Abstract
Previous studies including ours have demonstrated a critical function of the transcription factor ETV2 (ets variant 2; also known as ER71) in determining the fate of cardiovascular lineage development. However, the underlying mechanisms of ETV2 function remain largely unknown. In this study, we demonstrated the novel function of the miR (micro RNA)-126-MAPK (mitogen-activated protein kinase) pathway in ETV2-mediated FLK1 (fetal liver kinase 1; also known as VEGFR2)+ cell generation from the mouse embryonic stem cells (mESCs). By performing a series of experiments including miRNA sequencing and ChIP (chromatin immunoprecipitation)-PCR, we found that miR-126 is directly induced by ETV2. Further, we identified that miR-126 can positively regulate the generation of FLK1+ cells by activating the MAPK pathway through targeting SPRED1 (sprouty-related EVH1 domain containing 1). Further, we showed evidence that JUN/FOS activate the enhancer region of FLK1 through AP1 (activator protein 1) binding sequences. Our findings provide insight into the novel molecular mechanisms of ETV2 function in regulating cardiovascular lineage development from mESCs.
Keywords: ETV2/ER71, FLK1+ cells, miR-126, EGFL7, Embryonic stem cells
Introduction
The ETS (E26 transformation-specific or E-twenty-six specific sequence) transcription factor family members play key roles in diverse biological events including cell survival, cancer, vascular-angiogenesis and hematopoiesis mainly through the interaction between their conserved ETS DNA binding domain and the consensus sequence (5′-GGAA/T-3′) present in the regulatory elements of their target genes [1, 2]. ETV2 (ets variant 2; also known as ER71), a member of the ETS transcription factor family, has been reported as an indispensable factor in establishing the cardiovascular system [3, 4]. We previously demonstrated that deficiency in Etv2 led to embryonic lethality due to a complete lack of both vascular and hematopoietic compartments [5, 6]. We also showed that ETV2 can directly bind the promoters/enhancers of endothelial and hematopoietic genes including Flk1 (fetal liver kinase 1; also known as VEGFR2) [5–7]. Together with the findings that ETV2 can induce de novo generation of FLK1+ cells, the multipotent progenitor for blood, endothelial and cardiac lineages from mouse embryonic stem cells (mESCs) [5], these results strongly suggest the critical function of ETV2 for the establishment of the cardiovascular system. Reports from other groups further support the importance of ETV2 in this process [8–11]. Regarding the regulatory mechanisms of ETV2 functions, several studies examining the ETV2 binding proteins have been reported. For example, it was shown that the interaction between ETV2 and FOXC2 (forkhead box protein C2) plays an important role in regulating several key genes of the endothelial and hematopoietic lineages [12, 13]. Also, our recent study revealed the functional significance of the ETV2-OVOL2 (ovo-like zinc finger 2) interaction in generating FLK1+ cells and its further differentiation into the hematopoietic and endothelial cells [14]. However, the detailed molecular insight into the ETV2 function remains largely unknown.
To better understand the machinery of ETV2 that regulates the FLK1+ cell generation from mESCs, we profiled miRNAs (micro RNAs) that are differentially regulated by ETV2 and found miR-126 as one of the direct downstream players of ETV2. We subsequently investigated the molecular mechanism of the miR-126/MAPK (mitogen-activated protein kinase) pathway in ETV2-mediated FLK1+ cell generation.
Materials and methods
Complete materials and methods are presented in Additional file 1: Supplemental materials and methods.
Results
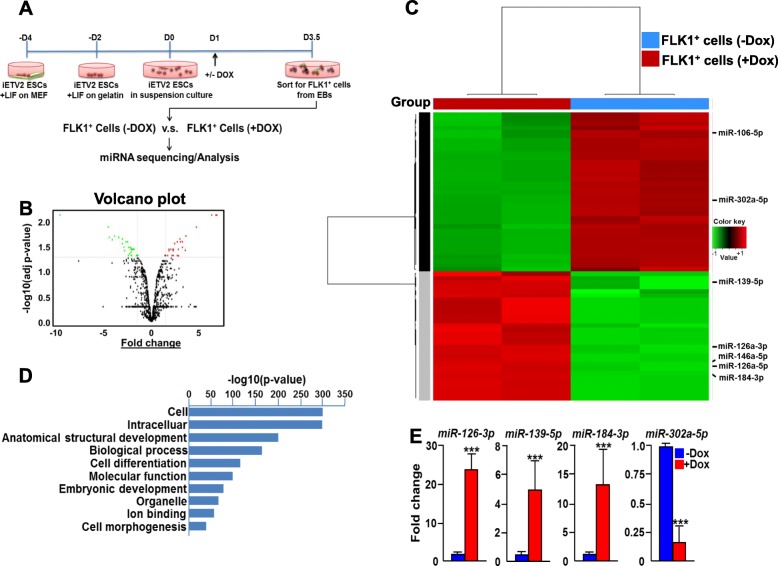
Analysis of ETV2-mediated miRNAs
To gain a novel insight into the molecular mechanisms of ETV2 function in FLK1+ cell generation, we performed miRNA profiling analysis. FLK1+ cells from doxycycline (Dox)-inducible ETV2 in mESC (herein, iFLAG-ETV2 ESCs) [14] at day 3.5 of differentiation ± Dox were FACS (fluorescence-activated cell sorting)-sorted and subjected to miRNA sequencing (Fig. 1a). The miRNAs with ≥ 1.5 fold change and a false discovery rate (FDR) ≤ 0.05 were considered to be significantly differentially expressed, resulting in a total of 67 miRNAs of interest that were subsequently subjected to unsupervised hierarchical clustering (Fig. 1b, c). GO (gene ontology) term analysis indicated that the ETV2-mediated miRNAs could be involved in diverse biological events with embryo development, cell differentiation and anatomical structure development being top ranked (Fig. 1d). Signaling pathways such as MAPK, RAP1 (ras-associated protein 1) and WNT (wingless-related integration site) were identified as the major regulatory network of the miRNAs, all of which are critical for cardiovascular development (Additional file 2: Tables S1 and S2). Some of the differentially expressed miRNAs were validated by qRT-PCR (Fig. 1e and Additional file 3: Figure S1).
Fig. 1.
Analysis of ETV2-regualted miRNA expression in FLK1+ cells. a Schematic diagram of miRNA sequencing experiment. Doxycycline-inducible (iFLAG-ETV2) mESCs were differentiated, treated with ± Doxycycline (Dox) at day 1 and sorted for FLK1+ cells at day 3.5. RNAs from the sorted cells were subjected to miRNA sequencing and analysis. b Volcano plot showing the log2 fold change between +Dox versus −Dox against the −log10 FDR-adjusted p value for each miRNA. miRNAs (FDR ≤ 0.05) with fold change of ≥ 1.5 (in red; upregulated) and ≤ − 1.5 (in green; downregulated) were highlighted and selected. c Heatmap of the selected miRNAs in response to overexpression of ETV2. miRNAs upregulated and downregulated in +DOX were indicated with gray and black bars, respectively. d Gene Ontology (GO) categories of selected miRNAs by DIANA miRpath analysis. Bars indicate the significance level of miRNA target genes and interactions. e Differentiated iFLAG-ETV2 mESCs at day 3.5 were subjected to qRT-PCR analysis. n = 3, ***p < 0.001
Among the differentially regulated miRNAs, miRNA-126 drew our attention as an important player of ETV2-mediated FLK1+ cell generation due to its function in vascular development and hematopoiesis [15–17]. Independently, our previous reports showed that Egfl7 (egf-like domain multiple 7) [18], the host gene of miR-126, is one of the most upregulated genes upon ETV2 overexpression [6, 7]. Accordingly, we sought to determine the functional consequence of miR-126 in regulating ETV2-mediated FLK1+ cell generation.
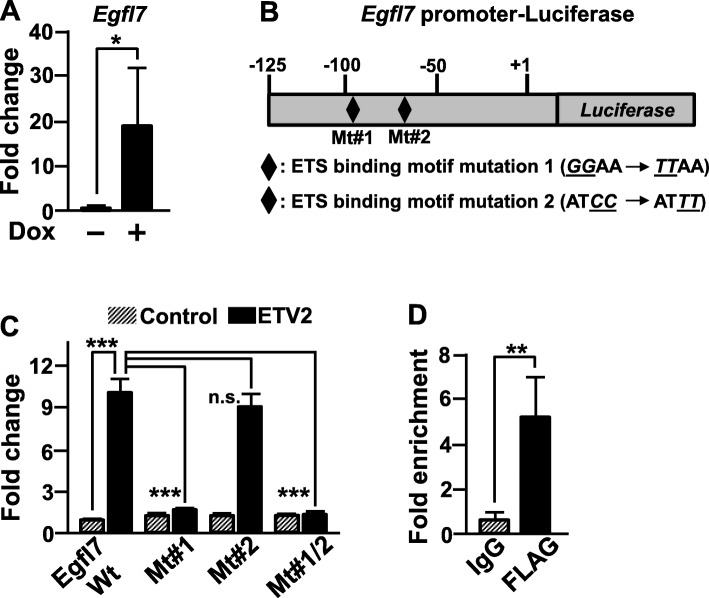
ETV2 directly activates the expression of miR-126 through Egfl7 promoter
First, we found a significant increment of the expression of both miR-126 and Egfl7 in differentiating iFLAG-ETV2 ESCs upon Dox treatment (Figs. 1e and 2a). Next, we examined whether ETV2 can directly activate the promoter of Egfl7, thus inducing the expression of miR-126. From the literature search and our own investigation, we found the highly conserved upstream region of the transcription start site in mouse and human EGFL7 that contains two conserved potential ETS binding sites (Fig. 2b). By performing the promoter assay, we revealed that the overexpression of Etv2 significantly increased the activity of the Egfl7 promoter. However, the Egfl7 promoter construct with mutations on one putative ETS site failed to respond to ETV2 (Fig. 2c). The results were further corroborated by chromatin immunoprecipitation (ChIP)-PCR assay, confirming in vivo occupancy of ETV2 in Egfl7 promoter (Fig. 2d). Taken together, we conclude that the expression of Egfl7 and thus miR-126 is directly regulated by ETV2 in differentiating mESCs.
Fig. 2.
ETV2 upregulates miR126 expression through direct binding on Egfl7 promoter. a Expression analysis. iFLAG-ETV2 mESCs were differentiated for 3.5 days ± Dox treatment and were subjected to gene expression analysis. n = 3, *p < 0.05. b Schematic diagram of Egfl7 promoter-luciferase plasmid. Two potential ETS binding elements were marked as diamonds, and their mutant sequences were shown in the bottom. c HEK/293T cells were transiently co-transfected with pCMV-Etv2 and pGL3-luciferase constructs carrying wild type (Wt), or mutants of Egfl7 promoter. Firefly luciferase activity was normalized by Renilla luciferase activity. n = 3. ***p < 0.001. d iFLAG-ETV2 mESCs were differentiated in the presence of Dox for 3.5 days and then subjected to ChIP-PCR assay. Rabbit anti-FLAG or IgG antibody was used for the immunoprecipitation. n = 3, **p < 0.01
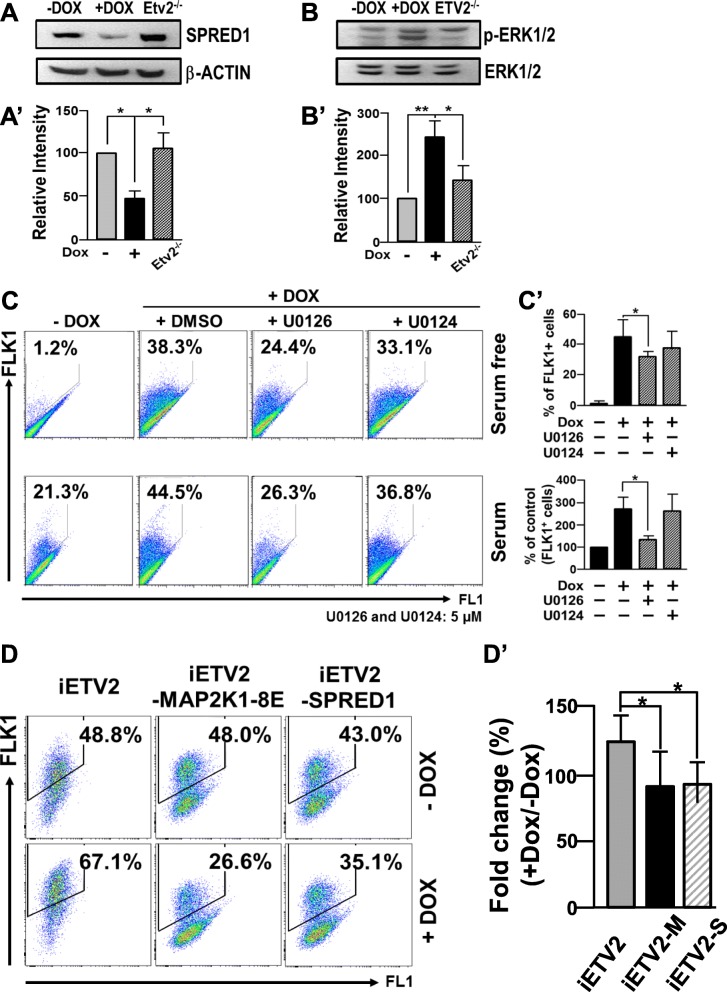
The miR-126/MAPK pathway plays an important role for ETV2-induced FLK1+ cell generation
MiR-126 (especially, miR-126-3p) can regulate the MAPK pathway through the suppression of the SPRED1 expression, a negative regulator of the MAPK pathway [15, 19]. Together with the report that treatment of bFGF (basic fibroblast growth factor), an agonist of FGF receptor-mediated signaling including MAPK [20], increases the generation of the FLK1+ cells from mESCs [21], we hypothesized that ETV2 induces FLK1+ cells partly through the miR-126-MAPK pathway by suppressing the expression of SPRED1. As shown in Fig. 3a and b, while SPRED1 (sprouty related EVH1 domain containing 1) was significantly reduced in response to ETV2, augmented phospho-ERK1/2 (extracellular signal-regulated kinases 1/2) level was evident upon the ETV2 overexpression in differentiating mESCs. Interestingly, Etv2−/− ESCs showed an increased level of SPRED1 expression with concomitant decrease of phosphorylated ERK1/2. Further, we found that ETV2-induced FLK1+ cell generation was significantly reduced in the presence of U0126, a MAPK inhibitor, compared to the control (Fig. 3c). To corroborate the findings, we transfected Dox-inducible MAP2K1-8E, a catalytically inactive form of MAP2K1 [22] into iFLAG-ETV2 mESCs in which both ETV2 and MAP2K1-8E are co-overexpressed upon Dox treatment. In agreement with the inhibitor treatment (Fig. 3c), the co-expression of MAPK2K1-8E and ETV2 led to a decreased generation of FLK1+ cells, compared to the group in which ETV2 only is overexpressed (Fig. 3d). Additionally, we went on to show that overexpression of SPRED1 was able to inhibit generation of FLK1+ cells upon the overexpression of ETV2 (Fig. 3d). These results clearly suggest that the SPRED1/MAPK pathway plays an important role for ETV2-induced FLK1+ cell generation from mESCs.
Fig. 3.
ETV2 increases FLK1+ cells through the miR-126/MAPK pathway. A, B iFLAG-ETV2 mESCs were differentiated with ± Dox for 3.5 days and subjected to western blot analysis for SPRED1 (A) and phosphorylated ERK1/2 (B). β-ACTIN and ERK1/2 were used as loading controls. (A’, B’) The relative protein expression of SPRED1 and p-ERK1/2 was normalized against β-ACTIN and total ERK1/2, respectively. Differentiated Etv2−/− mESCs were also included for the analysis. n = 3, *p < 0.05, **p < 0.01. (C) iFLAG-ETV2 mESCs were differentiated for 4 days in serum-free (upper) or for 3.5 days in serum (lower) conditions in the presence or absence of Dox. The resulting cells were analyzed for FLK1 expression by flow cytometry. U0126 (5 μM) or U0124 (5 μM) was treated during the differentiation. C’ Quantification. n = 3, *p < 0.05. D Differentiated iFLAG-ETV2, iFLAG-ETV2-MAP2K1-8E (iFLAG-ETV2-M) or iFLAG-ETV2-SPRED1 (iFLAG-ETV2-S) mESC ± Dox were analyzed for FLK1 expression by flow cytometry. D’ Quantification. n = 3, *p < 0.05
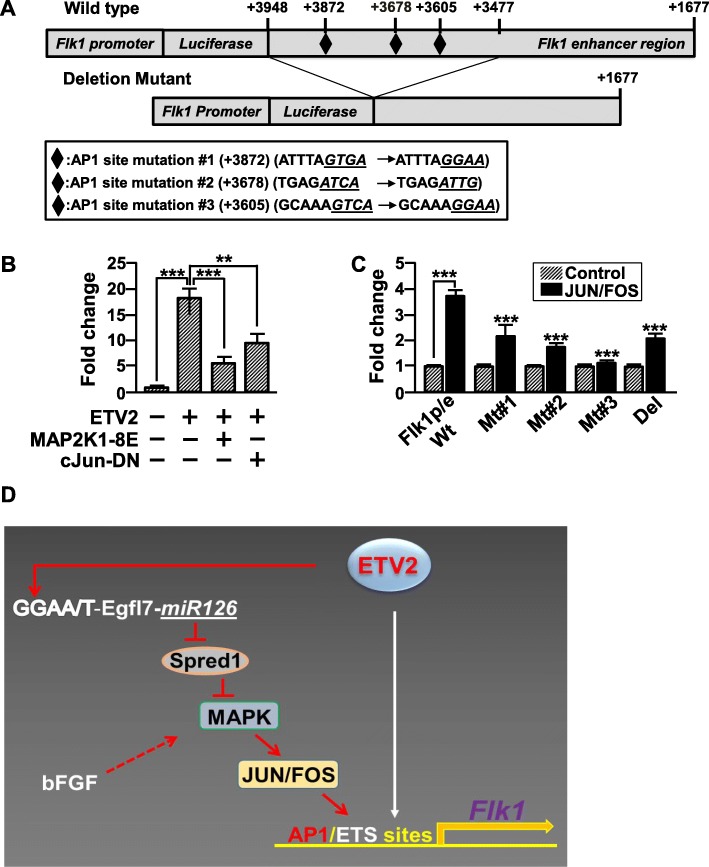
Direct activation of FLK1 gene expression by the MAPK pathway
To get a detailed insight into how the MAPK pathway activated by ETV2 regulates the generation of FLK1+ cells, we examined the regulatory elements such as promoter and enhancer of Flk1 gene [23] and found several potential activator protein 1 (AP1) binding sequences in Flk1 enhancer which is critical for controlling the endogenous expression of Flk1 (Fig. 4a). AP1 (activator protein 1), a heterodimeric transcriptional complex consisting of JUN and FOS proteins, conveys the important functions for multiple biological processes such as differentiation, proliferation and apoptosis [24]. Given the findings that AP1 complex acts as a downstream target of the MAPK pathway [25], these suggest an important mechanistic link between ETV2 and FLK1 gene expression via the MAPK-AP1 pathway. Therefore, we first performed a luciferase-based promoter assay and revealed that the activity of the Flk1-promoter/enhancer (p/e) was increased in response to ETV2 or c-JUN/FOS, a downstream of the MAPK pathway (Fig. 4b, c). In contrast, both MKAP2K1-8E and the JUN dominant negative mutant, c-JUN DN [26], inhibited ETV2 function in activating Flk1-p/e (Fig. 4b). Further, we showed that mutations on the putative AP-1 binding site in the Flk1-p/e led to a significantly reduced luciferase activity induced by c-JUN/FOS (Fig. 4c). Collectively, these results suggest that the MAPK pathway can activate the expression of Flk1 gene partly through c-JUN/FOS.
Fig. 4.
ETV2 activates FLK1 expression through AP-1 binding sites in Flk1 enhancer region. a Schematic diagram of Flk1-promoter-enhancer (p/e)-luciferase plasmid. Three potential AP-1 binding elements were marked as diamonds. b, c Luciferase-based promoter assay. b pGL3-Flk1 p/e was transiently co-transfected with pCMV-Etv2, pMCL-MAP2K1-8E, pCMV-c-Jun ND (dominant negative form of c-Jun) into HEK/293T cells. n = 3, **p < 0.01, ***p < 0.001. c pGL3-luciferase construct carrying wild type (Wt), putative AP-1 binding site mutants (Mt #1, Mt #2, Mt #3) or enhancer deletion mutant (del) of Flk1 p/e was co-transfected with pCMV-c-Jun and c-Fos into HEK/293T cells. Firefly luciferase activity was normalized by Renilla luciferase activity. n = 3, ***p < 0.001. d A working model for ETV2-miR126/MAPK in regulating FLK1 expression. In addition to the direct binding of ETV2 on the ETS binding sites in FLK1 gene, ETV2 activates miR-126, which can target SPRED1, thereby activating the MAPK pathway. JUN/FOS, a downstream signaling complex of the MAPK pathway, subsequently activates gene transcription of FLK1 via the AP1 binding sites
Discussion
In our study, several layers of novel findings on ETV2 function were made. First, we reported genome-wide miRNA profiles in FLK1+ cells generated in response to ETV2, providing a new research resource. Second, we demonstrated the functional significance of the miR-126/MAPK pathway in ETV2-mediated FLK1+ cell generation. Third, we also showed a direct activation of Flk1 enhancer by the JUN/FOS complex. Overall, our results reveal that the miR-126/MAPK pathway constitutes a novel mechanism responsible for ETV2-mediated Flk1 expression (Fig. 4d).
Our miRNA profiling results suggest that ETV2 regulates development of FLK1+ cells, hematopoietic and endothelial cell lineages as well as cardiomyocytes through miRNAs. For example, miR-10b, one of the downregulated miRNAs by ETV2, is a critical regulator for vessel development through targeting FLT-1 (fms-related tyrosine kinase 1), which can inhibit VEGF (vascular endothelial growth factor)-FLK1 signaling [27]. In contrast, miR-146a was upregulated in response to ETV2 expression. Interestingly, miR-146 can target CXCR4 (C-X-C chemokine receptor type 4), a receptor of SDF1 (stromal cell-derived factor 1), whose signaling is important during heart development and promotes cardiogenesis from pluripotent stem cells [28–31]. Another downregulated miRNA by ETV2, miR-106, has been reported as a critical player for cardiac development as evidenced by in utero lethality displaying severe cardiac defects in compound knockout of mouse miR-106~25 and miR-17~92 cluster [32]. Thus, determining biological consequences of the links between ETV2 and ETV2-dependent miRNAs for cardiovascular lineage development would be an interesting topic for further studies.
Studies have shown that miR-126 plays important functions in cardiovascular development and angiogenesis [15–17]. In this study, we provided evidence of the unknown function of miR-126 in generating the first emerging FLK1+ cells. Our results showed that ETV2 via a direct binding on the Egfl7 promoter can induce the expression of miR-126. In the subsequent studies, inhibition of the miR-126/MAPK pathway leads to impairment of ETV2-mediated FLK1+ cell generation. Thus, our results suggest a novel post-transcriptional regulatory mechanism of ETV2 in generating FLK1+ cell. Although EGFL7 is co-transcribed with miR-126 upon ETV2 expression, we rule out the potential contribution of EGFL7 to inducing FLK1+ cell generation due to the dispensable function of EGFL7 in early stages of embryogenesis and embryonic vasculature formation as demonstrated by knockout studies in mice and knockdown experiments in differentiating ESCs [18, 33]. Taken together, it is evident that miR-126 acting downstream of ETV2 plays important roles in the establishment of the cardiovascular system.
The molecular insights into Flk1 gene expression (i.e., determining its upstream regulators) has not been an active research area, despite its critical function in hematopoietic and vascular system. Regarding this, we have demonstrated that ETV2 directly binds ETS binding elements present on both promoter and enhancer of Flk1 and activates its expression [5]. The subsequent ChIP sequencing analysis demonstrated that a wide range of genes critical for endothelial cell and blood cells are direct downstream targets of ETV2 [7]. In this current study, we revealed an important function of MAPK activity in regulating Flk1 gene expression. Overexpression of ETV2 activates the MAPK activity, while inhibition of the MAPK pathway decreases ETV2-mediated FLK1+ cell generation. Further, we showed that the MAPK pathway is able to directly upregulate the expression of Flk1 via a direct binding of JUN/FOS on Flk1 enhancer. Thus, we envision that ETV2 induces Flk1 gene expression in a bimodal manner, one by activating the miR-126/MAPK pathway and the other by its direct binding on Flk1 gene regulatory elements. In mouse embryos, activated ERK1/2 is detected in the blood islands, the first sites of blood and endothelial cell development and later in the dorsal aorta and intersomitic vessels [34]. Further, Erk2-deficient mouse embryos are incompatible with proper mesoderm formation [35, 36]. Sprouty/Spred proteins, the negative effectors for Ras/MAPK pathway [19], have inhibitory functions in generating mesoderm and blood as well as vessel development [37–40], further suggesting important functions of the MAPK pathway in emergence of mesoderm with hematopoietic and endothelial potential. Considering that the enhanced protein stability of ETV2 by OVOL2 is thought to be one mechanism for ETV2-mediated Flk1 gene transcription [14], it would be interesting to see if the interaction between ETV2 and OVOL2 can positively regulate the miR-126/MAPK pathway in this process. In addition, the findings that ETV2 interacts with OVOL2 [14] or FOXC2 [12] and that ETV2 and JUN/FOS acts on Flk1 promoter and enhancer suggest a hypothesis that ETV2 can form a transcriptional complex with OVOL2, FOXC2, and/or AP1 to regulate the expression of Flk1, which could provide an in-depth and novel insight into molecular mechanisms behind the ETV2-FLK1 axis.
In conclusion, we reported miRNA profiles regulated by ETV2 in generating FLK1+ cells from mESCs. Further, we showed that ETV2 can regulate the expression of Flk1 through the miR126/MAPK pathway. These findings could provide a novel insight into the mechanisms of how ETV2 regulates the development of the cardiovascular system. Studying the functions of other miRNAs identified in this research would be important for further deciphering the ETV2-miRNA regulatory mechanisms in FLK1+ cell and cardiovascular lineage generation. Since embryonic events can often play a critical role in adults, examination of our findings in pathophysiological angiogenesis would be warranted.
Supplementary information
Additional file 1. Supplemental materials and methods
Additional file 2: Table S1. Gene Ontology (GO) categories for genes targeted by significant differentially expressed miRNA’s. Table S2. KEGG categories for genes targeted by significant differentially expressed miRNA’s. Table S3. Primer sequences.
Additional file 3: Figure S1. Analysis on miR-126-5p in response to ETV2. Differentiated iFLAG-ETV2 mESCs at day 3.5 were subjected to qRT-PCR analysis. n=3, **p<0.01.
Acknowledgements
We would like to thank Dr. Seyoung Oh for initial technical support. We also would like to thank Emory’s Pediatrics/Winship Flow Cytometry Core.
Abbreviations
- AP1
Activator protein 1
- ChIP
Chromatin immunoprecipitation
- c-JUN DN
JUN dominant negative mutant
- CXCR4
C-X-C chemokine receptor type 4
- Dox
Doxycycline
- EGFL7
Egf-like domain multiple 7
- ERK1/2
Extracellular signal-regulated kinases 1/2
- ETS
E26 transformation-specific
- ETV2
Ets variant 2
- FACS
Fluorescence-activated cell sorting
- FDR
False discovery rate
- FLK1
Fetal liver kinase 1
- Flk1-p/e
Flk1-promoter/enhancer
- FLT-1
Fms-related tyrosine kinase 1
- FOXC2
Forkhead box protein C2
- GO
Gene ontology
- iFLAG-ETV2
Doxycycline-inducible FLAG-ETV2
- MAPK
Mitogen-activated protein kinase
- mESCs
Mouse embryonic stem cells
- OVOL2
Ovo-like zinc finger 2
- RAP1
Ras-associated protein 1
- SDF1
Stromal cell-derived factor 1
- SPRED1
Sprouty-related EVH1 domain containing 1
- VEGF
Vascular endothelial growth factor
- WNT
Wingless-related integration site
Authors’ contributions
JYK, DHL, JKK, HSC, HS, WJP, JYC, and TMK contributed to the collection and assembly of data. JYK and DHL contributed to the design, data analysis, interpretation, and manuscript writing. BD, MR, JK, and SJG contributed to the data analysis. CP contributed to the conception, design, collection and assembly of data, financial support, data interpretation, and manuscript writing. JYK and CP contributed to the final approval of manuscript. All authors read and approved the final manuscript.
Funding
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA13829. This work was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1A02085481) (T.M.K) and the March of Dimes Foundation, 5-FY12-44 (to C.P.), the American Heart Association, 11SDG7390074 (to C. P.), the Children’s Miracle Network, 660085–1116 (to C.P.), Children’s Heart Research and Outcomes Center and Children’s Healthcare of Atlanta 00060337 (to C.P.) and NIH HL119291 (to C.P.).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13287-019-1466-8.
References
- 1.Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem. 2004;91(5):896–903. doi: 10.1002/jcb.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Findlay VJ, LaRue AC, Turner DP, Watson PM, Watson DK. Understanding the role of ETS-mediated gene regulation in complex biological processes. Adv Cancer Res. 2013;119:1–61. doi: 10.1016/B978-0-12-407190-2.00001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh SY, Kim JY, Park C. The ETS factor, ETV2: a master regulator for vascular endothelial cell development. Mol Cells. 2015;38(12):1029–1036. doi: 10.14348/molcells.2015.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DH, Kim TM, Kim JK, Park C. ETV2/ER71 transcription factor as a therapeutic vehicle for cardiovascular disease. Theranostics. 2019;9(19):5694–5705. doi: 10.7150/thno.35300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2(5):497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Kang I, Park C, Chang LW, Wang W, Lee D, Lim DS, Vittet D, Nerbonne JM, Choi K. ER71 specifies Flk-1+ hemangiogenic mesoderm by inhibiting cardiac mesoderm and Wnt signaling. Blood. 2012;119(14):3295–3305. doi: 10.1182/blood-2012-01-403766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F, Li D, Yu YY, Kang I, Cha MJ, Kim JY, Park C, Watson DK, Wang T, Choi K. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO Rep. 2015;16(5):654–669. doi: 10.15252/embr.201439939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci U S A. 2009;106(3):814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa S, Jakt ML, Tarui H, Nishikawa S. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRalpha+ primitive mesoderm. Blood. 2011;118(26):6975–6986. doi: 10.1182/blood-2011-05-352658. [DOI] [PubMed] [Google Scholar]
- 10.Neuhaus H, Muller F, Hollemann T. Xenopus er71 is involved in vascular development. Dev Dyn. 2010;239(12):3436–3445. doi: 10.1002/dvdy.22487. [DOI] [PubMed] [Google Scholar]
- 11.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4(1):e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, Pennacchio LA, Dubchak I, Krieg PA, Stainier DY, Black BL. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135(6):1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldman MB, Lin S. Etsrp/Etv2 is directly regulated by Foxc1a/b in the zebrafish angioblast. Circ Res. 2012;110(2):220–229. doi: 10.1161/CIRCRESAHA.111.251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Lee RH, Kim TM, Kim DW, Jeon YJ, Huh SH, Oh SY, Kyba M, Kataoka H, Choi K, Ornitz DM, Chae JI, Park C. OVOL2 is a critical regulator of ER71/ETV2 in generating FLK1+, hematopoietic, and endothelial cells from embryonic stem cells. Blood. 2014;124(19):2948–2952. doi: 10.1182/blood-2014-03-556332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sturgeon CM, Chicha L, Ditadi A, Zhou Q, McGrath KE, Palis J, Hammond SM, Wang S, Olson EN, Keller G. Primitive erythropoiesis is regulated by miR-126 via nonhematopoietic Vcam-1+ cells. Dev Cell. 2012;23(1):45–57. doi: 10.1016/j.devcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135(24):3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 19.Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K, Tsuneoka M, Komiya S, Baron R, Yoshimura A. Spred is a Sprouty-related suppressor of Ras signalling. Nature. 2001;412(6847):647–651. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- 20.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faloon P, Arentson E, Kazarov A, Deng CX, Porcher C, Orkin S, Choi K. Basic fibroblast growth factor positively regulates hematopoietic development. Development. 2000;127(9):1931–1941. doi: 10.1242/dev.127.9.1931. [DOI] [PubMed] [Google Scholar]
- 22.Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265(5174):966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 23.Kappel A, Ronicke V, Damert A, Flamme I, Risau W, Breier G. Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood. 1999;93(12):4284–4292. doi: 10.1182/blood.V93.12.4284. [DOI] [PubMed] [Google Scholar]
- 24.Bejjani F, Evanno E, Zibara K, Piechaczyk M, Jariel-Encontre I. The AP-1 transcriptional complex: local switch or remote command? Biochim Biophys Acta Rev Cancer. 2019;1872(1):11–23. doi: 10.1016/j.bbcan.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Maik-Rachline G, Hacohen-Lev-Ran A, Seger R. Nuclear ERK: mechanism of translocation, substrates, and role in cancer. Int J Mol Sci. 2019;20(5):1194. doi: 10.3390/ijms20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang ZY, Sato H, Kusam S, Sehra S, Toney LM, Dent AL. Regulation of IL-10 gene expression in Th2 cells by Jun proteins. J Immunol. 2005;174(4):2098–2105. doi: 10.4049/jimmunol.174.4.2098. [DOI] [PubMed] [Google Scholar]
- 27.Hassel D, Cheng P, White MP, Ivey KN, Kroll J, Augustin HG, Katus HA, Stainier DY, Srivastava D. MicroRNA-10 regulates the angiogenic behavior of zebrafish and human endothelial cells by promoting vascular endothelial growth factor signaling. Circ Res. 2012;111(11):1421–1433. doi: 10.1161/CIRCRESAHA.112.279711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiriac A, Terzic A, Park S, Ikeda Y, Faustino R, Nelson TJ. SDF-1-enhanced cardiogenesis requires CXCR4 induction in pluripotent stem cells. J Cardiovasc Transl Res. 2010;3(6):674–682. doi: 10.1007/s12265-010-9219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95(16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 31.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 32.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durrans A, Stuhlmann H. A role for Egfl7 during endothelial organization in the embryoid body model system. J Angiogenes Res. 2010;2:4. doi: 10.1186/2040-2384-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130(19):4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- 35.Saba-El-Leil MK, Vella FD, Vernay B, Voisin L, Chen L, Labrecque N, Ang SL, Meloche S. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4(10):964–968. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Y, Li W, Wu J, Germann UA, Su MS, Kuida K, Boucher DM. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci U S A. 2003;100(22):12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muhl B, Hagele J, Tasdogan A, Loula P, Schuh K, Bundschu K. SPREDs (Sprouty related proteins with EVH1 domain) promote self-renewal and inhibit mesodermal differentiation in murine embryonic stem cells. Dev Dyn. 2015;244(4):591–606. doi: 10.1002/dvdy.24261. [DOI] [PubMed] [Google Scholar]
- 38.Nobuhisa I, Kato R, Inoue H, Takizawa M, Okita K, Yoshimura A, Taga T. Spred-2 suppresses aorta-gonad-mesonephros hematopoiesis by inhibiting MAP kinase activation. J Exp Med. 2004;199(5):737–742. doi: 10.1084/jem.20030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev Cell. 2005;8(5):689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi K, Kohno R, Ayada T, Kato R, Ichiyama K, Morisada T, Oike Y, Yonemitsu Y, Maehara Y, Yoshimura A. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol Cell Biol. 2007;27(12):4541–4550. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental materials and methods
Additional file 2: Table S1. Gene Ontology (GO) categories for genes targeted by significant differentially expressed miRNA’s. Table S2. KEGG categories for genes targeted by significant differentially expressed miRNA’s. Table S3. Primer sequences.
Additional file 3: Figure S1. Analysis on miR-126-5p in response to ETV2. Differentiated iFLAG-ETV2 mESCs at day 3.5 were subjected to qRT-PCR analysis. n=3, **p<0.01.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.