Abstract
Mucin-1 (MUC1) is a highly attractive antigenic target for anticancer vaccines. Naturally existing MUC1 can contain multiple types of O-linked glycans, including the Thomsen–Friedenreich (Tf) antigen and the Sialyl Thomsen-nouveau (STn) antigen. In order to target these antigens as potential anticancer vaccines, MUC1 glycopeptides SAPDT*RPAP (T* is the glycosylation site) bearing the Tf and the STn antigen, respectively, have been synthesized. The bacteriophage Qβ carrier is a powerful carrier for antigen delivery. The conjugates of MUC1-Tf and -STn glycopeptides with Qβ were utilized to immunize immune-tolerant human MUC1 transgenic (MUC1.Tg) mice, which elicited superior levels of anti-MUC1 IgG antibodies with titers reaching over 2 million units. The IgG antibodies recognized a wide range of MUC1 glycopeptides bearing diverse glycans. Antibodies induced by Qβ-MUC1-Tf showed strongest binding, with MUC1-expressing melanoma B16-MUC1 cells, and effectively killed these cells in vitro. Vaccination with Qβ-MUC1-Tf first followed by tumor challenge in a lung metastasis model showed significant reductions of the number of tumor foci in the lungs of immunized mice as compared to those in control mice. This was the first time that a MUC1-Tf-based vaccine has shown in vivo efficacy in a tumor model. As such, Qβ-MUC1 glycopeptide conjugates have great potential as anticancer vaccines.
Graphical Abstract
Mucin-1 (MUC1) is a cell surface glycoprotein overexpressed on a range of cancer cells including breast, lung, pancreatic, colon, prostate, and ovarian cancers with a key role in cancer development.1,2 MUC1 contains an extracellular domain, which comprises a variable number (30–200) of 20 amino acid tandem repeats with the sequence of SAPDTRPAPGSTAPPAHGVT.3,4 The serine and threonine residues in the tandem repeat can be glycosylated. The O-linked glycans of tumor-associated MUC1 are truncated and less branched, differentiating MUC1 from tumor versus normal cells.1,2,5 The level of MUC1 on tumor cells can be 100 times higher than that on normal cells, rendering it an attractive target for vaccines. Clinical studies have shown that patients with high levels of anti-MUC1 IgG antibodies are associated with better prognosis in a variety of cancers. For example, a significantly higher 1 year survival rate (91% vs 21%, p < 0.001) was observed in nonresectable non-small cell lung cancer patients with high anti-MUC1 IgG titers than those with low antibody levels.6 The amounts of anti-MUC1 IgG but not IgM antibodies in patients with invasive ductal pancreatic carcinoma correlated significantly with survival time (p = 0.0004).7 Therefore, if high anti-MUC1 antibody titers can be generated through vaccination, the vaccines can potentially protect the host from tumor development.
Earlier strategies for MUC1-based vaccines typically utilized MUC1 peptide as the antigen.8,9 As MUC1 is an endogenous protein in humans, B cells reacting strongly to MUC1 are commonly deleted during development. As a result, MUC1 is well-tolerated by the body, rendering it more challenging to elicit powerful anti-MUC1 antibody responses. One strategy to enhance the levels of antibodies generated by MUC1 in vaccine design is by introducing glycosylation into MUC1, such as the Thomsen-nouveau antigen (Tn antigen, αGalNAc-Ser/Thr).5,10–12 Immunization with human MUC1 transgenic mice, which are capable of mimicking MUC1 immunotolerance in humans, with MUC1-Tn glycopeptide has been shown to produce higher levels anti-MUC1 antibodies or T cell responses compared to the levels of the corresponding MUC1 peptide.10,12 The immune responses induced by MUC1-Tn can kill MUC1-expressing tumor cells and protect the host from tumor-induced death in MUC1.Tg mouse models.
Besides the Tn antigen, tumor-associated MUC1 can contain disaccharides such as STn (αNeu5Ac-(2,6)-αGal-NAc-Ser/Thr) and Thomsen–Friedenreich (Tf) antigen (βGal-(1,3)-αGalNAc-Ser/Thr).4,13–15 Studies have been carried out targeting these antigens using innovative platforms including protein carriers such as tetanus toxoid and bovine serum albumin,16–18 fully synthetic self-adjuvanting multi-component constructs,19,20 multivalent antigen display,21 as well as fluorinated analogues of the carbohydrate.22,23 Several such constructs have been evaluated in MUC1.Tg mice,24 which produced anti-MUC1 IgG antibodies with typical titers of several thousand enzyme-linked immunosorbent assay (ELISA) units.16,18,19 However, to the best of our knowledge, the abilities of these MUC1 constructs to protect the immunized host from tumor development in vivo have not been reported.
Herein, we report the synthesis of MUC1 glycopeptides SAPDT*RPAP bearing Tf and STn antigens, respectively. The glycopeptides were conjugated with bacteriophage Qβ virus-like particle, and the immunogenicities of these conjugates were evaluated in immunotolerant MUC1.Tg mice. High levels of IgG antibodies capable of binding strongly to tumor cells were induced, with antibody titers reaching over 2 million ELISA units. For B16-MUC1 melanoma cells, antibodies produced by Qβ-MUC1-Tf bound strongest compared to those elicited by the corresponding conjugates with unglycosylated MUC1 peptide or glycopeptides with other glycoforms. Furthermore, immunization of MUC1.Tg mice with Qβ-MUC1-Tf significantly protected mice from challenges by B16-MUC1 cells in a tumor metastasis model.
RESULTS AND DISCUSSION
Synthesis of Qβ-MUC1 Conjugates Carrying STn or Tf Antigens.
The bacteriophage Qβ virus-like particle is a powerful platform for glycoconjugate-based anticancer vaccine development.25–28 A major factor for the superior abilities of Qβ to elicit anticancer antibodies against cancer-associated carbohydrate antigens is its highly organized three-dimensional structure for ordered antigen display, leading to effective cross-linking of B cell receptors and powerful activation of antibody-producing B cells.29,30 When conjugated with Qβ, a MUC1 peptide with the sequence SAPDTRPAP has been found to be the critical protective epitope for anticancer immunity.12 Extending the peptide beyond the protective epitope decreased the binding of IgG antibodies to MUC1-expressing tumor cells. Glycosylated MUC1 vaccines can induce immune responses stronger than those of the unglycosylated control.5,31,32 Consistent with literature reports, glycosylation of the threonine residue within the SAPDTRPRP with N-acetyl galactosamine (i.e., the Tn antigen) significantly enhanced the anticancer antibody responses compared to the corresponding unglycosylated MUC1 peptide.12 The Qβ-MUC1-Tn construct provided better protection to immunized animals against cancer development than the corresponding conjugate of MUC1-Tn with Keyhole Limpet Hemocyanin (KLH), a gold standard carrier commonly utilized in anticancer conjugate vaccines.
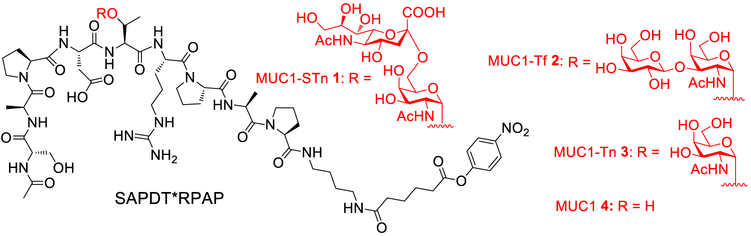
In order to study MUC1 glycopeptide antigens bearing disaccharides, MUC1 glycopeptides 1 and 2 bearing STn and Tf, respectively, were designed (Scheme 1). Enzymatic synthesis can be an efficient strategy for glycopeptide assembly.33,34 We first explored enzymatic extension of the glycan of MUC1-Tn glycopeptide 328 with an α−2,6-sialyltransferase. However, incubation of MUC1-Tn 3 with Photobacterium damselae α−2,6-sialyltransferase (Pd2,6ST) and CMP-Neu5Ac as the sialic acid donor did not lead to any glycopeptide product 1. Previously, another MUC1-Tn sequence APGS*TAPPA (* denotes GalNAc) was reported to be successfully sialylated with Pd2,6ST,34 which was reproduced by us (data not shown). Thus, the difficulty encountered in sialylating MUC1-Tn 3 was presumably because MUC1 peptide backbone SAPDTRPAP interfered with the glycosylation by Pd2,6ST.
Scheme 1.
Structures of MUC1 (Glyco)peptides 1–4
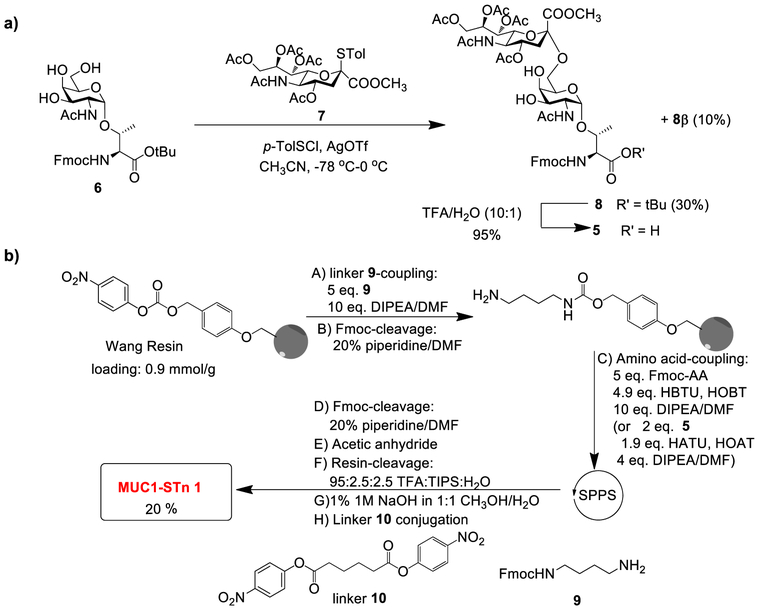
Rather than screening other enzymes to synthesize MUC1-STn 1,35 we resorted to the chemical strategy to access the target glycopeptide by solid-phase peptide synthesis (SPPS), which required the Fmoc-protected STn antigen building block 5. The synthesis of 5 started from sialylation of the galactosamine threonine ester 636,37 with sialyl donor 726 using the p-TolSCI/AgOTf promoter system38 (Scheme 2a). The desired Fmoc-Neu5Ac-α−2,6-GalNAc-a-Thr 8 was isolated in 30% yield in addition to 10% of its β-isomer 8β. The stereochemistry of the newly formed glycosyl linkage of 8 was assigned based on the 3-bond coupling constant between C1 and H3ax of sialic acid (3Jci,H3ax = 6.4 Hz), as well as that between H7 and H8 of the sialic acid unit (3JH7,H8 = 8.3 Hz).39,40 Recently, sialyl donors modified with groups such as 4-O,5-N-oxazolidinone and 5-azide have been shown to give high yields and stereoselectivities in sialylation reactions.41–43 Although the sialylation yield using donor 7 was modest, it was advantageous to use donor 7 as it took much fewer steps to prepare, and no additional synthetic steps were needed to adjust the protecting groups on C5 of sialic acid back to acetamide following sialylation (saving at least five synthetic steps overall). Acid treatment of 8 cleaved its tert-butyl ester to yield the Fmoc-protected STn antigen building block 5 for solid-phase synthesis.
Scheme 2.
(a) Synthesis of Fmoc-Neu5Ac-α−2,6-GaINAc-α-Thr 5 and (b) Solid-Phase Synthesis of MUC1-STn Glycopeptide 1
The free C-terminus of MUC1 can be immunodominant,12 prompting us to synthesize MUC1 glycopeptide 1, which is conjugatable through a C-terminal aminoalkyl amide.12,44 MUC1 glycopeptide 1 was assembled through the SPPS approach starting from the p-nitrophenyl carbonate functionalized Wang resins preloaded with mono-Fmoc-protected 1,4-diaminobutane 9 followed by glycopeptide elongation (Scheme 2b). The coupling of Fmoc-protected amino acids to peptide chains was carried out with (2-(1H-benzotriazol-1-yl)−1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)/hydroxybenzotriazole (HOBt). For coupling of the glycosyl amino acid 5, 1 -[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU)/1-hydroxy-7-azabenzotriazole (HOAt) were utilized as the coupling agents. Following capping of the N-terminus with an acetyl group, the glycopeptide was cleaved from the resins followed by methyl ester and O-acetate cleavage using 1% 1 M NaOH (aq) in methanol/H2O (1:1). The resulting glycopeptide bearing a free amine at its C-terminus was incubated with adipate bis(4-nitrophenyl) ester10.45 C18 reverse-phase HPLC purification produced the desired MUC1-STn glycopeptide 1 in 20% overall yield from the resin.
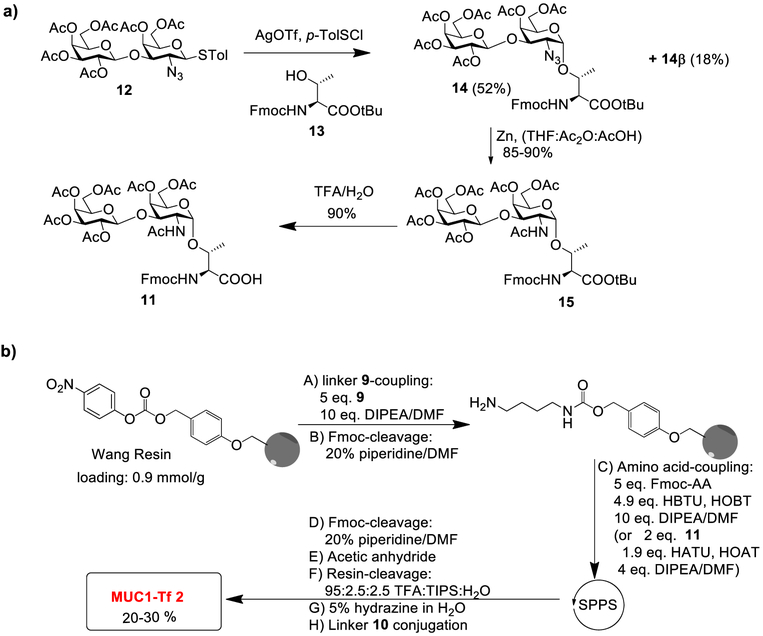
We next synthesized MUC1 glycopeptide 2 bearing the Tf antigen. The Fmoc-protected Tf antigen building block 11 was obtained through the glycosylation of disaccharide donor 1246 and acceptor 13 promoted by p-TolSCl/AgOTf (Scheme 3a). This reaction gave the desired Fmoc-protected Tf antigen building block 14 in 52% yield, which was separated from its β-anomer 14β (18%). After reduction of azide and acidolysis of the tert-butyl ester of 14, the Fmoc-protected Tf antigen building block 11 was isolated. Following a similar SPPS approach as in the synthesis of MUC1-STn 1, the desired MUC1-Tf 2 was produced in 20–30% overall yield (Scheme 3b).
Scheme 3.
(a) Synthesis of Fmoc-Gal-β-1,3-GalNAc-α-Thr 11 and (b) Solid-Phase Synthesis of MUC1-Tf Glycopeptide 2
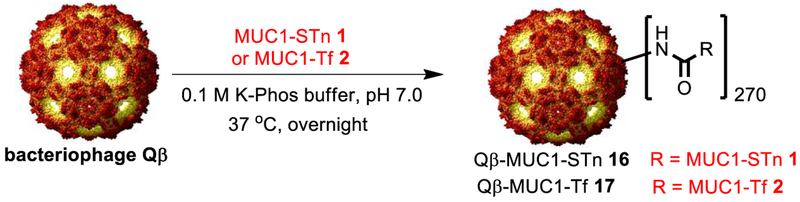
For Qβ-based vaccines, it is critical that the antigen is covalently conjugated with the carrier, as an admixture of MUC1 glycopeptide and Qβ was ineffective in generating anti-MUC1 antibodies compared to the covalent conjugate of Qβ-MUC1.28 The ligations of MUC1 glycopeptides 1 and 2 with recombinant bacteriophage Qβ were carried out in K-Phos buffer (0.1 M, pH 7) overnight at 37 °C to give Qβ-MUC1-STn 16 and Qβ-MUC1-Tf 17 (Scheme 4). Mass spectrometry analysis of the capsid showed that the numbers of glycopeptides per Qβ particle were 270 on average (Supporting Information Figure S1).12 The conjugates had an average hydrodynamic radius of 17 nm with low polydispersity (Supporting Information Figure S2).
Scheme 4.
Synthesis of Qβ-MUC1 Conjugates 16 and 17
Qβ-HVIUC1 Conjugates Elicited High Titers of Anti-MUC1 IgG Antibodies.
With Qβ-MUC1 constructs in hand, their immunogenicities were evaluated in MUC1.Tg mice. Compared to the commonly used wild-type mice, MUC1.Tg mice are a more suitable model for evaluation of MUC1-based vaccines as human MUC1 has difference sequences from mouse MUC1, and the MUC1.Tg mice can better mimic the MUC1 immunotolerance encountered in humans compared to wild-type mice.24 MUC1.Tg mice were immunized with Qβ-MUC1-STn 16 and Qβ-MUC1-Tf 17 (8.6 nmol of MUC1) using MPLA (monophosphosphoryl lipid A from Salmonella enterica serotype Minnesota Re 595, Re mutant) as the adjuvant (day 0), which is a TLR-4 agonist approved by FDA for use in human patients to enhance immune responses.47 Two booster injections were administered to mice on days 14 and 28. On day 35, the sera were collected.
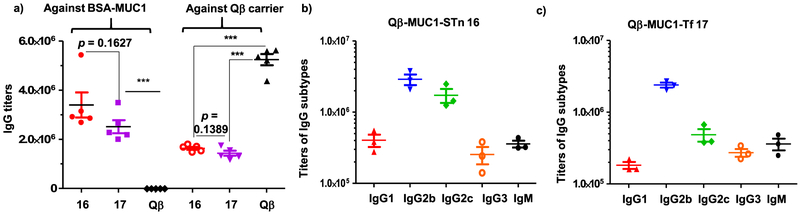
To analyze the levels of induced anti-MUC1 antibodies, the MUC1 glycopeptides were conjugated with bovine serum albumin (BSA) to generate BSA-MUC1 conjugates 18 and 19 (Supporting Information Scheme S1 and Figure S3). ELISA was performed using BSA-MUC1 conjugates to analyze the levels of anti-MUC1 antibodies in postimmune sera from Qβ-MUC1 16 and 17 immunized MUC1.Tg mice. Strong anti-MUC1 IgG responses were elicited by both constructs with mean IgG titers of 3,399,000 and 2,510,000, respectively (Figure 1a), with the titer number reported as the highest fold of dilution giving the optical density (OD) value of 0.1 over those of the preimmune control sera (OD ~ 0.2), and the induced midpoint (EC50) IgG titers were 336,358 from 16 and 253,953 from 17 when determined from titration curves corresponding to the dilution that induces the half-maximal absorbance values (Supporting Information Figure S4). Furthermore, the attachment of MUC1 glycopeptides on Qβ significantly reduced the titers of antibodies elicited against the Qβ carrier itself presumably because MUC1 glycopeptides partially shielded the surface Qβ in the Qβ-MUC1 conjugate from immune recognition (Figure 1). The anti-MUC1 IgG levels induced by the Qβ conjugates compare favorably with the titers (typically several thousand ELISA units) of antibodies produced by other reported MUC1-Tf and MUC1-STn constructs in MUC1.Tg mice,16,18,19 which demonstrates the advantages of the Qβ approach.
Figure 1.
(a) Titers of anti-MUC1 and anti-Qβ IgG antibodies from MUC1.Tg mice immunized with Qβ-MUC1 conjugates 16 and 17. For determination of anti-MUC1 IgG titers, the ELISA measurements were performed against the corresponding BSA-MUC1 conjugates 18 and 19. For testing levels of anti-Qβ IgG, the ELISA was performed against Qβ. Each symbol represents one mouse (n = 5 mice for each group). The titer was determined by regression analysis with log10 dilution plotted with optical density and reported as the highest fold of dilution giving the optical absorbance value of 0.1 over those of the preimmune control sera (OD = 0.2). IgG subtypes of MUC1.Tg mice immunized (b) Qβ-MUC1-STn 16 and (c) Qβ-MUC1-Tf 17 assayed against the corresponding BSA-MUC1 conjugates 18 and 19, respectively; ***p < 0.001. The p values were determined through a two-tailed unpaired Student’s t-test using GraphPad Prism.
To better understand the profile of antibody responses, the titers of IgG antibody subtype were determined via ELISA. Among the IgG subtypes, the titers of IgG2b and IgG2c antibodies were highest, reaching 2,896,000 and 1,729,000, respectively, for Qβ-MUC1-STn 16 (Figure 1b) and 2,391,000 and 485,000 for Qβ-MUC1-Tf 17 (Figure 1c), suggesting the main type of immune responses induced was biased toward Th1. Significant amounts of anti-MUC1 IgM antibodies have also been induced (Figure 1).
Antibodies Elicited by Qβ-MUC1-STn 16 and Qβ-MUC1-Tf 17 Bound Strongly with MUC1-Expressing Cancer Cells.
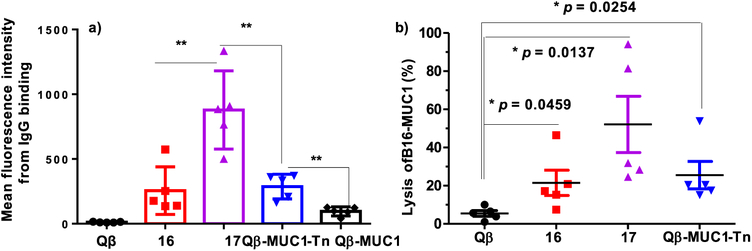
We evaluated next the abilities of postimmune sera to recognize MUC1 expressed in the native environment, that is, on the surface of MUC1-expressing tumor cells. B16-MUC1 mouse melanoma cells and MCF-7 human breast cancer cells were incubated with sera from MUC1.Tg mice immunized with various Qβ-MUC1 conjugates, and IgG antibody bindings to cancer cells were tested using flow cytometry. As shown in Figure 2 and Supporting Information Figure S5, significant enhancements in cellular binding to cancer cells were observed with both sera compared to those from control mice immunized with Qβ, suggesting the successful generation of anticancer antibodies. Sera induced by Qβ-MUC1-Tf 17 were found to bind B16-MUC1 cells stronger than those by Qβ-MUC1-STn 16, as well as those elicited by Qβ-MUC1-Tn and Qβ-MUC1 (Figure 2 and Supporting Information Figure S5). The stronger binding of sera induced by Qβ-MUC1-Tf 17 is possibly due to higher expression of Tf antigen on B16-MUC1 cells. Antibodies from mice immunized with both Qβ-MUC1-STn 16 and Qβ-MUC1-Tf 17 also bound well with human breast cancer cells MCF-7 (Supporting Information Figure S6a). Importantly, all postimmune sera had low bindings with the normal breast cell MCF-10A similar to those from the control mice (Supporting Information Figure S6b), suggesting high cancer selectivity of anti-MUC1 antibodies generated.
Figure 2.
(a) Flow cytometry analysis of anti-MUC1 IgG antibodies showed Qβ-MUC1-Tf 17 elicited antibodies with significantly stronger binding to tumor cells compared with Qβ-MUC1-Tn elicited antibodies. Binding to B16-MUC1 cells was tested with 1:20 dilution of the corresponding sera, (b) Antibodies induced by Qβ-MUC1 conjugates exhibited significantly high CDC toward tumor cells. CDC toward B16-MUC1 cells was determined by a MTS assay. Each symbol represents one mouse (n = 5 mice for each group); *p < 0.05, **p < 0.01. The p values were determined through a two-tailed unpaired Student’s t-test using GraphPad Prism.
It should be noted that relatively concentrated sera are needed for statistically significant enhancement in B16MUC1 binding in flow cytometry analysis (less than 500-fold dilution, Supporting Information Figure S5c) compared to the high ELISA titers (on the order of hundreds of thousands to millions). There can be several potential reasons: (1) ELISA assay uses secondary antibodies conjugated with the enzyme horseradish peroxidase (HRP) for detection. HRP can catalytically turn over its substrate, greatly enhancing the detection sensitivity. (2) It is known that anti-MUC1 antibodies can be internalized upon binding with MUC1-bearing tumor cells,48 thus possibly reducing the amounts of cell surface antibodies for fluorescence detection. (3) The tumor-binding antibodies may be a small subset of the total pool of antibodies elicited. Although further studies are needed to gain a better understanding, the abilities of the postimmune sera to recognize MUC1-bearing tumor cells prompted function analysis of the postimmune sera.
Antibodies Induced by Qβ-MUC1-Tf 17 Killed MUC1-Expressing Tumor Cells in Vitro and Immunization with Qβ-MUC1-Tf Significantly Protected Mice in a Metastasis Model in Vivo.
With the high levels of IgG elicited by Qβ-MUC1-Tf 17 and strong tumor binding by the IgG antibodies elicited, their abilities to kill the tumor cells were measured in vitro. Upon incubation of B16-MUC1 cells (Figure 2b) with postimmune sera and rabbit complement, significantly higher percentages of tumor cells were killed by Qβ-MUC1 conjugate immunized sera as compared to cells treated with sera from Qβ immunized mice. Consistent with the flow cytometry result (Figure 2a), Qβ-MUC1-Tf 17 immunization produced the highest lytic activities toward B16-MUC1 cancer cells.
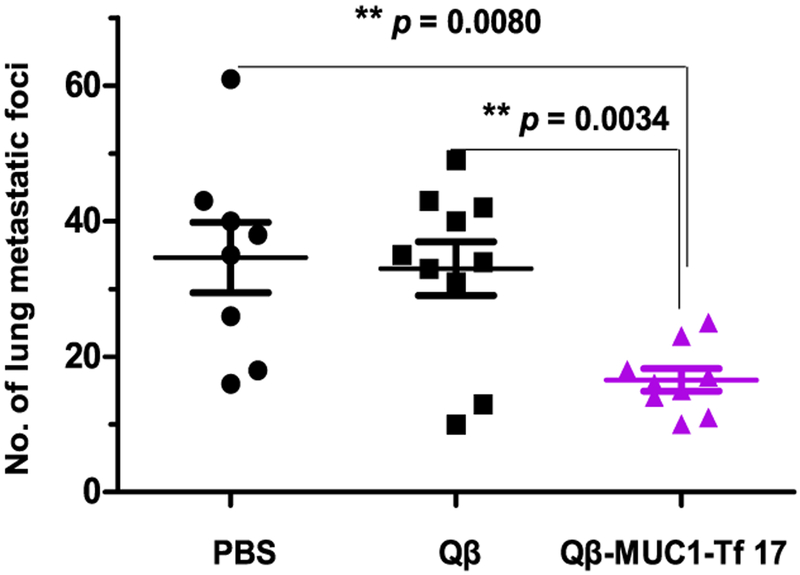
As Qβ-MUC1-Tf 17 elicited antibodies for stronger binding and killing of B16-MUC1 cells than Qβ-MUC1-STn 16, the in vivo tumor protection testing focused on Qβ-MUC1-Tf 17. We evaluated tumor protection in a metastasis model as tumor metastasis is a major hurdle to patient survival. MUC1.Tg mice were immunized with Qβ-MUC1-Tf 17, Qβ, and phosphate-buffered saline (PBS, the control groups) with MPLA adjuvant. B16-MUC1 melanoma cells were injected via tail vein, and the numbers of tumor foci in lungs were determined 21 days after tumor inoculation. Excitingly, Qβ-MUC1-Tf 17 brought a notable reduction in tumor load versus the PBS control (p = 0.0080) and Qβ control (p = 0.0034) (Figure 3 and Figure S7).
Figure 3.
Vaccination of Qβ-MUC1-Tf 17 significantly protected MUC1.Tg mice from formation of metastatic-like tumor foci in the lungs. MUC1.Tg mice were immunized with Qβ-MUC1-Tf 17, Qβ, or PBS, respectively, on days 0, 14, and 28 with MPLA as the adjuvant. On day 35, the immunized mice were challenged with 1 × 105 B16-MUC1 cells via tail vein injection, followed by a fourth immunization. On day 45, the mice were given the last immunization. Twenty-one days after tumor inoculation, the mice were sacrificed and the number of tumor foci in the lungs were counted. Each symbol represents one mouse (n = 8–10 mice for each group); **p < 0.01. The p values are determined through two-way ANOVA with Bonferroni post-test using GraphPad Prism.
Glycopeptide Microarray Screening Revealed the Recognition of Multiple MUC1 Glycoforms by Antibodies Induced by Qβ-MUC1 Conjugates.
With the promising anticancer activities observed, we profiled the epitope structures recognized by antibodies generated to gain a deeper understanding of the epitope profile. Postimmune sera from MUC1.Tg mice immunized with Qβ-MUC1-Tf 17 were screened against a MUC1 glycopeptide microarray.49 This glycopeptide array consisted of 72 MUC1 glycopeptides with the common backbone sequence of PAHGVTSAPDTRPAPGSTAP within one tandem repeat region. The MUC1 glycopeptides were glycosylated with Tn, Tf, or cores 1–4 glycans at various locations of serines and threonines. Furthermore, other glycoproteins including mucin-5B (MUC5B) glycopeptides, fetuin, transferrin, mucins from porcine stomach, and bovine submaxillary glands were also included on the microarray. The arrays were incubated with individual mouse serum, followed by removal of unbound antibodies through thorough washing. A fluorescently labeled anti-mouse IgG secondary antibody was subsequently added to the microarray to semiquantify the amounts of serum IgG antibodies bound to individual array components.
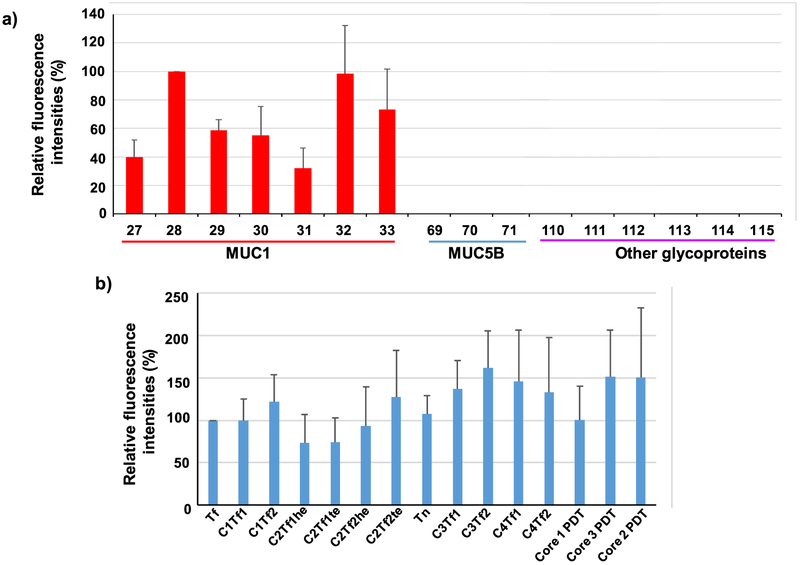
As can be seen from Figure 4a, no cross-reactivities were observed to MUC5B glycopeptides 69–71 or glycoproteins 110–115 (poly(LacNAc)-BSA, fetuin, transferrin, ICAM-1, porcine stomach mucin, and bovine submaxillary mucin), highlighting that antibodies generated were specific to MUC1 glycopeptide rather than glycan only. Interesting structural dependence of binding was observed on MUC1 glycopeptides. Glycopeptides bearing Tf in its PDTR region were bound stronger than those lacking glycosylation in this region. For example, glycopeptides 27–33 all contain the same protein backbone and Tf glycan, with Tf located at various locations of the peptide. Glycopeptide 28, which has Tf in its PDTR region, gave the strongest binding to postimmune sera compared to that with 27 and 29 (Figure 4a). Glycopeptides 32 and 33 contain multiple Tfs in the backbone including a Tf in its PDTR region. They were recognized well by postimmune sera. These results indicate that the presence of Tf in the PDTR region is important for antibody recognition, and antibodies induced are site-selective toward the PDT*R region contained in the immunizing antigen Qβ-MUC1-Tf 17. The site selectivity is possibly due to differential glycopeptide conformations bestowed by the glycans at various positions of the glycopeptides.11
Figure 4.
Representative results of MUC1 glycopeptide microarray screening of antisera from Qβ-MUC1-Tf 17 immunized mice, (a) Comparison of fluorescence intensities of microarray components containing MUC1 glycopeptides bearing Tf antigen at various locations showed that glycosylation at that PDT*R region led to the strongest recognition by postimmune sera. Glycopeptide 27: PAHGVT*SAPDTRPAPGSTA; 28: PAHGVTSAPDT*RPAPGSTA; 29: PAHGVTSAPDTRPAPGST*A; 30: PAHGVT*SAPDT*RPAPGSTA; 31: PAHGVT*SAPDTRPAPGST*A 32: PAHGVTSAPDT*RPAPGST*A; 33: PAHGVT*SAPDT*RPAPGST*A. Glycopeptides 69–71 are various MUC5B glycopeptides. 110–115 are poly(LacNAc)-BSA, fetuin, transferrin, ICAM-1, porcine stomach mucin, and bovine submaxillary mucin, respectively, (b) Comparison of fluorescence intensities of microarray components containing MUC1 glycopeptides bearing various glycans at PAHGVTSAPDT*RPAPGSTA showed that, although Tf gave the strongest recognition, other glycans can be recognized, as well. Glycan structures: glycopeptide 28: Tf (for abbreviations and structures, see Supporting Information Scheme S2 and Figure S8); 35: C1Tf1; 42: C1Tf2; 56: C2Tf1he; 49: C2Tf1te; 73: C2Tf2he; 63: C2Tf2te; 21: Tn; 80: C3Tf1 87: C3Tf2; 94: C4Tf1; 101: C4Tf2; 109: core 1 PDT; 108: core 3 PDT; 107: core 2 PDT. The error bars represent standard deviation (SD) of eight replicates.
Comparison of PAHGVTSAPDT*RPAPGSTA with varying glycan structures showed that Qβ-MUC1-Tf 17 induced antibodies bound to glycopeptides bearing Tf and other glycans ranging from core 1 to core 4 pentasaccharide (Figure 4b), indicating a wide repertoire of anti-MUC1 antibodies generated presumably through binding with the Tf core. Interestingly, compared to antibodies produced by Qβ-MUC1-Tn,12 those generated by Qβ-MUC1-Tf 17 recognized a wider range of glycoforms based on the microarray analysis. As tumor-associated MUC1 can have diverse glycosylations,14,50,51 the abilities of Qβ-MUC1-Tf 17 to induce antibodies recognizing multiple MUC1 glycopeptides bode well for cancer treatment.
In addition to sera from Qβ-MUC1-Tf 17 immunized mice, those from mice receiving Qβ-MUC1-STn 16 were analyzed on the glycopeptide microarray. Similarly, the sera exhibited much stronger binding to MUC1 glycopeptide bearing STn in its PDTR region (glycopeptide 117) than those lacking STn in this region (glycopeptides 116, 118–121 Figure S9a). Little cross-reactivities were observed to MUC5B glycopeptides 69–71 or non-MUC1 glycoproteins 110–115. When sera binding to glycopeptides bearing the backbone sequence of PAHGVT-SAPDT*RPAPGSTA and a glycan at the PDTR region were measured, the postimmune sera recognized a wide range of glycopeptides (Figure S9b).
It should be pointed out that, although we focused on the induction of anti-MUC1 IgG antibodies in the current study, cytotoxic T cells can be another important mechanism for the observed anticancer effects. Studies are ongoing to generate and analyze MUC1 specific cytotoxic T cells through immunization.
CONCLUSIONS
Whereas MUC1 peptides and MUC1-Tn glycopeptides have been evaluated as antigens for tumor protection in vivo, a MUC1 glycopeptide bearing disaccharides such as Tf antigen has not been tested in tumor models. We have developed an effective synthesis of MUC1-Tf and -STn glycopeptides and covalently conjugated them with a powerful carrier bacteriophage Qβ. The resulting conjugates were used to immunize MUC1.Tg mice, which elicited superior levels of anti-MUC1 IgG antibodies (igG titers over 2 million ELISA units), highlighting that Qβ can be an effective carrier, boosting antibodies against multiple glycoforms. The antibodies induced have a preference toward the specific MUC1 glycan sequence utilized for immunization and, at the same time, could bind a range of MUC1 glycoforms while sparing any non-MUC1 glycoproteins as demonstrated in glycopeptide microarray studies. As MUC1 glycosylation on tumor cells is highly heterogeneous, the ability to recognize multiple MUC1 glycoforms is advantageous. This is reflected in strong recognition of MUC1-bearing tumor cells, with antibodies produced by Qβ-MUC1-Tf 17 capable of binding the strongest with B16-MUC1 melanoma cells. MUC1.Tg mice preimmunized with Qβ-MUC1-Tf 17 first showed significant reductions in tumor load in the lungs when subjected to a metastatic tumor model, suggesting the translational potential of this construct as anticancer vaccines.
MATERIALS AND METHODS
Mouse Immunization.
MUC1.Tg mice were generated by breeding C57BL/6 wild-type female mice and MUC1.Tg male mice with a 10.6 kb genomic Sac II fragment of the human MUC1 gene and maintained in the University Laboratory Animal Resources facility of Michigan State University. Pathogen-free MUC1.Tg female mice aged 6–10 weeks were used for studies. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Michigan State University.
In all studies, MUC1.Tg mice were subcutaneously injected under the scruff on day 0 with 0.2 mL of various Qβ-MUC1 vaccines in PBS containing MPLA (20 μL, 1 mg mL−1 in DMSO) for each mouse. Boosters were given subcutaneously at the same amounts of vaccines with MPLA under the scruff on days 14 and 28. All Qβ-MUC1 conjugates administered have the same amounts of MUC1 (8.6 nmol). Sera samples were collected on days 0 (before immunization) and 35. The final bleeding was done by cardiac bleed.
Cancer Immunotherapy Study.
For the lung metastasis model, MUC1.Tg female mice aged 6–10 weeks were subcutaneously immunized under the scruff on day 0 with 0.2 mL of PBS, Qβ, or Qβ-MUC1-Tf 17 in PBS (all injections contained MPLA (20 μL, 1 mg mL−1 in DMSO)). Boosters were given subcutaneously with the same amounts of vaccines mixed with MPLA under the scruff on days 14 and 28. On day 35, vaccinated mice were challenged with 1 × 105 B16-MUC1 cells per mouse by intravenous injection, followed by a fourth vaccination of conjugates mixed with MPLA. On day 45, the mice were given the last vaccination of conjugates mixed with MPLA. On day 56, pulmonary metastases were enumerated by intratracheal injection of black ink (50% in PBS). Black ink injected lungs were washed in Feket’s solution (300 mL 70% EtOH, 30 mL 37% formaldehyde, 5 mL glacial acetic acid) and then placed in fresh Feket’s solution overnight. White tumor nodules against a black lung background were then counted.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the National Cancer Institute, National Institutes of Health (Grant R01 CA225105), Michigan State University Foundation, the Michigan Economic Development Corporation through the MTRAC program, the Deutsche Forschungsgemeinschaft, DFG (WE 4751/2-1), Fonds der Chemischen Industrie (Liebig fellowship to U.W., Li 184/01), Ministerium für Kultur und Wissenschaft des Landes Nordrhein-Westfalen and the Bundesministerium für Bildung und Forschung for financial support of our work. We would like to thank O.J. Finn (University of Pittsburgh), H. Clausen (University of Copenhagen), and S.J. Gendler (Mayo Clinic) for kindly providing us the cells as well as the MUC1.Tg mice.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.9b00381.
Detailed experimental procedures, Figures S1–S9, and Schemes S1 and S2 (PDF)
The authors declare the following competing financial interest(s): Xuefei Huang is the founder of Iaso Therapeutics Inc., which is dedicated to the development of next generation of carbohydrate and glycoconjugate-based anticancer vaccines.
REFERENCES
- (1).Nath S, and Mukherjee P (2014) MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med 20, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hattrup CL, and Gendler SJ (2008) Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol 70, 431–457. [DOI] [PubMed] [Google Scholar]
- (3).Apostolopoulos V, Hu XF, Pouniotis DS, and Xing PX (2004) MUC1: a molecule of many talents. Curr. Trends Immunol. 12, 629–639. [Google Scholar]
- (4).Fontenot JD, Santhana Mariappan SV, Catasti P, Domenech N, Finn OJ, and Gupta G (1995) Structure of a tumor associated antigen containing a tandemly repeated immunodominant epitope. J. Biomol. Struct. Dyn 13, 245–260. [DOI] [PubMed] [Google Scholar]
- (5).von Mensdorff-Pouilly S, Moreno M, and Verheijen RHM (2011) Natural and induced humoral responses to MUC1. Cancers 3, 3073–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hirasawa Y, Kohno N, Yokoyama A, Kondo K, Hiwada K, and Miyake M (2000) Natural autoantibody to MUC1 is a prognostic indicator for non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 161, 589–594. [DOI] [PubMed] [Google Scholar]
- (7).Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, and Hinoda Y (2003) Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int. J. Cancer 103, 97–100. [DOI] [PubMed] [Google Scholar]
- (8).Soares MM, Mehta V, and Finn OJ (2001) Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J. Immunol 166, 6555–6563. [DOI] [PubMed] [Google Scholar]
- (9).Vassilaros S, Tsibanis A, Tsikkinis A, Pietersz GA, McKenzie IF, and Apostolopoulos V (2013) Up to 15-year clinical follow-up of a pilot phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy 5, 1177–1172. [DOI] [PubMed] [Google Scholar]
- (10).Ryan SO, Turner MS, Gariépy J, and Finn OJ (2010) Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses. Cancer Res. 70, 5788–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kinarsky L, Suryanarayanan G, Prakash O, Paulsen J, Clausen H, Hanisch FA, Hollingsworth MA, and Sherman S (2003) Conformational studies on the MUC1 tandem repeat glycopeptides: implication for the enzymatic O-glycosylation of the mucin protein core. Glycobiology 13, 929–939. [DOI] [PubMed] [Google Scholar]
- (12).Wu X, Yin Z, McKay C, Pett C, Yu J, Schorlemer M, Gohl T, Sungsuwan S, Ramadan S, Baniel C, Allmon A, Das R, Westerlind U, Finn MG, and Huang X (2018) Protective epitope discovery and the design of MUC1 based vaccine for effective tumor protections in immunotolerant mice. J. Am. Chem. Soc 140,16596–16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kanitakis J, Al-Rifai I, Faure M, and Claudy A (1998) Differential expression of cancer associated antigens T (Thomsen-Friedenreich) and Tn to the skin in primary and metastatic carcinomas. J. Clin. Pathol 51, 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hanisch FG, Stadie TR, Deutzmann F, and Peter-Katalinic J (1996) MUC1 glycoforms in breast cancer - cell line T47D as a model for carcinoma-associated alterations of O-glycosylation. Eur. J. Biochem 236, 318–327. [DOI] [PubMed] [Google Scholar]
- (15).Beatson R, Maurstad G, Picco G, Arulappu A, Coleman J, Wandell HH, Clausen H, Mandel U, Taylor-Papadimitriou J, Sletmoen M, and Burchell JM (2015) The breast cancer-associated glycoforms of MUC1, MUC1-Tn and sialyl-Tn, are expressed in COSMC wild-type cells and bind the C-type lectin MGL. PLoS One 10, e0125994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Stergiou N, Gaidzik N, Heimes A-S, Dietzen S, Besenius P, Jäkel J, Brenner W, Schmidt M, Kunz H, and Schmitt E (2019) Reduced breast tumor growth after immunization with a tumor-restricted MUC1 glycopeptide conjugated to tetanus toxoid. Cancer Immunol. Res 7, 113–122. [DOI] [PubMed] [Google Scholar]
- (17).Cai H, Huang Z-H, Shi L, Zou P, Zhao YF, Kunz H, and Li YM (2011) Synthesis of Tn/T antigen MUC1 glycopeptide BSA conjugates and their evaluation as vaccines. Eur. J. Org. Chem 2011, 3685–3689. [Google Scholar]
- (18).Stergiou N, Glaffig M, Jonuleit H, Schmitt E, and Kunz H (2017) Immunization with a synthetic human MUC1 glycopeptide vaccine against tumor-associated MUC1 breaks tolerance in human MUC1 transgenic mice. ChemMedChem 12, 1424–1428 and references cited therein. [DOI] [PubMed] [Google Scholar]
- (19).Thompson P, Lakshminarayanan V, Supekar NT, Bradley JM, Cohen PA, Wolfert MA, Gendler SJ, and Boons G-J (2015) Linear synthesis and immunological properties of a fully synthetic vaccine candidate containing a sialylated MUC1 glycopeptide. Chem. Commun 51, 10214–10217 and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wilkinson BL, Day S, Malins LR, Apostolopoulos V, and Payne RJ (2011) Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated MUC1 glycopeptides and the Toll-like receptor 2 agonist Pam3CysSer. Angew. Chem.j Int. Ed 50, 1635–1639. [DOI] [PubMed] [Google Scholar]
- (21).Cai H, Sun ZY, Chen MS, Zhao YF, Kunz H, and Li YM (2014) Synthetic multivalent glycopeptide-lipopeptide anti-tumor vaccines: impact of the cluster effect on the killing of tumor cells. Angew. ChemInt. Ed 53, 1699–1703. [DOI] [PubMed] [Google Scholar]
- (22).Hoffmann-Röder A, Kaiser A, Wagner S, Gaidzik N, Kowalczyk D, Westerlind U, Gerlitzki B, Schmitt E, and Kunz H (2010) Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen-Friedenreich antigen and a fluorine-substituted analogue. Angew. Chem., Int. Ed 49, 8498–8503. [DOI] [PubMed] [Google Scholar]
- (23).Hoffmann-Röder A, and Johannes M (2011) Synthesis of a MUC1-glycopeptide–BSA conjugate vaccine bearing the 3-deoxy-3-fluoro-Thomsen–Friedenreich antigen. Chem. Commun 47, 9903–9905. [DOI] [PubMed] [Google Scholar]
- (24).Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, and Gendler SJ (1998) Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 58, 315–321. [PubMed] [Google Scholar]
- (25).Yin Z, Comellas-Aragones M, Chowdhury S, Bentley P, Kaczanowska K, BenMohamed L, Gildersleeve JC, Finn MG, and Huang X (2013) Boosting immunity to small tumor-associated carbohydrates with bacteriophage Qβ capsids. ACS Chem. Biol 8, 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Yin Z, Dulaney S, McKay C, Baniel C, Kaczanowska K, Ramadan S, Finn MG, and Huang X (2016) Chemical synthesis of GM2 glycans, bioconjugation with bacteriophage Qβ and the induction of anti-cancer antibodies. ChemBioChem 17, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Yin Z, Chowdhury S, McKay C, Baniel C, Wright WS, Bentley P, Kaczanowska K, Gildersleeve JC, Finn MG, BenMohamed L, and Huang X (2015) Significant impact of immunogen design on the diversity of antibodies generated by carbohydrate-based anti-cancer vaccine. ACS Chem. Biol 10, 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Yin Z, Wu X, Kaczanowska K, Sungsuwan S, Comellas-Aragones M, Pett C, Yu J, Baniel C, Westerlind U, Finn MG, and Huang X (2018) Antitumor humoral and T cell responses by Mucin-1 conjugates of bacteriophage Qβ in wild-type mice. ACS Chem. Biol 13, 1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Mohsen MO, Zha L, Cabral-Miranda G, and Bachmann MF (2017) Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin. Immunol 34, 123–132. [DOI] [PubMed] [Google Scholar]
- (30).Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, and Zinkernagel RM (1993) The influence of antigen organization on B cell responsiveness. Science 262, 1448–1451. [DOI] [PubMed] [Google Scholar]
- (31).Cai H, Huang Z-H, Shi L, Sun Z-Y, Zhao Y-F, Kunz H, and Li Y-M (2012) Variation of the glycosylation pattern in MUC1 glycopeptide BSA vaccines and its influence on the immune response. Angew. Chem., Int. Ed 51, 1719–1723. [DOI] [PubMed] [Google Scholar]
- (32).Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA, Gendler SJ, and Boons G-J (2012) Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. U. S. A 109, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, Burchell J, and Clausen H (2006) Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology 16, 96–107. [DOI] [PubMed] [Google Scholar]
- (34).Malekan H, Fung G, Thon V, Khedri Z, Yu H, Qu J, Li Y, Ding L, Lam K, and Chen X (2013) One-pot multi-enzyme (OPME) chemoenzymatic synthesis of sialyl-Tn-MUC1 and sialyl-T-MUC1 glycopeptides containing natural or non-natural sialic acid. Bioorg. Med. Chem 21, 4778–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kurosawa N, Takashima S, Kono M, Ikehara Y, Inoue M, Tachida Y, Narimatsu H, and Tsuji S (2000) Molecular cloning and genomic analysis of mouse GalNAc alpha 2,6-sialyltransferase (ST6GalNAc I). J. Biochem 127, 845–854. [DOI] [PubMed] [Google Scholar]
- (36).Liebe B, and Kunz H (1997) Solid-phase synthesis of a tumor-associated sialyl-T, antigen glycopeptide with a partial sequence of the “tandem repeat” of the MUC-1 mucin. Angew. Chem., Int. Ed. Engl 36, 618–621. [Google Scholar]
- (37).Sungsuwan S, Yin Z, and Huang X (2015) Lipopeptide coated iron oxide nanoparticles as potential glyco-conjugate based synthetic anti-cancer vaccines. ACS Appl. Mater. Interfaces 7, 17535–17544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Huang X, Huang L, Wang H, and Ye X-S (2004) Iterative one-pot oligosaccharide synthesis. Angew. Chem., Int. Ed 43, 5221–5224. [DOI] [PubMed] [Google Scholar]
- (39).Ye X-S, Huang X, and Wong C-H (2001) Conversion of the carboxy group of sialic acid donors to a protected hydroxymethyl group yields an efficient reagent for the synthesis of the unnatural beta-linkage. Chem. Commun, 974–975. [Google Scholar]
- (40).Boons G-J, and Demchenko AV (2000) Recent advances in O-sialylation. Chem. Rev 100, 4539–4566. [DOI] [PubMed] [Google Scholar]
- (41).Zhang X-T, Gu Z-Y, and Xing G-W (2014) Comparative studies on the O-sialylation with four different α/β-oriented (N-acetyl)-5-N,4-O-carbonyl-protected p-toluenethiosialosides as donors. Carbohydr. Res 388, 1–7 and references cited therein. [DOI] [PubMed] [Google Scholar]
- (42).Dhakal B, Buda S, and Crich D (2016) Stereoselective synthesis of 5-epi-α-sialosides related to the pseudaminic acid glycosides. Reassessment of the stereoselectivity of the 5-azido-5-deacetamidosialyl thioglycosides and use of triflate as nucleophile in the zbiral deamination of sialic acids.J. Org. Chem 81, 10617–10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).De Meo C, Farris M, Ginder N, Gulley B, Priyadarshani U, and Woods M (2008) Solvent effect in the synthesis of sialosyl α(2–6) galactosides: is acetonitrile the only choice? Eur.J. Org. Chem 2008, 3673–3677. [Google Scholar]
- (44).Glaffig M, Stergiou N, Hartmann S, Schmitt E, and Kunz H (2018) A synthetic MUC1 anticancer vaccine containing mannose ligands for targeting macrophages and dendritic cells. ChemMedChem 13, 25–29. [DOI] [PubMed] [Google Scholar]
- (45).Wu X, Ling C-C, and Bundle DR (2004) A new homobifunctional p-nitro phenyl ester coupling reagent for the preparation of neoglycoproteins. Org. Lett 6, 4407–4410. [DOI] [PubMed] [Google Scholar]
- (46).Nilsson J, Brinkmalm G, Ramadan S, Gilborne L, Noborn F, Blennow K, Wallin A, Svensson J, Abo-Riya MA, Huang X, and Larson G (2019) Synthetic standard aided quantification and structural characterization of amyloid-beta glycopeptides enriched from cerebrospinal fluid of Alzheimer’s disease patients. Sci. Rep 9, 5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Vacchelli E, Galluzzi L, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, and Kroemer G (2012) Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology 1, 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Thie H, Toleikis L, Li J, von Wasielewski R, Bastert G, Schirrmann T, Esteves IT, Behrens CK, Fournes B, Fournier N, de Romeuf C, Hust M, and Dübel S (2011) Rise and fall of an anti-MUC1 specific antibody. PLoS One 6, e15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Pett C, Cai H, Liu J, Palitzsch B, Schorlemer M, Hartmann S, Stergiou N, Lu M, Kunz H, Schmitt E, and Westerlind U (2017) Microarray analysis of antibodies induced with synthetic antitumor vaccines: specificity against diverse mucin core structures. Chem. - Eur. J 23, 3875–3884. [DOI] [PubMed] [Google Scholar]
- (50).Storr SJ, Royle L, Chapman CJ, Hamid UM, Robertson JF, Murray A, Dwek RA, and Rudd PM (2008) The O-linked glycosylation of secretory/shed MUC1 from an advanced breast cancer patient’s serum. Glycobiology 18, 456–462. [DOI] [PubMed] [Google Scholar]
- (51).Beatty P, Hanisch FG, Stolz DB, Finn OJ, and Ciborowski P (2001) Biochemical characterization of the soluble form of tumor antigen MUC1 isolated from sera and ascites fluid of breast and pancreatic cancer patients. Clin. Cancer Res. 7, 781s–787s. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.