti C., Piras S., Moretta L., Marinari U.M., Pronzato M.A., Nitti M. The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid. Med. Cell. Longev. 2015;2016:1958174. doi: 10.1155/2016/1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X., Zou Z., Tang J., Zheng Y., Liu Y., Luo Y., Liu Q., Wang Y. NOS1 upregulates ABCG2 expression contributing to DDP chemoresistance in ovarian cancer cells. Biol. Pharm. Bull. 2018;41:1237–1242. doi: 10.1248/bpb.b18-00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu C., Jiao Y., Xue J., Zhang Q., Yang H., Xing L., Chen G., Wu J., Zhang S., Zhu W., et al. Metformin sensitizes non-small cell lung cancer cells to an epigallocatechin-3-gallate (EGCG) treatment by suppressing the Nrf2/HO-1 signaling pathway. Int. J. Biol. Sci. 2017;13:1560–1569. doi: 10.7150/ijbs.18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayakawa S., Saito K., Miyoshi N., Ohishi T., Oishi Y., Miyoshi M., Nakamura Y. Anti-cancer effects of green tea by Either anti- or pro- oxidative mechanisms. Asian Pac. J. Cancer Prev. 2016;17:1649–1654. doi: 10.7314/apjcp.2016.17.4.1649. [DOI] [PubMed] [Google Scholar]
- 124.Blaser H., Dostert C., Mak T.W., Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 125.Li J., Sapper T.N., Mah E., Moller M.V., Kim J.B., Chitchumroonchokchai C., Mcdonald J.D., Bruno R.S. Green tea extract treatment reduces NFκB activation in mice with diet-induced nonalcoholic steatohepatitis by lowering TNFR1 and TLR4 expression and ligand availability. J. Nutr. Biochem. 2017;41:34–41. doi: 10.1016/j.jnutbio.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 126.Santamarina A.B., Carvalho-Silva M., Gomes L.M., Okuda M.H., Santana A.A., Streck E.L., Seelaender M., Nascimento C.M.O.D., Ribeiro E.B., Lira F.S. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. J. Nutr. Biochem. 2015;26:1348–1356. doi: 10.1016/j.jnutbio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 127.Mitchell T.S., Moots R.J., Wright H.L. Janus kinase inhibitors prevent migration of rheumatoid arthritis neutrophils towards interleukin-8, but do not inhibit priming of the respiratory burst or reactive oxygen species production. Clin. Exp. Immunol. 2017;189:250–258. doi: 10.1111/cei.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pal D., Sur S., Mandal S., Das S., Pandaa C.K. Regular black tea habit could reduce tobacco associated ROS generation and DNA damage in oral mucosa of normal population. Food Chem. Toxicol. 2012;50:2996–3003. doi: 10.1016/j.fct.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 129.Li J., Sapper T.N., Mah E., Rudraiah S., Schill K.E., Chitchumroonchokchai C., Moller M.V., Mcdonald J.D., Rohrer P.R., Manautou J.E., et al. Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NF-κB pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol. Nutr. Food Res. 2016;60:858–870. doi: 10.1002/mnfr.201500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li W., Khor T.O., Xu C., Shen G., Jeong W.S., Yu S., Kong A.N. Activation of Nrf2-antioxidant signaling attenuates NF-κB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Das D.N., Panda P.K., Naik P.P., Mukhopadhyay S., Sinha N., Bhutia S.K. Phytotherapeutic approach: A new hope for polycyclic aromatic hydrocarbons induced cellular disorders, autophagic and apoptotic cell death. Toxicol. Mech. Methods. 2017;27:1–17. doi: 10.1080/15376516.2016.1268228. [DOI] [PubMed] [Google Scholar]
- 132.Wang Z.Y., Khan W.A., Bickers D.R., Mukhtar H. Protection against polycyclic aromatic hydrocarbon-induced skin tumor initiation in mice by green tea polyphenols. Carcinogenesis. 1989;10:411–415. doi: 10.1093/carcin/10.2.411. [DOI] [PubMed] [Google Scholar]
- 133.Wilk A., Rski P.W., Lassak A., Vashistha H., Lirette D., Tate D., Zea A.H., Koochekpour S., Rodriguez P., Meggs L.G., et al. Polycyclic aromatic hydrocarbons-induced ROS accumulation enhances mutagenic potential of T-antigen from human polyomavirus JC. J. Cell Physiol. 2013;228:2127–2138. doi: 10.1002/jcp.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Libalova H., Milcova A., Cervena T., Vrbova K., Rossnerova A., Novakova Z., Topinka J., Rossner P., Jr. Kinetics of ROS generation induced by polycyclic aromatic hydrocarbons and organic extracts from ambient air particulate matter in model human lung cell lines. Mutat. Res. Toxicol. Environ. Mutagen. 2018;827:50–58. doi: 10.1016/j.mrgentox.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Fukuda I., Nishiumi S., Mukai R., Yoshida K., Ashida H. Catechins in tea suppress the activity of cytochrome P450 1A1 through the aryl hydrocarbon receptor activation pathway in rat livers. Int. J. Food Sci. Nutr. 2015;66:300–307. doi: 10.3109/09637486.2014.992007. [DOI] [PubMed] [Google Scholar]
- 136.Yang C.S., Lambert J.D., Hou Z., Ju J., Lu G., Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol. Carcinog. 2010;45:431–435. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- 137.Hider R.C., Liu Z.D., Khodr H.H. Metal chelation of polyphenols. Methods Enzym. 2001;335:190–203. doi: 10.1016/s0076-6879(01)35243-6. [DOI] [PubMed] [Google Scholar]
- 138.Zhou Y., Que K.-T., Zhang Z., Zhao P.X., You Y., Gong J.-P., Liu Z.-J. Iron ion polarizes macrophages to M1 subtype through ROS-acetyl-P53. J. Third Mil. Med. Univ. 2018;7:4012–4022. doi: 10.1002/cam4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gumiennakontecka E., Pyrkoszbulska M., Szebesczyk A., Ostrowska M. Iron chelating strategies in systemic metal overload, neurodegeneration and cancer. Curr. Med. Chem. 2014;21:3741–3767. doi: 10.2174/0929867321666140706143402. [DOI] [PubMed] [Google Scholar]
- 140.Yang W.-H., Fong Y.-C., Lee C.-Y., Jin T.-R., Tzen J.T., Li T.-M., Tang C.-H. Epigallocatechin-3-gallate induces cell apoptosis of human chondrosarcoma cells through apoptosis signal-regulating kinase 1 pathway. J. Cell. Biochem. 2011;112:1601–1611. doi: 10.1002/jcb.23072. [DOI] [PubMed] [Google Scholar]
- 141.Alshatwi A.A., Periasamy V.S., Athinarayanan J., Elango R. Synergistic anticancer activity of dietary tea polyphenols and bleomycin hydrochloride in human cervical cancer cell: Caspase-dependent and independent apoptotic pathways. Chem. Biol. Interact. 2016;247:1–10. doi: 10.1016/j.cbi.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 142.Tsai C.Y., Chen C.Y., Chiou Y.H., Shyu H.W., Lin K.H., Chou M.C., Huang M.H., Wang Y.F. Epigallocatechin-3-gallate suppresses human herpesvirus 8 replication and induces ROS leading to apoptosis and autophagy in primary effusion lymphoma cells. Int. J. Mol. Sci. 2018;19:16. doi: 10.3390/ijms19010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Satoh M., Takemura Y., Hamada H., Sekido Y., Kubota S. EGCG induces human mesothelioma cell death by inducing reactive oxygen;species and autophagy. Cancer Cell Int. 2013;13:19. doi: 10.1186/1475-2867-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ling T., Forester S.C., Lambert J.D. Pro-oxidant effects of the green tea catechin, (−)-epigallocatechin-3-gallate in oral cancer cells: A role for the mitochondria. Cancer Res. 2013;73:3667. [Google Scholar]
- 145.Tao L., Park J.Y., Lambert J.D. Differential prooxidative effects of the green tea polyphenol, (−)-epigallocatechin-3-gallate, in normal and oral cancer cells are related to differences in sirtuin 3 signaling. Mol. Nutr. Food Res. 2015;59:203–211. doi: 10.1002/mnfr.201400485. [DOI] [PubMed] [Google Scholar]
- 146.Xie L., Luo Z., Zhao Z., Chen T. Anticancer and antiangiogenic Iron(II) complexes that target thioredoxin reductase to trigger cancer cell apoptosis. J. Med. Chem. 2016;60:202–214. doi: 10.1021/acs.jmedchem.6b00917. [DOI] [PubMed] [Google Scholar]
- 147.Zhang H., Cao D., Cui W., Ji M., Qian X., Zhong L. Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (−)-epigallocatechin-3-gallate. Free Radic. Biol. Med. 2010;49:2010–2018. doi: 10.1016/j.freeradbiomed.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 148.Park B.J., Kang S.S., Hong S.G., Lee J.H., Kim H.S., Chun Y.K., Hong S.R., Kang Y.S., Moon I.G., Lee S.K., et al. Telomerase Activity in Human Breast Tumors. J. Natl. Cancer Inst. 1998;1:203–207. doi: 10.4048/jkbcs.1998.1.2.203. [DOI] [Google Scholar]
- 149.Na Y.M., Kim J.H., Choi J.H., Wen L., Ko Y.J., Rhee S., Bang H., Ham S.W., Park A.J., Lee K.H. Selective death of cancer cells by preferential induction of reactive oxygen species in response to (−)-epigallocatechin-3-gallate. Biochem. Biophys. Res. Commun. 2012;421:91–97. doi: 10.1016/j.bbrc.2012.03.120. [DOI] [PubMed] [Google Scholar]
- 150.Gonzalez-Donquiles C., Alonso-Molero J., Fernandez-Villa T., Vilorio-Marqués L., Molina A.J., Martín V. The NRF2 transcription factor plays a dual role in colorectal cancer: A systematic review. PLoS ONE. 2017;12:e0177549. doi: 10.1371/journal.pone.0177549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Amin A.R.M.R., Wang D., Zhang H., Peng S., Shin H.J.C., Brandes J.C., Tighiouart M., Khuri F.R., Chen Z.G., Shin D.M. Enhanced anti-tumor activity by the combination of the natural compounds (−)-epigallocatechin-3-gallate and luteolin: Potential role of p53. J. Biol. Chem. 2010;285:34557–34565. doi: 10.1074/jbc.M110.141135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Khan H.Y., Zubair H., Ullah M.F., Ahmad A., Hadi S.M. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: Implications for a cancer preventive mechanism. Biometals. 2011;24:1169–1178. doi: 10.1007/s10534-011-9475-9. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y., Yang N.D., Zhou F., Shen T., Duan T., Zhou J., Shi Y., Zhu X.Q., Shen H.M. (−)-Epigallocatechin-3-gallate induces non-apoptotic cell death in human cancer cells via ROS-mediated lysosomal membrane permeabilization. PLoS ONE. 2012;7:e46749. doi: 10.1371/journal.pone.0046749. [DOI] [PMC free article] [PubMed] [Google Scholar]