Abstract
Purpose:
This study tested the feasibility and efficacy of using a text-based intervention to increase initiation, decrease discontinuation, and improve adherence as prescribed to adjuvant hormone therapy (AHT) among hyphenate post-menopausal breast cancer survivors.
Methods:
The 3-month intervention consisted of daily text message reminders to take medication, coupled with a dynamic (eg, feedback on progress) tailored intervention using weekly interactive surveys delivered by a smartphone app. Five clinic sites within the Alliance for Clinical Trials in Oncology participated. Hormone levels were measured prior to AHT initiation and at study exit.
Results:
Of the 39 patients recruited to the pilot study, 27 (69.2%) completed all study requirements (completed both the baseline and the exit surveys, both blood draws, and did not miss more than 2 weekly surveys). Significant improvements were observed pre- to postintervention for self-reported medication adherence (P = .015), mental health functioning (P = .007), and perceived stress (P = .04). Significant decreases in estradiol, estrogen, and estrone hormone levels were observed from baseline to study exit (P < .001), indicating the accuracy of self-reported AHT adherence. Participants (91.9%) and physicians (100%) agreed that participant participation in the intervention was beneficial.
Conclusions:
The results of this pilot study established the general feasibility and efficacy of an app-based intervention to support patient AHT adherence. Larger controlled, randomized trials are needed to examine the effectiveness of the app-based intervention in improving AHT and quality of life among breast cancer survivors.
Keywords: adjuvant hormone therapy, mobile apps, breast cancer, intervention
Introduction
Breast cancer is the most common cancer diagnosed in women and is the second leading cause of cancer deaths in women in the United States.1 In the United States in 2018, there were over 266,120 new cases of female breast cancer diagnosed and approximately 40,920 deaths from breast cancer.1 Considerable progress has been made in the recent decades in the detection, prevention, and treatment of cancer. One of the most important additions to breast cancer treatment has been adjuvant hormone therapy (AHT), including tamoxifen and aromatase inhibitors.2 The continued use of AHT is critical to the transition from active treatment to survivorship care, because it has been shown to reduce disease recurrence and mortality among patients having breast cancer with hormone receptor-positive disease.3,4
Despite proven clinical efficacy of AHT, evidence from previous studies suggests up to 50%5-8 of patients do not complete treatment as recommended. Murphy et al reported prevalence of adherence ranged from 41% to 72% and discontinuation ranged from 31% to 73% across the included studies.9 Discontinuation rates vary by type of AHT, with rates being higher for tamoxifen than those for aromatase inhibitors.10 Previous studies have found a variety of factors including extremes of age, being non-white, married, receipt of a lumpectomy versus mastectomy, presence of comorbidities, higher out-of-pocket costs, history of switching AHT type, and negative beliefs about efficacy of treatment as being associated with nonadherence to AHT.2,9,11-13 It is important to not only identify factors associated with nonadherence but also explore new methods to increase and maintain adherence to AHT.
One strategy to improve AHT nonadherence is the use of technology-based interventions. In recent years, increasing mobile phone ownership has made mobile phones a promising tool to improve the implementation and delivery of evidence-based health-care interventions.14,15 In a meta-analysis by Thakkar and colleagues,15 mobile phone text messaging was found to approximately double the odds of medication adherence among patients with chronic disease. Mobile phones with Internet capability (eg, smartphones) can also access software applications that offer patients a range of medication information through customized videos, assessments, data logs, reminders, monitoring, and interactivity with their health-care provider(s).14,16 Wilcox et al recently reported that their interactive tool for medication tracking was important in not only fostering patient participation in their own care but also improved patient–provider communication.
In the cancer context, there is a recent proliferation of efforts in the literature to develop smartphone app-based medication adherence interventions.17,18 Ali and colleagues18 found that among respondents with smartphones, 66% were interested in an app for AHT adherence, and all valued the inclusion of education and behavioral interventions in an AHT adherence app. Mougalian et al19 reported a positive reaction to their text-message application that simultaneously tracked AHT adherence, recorded symptoms, and alerted the clinical team for patient follow-up. However, more research is needed to examine daily adherence to AHT and potential changes in adherence over time in relation to symptoms, health-related quality of life, social support, and other factors known to influence AHT adherence.2,9,11-13 Thus, this study sought to test the feasibility and effectiveness of using a text-based intervention to increase initiation, decrease discontinuation, and improve adherence as prescribed to AHT among postmenopausal women with breast cancer.
Methods
Recruitment
This pilot study involved 5 sites within the Alliance for Clinical Trials in Oncology (ALLIANCE) including the Ohio State University Comprehensive Cancer Center (OSUCCC), the University of Vermont Medical Center, the Wake Forest University Comprehensive Cancer Center, the Southeast Medical Oncology Center in North Carolina, and Novant Health Oncology Specialists, North Carolina. Patients were eligible if they were (1) female adults aged 18 or older; (2) post-menopausal; (3) diagnosed with primary breast cancer (stages 0-III); (4) eligible to receive AHT (tamoxifen or an aromatase inhibitor) for the first time; (5) completed all primary treatment; (6) smartphone owners; (7) agreeable to receiving text messages on their smartphone over a 3-month period; and (8) willing to provide consent and permission to review their medical records.
At all sites, recruitment occurred after the end of initial treatment and during the clinic visit prior to the initiation of AHT. Recruitment began in October 2014, and follow-up with patients ended in June 2016. Potential participants were screened by designated staff members at each study site via an eligibility form. Oncologists or a research staff member (based on provider preference) provided a brief study introduction and inquired about the patients’ interest in participating. If a patient expressed interest, the study was explained, and the patient was given an opportunity to ask questions. Once the patient’s questions were answered and they met all eligibility criteria, a written informed consent was obtained. Finally, the study coordinator at the coordinating center registered all patients into one database. The study protocol was approved by all study sites’ institutional review board. The authors confirm that all ongoing and related trials for this intervention are registered.
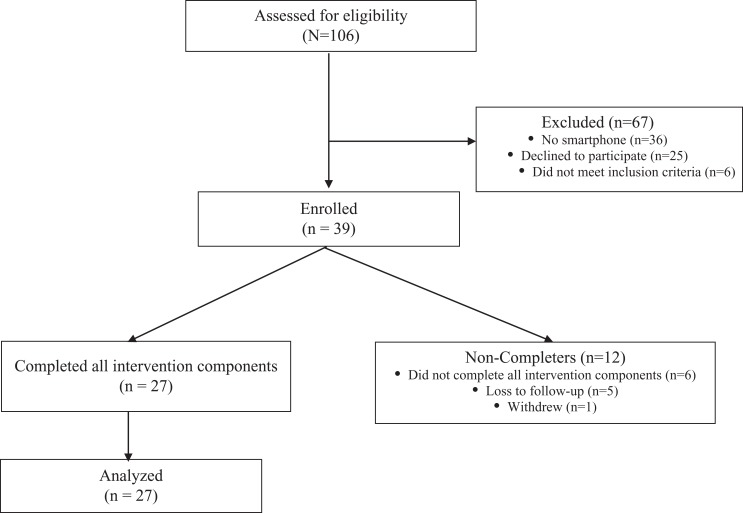
After signing the informed consent, participants completed a blood draw and viewed a DVD video in the clinic setting about the importance of adhering to the medication and communicating with their health-care team about symptoms, challenges associated with, and barriers to adherence. The video also taught participants how to use PACE (Presenting detailed information, Asking questions, Checking your understanding, and Expressing concerns) communication skills with their health-care provider. PACE focuses on training patients to effectively communicate with health-care providers and has been successfully tested in previous studies.20,21 In addition, participants received training on the text messaging system and smartphone app. Finally, the participants were asked to complete a blood draw to document changes in estradiol, estrogen, and estrone hormone levels while taking the AHT medication. All of these activities occurred prior to AHT initiation. The schema of the study recruitment is presented in Figure 1.
Figure 1.
CONSORT diagram.
Questionnaires
After the clinic visit, participants were contacted to schedule a date and time for a baseline phone interview conducted by a trained interviewer at the OSUCCC. The baseline interview was conducted as closely as possible to the initiation of AHT and was completed over the phone due to time constraints. The length of time to administer the questionnaire ranged from 40 minutes to 1 hour. The measures assessed in the interview were as follows:
Physical symptoms
Physical symptoms and side effects associated with the treatment and prevention of breast cancer were assessed by the Breast Cancer Prevention Trial Symptom Checklist.22 The measure lists 25 physical and psychological symptoms (e.g., hot flashes, nausea, and short temper) that are commonly reported among women in cancer treatment or prevention trials. Both a total score and subscale scores can be calculated from this measure.
Concern about recurrence
Fear of cancer recurrence was measured by the Concerns of Recurrence Scale.23 Four items assess overall fear (frequency, potential for upset, consistency, and intensity of fears). Individual items are scored from 0 (not at all) to 4 (extremely) and summed to calculate a total score. Higher scores indicate greater concerns about cancer recurrence.
Self-efficacy
The 12-item Communication and Attitudinal Self-Efficacy (CASE)–Cancer scale was used in this study. It assesses 3 domains: (1) understanding and participating in care; (2) maintaining a positive attitude; and (3) seeking and obtaining information.24 Item scores for CASE–Cancer are on a 4-point scale and then are summed to provide domain scores. Higher scores denote higher self-efficacy
Depression
Depressive symptoms were measured by the Center for Epidemiologic Studies–Depression Scale.25 Responses are on a Likert-type scale ranging from 0 (none or rarely) to 3 (most or all the time) and include 20 common affective and somatic symptoms of depression experienced in the past week. Item scores are summed together to produce a scale score (range: 0-63). Higher scores indicate higher depressive symptomatology.
Pain interference
Pain interference was assessed using 7 items from the Brief Pain Inventory (BPI)-Interference subscale.26 The BPI-Interference measures how much pain has interfered with 7 daily activities, including general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Responses are on a Likert-type scale ranging from 0 (does not interfere) to 10 (completely interferes), with higher scores denoting more pain interference.
Fatigue interference
Fatigue interference was assessed with the 7-item Fatigue Symptom Inventory (FSI)-interference subscale.27 The FSI-interference measures how much fatigue has interfered with general activity, ability to bathe and dress oneself, normal work activity, ability to concentrate, relations with others, enjoyment of life, and mood. Responses are on an 11-point Likert-type 0 to 10 scale. Higher scores denote greater fatigue interference.
Health-related quality of life
The Short Form-8 (version 2)28 was used to assess health-related quality of life in the past month. This 8-item measure produces 2 subscale scores: the physical component summary (PCS-8) and the mental health component summary (MCS-8). Both scores range from 0 to 100, with 100 being the more favorable score.
Perceived stress
The 14-item Perceived Stress Scale29 was used to measure perception of stress and the degree to which situations in one’s life are appraised as stressful. Individual items are on a Likert-type scale ranging from 0 = never to 4 = very often, which are summed together for a total scale score. Higher scores indicate more perceived stress.
Social desirability
The 5-item Social Desirability Response Set (SDRS)30 assessed social desirability in responding to questionnaire items. The SDRS items are on a Likert-type scale ranging from 1 = definitely true to 5 = definitely false, with higher scores denoting higher social desirability tendencies.
Social support
The 20-item MOS-Social Support Questionnaire31 measured 4 types of social support: emotional/informational support, tangible support, affectionate support, and positive social interactions. The items are on a Likert-type scale ranging from 1 = none of the time to 5 = all of the time, with higher scores indicating higher social support. Subscale scores of the 4 types of social support, as well as a total score comprised of all items, are calculated.
Medication adherence
A single item from the Morisky Medication Adherence Scale32 assessed medication adherence on the weekly app surveys: “Over the past 7 days, on how many days did you take a dose of your AHT medication?”
Demographic characteristics
Participant demographic information was collected, including age, race, ethnicity, marital status, education level, employment status, income, and type of health insurance.
Text-Based Intervention
Following completion of the first clinic visit and baseline interview, participants received daily text messaging and weekly app surveys for 90 days. Messaging focused on 3 behaviors: initiation, continuation, and adherence to the prescribed dose, as appropriate. Each day, at a time agreed upon at baseline by the participants, a text message was sent to their smartphones reminding them to take their AHT medication. A library of 14 distinct, positive messages (Supplementary Appendix 1) were created to ensure participants received a new message every day for 14 days, at which point the messages would recycle.
In addition to the daily text messaging, participants received a preprogrammed text message, once a week, saying, “Your weekly adherence survey is waiting for you. Please log into the Adherence app to complete it.” The survey was active for 24 hours. The survey asked, “Over the past 7 days, on how many days did you take a dose of your AHT medication?” Response options ranged from 0 to 7. The dynamic tailoring of the app provided specific feedback to participants. A response of 6 and above triggered a message (Supplementary Appendix 2) that encouraged patients to keep taking the medication. A response of 5 or lower triggered a message asking about reasons for the missed doses followed by problem-solving based tips to improve adherence. The treating physician was notified via e-mail of patients who missed more than 1 dose as well as the reason(s) the patient provided. If a patient reported symptoms as the reason for lowered AHT adherence, the system provided an option for patients to leave an audio message within the app with the option to share that message with their physician via e-mail.
Approximately 3 months after the start of AHT, participants were asked to return to their provider for a second blood draw. Trained interviewers at OSUCCC then contacted participants to complete the follow-up interview survey over the phone. The second survey included the measures assessed at baseline as well as medication adherence and reflections on the study intervention (e.g., likes and dislikes). Participants were mailed a letter with a $25 gift card after completing each survey (baseline and exit) as a thank-you for their time. Participating physicians were also contacted via phone to complete an end-of-study survey about the intervention and the study participation of their patients.
Clinical Characteristics
Clinic staff at the participating centers completed a survey of the patients’ cancer and treatments. Data elements recorded were stage at diagnosis, size of breast tumor, number of nodes evaluated, number of positive nodes, estrogen and progesterone receptor status, primary treatment (lumpectomy, mastectomy, radiation therapy, adjuvant chemotherapy, neoadjuvant chemotherapy, trastuzumab), and type of AHT (tamoxifen).
Analyses
Data were summarized using means (Ms) and standard deviations (SDs) or frequencies. Scores assessed at baseline and exit were compared using paired t tests. Wilcoxon’s signed rank tests were used to compare the changes in estradiol, estrogen, and estrone from baseline to study exit. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Sample Characteristics
The majority of participants were recruited from the Ohio State University’s Comprehensive Cancer Center (66.7%), followed by the Wake Forest University Comprehensive Cancer Center (15.4%), the University of Vermont Medical Center (10.3%), Novant Health (5.1%), and the Southeast Medical Oncology Center in North Carolina (2.6%). Figure 1 presents a CONSORT diagram of participants (n = 39). Twelve consented participants did not complete all elements in the intervention. Reasons for noncompletion, as reported by the patients, included being busy, not feeling well, or forgetfulness.
The demographic and clinical characteristics of participants by protocol completion (defined as having completed both the baseline and the exit surveys, both blood draws, and not missing more than 2 weekly surveys) are presented in Table 1. The mean age of the total sample was 59.7 (SD = 7.0), and the majority were non-Hispanic white and married. The majority of the sample had positive estrogen (100%) and progesterone (84.2%) status and received a lumpectomy (71.1%) and radiation (73.7%) as primary treatment. The demographic characteristics of the participants who did and did not complete the protocol were similar, except that the completers were older, had a higher level of education, and were more likely to be retired than the non-completers. In addition, the completers were more likely to have had a lumpectomy versus a mastectomy and radiation therapy than the non-completers. Finally, participants who completed the protocol were on the AHT tamoxifen at significantly higher rates (85.7%) than those participants who did not complete the protocol (14.3%; P = .04).
Table 1.
Participant Demographic and Clinical Characteristics by Protocol Completion.a
| Variable | Not Complete, n = 12 | Complete, n = 27 | Total, n = 39 | P Value |
|---|---|---|---|---|
| Age, mean (SD) | 56.5 (9.2) | 61.1 (5.4) | 59.7 (7.0) | .06 |
| Race | ||||
| White | 12 (16.7%) | 24 (66.7%) | 36 (92.3%) | 1.00 |
| Black | 0 (0%) | 2 (7.4%) | 2 (5.1%) | |
| Other | 0 (0%) | 1 (3.7%) | 1 (2.6%) | |
| Hispanic, Latino, or Spanish origin | 0 (0%) | 1 (3.7%) | 1 (2.6%) | 1.00 |
| Marital status | ||||
| Married | 9 (75.0%) | 17 (63.0%) | 26 (66.7%) | .49 |
| Divorced | 2 (16.7%) | 9 (33.3%) | 11 (28.2%) | |
| Widowed | 1 (8.3%) | 1 (3.7%) | 2 (5.1%) | |
| Education level | ||||
| High School or less | 2 (16.7%) | 1 (3.7%) | 3 (7.7%) | .33 |
| Some college/associate degree | 5 (41.7%) | 11 (40.7%) | 16 (41.0%) | |
| Bachelor’s degree or higher | 5 (41.7%) | 15 (55.6%) | 20 (51.3%) | |
| Employment status | ||||
| Full time | 4 (33.3%) | 10 (37%) | 14 (35.9%) | .15 |
| Part time | 0 (0.0%) | 3 (11.1%) | 3 (7.7%) | |
| Housework/child care | 2 (16.7%) | 0 (0.0%) | 2 (5.1%) | |
| Not working due to health | 3 (25.0%) | 3 (11.1%) | 6 (15.4%) | |
| Retired | 3 (25.0%) | 11 (40.7%) | 14 (35.9%) | |
| Income | ||||
| <$40 000 | 1 (8.3%) | 4 (14.8%) | 5 (12.8%) | .33 |
| $40 000-$70 000 | 2 (16.7%) | 9 (33.3%) | 11 (28.2%) | |
| >$70 000 | 9 (75.0%) | 11 (40.7%) | 20 (51.3%) | |
| Type of health insurance | ||||
| Private | 6 (50.0%) | 17 (63.0%) | 23 (59.0%) | .06 |
| Medicare | 3 (25.0%) | 9 (33.3%) | 12 (30.8%) | |
| Medi-gap | 0 (0.0%) | 1 (3.7%) | 1 (2.6%) | |
| Military | 3 (25.0%) | 0 (0.0%) | 3 (7.7%) | |
| Stage at diagnosis | ||||
| Stage I | 7 (63.6%) | 14 (51.9%) | 21 (55.2%) | .62 |
| Stage II | 2 (18.2%) | 9 (33.3%) | 11 (28.9%) | |
| Stage III | 2 (18.2%) | 4 (14.8%) | 6 (15.8%) | |
| Size of breast tumor, cm, mean (SD) | 1.6 (1.3) | 1.9 (1.5) | 1.8 (1.5) | .51 |
| Nodes evaluated, mean (SD) | 6.4 (6.7) | 6.3 (6.1) | 6.3 (6.2) | .96 |
| Nodes positive | ||||
| 0 | 7 (63.6%) | 20 (74.1%) | 27 (71.1%) | .62 |
| 1 | 1 (9.1%) | 1 (3.7%) | 2 (5.3%) | |
| 2 | 1 (9.1%) | 3 (11.1%) | 4 (10.5%) | |
| 4 | 1 (9.1%) | 1 (3.7%) | 2 (5.3%) | |
| 8 | 1 (9.1%) | 0 (0.0%) | 1 (2.6%) | |
| 11 | 0 (0.0%) | 1 (3.7%) | 1 (2.6%) | |
| 12 | 0 (0.0%) | 1 (3.7%) | 1 (2.6%) | |
| Positive estrogen receptor status | 11 (100.0%) | 27 (100.0%) | 38 (100.0%) | 1.00 |
| Positive progesterone receptor status | 10 (90.9%) | 22 (81.5%) | 32 (84.2%) | .65 |
| Positive HER2/neu receptor status | 2 (18.2%) | 5 (18.5%) | 7 (18.4%) | 1.00 |
| Included as primary treatment | ||||
| Lumpectomy | 6 (54.5%) | 21 (77.8%) | 27 (71.1%) | .24 |
| Mastectomy | 7 (63.6%) | 8 (29.6%) | 15 (39.5%) | .07 |
| Radiation therapy | 7 (63.6%) | 21 (77.8%) | 28 (73.7%) | .43 |
| Adjuvant chemotherapy | 6 (54.5%) | 6 (22.2%) | 12 (31.6%) | .07 |
| Neoadjuvant chemotherapy | 0 (0.0%) | 3 (11.1%) | 3 (7.9%) | .54 |
| Herceptin | 2 (18.2%) | 5 (18.5%) | 7 (18.4%) | 1.00 |
| Type of adjuvant hormone therapy | ||||
| Tamoxifen | 1 (9.1%) | 1 (3.7%) | 2 (5.3%) | .50 |
| Arimidex | 3 (27.3%) | 18 (66.7%) | 21 (55.3%) | .04 |
| Aromasin | 1 (9.1%) | 0 (0.0%) | 1 (2.6%) | 1.00 |
| Femara | 7 (63.6%) | 8 (29.6%) | 15 (39.5%) | .07 |
| Other | 1 (9.1%) | 0 (0.0%) | 1 (2.6%) | 1.00 |
Abbreviations: HER2, human epidermal growth factor receptor; SD, standard deviation.
a Protocol completion was defined as having completed both the baseline and the exit surveys, both blood draws and not missing more than 2 weekly surveys. The clinical questionnaire was not completed for 1 participant.
Hormone Status
Table 2 shows the quartiles and the minimum/maximum for the 3 hormones: estradiol, estrogen, and estrone at baseline and exit. Declines in the quartiles were observed, with all 3 hormone values at exit being lower than baseline (P’s < .0001), suggesting that participants were accurate about their self-reported AHT adherence.
Table 2.
Quartiles of Participants’ Hormones at Baseline and Exit.a
| Event | Hormone | N | Min | 25th Percentile | 50th Percentile | 75th Percentile | Max |
|---|---|---|---|---|---|---|---|
| Baseline | Estradiol by TMS | 38 | 1.2 | 3.6 | 5.1 | 7.6 | 38.3 |
| Estrogen total calculation | 38 | 4.6 | 15.3 | 19.7 | 33.6 | 85.1 | |
| Estrone by TMS | 38 | 2.0 | 11.0 | 15.2 | 23.7 | 63.4 | |
| Exit | Estradiol by TMS | 32 | −1.0 | 1.1 | 1.5 | 2.0 | 4.9 |
| Estrogen total calculation | 30 | −1.0 | −1.0 | −1.0 | −1.0 | 6.8 | |
| Estrone by TMS | 30 | −1.0 | −1.0 | −1.0 | −1.0 | 3.4 |
Abbreviations: TMS, tandem mass spectrometry; min, minimum; max, maximum.
a P-values from Wilcoxon signed rank test were significant for all 3 hormones at the P < .0001 level. −1 indicates below MDL.
Psychosocial and Quality-of-Life Concerns, Symptoms, and Medication Adherence
Participants’ unadjusted psychosocial, symptom, and medication adherence scores at baseline and study exit are reported in Table 3. Of these variables, the participants’ perceived stress scores significantly decreased from baseline (M = 17.17) to study exit (M = 15.64; P = .040). The MCS-8 subscale score also increased significantly from pre- (M = 49.95) to post-intervention (52.98; P = .007), indicating better mental health functioning. In addition, participants’ self-reported adherence to AHT, as measured by a single item on the Morisky Medication Adherence Scale, also improved significantly from baseline (M = 1.92) to study end (M = 1.17; P = .015). No other variables had significant changes from pre- to post-intervention.
Table 3.
Participants’ Unadjusted Psychosocial, Symptom, and Medication Adherence Scores at Baseline and Study Exit.
| Variable | N | Baseline, Mean (SD) | Exit, Mean (SD) | Mean Difference (95% CL) | P Value |
|---|---|---|---|---|---|
| BCPT total score | 37 | 0.71 (0.54) | 0.75 (0.51) | 0.04 (−0.06, 0.14) | .412 |
| Concerns about Recurrence score | 37 | 10.86 (6.55) | 9.73 (4.95) | −1.14 (−2.31, 0.04) | .057 |
| CASE 3-item score | 36 | 27.83 (3.20) | 27.78 (4.36) | −0.06 (−1.67, 1.56) | .944 |
| CES-D score | 31 | 6.90 (9.05) | 5.68 (7.96) | −1.23 (−3.47, 1.01) | .272 |
| Brief Pain Inventory (BPI) score | 11 | 2.84 (2.78) | 3.63 (2.61) | 0.79 (−0.44, 2.02) | .182 |
| Fatigue Symptom Inventory (FSI) score | 36 | 1.82 (2.20) | 1.57 (1.97) | −0.25 (−0.76, 0.26) | .322 |
| SF-8 MCS mental subscale score | 36 | 49.95 (7.81) | 52.98 (6.51) | 3.03 (0.87, 5.18) | .007 |
| SF-8 PCS physical subscale score | 36 | 45.44 (10.27) | 46.39 (10.58) | 0.95 (−1.71, 3.61) | .473 |
| Perceived Stress Scale (PSS) score | 36 | 17.14 (9.39) | 15.64 (8.24) | −1.50 (−2.93, −0.07) | .040 |
| Social Desirability Response score | 37 | 21.62 (1.75) | 21.41 (2.20) | −0.22 (−0.99, 0.56) | .577 |
| MOS Social Support score | 36 | 89.58 (10.23) | 90.94 (9.35) | 1.36 (−0.70, 3.42) | .189 |
| Morisky Adherence score | 36 | 1.92 (1.70) | 1.17 (1.32) | −0.75 (−1.35, −0.15) | .015 |
Abbreviations: BCPT, Breast Cancer Prevention Trial Symptom Checklist; CASE, Communication and Attitudinal Self-Efficacy; CES-D, Center for Epidemiological Studies Depression; MCS, Mental Health Component Summary; PSS, Perceived Stress Score; MOS, Medical Outcomes Study; SD, standard deviation.
Patient Postintervention Evaluation
Thirty-seven patients responded to the postintervention evaluation, and 97.3% reported a positive experience. More than 91% of patients believed that they benefited from study participation, and 81% agreed or strongly agreed that the daily reminder messages helped them to be more adherent to their AHT medication. Ninety-five percent of patients agreed or strongly agreed that the intervention would be helpful for future patients taking AHT medication. The most favorably rated intervention component was that the instructions for the text messaging system were helpful (97.3% agreed or strongly agreed).
Physician Post-intervention Evaluation
All participating physicians (n = 7) completed the post-intervention evaluation. All physicians reported that they agreed or strongly agreed that their patients were helped by participating in the study. More than 85% agreed/strongly agreed that (1) the video positively influenced their patient’s communication, (2) their patients valued participation in the study, and (3) being informed about their patient’s adherence helped them provide better care. All physicians agreed/strongly agreed that they would recommend that patients use the intervention while taking AHT medication.
Discussion
This study tested the feasibility of an app-based intervention program to improve AHT adherence and decrease discontinuation of AHT among postmenopausal breast cancer survivors. The study results indicated that not only did the app support AHT adherence, but it also improved parameters of patient well-being. Both subjective (self-report) and objective (blood samples) measures of AHT adherence suggested that participants were adherent to their AHT medication. This current investigation corresponds with results from previous studies.33-35 The few app-based interventions to improve AHT adherence among breast cancer survivors demonstrate feasibility and patient acceptance but are limited by lack of verification of medication adherence.18,19 A 2017 review by Ginossar et al36 indicated the promise of technology-based interventions among breast cancer survivors and the “extreme need” for apps that focus on survivorship, including medication adherence. This study and others18,37 that utilize app-based interventions to measure and improve health outcomes among older breast cancer survivors are addressing this need. The results of this study and the growing body of literature in this area suggest the acceptance and utility of app-based interventions in this population.
Mental health functioning (MCS-8) improved and perceived stress decreased significantly among the participants from baseline to the end of the study. Mental health and psychological status has been found to predict nonadherence to AHT in women with breast cancer.13,38-40 For example, a study by Hershman et al7 found that intrusive avoidant thoughts about a distressing event and cancer-specific emotional distress decreased the likelihood of adherence to AHT. Interventions to modify these cognitive and psychological factors can improve patient adherence and health outcomes.40
We found no significant changes in depressive symptoms, fatigue, pain interference, and physical functioning (PCS-8) during the conduct of this intervention. This study, however, was not powered to examine these differences. Depressive symptoms, fatigue, and pain interference were also relatively low at baseline, suggesting that these symptoms may not have been amenable to much change as a result of the intervention. The primary purpose of this study was to test the feasibility of recruitment and the general conduct of the intervention, which was established. Future research will need to examine the multilevel factors influencing AHT adherence among breast cancer survivors in randomized controlled trials, with sufficient study power to examine these end points.
Physicians and patients agreed that the AHT adherence app intervention was beneficial and should be used by future patients. This finding is encouraging as it suggests acceptability and utility of the AHT adherence app. Clinical practice has begun adopting app-based and other electronic-based interventions for their patients. Physicians “prescribing” mobile health apps to their patients introduces a new area of health care and creates new opportunities, including reinventing the way health-care providers connect with their patients.41 The time is ripe for the integration of mobile health apps in health care for several reasons, including increased smartphone ownership, a growing culture of instantaneous access to information, the ease and low cost of app development, increased patient empowerment, the want for personalized health information, and the growth of big data and analytics.42,43 However, there are numerous barriers and challenges that need to be addressed in future interventions, including the need for technology support, patterns of adoption and use for all parties, perceptions of impersonal interactions, security and privacy, and the impact of monitoring on the clinical workflow of health-care providers.41-43
There are several limitations to this study. First, this pilot study lacked a control group. Thus, it is difficult to determine the effect of the intervention on AHT adherence in contemporaneous controls not using the app. Second, the small sample size limits the statistical analyses and power of the study’s findings. In addition, the eligibility criteria mandated that patients have a smartphone in order to participate in this study. We excluded 36 potential participants who did not have smartphones, some of whom were interested in participating in the study. This created a disparity in a patient population that could benefit from intervention participation. Future interventions should find ways to provide such technology to all participants, particularly as this technology is being used increasingly in health care interventions. Finally, a major challenge in recruitment was that we needed to identify patients prior to their initiation of AHT in this multicenter study. This was necessary in order to complete the blood draw. Many patients were referred to us after they started their AHT, which was problematic. Completing the pilot study, however, has now enabled us to refine our recruitment procedures in order to locate patients prior to the start of AHT therapy as well as to make arrangements for smartphone distributions to participants at no cost to them for similar interventions.
Strengths of the study were that we were able to recruit participants from multiple clinical sites, use the app successfully, and efficiently track all participants. The app worked effectively in delivering the daily text message reminders to the participants as well as the weekly surveys as prescribed by the protocol. Both patients and their providers found benefit in the intervention in increasing and/or maintaining adherence to AHT. These are important findings, given the critical need for AHT adherence in this survivor population.
Conclusion
This pilot study of breast cancer survivors found significant improvements in AHT adherence, mental health functioning, and perceived stress with an app-based intervention. Feedback about the intervention and its usability were positive from both patients and health-care providers. The advantages of this app-based intervention are its relative simplicity, low cost, high levels of acceptance, and ability to reach a multisite patient population. A larger longer term, randomized controlled intervention is planned to establish the effectiveness of the app-based intervention in improving AHT adherence in this patient population.
Supplemental Material
supplemental_table_1 for Increasing Adherence to Adjuvant Hormone Therapy Among Patients With Breast Cancer: A Smart Phone App-Based Pilot Study by Jessica L. Krok-Schoen, Michelle J. Naughton, Gregory S. Young, Jennifer Moon, Ming Poi, Susan A. Melin, Marie E. Wood, Judith O. Hopkins, Electra D. Paskett and Douglas M. Post in Cancer Control
Supplemental Material
supplemental_table_2 for Increasing Adherence to Adjuvant Hormone Therapy Among Patients With Breast Cancer: A Smart Phone App-Based Pilot Study by Jessica L. Krok-Schoen, Michelle J. Naughton, Gregory S. Young, Jennifer Moon, Ming Poi, Susan A. Melin, Marie E. Wood, Judith O. Hopkins, Electra D. Paskett and Douglas M. Post in Cancer Control
Acknowledgments
ClinicalTrials.gov Identifier: NCT02400060.
Authors’ Note: Our study was approved by The Ohio State University Research Ethics Committee (approval no. 2016C0006). All patients provided written informed consent prior to enrollment in the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have full control of all primary data and the authors agree to allow the journal to review their data if requested.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant) and U10CA180850 and The Ohio State University Comprehensive Cancer Center Pharmacoanalytical Shared Resource, P30CA016058.
ORCID iD: Jessica L. Krok-Schoen, PhD  https://orcid.org/0000-0003-2484-5887
https://orcid.org/0000-0003-2484-5887
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet (London, England). 2013;381(9869):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camacho FT, Tan X, Alcala HE, Shah S, Anderson RT, Balkrishnan R. Impact of patient race and geographical factors on initiation and adherence to adjuvant endocrine therapy in medicare breast cancer survivors. Med. 2017;96(24): e7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kroenke C, Hershman DL, Adams S, Kwan ML, Kushi LH. Social support at diagnosis and noninitiation, discontinuation, and nonadherence to adjuvant hormonal therapy in the pathways study. J Clin Oncol. 2017;35(5 S):7–7. [Google Scholar]
- 7. Hershman DL, Kushi LH, Hillyer GC, et al. Psychosocial factors related to non-persistence with adjuvant endocrine therapy among women with breast cancer: the breast cancer quality of care study (BQUAL). Breast Cancer Res Treat. 2016;157(1):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. [DOI] [PubMed] [Google Scholar]
- 9. Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nekhlyudov L, Li L, Ross-Degnan D, Wagner AK. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat. 2011;130(2):681–689. [DOI] [PubMed] [Google Scholar]
- 12. Neugut AI, Hillyer GC, Kushi LH, et al. Non-initiation of adjuvant hormonal therapy in women with hormone receptor-positive breast cancer: the breast cancer quality of care study (BQUAL). Breast Cancer Res Treat. 2012;134(1):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells KJ, Pan TM, Vazquez-Otero C, et al. Barriers and facilitators to endocrine therapy adherence among underserved hormone-receptor-positive breast cancer survivors: a qualitative study. Support Care Cancer. 2016;24(10):4123–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santo K, Richtering SS, Chalmers J, Thiagalingam A, Chow CK, Redfern J. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4(4):e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Int Med. 2016;176(3):340–349. [DOI] [PubMed] [Google Scholar]
- 16. Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc. 2013;53(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fishbein JN, Nisotel LE, MacDonald JJ, et al. Mobile application to promote adherence to oral chemotherapy and symptom management: a protocol for design and development. JMIR Res Protocol. 2017;6(4): e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ali EE, Leow JL, Chew L, Yap KY. Patients’ perception of app-based educational and behavioural interventions for enhancing oral anticancer medication adherence. J Cancer Educ. 2017. [DOI] [PubMed] [Google Scholar]
- 19. Mougalian SS, Epstein LN, Jhaveri AP, et al. Bidirectional text messaging to monitor endocrine therapy adherence and patient-reported outcomes in breast cancer. JCO Clin Cancer Informat. 2017;In press. [DOI] [PubMed] [Google Scholar]
- 20. Cegala DJ, McClure L, Marinelli TM, Post DM. The effects of communication skills training on patients’ participation during medical interviews. Patient educat counsel. 2000;41(2):209–222. [DOI] [PubMed] [Google Scholar]
- 21. Cegala DJ, Post DM, McClure L. The effects of patient communication skills training on the discourse of older patients during a primary care interview. J Ame Geriat Soci. 2001;49(11):1505–1511. [DOI] [PubMed] [Google Scholar]
- 22. Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97(6):448–456. [DOI] [PubMed] [Google Scholar]
- 23. Vickberg SM. The concerns about recurrence scale (CARS): a systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25(1):16–24. [DOI] [PubMed] [Google Scholar]
- 24. Wolf MS, Chang CH, Davis T, Makoul G. Development and validation of the communication and attitudinal self-efficacy scale for cancer (CASE-cancer). Patient Educ Couns. 2005;57(3):333–341. [DOI] [PubMed] [Google Scholar]
- 25. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 26. Cleeland CS. Measurement of pain by self-report In: Chapman CR, Loeser JD, eds. Advances in Pain Research and Therapy. New York, NY: Raven Press; 1989:391–403. [Google Scholar]
- 27. Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the fatigue symptom inventory. Qual Life Res. 1998;7(4):301–310. [DOI] [PubMed] [Google Scholar]
- 28. Ware JE, Kosinski M, Dewey JE, Gandek B. How to score and interpret single-item health status measures: a manual for users of the SF-8 health survey. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 29. Cohen S, Kamarak T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 30. Hays RD, Hayashi T, Stewart AL. A Five-item measure of socially desirable response set. Educat Psychol Measure. 1989;49:629–636. [Google Scholar]
- 31. Sherbourne CD, Stewart AL. The MOS social support survey. Soci sci med. 1991;32(6):705–714. [DOI] [PubMed] [Google Scholar]
- 32. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med care. 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 33. Miloh T, Annunziato R, Arnon R, et al. Improved adherence and outcomes for pediatric liver transplant recipients by using text messaging. Pediatrics. 2009;124(5): e844–e850. [DOI] [PubMed] [Google Scholar]
- 34. Ting TV, Kudalkar D, Nelson S, et al. Usefulness of cellular text messaging for improving adherence among adolescents and young adults with systemic lupus erythematosus. J Rheumatol. 2012;39(1):174–179. [DOI] [PubMed] [Google Scholar]
- 35. Dowshen N, Kuhns LM, Johnson A, Holoyda BJ, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: a pilot study using personalized, interactive, daily text message reminders. J Med Int Res. 2012;14(2): e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ginossar T, Shah SF, West AJ, et al. Content, usability, and utilization of plain language in breast cancer mobile phone apps: a systematic analysis. JMIR Mhealth Uhealth. 2017;5(3):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCarroll ML, Armbruster S, Pohle-Krauza RJ, et al. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol oncol. 2015;137(3):508–515. [DOI] [PubMed] [Google Scholar]
- 38. Bender CM, Gentry AL, Brufsky AM, et al. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Foru. 2014;41(3):274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manning M, Bettencourt BA. Depression and medication adherence among breast cancer survivors: bridging the gap with the theory of planned behaviour. Psychol health. 2011;26(9):1173–1187. [DOI] [PubMed] [Google Scholar]
- 40. Lin C, Clark R, Tu P, Bosworth HB, Zullig LL. Breast cancer oral anti-cancer medication adherence: a systematic review of psychosocial motivators and barriers. Breast Cancer Res Treat. 2017;165(2):247–260. [DOI] [PubMed] [Google Scholar]
- 41. Austin RR, Hull S. The power of mobile health technologies and prescribing apps. Comput Inform Nurs. 2014;32(11):513–515. [DOI] [PubMed] [Google Scholar]
- 42. Elsevier Clinical Solutions. White Paper: Mobile applications and the future of healthcare.: Elsevier Clinical Solutions; 2015. [Google Scholar]
- 43. Ferguson C, Jackson D. Selecting, appraising, recommending and using mobile applications (apps) in nursing. J clin nurs. 2017;26(21-22):3253–3255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplemental_table_1 for Increasing Adherence to Adjuvant Hormone Therapy Among Patients With Breast Cancer: A Smart Phone App-Based Pilot Study by Jessica L. Krok-Schoen, Michelle J. Naughton, Gregory S. Young, Jennifer Moon, Ming Poi, Susan A. Melin, Marie E. Wood, Judith O. Hopkins, Electra D. Paskett and Douglas M. Post in Cancer Control
supplemental_table_2 for Increasing Adherence to Adjuvant Hormone Therapy Among Patients With Breast Cancer: A Smart Phone App-Based Pilot Study by Jessica L. Krok-Schoen, Michelle J. Naughton, Gregory S. Young, Jennifer Moon, Ming Poi, Susan A. Melin, Marie E. Wood, Judith O. Hopkins, Electra D. Paskett and Douglas M. Post in Cancer Control