Short abstract
Objective
Vitiligo is a common pigmentation disorder of the skin, and its pathogenesis remains unclear. We evaluated the effectiveness of compound glycyrrhizin combined with fractional carbon dioxide (CO2) laser and triamcinolone acetonide solution in patients with vitiligo.
Methods
Patients with stable vitiligo treated at our hospital between May 2016 and August 2017 were randomized to a control group or an observation group. Both groups were treated with fractional CO2 laser and triamcinolone acetonide solution, while the observation group also received compound glycyrrhizin. Clinical effectiveness, serum IL-17 and TGF-β levels, and adverse drug reactions were compared between the groups.
Results
Following treatment, the clinical effective rate was significantly higher in the observation group than in the control group (75.00% vs. 52.50%). Furthermore, IL-17 decreased in both groups but was significantly lower in the observation group, while TGF-β increased in both groups but was significantly higher in the observation group. The incidence of adverse drug reactions was not significantly different between the groups.
Conclusion
Compound glycyrrhizin in combination with fractional CO2 laser and triamcinolone acetonide solution can be used to treat vitiligo and appears to modulate cytokine levels.
Trial registration: Hebei Provincial Administration of Traditional Chinese Medicine (no. 2019366; http://www.hebwst.gov.cn/index.do?templet=cs_zyj)
Keywords: Vitiligo, compound glycyrrhizin, fractional CO2 laser, IL-17, TGF-β, triamcinolone acetonide
Introduction
Vitiligo is a common pigmentation disorder of the skin, and its specific clinical manifestations include porcelain-white plaque and white hair.1 Although the pathogeny and pathogenesis of vitiligo remain unclear, common pathogenic factors include autoimmunity, self-destruction of melanocytes, genetic factors, and nerve growth factors.2 An autoimmune hypothesis has been recognized, and inflammatory factors have been shown to damage melanocytes and promote a change in disease status.3 There is no definitive treatment for vitiligo available at present. Glucocorticoid has been shown to have a curative effect, but its use is limited by significant side effects.4 Vitiligo can also be treated with glycyrrhizin, the main component of compound glycyrrhizin, which is metabolized into glycyrrhetinic acid in vivo and shows similarities to glucocorticoid in structure but with fewer steroid adverse reactions.5 In the present study, we assessed changes in serum IL-17 and TGF-β levels in patients with vitiligo treated for 6 months with compound glycyrrhizin combined with fractional CO2 laser and triamcinolone acetonide solution to identify a novel effective therapeutic strategy for combined treatment of vitiligo.
Patients and methods
Ethical approval
The study was approved by the Institutional Ethics Committee of Baoding First Central Hospital, and written informed consent was obtained from all participants.
Patients
Patients (Chinese, Fitzpatrick Skin Type III) with vitiligo vulgaris in the stable phase who were treated at our hospital between May 2016 and August 2017 were included in this study. The inclusion criteria were (1) standard diagnosis of vitiligo vulgaris,6 with no development of rash within the 6 months prior to participation; (2) likelihood of good compliance and willingness to undergo an initial examination and subsequent study visits at regular intervals; and (3) no systematic or local use of immunosuppressors within the 3 months prior to participation. Patients were excluded from participation if they met the following criteria: (1) history of severe hepatic and renal function incompetence, hypertension, diabetes, or heart disease; (2) pregnancy or lactation; (3) history of skin cancer or photesthesia; and (4) allergy to the medications used in this study. Eligible patients were randomized to a control group or an observation group.
Treatment methods
Patients in both the control group and the observation group were treated by fractional CO2 laser (6–10 sessions; KL type laser, Jilin King Laser Technology Co., Ltd., China) and triamcinolone acetonide solution (40 mg/5 mL). Laser scanning was performed with laser selection according to the shape and size of the skin lesions, without cooling, and lidocaine was used as local anesthesia. The energy density was 20 to 40 mJ/pulse and the coverage was 10% to 20%. After laser treatment, triamcinolone acetonide solution (Transton™, Kuning Jida Pharmaceutical Co., Ltd., Kunming, China) was placed in a six-layer gauze at a concentration of 0.5 mL/cm2 and immediately applied as a wet compress for 10 minutes every 3 weeks. Following laser treatment, patients were advised to initially avoid contact with water, avoid irritation and friction in the treated area, keep the skin dry and clean, avoid exposure to sun, wear soft underwear, and avoid foods with a high vitamin C content. Halometasone cream (Aoneng™, Hong Kong Bright Future Pharmaceutical Co., Ltd., Hong Kong, China) was applied to the skin once per day.
In addition, patients in the observation group received compound glycyrrhizin tablets at a dose of 50 mg, three times per day (Meineng™, Minophagen Pharmaceutical Co., Ltd., Tokyo, Japan). Both groups received study treatment for 6 months. Patients who experienced adverse drug reactions and adverse reactions to laser treatment were discontinued from study treatment, and symptomatic treatment was administered where necessary.
Assessments
Clinical effect
The vitiligo standards of the pigment dermatology group under the Association of Integrative Dermatologic Medicine were used to evaluate the clinical effect of treatment,7 categorized as healed, significantly effective, effective, or ineffective. The categories were defined as follows: healed: complete fading of vitiligo and recovery of normal skin color; significantly effective: obvious decrease in or local fading of vitiligo, with the area in which normal skin color is recovered representing >50% of the total area of skin damage; effective: decrease in or local fading of vitiligo, with the area in which normal skin color is recovered representing 10% to 49% of the total area of skin damage; ineffective: no decrease in vitiligo area, or an expansion of the affected area. The effective rate of treatment was calculated for both groups at the clinical evaluation time point (6 months) and during follow-up (once per month for 6 months).
Serum IL-17 and TGF-β level
Peripheral venous blood (2–3 mL) was collected from fasting patients and centrifuged for 10 minutes at 2000 rpm and 4°C. The resulting supernatant was stored at −80°C until use. Human IL-17 and TGF-β ELISA kits (Suzhou Calvin Biotechnology Co., Ltd., Suzhou, China) were used to perform double antibody sandwich ELISA according to the manufacturer’s instructions. Optical density at a wavelength of 450 nm was measured using a microplate reader (HT21255; Zhengzhou Humanwell BIOCELL Biotech Co., Ltd., Zhengzhou, China) and used to calculate IL-17 and TGF-β concentration. Assays were performed in triplicate, and the levels of IL-17 and TGF-β were compared.
Adverse drug reactions
The incidence of adverse drug reactions, including hepatic and renal dysfunction, headache, nausea, vomiting, fever, and rash, was assessed by two experienced physicians and compared between the control and observational groups. Patient satisfaction was assessed using a questionnaire, with a score of 0 to 20 considered unsatisfactory, 20 to 40 not very satisfactory, 40 to 60 neutral, 60 to 80 satisfactory, and 80 to 100 very satisfactory.
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). A t-test was used for the comparison of measurement data, and a chi-squared test was used for categorical data. Values of P < 0.05 were considered statistically significant.
Results
Patient characteristics and clinical efficacy
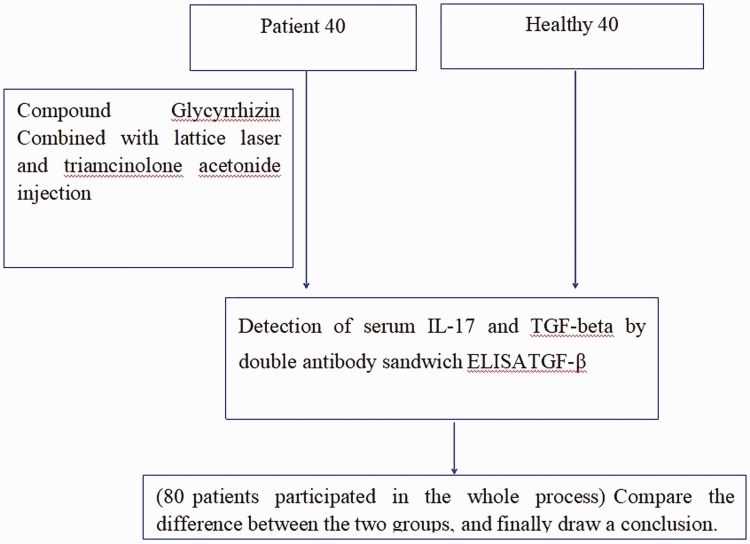
A study flow chart is shown in Figure 1. Eighty patients were enrolled in this study, with 40 randomized to the control group and 40 to the observation group. There were 20 male patients and 20 female patients in the control group, with an average age of 34.05 ± 12.45 years (range: 16–65 years) and average disease duration of 76.26 ± 27.23 months (range: 2–360 months). There were 22 male patients and 18 female patients in the observation group, with an average age of 32.61 ± 10.32 years (range: 18–62 years) and average disease duration of 78.32 ± 24.25 months (range: 3–360 months). All 80 patients participated in the entire study, with no drop outs. The patient characteristics were compared between the two groups, and no statistically significant differences were identified (Table 1).
Figure 1.
Study flow diagram.
Table 1.
Patient characteristics.
| Control group (n = 40) | Observation group (n = 40) | t/χ 2 | P | |
|---|---|---|---|---|
| Gender (male/female) | 20/20 | 22/18 | 0.201 | 0.654 |
| Age (years) | 34.05 ± 12.45 | 32.61 ± 10.32 | 0.565 | 0.574 |
| Height (cm) | 169.24 ± 10.31 | 168.57 ± 10.25 | 0.292 | 0.772 |
| BMI (kg/m2) | 22.25 ± 2.91 | 22.31 ± 2.90 | 0.092 | 0.927 |
| Duration of disease (m) | 76.26 ± 27.23 | 78.32 ± 24.25 | 0.357 | 0.722 |
| Skin involvementa (%) | ||||
| Mild | 22 | 25 | 0.484 | 0.922 |
| Moderate | 12 | 10 | ||
| Moderate-severe | 5 | 4 | ||
| Severe | 1 | 1 |
aSkin involvement: mild: skin involvement area < 1%, moderate 1% to 5%, moderate-severe 6% to 50%, severe >50%. Note: 1% of body area is approximately the size of the palm of the hand.
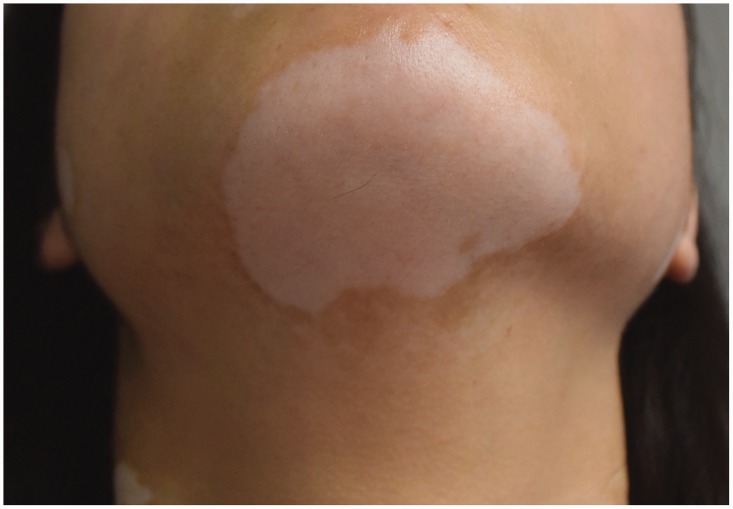
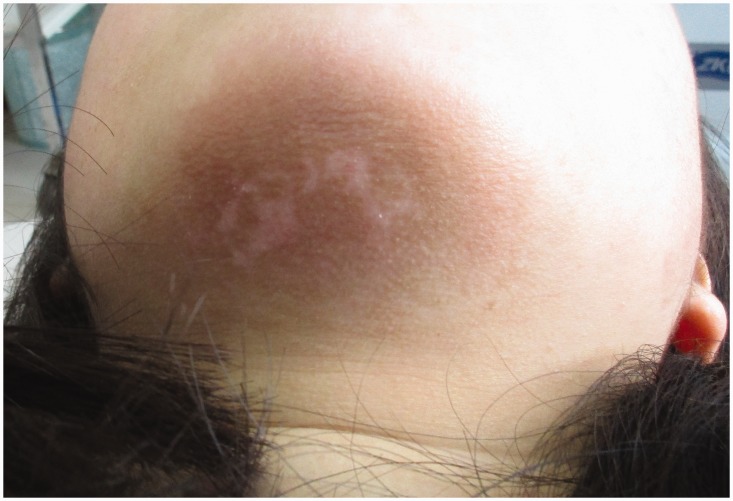
As shown in Table 2, the rate of clinical effectiveness in the observation group was significantly higher than that in the control group (75.00% vs. 52.50%, P < 0.05). Representative images taken before and after treatment in both groups are shown in Figure 2 and Figure 3.
Table 2.
Comparison of degree of improvement between the control and observation groups [n (%)].
| Group | No. | Healed | Significantly effective | Effective | Ineffective | Effective rate |
|---|---|---|---|---|---|---|
| Observation group | 40 | 5 (12.50) | 25 (62.50) | 6 (15.00) | 4 (10.00) | 30 (75.00) |
| Control group | 40 | 5 (12.50) | 16 (40.00) | 13 (32.50) | 6 (15.00) | 21 (52.50) |
| χ 2 | – | – | – | – | – | 4.381 |
| P | – | – | – | – | – | 0.036 |
Figure 2.
Large white spot with a clear border observed in a patient prior to treatment.
Figure 3.
Change in coloration following treatment.
Comparison of serum IL-17 and TGF-β levels
There was no significant difference in serum IL-17 and TGF-β levels between the two groups prior to treatment. After treatment, however, the IL-17 level decreased in both groups, and the IL-17 level in the observation group was significantly lower than that in the control group (P < 0.05). The TGF-β level increased in both groups after treatment, and was significantly higher in the observation group than in the control group (P < 0.05) (Table 3).
Table 3.
Comparison of serum IL-17 and TGF-b levels between the control and observation groups ( ± S).
| Indicator | Time | Control group (n = 40) | Observation group (n = 40) | t | P |
|---|---|---|---|---|---|
| IL-17 (ng/L) | Before treatment | 26.13 ± 4.52 | 26.27 ± 4.80 | 0.134 | 0.894 |
| After treatment | 19.25 ± 4.19a | 14.31 ± 1.57a | 6.983 | < 0.001 | |
| TGF-β (ng/L) | Before treatment | 458.76 ± 88.13 | 456.12 ± 90.57 | 0.132 | 0.895 |
| After treatment | 490.13 ± 92.09a | 582.25 ± 102.51a | 4.228 | < 0.001 |
aComparison with pre-treatment level within the same group, P < 0.05.
Comparison of adverse drug reactions
The incidence of adverse drug reactions was 17.50% in the observation group and 7.50% in the control group, and the difference between the two groups was not statistically significant (Table 4). Besides, both groups had relatively mild adverse drug reactions, and the adverse drug reaction disappeared after treatment. Patient satisfaction was assessed, and all patients were found to be satisfied with their treatment.
Table 4.
Comparison of adverse drug reactions between the control and observation groups [n (%)].
| Group | No. | Fever | Headache | Nausea and vomiting | Rash | Hepatic and renal dysfunction | Occurrence rate |
|---|---|---|---|---|---|---|---|
| Observation group | 40 | 1 | 1 | 3 | 1 | 1 | 7 (17.50) |
| Control group | 40 | 0 | 1 | 1 | 1 | 0 | 3 (7.50) |
| χ 2 | – | – | – | – | – | – | 1.829 |
| P | – | – | – | – | – | – | 0.176 |
Discussion
Vitiligo is considered an autoimmune disease that is mediated by T lymphocytes. CD4+T lymphocytes can be divided into multiple subpopulations, including Th1 cells, Th2 cells, Th17 cells, and regulatory T cells (Tregs), of which Th17 cells can secrete the cytokine IL-17 which plays an important role in multiple inflammatory diseases.8 Tregs mainly secrete the cytokine TGF-β to inhibit the immune response.9
TGF-β can inhibit Th1 and Th2 cell development, but its effect on promoting Th17 cell differentiation and development surpasses its inhibitory effect on Th1 and Th2 cells.10,11 When inflammation occurs, the innate immune system facilitates TH17 cell generation under the synergistic effect of TGF-β, and IL-17 secretion thus increases. TGF-β and IL-17 are closely related to the process of immune balance, and participate in the occurrence, development, and transition stages of vitiligo. IL-17 levels in the peripheral blood of vitiligo patients are higher than those in healthy individuals, especially in the early stage of the disease. In addition, the expression level of IL-17 is positively correlated with the area of skin damage.12 Melanocytes treated with IL-17A in vitro have been shown to shrink and to display a decrease in downstream gene expression and melanin synthesis, leading to reduced melanin production.13,14 TGF-β, as an inhibitor of melanocytes, inhibits melanocyte proliferation and DNA synthesis in healthy individuals.15 Serum TGF-β levels of vitiligo patients have been shown to be lower than those of a control group, and no TGF-β expression was detected by immunoassay in samples from vitiligo lesions and margins.16
Compound glycyrrhizin functions as a parahormone to promote immunoregulation, regulate T cell activation, induce interferon production, activate natural killer cells, enhance T lymphocyte differentiation, reduce melanocyte damage, recover melanocyte function, and restore normal skin color. Compound glycyrrhizin is also used to treat immunity-related diseases because of its ability to reduce the level of IL-17 in peripheral blood.17
The principle of vitiligo treatment by fractional CO2 laser is based on its ability to stimulate the damaged skin to secrete inflammatory cytokines so as to promote melanocyte proliferation and migration and facilitate drug delivery and absorption.18 Topical application of medications is an important dermatological treatment strategy. However, the structure of the skin and the barrier effect of the cuticle can significantly inhibit the effectiveness of topically applied medication.19 Fractional CO2 laser therapy can produce a thermal injury area (i.e., microscopic pores) which is beneficial to drug delivery. The subsequent use of triamcinolone acetonide solution can significantly improve the effectiveness of treatment and stimulate percutaneous immune induction.20 Serum TGF-β levels have been shown to increase in patients treated by fractional CO2 laser for scar tissue.21
In the present study, a clear clinical effect was noted in the observation group treated with compound glycyrrhizin combined with fractional CO2 laser and triamcinolone acetonide solution. Although the serum IL-17 level decreased in both patient groups after treatment, it was significantly lower in the observation group than in the control group. Similarly, while serum TGF-β level increased in both groups, it was significantly higher in the observation group than in the control group. Thus, the effective therapeutic mechanism of vitiligo appears to be related to a decrease in IL-17 level and corresponding increase in TGF-β level. IL-17 levels in the peripheral blood of vitiligo patients are elevated and TGF-β levels reduced compared with those in healthy individuals, indicating the presence of an autoimmune disorder. Furthermore, both groups showed a low incidence of adverse drug reactions, supporting the safety of compound glycyrrhizin combined with fractional CO2 laser and triamcinolone acetonide solution for vitiligo treatment. A limitation of the present study is its small sample size, meaning that the conclusions should be interpreted with caution. Further studies in larger patient cohorts are required to confirm whether the effective therapeutic mechanism of compound glycyrrhizin combined with fractional CO2 laser and triamcinolone acetonide solution in vitiligo treatment is related to IL-17 and TGF-β.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research was funded by Hebei Provincial Administration of Traditional Chinese Medicine (no. 2019366).
References
- 1.Bian Z. Chinese clinical dermatology. Phoenix Science Press, 2010, pp.1270–1271. [Google Scholar]
- 2.Kemp EH , Waterman EA, Weetman AP, et al. Autoimmune aspects of vitiligo. [J]. Autoimmunity 2001; 34: 65–77. [DOI] [PubMed] [Google Scholar]
- 3.Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. [J]. Pigment Cell Res 2003; 16: 208–214. [DOI] [PubMed] [Google Scholar]
- 4.Kanwar A J, Mahajan R, Parsad D. Low-dose oral min-pulse dexamethasone therapy in progressive unstable vitiligo [J]. J Cutan Med Surg 2013, 17: 259–268. [DOI] [PubMed] [Google Scholar]
- 5.Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds[J]. Phytother Res 2008, 22: 709–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pigmentology Group of the Professional Committee of Dermatology and Venereal Diseases of the Chinese Society of Integrated Traditional Chinese and Western Medicine. Standards for diagnosis and treatment of chloasma and vitiligo (2010 edition). Chinese J of Dermatology 2010; 43: 373. [Google Scholar]
- 7.Taieb A., Alomar A, Böhm M, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus[J]. Br J Dermatol 2012, 16: 5–19. [DOI] [PubMed] [Google Scholar]
- 8.Kurasawa K, Hirose K, Sano H, et al. Increasing interleukin-17 production in patients with patients with systemic sclerosis. Arthritis Rheum 2000; 43: 2455–2463. [DOI] [PubMed] [Google Scholar]
- 9.Peterson R A. Regulatory T-Cell: Diverse Phenotypes Integral to Immune Homeostasis and Suppression. [J]. Toxicologic Pathology 2012; 40: 186–204. [DOI] [PubMed] [Google Scholar]
- 10.Kotobuki Y, Tanemura A, Yang L, et al. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res 2012; 25: 219–230. [DOI] [PubMed] [Google Scholar]
- 11.Alanko T, Saksela O. Transforming growth factor beta 1 induces apoptosis in normal melanocytes but not in nevus cells grown in type 1 collagen gel. J Invest Dermatol 2000; 115: 286–291. [DOI] [PubMed] [Google Scholar]
- 12.Moretti S, Spallanzani A, Amato L, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res 2002; 15: 87–92. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, An X, Dong J, et al. IL-17 induces cellular stress microenvironment of melanocytes to promote autophagic cell apoptosis in vitiligo. [J]. FASEB J 2018; 32: 4899–4916. [DOI] [PubMed] [Google Scholar]
- 14.Singh RK, Lee Km, Vujkovic-Cvijin L, et al. The role of IL-17 in vitiligo: A review. [J]. Autoimmun Rev 2016; 15: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krasagakis K, Garbe C, Schriee PI, et al. Paracrine and autocrine regulation of human melanocyte and melanoma cell growth by transforming growth factor beta in vitro. [J]. Anticancer Res 1994; 16: 2565–2571. [PubMed] [Google Scholar]
- 16.van Beelen AJ, Teunissen MB, Kapsenberg ML, et al. Interleukin-17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol 2007; 7: 374–381. [DOI] [PubMed] [Google Scholar]
- 17.Yu JJ, Zhang CS, Coyle ME, et al. Compound glycyrrhizin plus conventional therapy for psoriasis vulgaris: a systematic review and meta-analysis of randomized controlled trials. [J]. Curr Med Res Opin 2017; 33: 279–287. [DOI] [PubMed] [Google Scholar]
- 18.Basak PY, Adiloglu AK, Ceyhan AM, et al. The role of helper and regulatory T Cells in the pathogenesis of vitiligo. J Am Acad Dermatol 2009; 60: 256–260. [DOI] [PubMed] [Google Scholar]
- 19.Wohlrab J. Topical preparations and their use in dermatology. [J]. J Dtsch Dermatol Ges 2016; 14: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 20.Suntiparpluacha M, Tammachote N, Tammachote R. Triamcinolone acetonide reduces viability, induces oxidative stress, and alters gene expressions of human chondrocytes. [J]. 2016; 20: 4985–4992 [PubMed] [Google Scholar]
- 21.Makboul M, Makboul R, Abdelhafez AH, et al. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. [J]. 2014; 13: 169–179. [DOI] [PubMed] [Google Scholar]