Abstract
Background
Though patients with chronic rhinosinusitis without nasal polyps (CRSsNP) represent a majority of the CRS population, they have not been completely characterized phenotypically.
Objective
To perform a comprehensive phenotypic characterization of subjects with CRSsNP, using CRS with nasal polyps (CRSwNP) as a comparator.
Methods
Patients with a history of CRS with positive sinus CT (>18 y.o.) evaluated in the Allergy/Immunology or Otolaryngology clinics of an academic center between 2002-2012 were identified via ICD-9 codes. Retrospective chart review was performed on a subset of 507 patients with CRSsNP and 874 with CRSwNP. Characteristics analyzed included demographics, comorbid conditions, and radiologic sinus severity.
Results
Of the total CRS population, approximately 82% had CRSsNP and 18% had CRSwNP. 319/507 (63%) were female in the CRSsNP group compared to 393/847 (45%) in the CRSwNP group. Prevalence of atopy was 52% in CRSsNP versus 76% in CRSwNP (p<0.0001). In CRSsNP, atopic patients had more severe radiographic disease compared to non-atopic patients (p<0.005). Prevalence of asthma was 36% in CRSsNP versus 56% in CRSwNP (p<0.0001). Comorbid asthma was not associated with radiographic sinus disease severity in CRSsNP but was associated with severity in CRSwNP (p<0.0001).
Conclusion
The relative prevalence of CRS phenotypes in the Western population is approximately 80% CRSsNP and 20% CRSwNP. CRSsNP patients were predominantly female whereas CRSwNP patients were predominantly male. The prevalence of asthma was higher in our cohort of CRSsNP patients than previously described. Atopy was associated with more severe radiographic sinonasal disease in CRSsNP while asthma was not associated with radiographic sinonasal disease severity.
Keywords: Chronic Rhinosinusitis, Chronic Rhinosinusitis without Nasal Polyps, Nasal Polyps, Asthma, Allergic Rhinitis
Introduction
Chronic rhinosinusitis (CRS) is a common disease, impacting approximately 5 to 15 percent of the population in the United States and Europe (1). CRS is defined as persistent sinonasal symptoms lasting for at least 12 weeks along with objective findings of sinonasal inflammation via computed tomography (CT) scan of paranasal sinuses or nasal endoscopy (2, 3). CRS has been divided into two different phenotypes based on the presence of nasal polyps; CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). Although it is well accepted that the majority of patients with CRS do not have nasal polyps, the relative proportions of CRS phenotypes in the western population are not known, as there is a paucity of accurate epidemiologic literature on the prevalence of CRS phenotypes. This is believed to be due to heterogeneity in study methodologies and variations in the definition of CRS diagnosis (1).
CRSsNP and CRSwNP subgroups not only differ phenotypically, but also appear to have distinct pathogenesis and clinical presentations (1). On histological evaluation, the mucosal lining in patients with CRSsNP shows basement membrane thickening, goblet cell hyperplasia, limited subepithelial edema, prominent fibrosis, and mononuclear cell infiltration. In patients with CRSwNP, histologic evaluation shows frequent epithelial damage, basement membrane thickening, edematous and fibrotic stromal tissue, and reduced numbers of vessels and glands (4, 5). The inflammatory cell profile tends to be type 1 and type 2 in CRSsNP, while CRSwNP tends to have a type 2 skewed inflammatory profile with increased eosinophils (1, 4). However, recent studies have demonstrated heterogeneity in the cellular and molecular endotypes of disease (6, 7). Clinically, patients with CRSsNP generally tend to present with facial pain and purulent drainage, whereas those with CRSwNP present with symptoms of nasal obstruction and hyposmia/anosmia (4). There are extensive studies that phenotypically characterize subjects with CRSwNP or CRS as a whole, but CRSsNP alone has not been well characterized even though CRSsNP accounts for the majority of CRS.
The goal of this study is to perform a comprehensive phenotypic characterization of subjects with CRSsNP and, in doing so, compare patients with CRSsNP to those with CRSwNP to help elucidate differences in phenotypic expression that may reveal differences in underlying pathophysiology. Improved knowledge about phenotype can potentially catalyze improvements in disease treatments and outcomes. Furthermore, by utilizing objective diagnostic criteria, we provide accurate prevalence of CRS phenotypes in the western population.
Methods
Identification of Subjects
Patients with CRS were identified via the Enterprise Database Warehouse (EDW) at an academic hospital. The EDW is a large repository of all out-patient and inpatient health records of patients treated within the hospital system since 2001. Patients were identified based on one or more of the following criteria: (1) had at least one ICD-9 code for acute sinusitis (461.x); (2) had at least one ICD-9 code of chronic sinusitis (473.x); (3) had at least one ICD-9 code for nasal polyps (471.x); or (4) had at least one Current Procedural Terminology (CPT) code associated with surgery for chronic sinusitis or nasal polyps (30110, 30115, 31254, 31255, 31256, 31267, 31287, 31288, 31296, 31297). The EDW provided the authors with a random list of patients meeting these criteria.
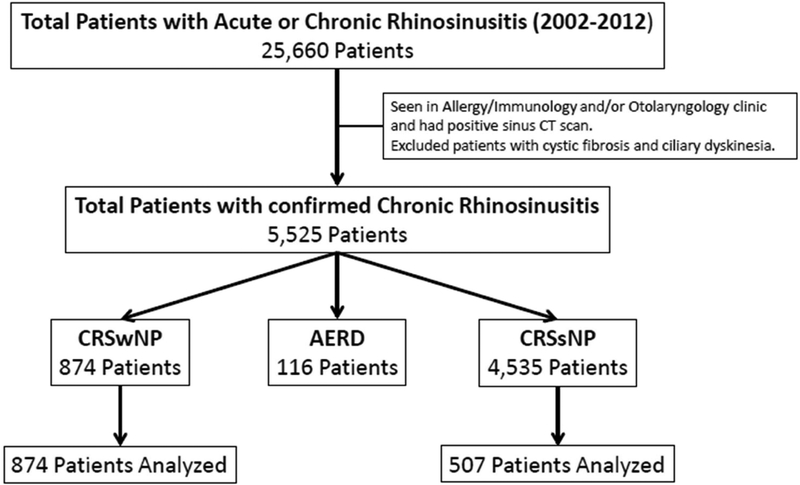
Within this cohort, we identified patients with chronic rhinosinusitis with and without nasal polyps who were treated in the Allergy/Immunology or Otolaryngology clinics at our academic outpatient center between 2002 and 2012 (Figure 1). We then manually reviewed the medical records of 507 patients with CRSsNP and 847 patients with CRSwNP who were randomly selected by three of the authors. These authors started in non-overlapping locations of the list provided by the EDW and then reviewed consecutive patients. Characteristics analyzed were age, gender, ethnicity, allergic rhinitis, radiologic sinus severity, number of sinus surgeries, diagnosis of asthma, and history of comorbid or autoimmune disease. Of note, data including some of the same CRSwNP subjects has been previously published by Stevens et. al. (8). This review was approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine.
Figure 1:
Algorithm for patient selection. Criteria for confirmed diagnosis of chronic rhinosinusitis is detailed in the methods section.
Abbreviations: AERD, Aspirin-Exacerbated Respiratory Disease; CRSsNP, Chronic Rhinosinusitis without Nasal Polyps; CRSwNP, Chronic Rhinosinusitis with Nasal Polyps.
Confirmation of CRS Diagnosis
All subjects had an abnormal sinus CT scan that was obtained upon initial evaluation in the otolaryngology or allergy clinic and met criteria for CRS as defined by internationally recognized consensus statements (1, 9). Patients were classified as CRSsNP or CRSwNP based on results of rhinoscopy or surgical pathology specimens. Patients with aspirin-exacerbated respiratory disease were excluded from the comparison analyses of CRSwNP patients versus CRSsNP patients, but were included in epidemiologic data of CRS phenotypes.
Determination of sinonasal disease severity
Radiologic sinus score was determined by the interpretation of sinus mucosal thickening on sinus CT imaging by clinical radiologists who were not affiliated with this study. The images were scored on a scale of 1 to 5 as mild (1), mild-moderate (2), moderate (3), moderate-severe (4), and severe (5). For several analyses these scores were further separated into the categories of mild (including scores between 1 to 2) and moderate to severe (including scores between 3 to 5).
Identification of subjects with allergic rhinitis
Allergic rhinitis was determined if the diagnosis was documented in the medical record by the treating allergist or otolaryngologist.
Identification of subjects with asthma and determination of asthma severity
Asthma diagnosis and severity were determined using the 2007 NAEPP Expert Panel Report 3 guidelines using FEV1% values (10).
Statistical analysis
All statistical calculations were performed using GraphPad Prism v7.04 (Graphpad Software, Inc., La Jolla, CA). Associations between CRSsNP and CRSwNP subgroup categorical variables were tested using chi-squared tests. The Mann Whitney test was used to compare continuous values including the mean radiologic sinus score and FEV1 values. The CRSsNP and CRSwNP groups were further subdivided into those with asthma and those without, and associations between these subgroups were examined using the Kruskal-Wallis test.
Results
Prevalence of CRS Phenotypes
The initial EDW search for acute or chronic sinusitis identified a population of 25,660 patients. Out of these patients, 4,535 patients (82%) fulfilled diagnostic criteria for CRSsNP and 990 patients (18%) for CRSwNP. Of those with CRSwNP, 116 patients were excluded from further analyses due to history of aspirin-exacerbated respiratory disease. A total of 507 patients with CRSsNP and 874 patients with CRSwNP were evaluated.
Baseline Characteristics
The mean age was similar between the two groups (Table 1). Patients with CRSsNP were predominantly female, compared to CRSwNP patients who were predominantly male (63% female vs. 45% female, respectively, p<0.0001). The prevalence of allergic rhinitis was significantly higher in patients with CRSwNP than those with CRSsNP (Table 1). Physician diagnosis of allergic rhinitis was not available in the medical records for 81 patients with CRSsNP and 133 patients with CRSwNP.
Table 1:
Baseline characteristics of subjects with chronic rhinosinusitis without nasal polyps (CRSsNP) and chronic rhinosinusitis with nasal polyps (CRSwNP).
| CRSsNP (n = 507) | CRSwNP (n = 874) | p value | |
|---|---|---|---|
| Mean Age (yrs ± SD) | 50.77 ± 14.21 | 50.35 ± 14.42 | 0.59 |
| Female Sex - no. (%) | 319 (63) | 393 (45) | <0.0001 |
| Current or Former Smoker - no. (%) | 143 (28) | 231 (26) | 0.47 |
| Asthma – no. (%) | 183 (36) | 490 (56) | <0.0001 |
| Allergic Rhinitis – no. (%) * | 216 (52) | 521 (76) | <0.0001 |
| FEV1 Percent Predicted, Prebronchodilation (mean ± SD) † | 83.17 ± 20.51 | 83.60 ± 17.92 | 0.76 |
| Number of Sinus Surgeries – (mean ± SD) | 0.94 ± 0.97 | 1.42 ± 1.56 | <0.0001 |
| Radiologic Sinus CT Score – (mean ± SD) ‡ | 1.84 ± 1.05 | 3.08 ± 1.42 | <0.0001 |
n = 412 for CRSsNP, n = 685 for CRSwNP
n = 162 for CRSsNP, n = 338 for CRSwNP
n = 474 for CRSsNP, n = 751 for CRSwNP
Asthma Prevalence and Severity
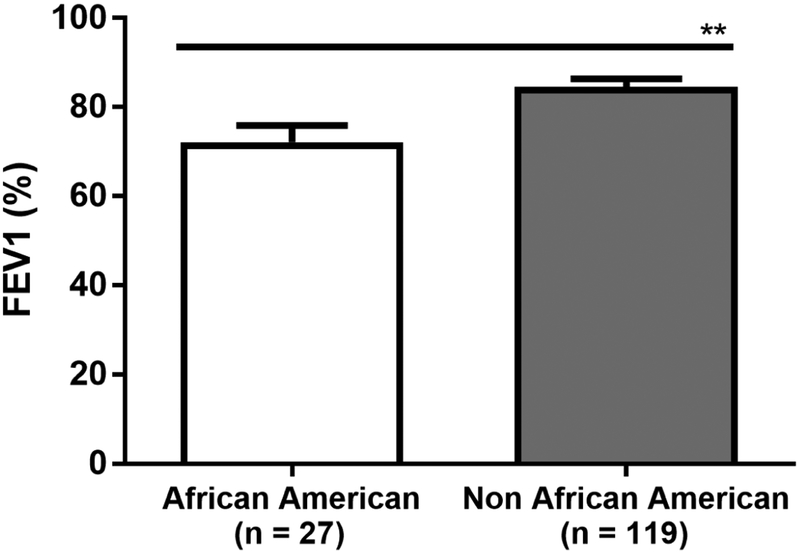
The prevalence of asthma in CRSsNP was 36%, versus 56% in those with CRSwNP (p<0.0001) (Table 1). Severity of asthma, as measured by FEV1 percent predicted, was similar between CRSsNP and CRSwNP patients (Table 1). There was an increased prevalence of allergic rhinitis by physician diagnosis in patients with comorbid asthma compared to those without asthma in both CRSsNP (68% vs 42%, p<0.0001) and CRSwNP (82% vs 66%, p=0.0007). Though the majority of CRSsNP patients were female, the prevalence of asthma was similar in females and males with CRSsNP (38% vs 32% respectively, p=0.17). The severity of asthma, as measured by FEV1 percent predicted, was also similar in females and males with CRSsNP (82.3 ± 22.4% vs 83.1 ± 19.5% respectively, p=0.71). In contrast in patients with CRSwNP, females were more likely than males to have comorbid asthma (67% vs 47% respectively, p<0.0001). Additionally, although African American patients with CRSsNP had similar sinonasal disease severity radiographically compared to Non-African American patients (radiologic sinus score of 1.93 ± 1.15 vs 1.81 ± 1.04 respectively, p=0.438), they had significantly worse lung function, as measured by mean FEV1 percent predicted, compared to Non-African American patients (72.15 ± 19.28% vs 84.51 ± 20.11% respectively, p=0.0018) (Figure 2).
Figure 2:
Forced expiratory volume in one second (FEV1) percent predicted in African American patients compared to Non-African American patients with CRSsNP. Statistical significance determined by Mann Whitney test, P = 0.002.
Sinus Disease Severity
The number of sinus surgeries was significantly higher in those with CRSwNP compared to CRSsNP (Table 1). Patients with CRSsNP with or without comorbid asthma had a similar number of sinus surgeries in their history (0.93 ± 1.0 vs 0.96 ± 0.91 respectively). Conversely those with CRSwNP with comorbid asthma had undergone significantly more surgeries than those with CRSwNP without asthma (1.64 ± 1.67 vs 1.15 ± 1.35, respectively, p<0.0001).
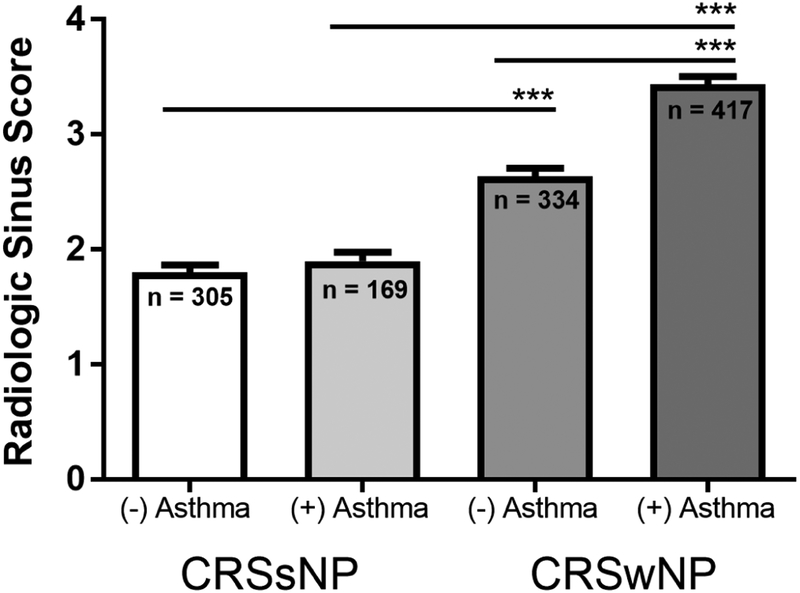
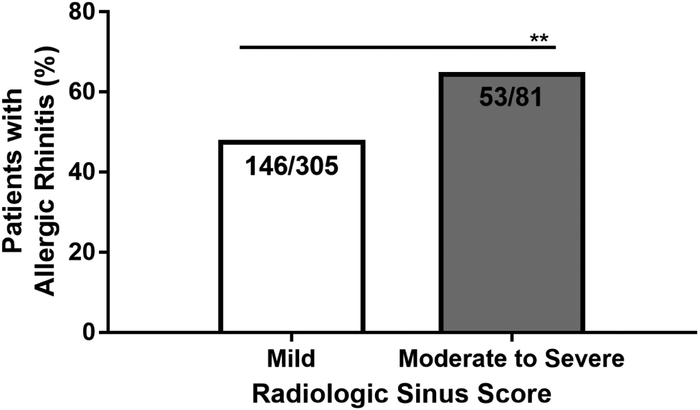
The severity of sinus disease was evaluated using the radiologic sinus CT score. Patients with CRSwNP had significantly higher scores than those with CRSsNP (Table 1). There was no significant difference in mean radiologic sinus score between males and females with CRSsNP (1.82 ± 1.06 in males vs 1.87 ± 1.06 in females, p>0.05). In CRSwNP, the mean radiologic sinus score was slightly higher in women than men, but this did not reach statistical significance (2.99 ± 1.38 in males vs 3.19 ± 1.47 in females, p=0.0501). The radiologic sinus score was not significantly affected by comorbid asthma in CRSsNP (mean CT severity score of 1.8 vs 1.9) (Figure 3). Conversely, having a diagnosis of asthma was significantly associated with increased radiologic sinus disease severity in those with CRSwNP (mean CT severity score of 2.5 vs. 3.3, p<0.0001). There was a greater prevalence of comorbid allergic rhinitis in patients with CRSsNP with moderate to severe radiologic sinus disease severity (53/81 (65%)) compared to those with mild radiologic sinus disease severity (146/305 (48%)) (p=0.0049) (Figure 4). CRSsNP patients who were current or former smokers accounted for 31% of those with mild/moderate radiologic sinus scores (106/345) and 35% of those with moderate/severe radiologic sinus scores (37/106) (p=0.42).
Figure 3:
Radiologic sinus score in patients with chronic rhinosinusitis without nasal polyps (CRSsNP) and chronic rhinosinusitis with nasal polyps (CRSwNP), subdivided by presence of comorbid asthma. Statistical significance determined by Kruskal-Wallis test, *** P<0.001.
Figure 4:
Prevalence of comorbid allergic rhinitis in patients with chronic rhinosinusitis without nasal polyps of either mild or moderate to severe status. Mild indicates radiologic sinus scores of 1-2 and moderate to severe indicates scores of 3-5. Statistical significance determined by Chi-square test, ** P<0.005.
Prevalence of Other Comorbid Conditions
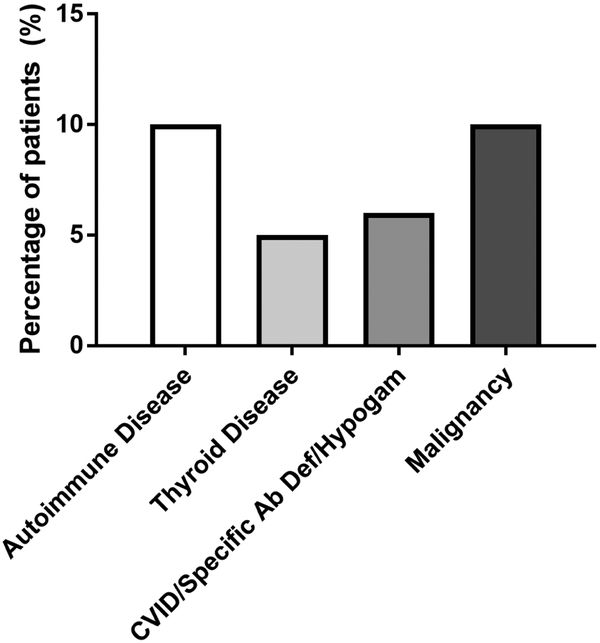
Physician diagnosis of comorbid conditions was also evaluated in CRSsNP only (Figure 5). Autoimmune disease was defined broadly as any pathologic condition thought to be caused by an adaptive autoimmune response directed against a self-antigen, excluding thyroid disease which was evaluated separately. In this population, 48/505 patients (10%) were diagnosed with an autoimmune disease. An additional 24/505 patients (5%) were diagnosed with a thyroid disorder, including hypo- or hyperthyroidism. Humoral immunodeficiency, including common variable immune deficiency, hypogammaglobulinemia or specific antibody deficiency, was documented in 31/505 patients (6%). Finally, history of malignancy, solid or hematologic, was noted in 52/505 patients (10%) with CRSsNP.
Figure 5:
Physician diagnosis of comorbid conditions in patients with chronic rhinosinusitis without nasal polyps.
Abbreviations: CVID, Common Variable Immunodeficiency; Specific Ab Def, Specific Antibody Deficiency; Hypogam, Hypogammaglobulinemia.
Discussion
The landmark European Position Paper on Rhinosinusitis and Nasal polyps published in 2012 highlighted the need for epidemiologic research exploring the prevalence and incidence of CRS phenotypes (1). This retrospective chart review of patients with CRS addresses this paucity of accurate epidemiologic data on prevalence of CRS phenotypes. All charts were manually reviewed to confirm that the diagnosis of CRS was accurate and based on objective evaluation by CT scan and often endoscopy. Based on this large cohort of patients with CRS, we estimate the prevalence of CRSsNP and CRSwNP to be approximately 80% and 20% respectively in the United States. To our knowledge, there is one additional study with fewer patients that used objective evaluation and found a similar prevalence of CRS subtypes (11).
This study, which presents data on the largest cohort of CRSsNP patients characterized to date, is unique in that it includes both medically and surgically managed patients, whereas most prior studies focused only on surgical cohorts. Surprisingly, even though CRSsNP accounts for the majority of patients with CRS, much of the literature focuses on patients with CRSwNP. By utilizing our large cohort to compare CRSsNP patients to CRSwNP patients, we were able to elucidate important differences that may have an impact in management of these conditions. This is important as CRSsNP presents a greater burden due to its much higher prevalence.
Previous studies have shown that CRSwNP is more prevalent in males than females, and our findings confirm this conclusion (12, 13). Interestingly, the majority of patients with CRSsNP in our cohort were females. There was a higher percentage of females (63%) in this population than expected from prior surgical studies which have shown a prevalence of 53-55% (12, 14). Women with CRSsNP have been shown to report a greater symptom burden and worse quality of life than men, though the reason for this disparity is unclear (15). The severity of sinus disease as measured by CT scan and the severity of asthma as measured by FEV1 percent predicted were similar between males and females with CRSsNP in this cohort.
The prevalence of asthma among patients with CRSsNP in this cohort was 36%, which is higher than expected from previously published cohorts. Stevens et. al. reported an asthma prevalence of 24.3% in a surgical cohort of CRSsNP patients, as documented in the medical record by the operating otolaryngologist (12). Promsopa et. al. reported a 16.5% prevalence of asthma in a CRSsNP population from an otolaryngology clinic. Their diagnosis of asthma was based on the results of pulmonary function testing alone, though the criteria for diagnosis was not clear and may have missed some of those with disease not detected on baseline lung function testing (16). Batra et. al. showed a 22% prevalence of asthma in a group of CRSsNP patients who were undergoing revision endoscopic sinus surgery and had failed maximal medical therapy, and thus likely not truly representing the prevalence in the whole CRSsNP population (14). Finally, also at our institution, Tan et. al. also evaluated a small group of 63 CRSsNP patients who had failed medical therapy and subsequently had undergone endoscopic sinus surgery and found a 30.2% prevalence of asthma (17). Some of these same patients potentially are included in this retrospective review. Patients in surgical cohorts may be less likely to have diagnosed asthma as they may not have had a comprehensive evaluation for asthma or severe asthma may preclude them from being considered for surgery. These data have significant clinical implications, as patients with asthma and comorbid CRS tend to have lower lung function as measured by FEV1, increased asthma symptoms and worse quality-of-life scores than those with asthma alone (18). In a study of patients with severe exacerbation-prone asthma, Delinger et. al. found that comorbid CRS was associated with increased frequency of asthma exacerbations (19). Thus, the association between asthma and CRS is not only of pathological relevance but may also be of value in clinical decision making.
A diagnosis of comorbid asthma did not affect radiologic disease severity in CRSsNP patients in our population but was associated with more severe disease in the CRSwNP group. Additionally, African American patients with CRSsNP had more severe asthma as measured by FEV1 despite having similar sinonasal disease. Previously published data has shown that CRS patients with asthma had significantly higher endoscopy and sinus CT severity scores than those without asthma, along with a strong relationship between asthma severity and increasing radiologic severity of sinus disease (14, 20-22). These studies often did not separate CRS into the CRSsNP and CRSwNP subtypes and usually used the Lund-Mackay scoring system in contrast to the scoring system used in this study, which was based upon radiologist interpretation of disease severity.
The prevalence of atopy in CRSsNP patients has previously been reported to be between 22-79% (14, 17, 23). Allergic rhinitis was diagnosed in a little over half of the patients with CRSsNP in the present population. These patients had a higher prevalence of severe sinus disease radiographically compared to non-atopic patients with CRSsNP. Robinson et. al. studied the relationship between atopy and CRS in a small group of CRS patients who were scheduled for endoscopic sinus surgery. They noted a higher mean CT score in atopic patients than nonatopic patients when evaluating all CRS patients, but when they analyzed CRSsNP and CRSwNP groups separately, this difference disappeared (23). Two other studies also found a higher prevalence of severe sinus CT scores in CRS patients with comorbid allergies, but they did not analyze CRSwNP or CRSsNP clinical subgroups (14, 24). On the other hand, other studies have shown no significant difference between the mean Lund-Mackay score in atopic versus nonatopic CRSsNP patients (17, 25).
CRSsNP was previously thought to be characterized by type 1 inflammation on the basis of elevation in interferon-gamma (IFN-γ) levels seen in early studies (26). We found a high prevalence of comorbid type 2 diseases, such as asthma and allergic rhinitis, in our CRSsNP population. Recent data has supported that type 2 inflammation is also important in the pathogenesis of CRSsNP. Tan et. al. showed that the overall frequency of type 2 inflammation (elevated levels of ECP, IL-5, IL-13) in sinus tissue from patients with CRSsNP was approximately 40%, which was higher than the frequency of type 1 inflammation seen (around 20%) (7).
The prevalence of autoimmune disease in the general population is estimated to be between 7.6-9.4% (27). In the group of CRSsNP patients studied here, there was a prevalence of around 15%, if thyroid disease was included. There were a few patients with granulomatosis with polyangiitis, which is known to be associated with sinus disease, but this did not account for most of the autoimmune conditions seen. A previous study evaluating the prevalence of CRS in patients with autoimmune conditions compared to patients with hypertension showed that only patients with inflammatory bowel disease had a significantly greater prevalence of CRS (28). The association between autoimmune disease and CRS is not known, but barrier dysfunction associated with autoimmune inflammation or skewing of the underlying Th1/Th2 status may contribute to CRS pathology (29). In patients with CRSwNP, there is evidence that autoantibodies, particularly those against nuclear antigens, are increased locally in nasal polyp tissue (30). There is less data for autoantibodies in CRSsNP, though one study did report increased IgA class anti-cytokine autoantibodies, which are of unclear pathophysiologic significance (31).
The prevalence of humoral immunodeficiency in our CRSsNP population was 6%. A recent study done by Keswani et. al. looked retrospectively at patients with CRS who had been evaluated for humoral immunodeficiency and found a diagnosis of CVID in 5.6% of patients (32). Additionally, a meta-analysis by Schwitzguebel et. al. evaluated the prevalence of immunoglobulin deficiencies in patients with CRS and showed pooled IgG, IgA, and IgM deficiencies in 13% of patients with recurrent CRS and 23% of patients with difficult-to-treat CRS (33). The prevalence in our cohort may have been lower as we evaluated the whole CRS population, not just those who were being screened for immunodeficiency.
There are several limitations in this study. The data is retrospective based on chart review. There was not a specific process of patient randomization, which may have led to some bias. Additionally, given that most patients were evaluated in the allergy clinic, the prevalence of allergic rhinitis and asthma may be affected by selection bias. The amount of data that was available for each subject was variable based on the frequency of clinic visits and the detail of documentation by the treating physicians. All patients seen in the allergy clinic at our institution underwent skin prick testing to confirm the diagnosis of allergic rhinitis, but those patients only seen in otolaryngology clinic may have had skin prick testing at an outside institution, the results of which were not available for review. For measurement of sinus disease severity, we used a radiology-based scoring system that has not been validated but has nonetheless been utilized in other CRS studies within our institution (8, 12). We believe this system allows for a more expanded classification of disease severity than the most commonly used Lund-Mackay scoring method which does not delineate between degrees of partial sinus opacification. We also reported that 10% of our CRSsNP patients were diagnosed with malignancy, but we do not know the rate of malignancy in the general EDW population and thus are not able to make a comparison. Due to the design of this study, we also were not able to establish the temporality of diagnosis of comorbid conditions, such as malignancy, relative to the diagnosis of CRS. Finally, the patients in this study were all seen at an academic institution and may not be representative of some non-academic settings. However, due to the location of our institution, the majority of our patients are not tertiary care referrals and include a significant number of patients who are self-referrals or are referred directly by primary care physicians.
Conclusion
In summary, CRSsNP and CRSwNP are distinct subgroups of CRS which differ in clinical presentation and pathophysiology. By utilizing objective diagnostic criteria, we were able to provide accurate epidemiologic data regarding the relative prevalence of patients with CRSsNP and CRSwNP phenotypes in the United States. This study confirms and further extends our understanding of heterogeneity of disease among patients with CRS, specifically those with CRSsNP. There is a higher prevalence of comorbid asthma in patients with CRSsNP than previously described and therefore, physicians treating patients with CRSsNP need to be more aware of this strong association and consider screening for asthma. In addition, based upon our study of this large cohort of patients with CRSsNP, allergic rhinitis appears to affect sinus disease severity. Although the prevalence of comorbid autoimmune diseases in CRSsNP was higher than expected, the contribution of these comorbidities to CRS disease severity is not known. Future studies are needed to evaluate the impact of molecular endotypes, as well as examine the effect of comorbidities such as asthma, allergic rhinitis, humoral immunodeficiency and autoimmune diseases, on the clinical expression and severity of CRSsNP.
Highlights box:
What is already known about this topic?
Chronic rhinosinusitis (CRS) is a common chronic disease that can be phenotypically divided into two subgroups based on presence of nasal polyps: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).
What does the article add to our knowledge?
Prior to this study, the prevalence of CRS phenotypes was not well described. This study comprehensively characterized one of the largest cohorts of patients with CRSsNP to date, and compared them to patients with CRSwNP.
How does this study impact current management guidelines?
Understanding the phenotypic characteristics of patients with CRSsNP will assist physicians in management of this subgroup of CRS that accounts for the greatest burden of sinonasal disease in the western population.
Acknowledgments
Funding Source: Ernest S. Bazley Trust, U19 AI1016683
Abbreviations:
- AERD
Aspirin-Exacerbated Respiratory Disease
- CRS
Chronic Rhinosinusitis
- CRSsNP
Chronic Rhinosinusitis without Nasal Polyps
- CRSwNP
Chronic Rhinosinusitis with Nasal Polyps
- CT
computed tomography
- CVID
Common Variable Immunodeficiency
- EDW
Enterprise Database Warehouse
- FEV1
Forced Expiratory Volume in One Second
- Hypogam
Hypogammaglobulinemia
- Specific Ab Def
Specific Antibody Deficiency
Footnotes
Conflicts of Interest:
L. C. Grammer reports grants from NIH, grants from Bazley Foundation, during the conduct of the study; reports personal fees from consultancy with Astellas Pharmaceuticals, lectures including service on speakers’ bureaus from AAAAI, Mount Sinai, New York, NY, grants from NIH, Food Allergy Network and Bazley Foundation, and personal fees from Lippincott, UpToDate, BMJ, Elsevier, Kluwers Wolter, outside the submitted work. R. C. Kern reports serving as a consultant for Sanofi and 480 Biomedical, outside the submitted work. B. K. Tan reports grants from NIH, personal fees from Optinose, Inc, personal fees from Sanofi, and a patent pending for Treatment of Eosinophilic Inflammatory Disease pending, outside the submitted work. R. P. Schleimer Dr. Schleimer reports grants from NIH during the conduct of the study; reports personal fees from serving as a consultant for Intersect ENT, GlaxoSmithKline, Allakos, Aurasense, Merck, BioMarck, Sanofi, AstraZeneca/Medimmune, Genentech, Exicure Inc, Otsuka Inc, Aqualung Therapeutics Corp, ActoBio Therapeutics, outside the submitted work. In addition, R. P. Schleimer has Siglec-8 and Siglec-8 ligand related patents licensed to Allakos Inc. A. T. Peters previously served as a consultant for Sanofi-Regeneron. The rest of the authors declare that they have no relevant conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Not applicable
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;23:3 p preceding table of contents, 1-298. [PubMed] [Google Scholar]
- 2.Bhattacharyya N, Fried MP. The accuracy of computed tomography in the diagnosis of chronic rhinosinusitis. Laryngoscope. 2003;113(1):125–9. [DOI] [PubMed] [Google Scholar]
- 3.Wuister AM, Goto NA, Oostveen EJ, de Jong WU, van der Valk ES, Kaper NM, et al. Nasal endoscopy is recommended for diagnosing adults with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2014;150(3):359–64. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg. 2004;131(6 Suppl):S1–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ocampo CJ, Grammer LC. Chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2013;1(3):205–11; quiz 12-3. [DOI] [PubMed] [Google Scholar]
- 6.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–56 e4. [DOI] [PubMed] [Google Scholar]
- 7.Tan BK, Klingler AI, Poposki JA, Stevens WW, Peters AT, Suh LA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2017;139(2):699–703 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens WW, Peters AT, Hirsch AG, Nordberg CM, Schwartz BS, Mercer DG, et al. Clinical Characteristics of Patients with Chronic Rhinosinusitis with Nasal Polyps, Asthma, and Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2017;5(4):1061–70 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlandi RR, Kingdom TT, Hwang PH. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis Executive Summary. Int Forum Allergy Rhinol. 2016;6 Suppl 1:S3–21. [DOI] [PubMed] [Google Scholar]
- 10.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharyya N Clinical and symptom criteria for the accurate diagnosis of chronic rhinosinusitis. Laryngoscope. 2006;116(7 Pt 2 Suppl 110): 1–22. [DOI] [PubMed] [Google Scholar]
- 12.Stevens WW, Peters AT, Suh L, Norton JE, Kern RC, Conley DB, et al. A retrospective, cross-sectional study reveals that women with CRSwNP have more severe disease than men. Immun Inflamm Dis. 2015;3(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins MM, Pang YT, Loughran S, Wilson JA. Environmental risk factors and gender in nasal polyposis. Clin Otolaryngol Allied Sci. 2002;27(5):314–7. [DOI] [PubMed] [Google Scholar]
- 14.Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope. 2013;123 Suppl 7:S1–11. [DOI] [PubMed] [Google Scholar]
- 15.Lal D, Rounds AB, Divekar R. Gender-specific differences in chronic rhinosinusitis patients electing endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016;6(3):278–86. [DOI] [PubMed] [Google Scholar]
- 16.Promsopa C, Kansara S, Citardi MJ, Fakhri S, Porter P, Luong A. Prevalence of confirmed asthma varies in chronic rhinosinusitis subtypes. Int Forum Allergy Rhinol. 2016;6(4):373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan BK, Zirkle W, Chandra RK, Lin D, Conley DB, Peters AT, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1(2):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ek A, Middelveld RJ, Bertilsson H, Bjerg A, Ekerljung L, Malinovschi A, et al. Chronic rhinosinusitis in asthma is a negative predictor of quality of life: results from the Swedish GA(2)LEN survey. Allergy. 2013;68(10):1314–21. [DOI] [PubMed] [Google Scholar]
- 19.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoover GE, Newman LJ, Platts-Mills TA, Phillips CD, Gross CW, Wheatley LM. Chronic sinusitis: risk factors for extensive disease. J Allergy Clin Immunol. 1997;100(2):185–91. [DOI] [PubMed] [Google Scholar]
- 21.Newman LJ, Platts-Mills TA, Phillips CD, Hazen KC, Gross CW. Chronic sinusitis. Relationship of computed tomographic findings to allergy, asthma, and eosinophilia. JAMA. 1994;271(5):363–7. [DOI] [PubMed] [Google Scholar]
- 22.Lin DC, Chandra RK, Tan BK, Zirkle W, Conley DB, Grammer LC, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25(4):205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol. 2006;20(6):625–8. [DOI] [PubMed] [Google Scholar]
- 24.Ramadan HH, Fornelli R, Ortiz AO, Rodman S. Correlation of allergy and severity of sinus disease. Am J Rhinol. 1999;13(5):345–7. [DOI] [PubMed] [Google Scholar]
- 25.Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23(2): 145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11): 1280–9. [DOI] [PubMed] [Google Scholar]
- 27.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33(3-4):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandra RK, Lin D, Tan B, Tudor RS, Conley DB, Peters AT, et al. Chronic rhinosinusitis in the setting of other chronic inflammatory diseases. Am J Otolaryngol. 2011;32(5):388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017;12:331–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128(6): 1198–206 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsybikov NN, Egorova EV, Kuznik BI, Fefelova EV, Magen E. Anticytokine autoantibodies in chronic rhinosinusitis. Allergy Asthma Proc. 2015;36(6):473–80. [DOI] [PubMed] [Google Scholar]
- 32.Keswani A, Dunn NM, Manzur A, Kashani S, Bossuyt X, Grammer LC, et al. The Clinical Significance of Specific Antibody Deficiency (SAD) Severity in Chronic Rhinosinusitis (CRS). J Allergy Clin Immunol Pract. 2017;5(4): 1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwitzguebel AJ, Jandus P, Lacroix JS, Seebach JD, Harr T. Immunoglobulin deficiency in patients with chronic rhinosinusitis: Systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523–31. [DOI] [PubMed] [Google Scholar]