Short abstract
Objective
To evaluate the effect of GSTA3 within the PI3K–Keap1/Nrf2 pathway in renal interstitial fibrosis (RIF).
Methods
An in vitro RIF model with TGF-β1 stimulation in NRK-52E cells was established to identify potential signaling pathways that modulate GSTA3. Changes in GSTA3 expression were observed in the RIF model after silencing or enhancing Nrf2 expression. Changes in GSTA3, Keap1, and Nrf2 expression were detected after blocking the upstream of the Keap1/Nrf2 signaling pathway (including MAPK and PI3K/Akt). The effect of Nrf2 on GSTA3 expression was evaluated by overexpressing Nrf2.
Results
Protein and mRNA levels of GSTA3, FN, Nrf2, and Keap1 were significantly increased after TGF-β1 stimulation. GSTA3 was also upregulated following overexpression of Nrf2. TGF-β1 activated the PI3K/Akt signaling pathway, leading to RIF. After blocking this pathway, the production of superoxide dismutase, reactive oxygen species, and fibronectin were reduced. The MAPK pathway was not involved in the development of RIF via regulating GSTA3 expression.
Conclusions
The PI3K–KEAP1/Nrf2–GSTA3 signaling pathway is a possible mechanism of resisting external stimulation of renal fibrosis factors, regulating oxidative stress, and preventing RIF.
Keywords: GSTA3, PI3K–Keap1/Nrf2, renal interstitial fibrosis, antioxidant, reactive oxygen species, TGF-β1
Introduction
Glutathione transferases (GSTs) are widely distributed in organisms and the biosphere and are dual functional metabolic and antioxidant enzymes.1 Glutathione S-transferase alpha 3 (GSTA3) is one of the most important members of the GST family, as it is involved in detoxification and cellular defense. GSTA3 plays a critical role in various diseases associated with oxidation-regulating proteins. Furthermore, murine GSTA3 knockout models suffer extensive liver fibrosis.2 Our previous studies have found that GSTA3 expression is significantly downregulated in the renal cortex of unilateral ureteral obstruction model rats and that GSTA3 can alleviate renal interstitial fibrosis (RIF) by downregulating fibronectin (FN) expression.3 However, the molecular mechanism underlying how GSTA3 alleviates renal tubulointerstitial fibrosis is not fully understood.
Nuclear factor-erythroid-2-related factor 2 (Nrf2, also known as NFE2L2) plays a central role in the basal activity and coordinated induction of more than 250 genes, such as those encoding phase II antioxidant detoxifying enzymes and related proteins.4 Nrf2 is distributed in the cytoplasm as an inactive complex bound to Kelch-like ECH-associated protein 1 (Keap1), a repressor molecule that facilitates Nrf2 ubiquitination. Electrophilic reagents or oxidative stress cause an uncoupling of Nrf2 and Keap1. Subsequent phosphorylation of Nrf2 allows its transfer into the nucleus, where it combines with antioxidant response elements (AREs) to improve the cellular capacity to deal with antioxidant stress.5–7 Many studies have indicated that the Keap1–Nrf2 signaling pathway is a pivotal mechanism of antioxidant defense, and Keap1–Nrf2 are thought to be upstream of GSTA3 and involved in its regulation.7,8 The mRNA levels of Nrf2-regulated genes are involved in the production and utilization of GSTA3 in the rat brain under oxidative stress.9 Additionally, genetic ablation of Nrf2 causes lupus-like autoimmune nephritis and exacerbates diabetes mellitus-induced oxidative stress, inflammation, and nephropathy in experimental animals.10 However, the effect of Nrf2 on GSTA3 has not been adequately evaluated in renal diseases.
Several signaling pathways are related to renal fibrosis, such as mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K) and Smad.11–14 Numerous studies have confirmed that MAPK and PI3K act as upstream regulators of Nrf2 that are involved in facilitating nuclear translocation and transcriptional activation of Nrf2.15,16 In human EA.hy926 endothelial cells, miltirone has been proven to resist oxidized low-density lipoprotein-derived oxidative stress and to activate Nrf2 via the MAPK-dependent signaling pathway.17 Cardiomyocytes are protected from anoxic damage through the MAPK–Nrf2 signaling pathway by 5-Aminolevulinic acid with sodium ferrous citrate.18 Additionally, anthocyanin supplementation through diet can mitigate oxidative stress, neuro-degeneration, and memory impairment in a mouse model of Alzheimer’s disease by activating the PI3K/Nrf2 pathway.19 Thus, we hypothesize that the anti-renal fibrotic function of GSTA3 is associated with the MAPK–Nrf2 or PI3K–Nrf2 pathways.
In this study we characterized the relationship between GSTA3 and Keap1/Nrf2 in RIF. We further investigated the role of the MAPK and PI3K signaling pathways in regulating GSTA3 expression, with the overall goal of identifying the underlying molecular mechanism through which GSTA3 alleviates the severity of RIF.
Materials and methods
Reagents
Recombinant human TGF-β1 was purchased from PeproTech Inc. (Rocky Hill, NJ, USA). LY294002 was purchased from Promega (Madison, WI, USA). Anti-FN and anti-α-Tubulin antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). RIPA lysis buffer and 100 mM PMSF were purchased from KeyGEN Biotech (Nanjing, China). Antibodies against p-ERK1/2, p-p38, and p-JNK were purchased from Cell Signaling Technology (Danvers, MA, USA). All MAPK inhibitors were purchased from Calbiochem (Darmstadt, Germany). Anti-GSTA3 and anti-Nrf2 antibodies were purchased from Abcam (Cambridge, UK). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA).
Cell culture
NRK-52E cells were obtained from Jinan University (Guangdong, China) and were cultured in DMEM (Gibco BRL, Life Technologies Inc., Gaithersburg, MD, USA) supplemented with 10% FBS (Gibco BRL), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C and 5% CO2.
Construction of the cellular RIF model
NRK-52E cells were seeded in a 6-well plate when the cells reached approximately 60% to 70% confluence, and then were transferred to serum-free medium and incubated for 24 hours before TGF-β1 stimulation. Recombinant human TGF-β1 was added into the culture medium at a final concentration of 5 ng/mL according to previously described procedures.3
Cell exposure
The obtained NRK-52E cells were subsequently treated with 10% FBS medium containing TGF-β1 for 6, 12, 24 and 48 hours.
Nrf2 plasmid or siRNA transfection
NRK-52E cells were transiently transfected with pcDNA3.1(+)-Nrf2 or pcDNA3.1(+) using Lipofectamine 2000 according to the manufacturer's instructions. The Nrf2 plasmid was constructed by Genepharma (Shanghai, China). The siRNAs against Nrf2 and the negative controls were designed and synthesized by Genepharma. These reagents were transfected into NRK-52E cells with Lipofectamine 2000 according to the product manual. Cells were collected at the indicated time points.
Cell viability
Cell viability was measured to evaluate the cytotoxic effects of treatments. NRK-52E cells were seeded (3 × 103 cells per well) in 96-well plates. After the cells had adhered, been transfected and treated with reagents, they were incubated at 37°C for 24 hours. Then, 10 µL CCK-8 solution (Invitrogen) was added to each well and a set of blank control wells, which were then further incubated at 37°C for 1.5 hours. Absorbance at 450 nm was measured using an automatic microplate reader (Bio-Rad, Hercules, CA, USA).
Screening signaling pathways
MAPK signaling includes the c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and P38 pathways. The P38 inhibitor SB203580, the JNK inhibitor SP600125, and the ERK inhibitor U0126 were used to individually block MAPK signaling. NRK-52E cells were pretreated with SB203580 (10 µM) for 1 hour, and subsequently treated with TGF-β1 for 48 hours. Additionally, cells were treated with U0126 (25 mM) for 1 hour in advance, and then TGF-β1 was added to the medium for 48 hours. SP600125 (20 µM) was dissolved to 2% FBS DMEM medium to treat NRK-52E cells for 1 hour. Then, TGF-β1 was supplemented to the medium and cultured for 48 hours. Afterward, the cells were harvested for further experiments. LY294002 is a potent and specific cell-permeable inhibitor of phosphatidylinositol 3-kinases (PI3K). NRK-52E cells were cultured for 24 hours in serum-free medium before blocking the PI3K/Akt signaling pathway. LY294002 was added to DMEM supplemented with 2% FBS at a final concentration of 20 µmol/L, and then 1 hour later, TGF-β1 was supplemented to the medium. Protein expression in treated cells was measured at 48 hours.
Real-time PCR
Total RNA of NRK-52E cells was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized using equal amounts of RNA from each specimen and the reverse transcription kit from Takara Bio Inc. (Shiga, Japan). The specific primers for target genes were designed from their GenBank sequences and synthesized by GENEray (Shanghai, China) as illustrated in Table 1. A mixture containing primers and cDNA was amplified by PCR. The reaction system was formulated in accordance with the specifications for SYBR Premix EX Taq kits (Takara Bio Inc.). The reaction conditions were as follows: pre-denaturation at 95°C for 30 seconds, denaturation at 95°C for 5 seconds, annealing at 58°C to 60°C (annealing temperature according to different primers), extension for 10 seconds (40 cycles), and then 95°C for 15 seconds. The PCR products were quantified, and then mRNA expression was calculated from the standard curve and normalized with β-actin.
Table 1.
Nucleotide sequences of the primers used for real-time PCR.
| Genes | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| GSTA3 | AACCGTTACTTTCCTGCCTTTG | GCCCTGCTCAGCCTATTGC |
| Fibronectin | GTGTCCTCCTTCCATCTTC | CAGACTGTCGGTACTCACG |
| Keap1 | TTCTGGGGATCCATGCAGCCAGATCCCAGG | AACAAACTCGAGTCACACGGTACAGTTCTG |
| Nrf2 | TTTGTAGATGACCATGAGTCGC | TCCTGCCAAACTTGCTCCAT |
| β-actin | CGTTGACATCCGTAAAGACC | GGAGCCAGGGCAGTAATCT |
Western blot
NRK-52E cells were lysed with RIPA Lysis Buffer on ice, and the lysates were centrifuged. Protein concentration was determined by enzyme linked immunoassay (Bio-Rad) and balanced with RIPA buffer. Proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a nitrocellulose membrane (Millipore, Billerica, MA, USA). Membranes were blocked in 5% fat-free milk for 2 hours at room temperature. After the membranes were washed with TBST three times, they were incubated with corresponding primary antibodies at 4°C overnight. After the membranes were washes with TBST three times again, they were incubated with horseradish peroxide-conjugated secondary antibodies for 2 hours. Protein expression was detected and images were generated using an Odyssey CLx Imaging System (LI-COR, Lincoln, NE, USA).
Detection of reactive oxygen species (ROS) and superoxide dismutase (SOD)
Changes in intracellular ROS levels were determined by measuring the oxidative conversion of cell-permeable 2’,7’-dichlorofluorescein diacetate (DCFH-DA) to fluorescent dichlorofluorescein (DCF) by flow cytometry. Briefly, treated cells were washed with PBS and incubated with DCFH-DA (5 mM, Invitrogen) at 37°C for 20 minutes. The cells were then re-suspended in 1 mL of PBS. DCF fluorescence was then measured by flow cytometry. Fluorescence data were expressed as the percentage change compared with untreated samples. SOD activity was determined using a kit from Nanjing Jiancheng Biological Company (Nanjing, China).
Statistical analysis
Data are expressed as the mean ± SEM. Statistical tests were performed using SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA). An unpaired Student's t-test was used to compare the mean values between two groups. One-way ANOVA with the LSD test were performed for multiple group comparisons. A P value less than 0.05 was considered as statistical significance.
Results
Expression of GSTA3, Nrf2, Keap1, and FN in NRK-52E cells after TGF-β1 stimulation
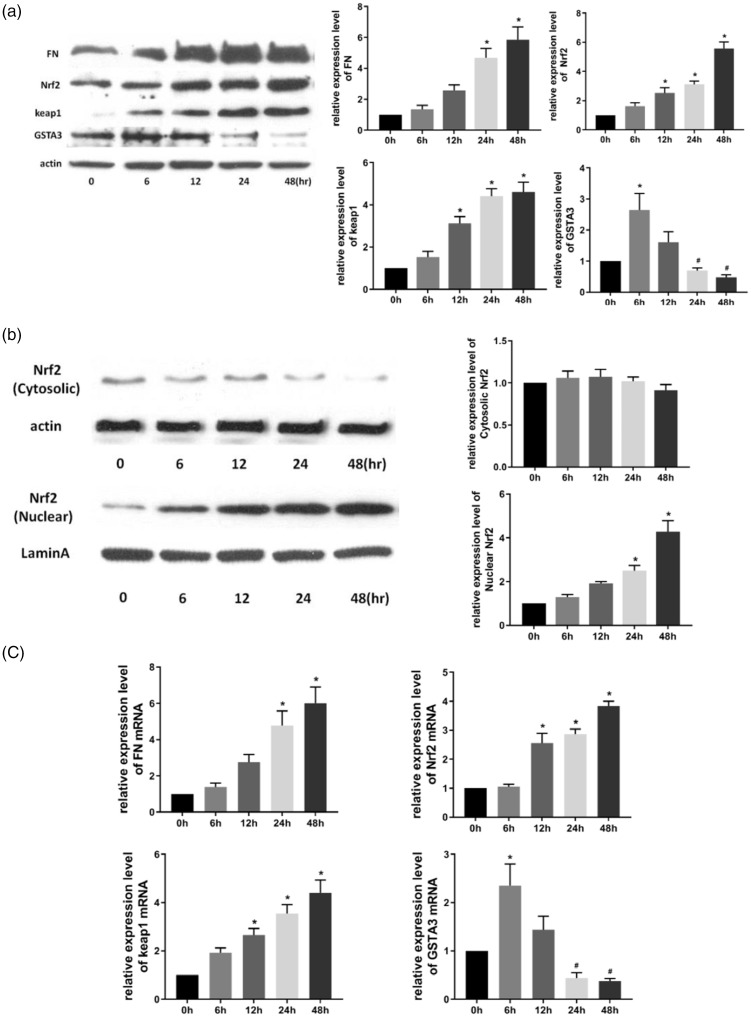
With prolonged TGF-β1 stimulation, FN levels were gradually increased; moreover, prolonged TGF-β1 treatment, increased the expression of FN, Nrf2, and Keap1 at the protein and mRNA levels, especially at 24 and 48 hours of TGF-β1 treatment (both P<0.05) (Figure 1a and c). GSTA3 expression at the protein and mRNA levels was upregulated within 6 hours, and subsequently downregulated in NRK-52E cells. GSTA3 expression was significantly upregulated at 6 hours, and significantly downregulated at 24 and 48 hours (all P<0.05) (Figure 1a and c). Nuclear Nrf2 levels were significantly upregulated after TGF-β1 treatment for 24 and 48 hours (both P<0.05) (Figure 1b), whereas cytoplasmic Nrf2 levels were unchanged.
Figure 1.
The expression of GSTA3, Nrf2, Keap1, and FN. (a) Effect of TGF-β1 stimulation in NRK-52E cells by western blot. After NRK-52E cells were treated with 5 ng/mL TGF-β1 for 0, 6, 12, 24, and 48 hours, GSTA3, FN, Nrf2, and Keap1 protein expression were analyzed by western blot; (b) NRK-52E cells were treated with 5 ng/mL TGF-β1 for 0, 6, 12, 24, and 48 hours, and then the expression of Nrf2 in cytoplasmic and nuclear fractions of NRK-52E cells were analyzed; (c) Effect of TGF-β1 stimulation on NRK-52E cells by RT-PCR. After NRK-52E cells were treated with 5 ng/mL TGF-β1 for 0, 6, 12, 24, and 48 hours, GSTA3, FN, Nrf2, and Keap1 mRNA levels were analyzed by real-time PCR. Results are presented as the mean ± standard error of five independent experiments. *P<0.05 vs. 0 hours, #P<0.05 vs. 6 or 12 hours.
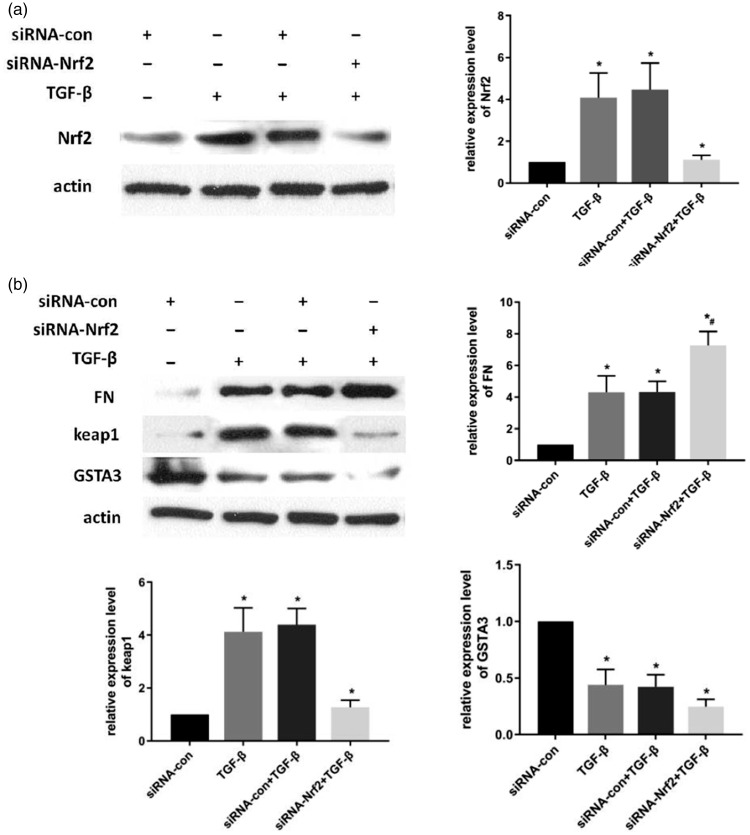
Effect of Nrf2 knockdown or overexpression on GSTA3 levels
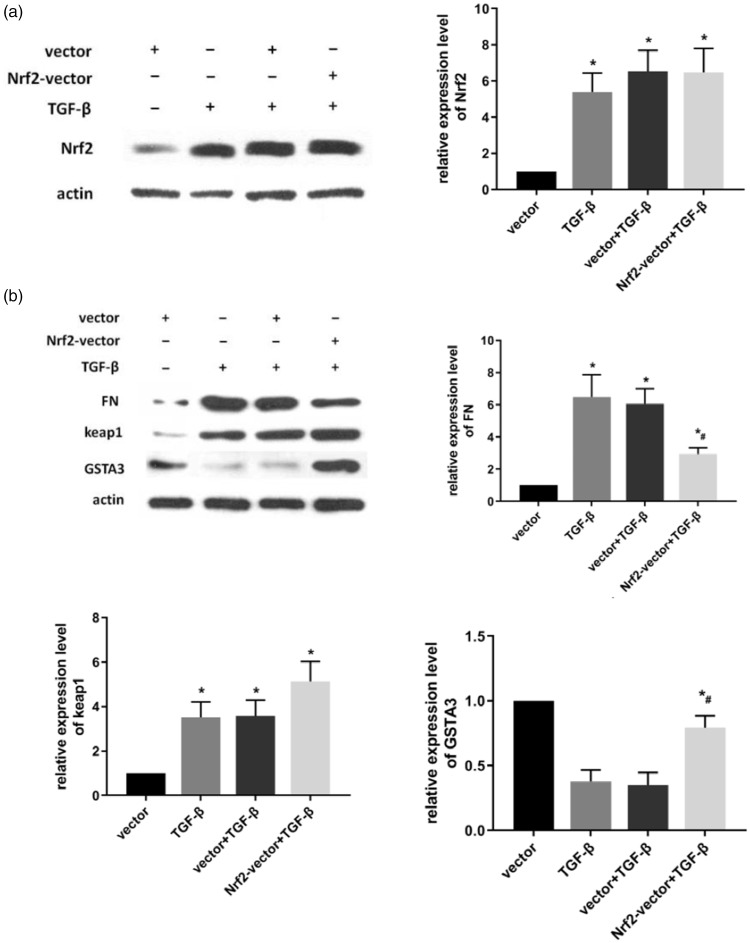
To demonstrate the relationship between Nrf2 and GSTA3 in RIF, TGF-β1 was used to stimulate NRK-52E cells that had been transfected with Nrf2 siRNA or Nrf2 plasmid. Western blot revealed that FN expression was significantly increased in the siRNA-transfected group compared with NRK-52E cells without Nrf2 siRNA treatment under TGF-β1 stimulation. Keap1 and GSTA3 were significantly downregulated at the protein and mRNA levels in the siRNA-transfected NRK-52E cells compared with NRK-52E cells without Nrf2 silencing (P<0.05) (Figure 3b). The result of Nrf2 overexpression was the opposite to that of Nrf2 knockdown. In the Nrf2 plasmid transfected group, Keap1 and GSTA3 levels were significantly upregulated compared with cells without Nrf2 overexpression after 24-hour TGF-β1 stimulation. Compared with the non-transfected Nrf2 cells, FN protein expression was significantly decreased in cells overexpressing Nrf2 following RIF induction (P<0.05) (Figure 2b).
Figure 3.
Effect of Nrf2 knockdown in NRK-52E cells. After 6-hour incubation with siRNA-Lipofectamine 2000 complexes, cells were maintained with normal DMEM for 18 hours. (a) Cells were treated with TGF-β1 and harvested for Nrf2 expression after 24 hours; (b) Cells were treated with TGF-β1 and harvested for FN, Keap1, and GSTA3 expression after 24 hours. Results are presented as the mean ± standard error of four independent experiments. *P<0.05. vs. siRNA-com, #P<0.05 vs. siRNA-com + TGF-β1.
Figure 2.
Effect of overexpressing Nrf2 in TGF-β- treated NRK-52E cells. (a) After the transfected cells were treated with 5 ng/mL TGF-β1 for 24 hours, Nrf2 protein expression was analyzed by western blot; (b) After the transfected cells were treated with 5 ng/mL TGF- β1 for 24 hours, FN, Keap1, and GSTA3 protein levels were analyzed by western blot. Results are presented as the mean ± standard error of four independent experiments. *P<0.05 vs. vector, #P<0.05 vs. vector + TGF-β1.
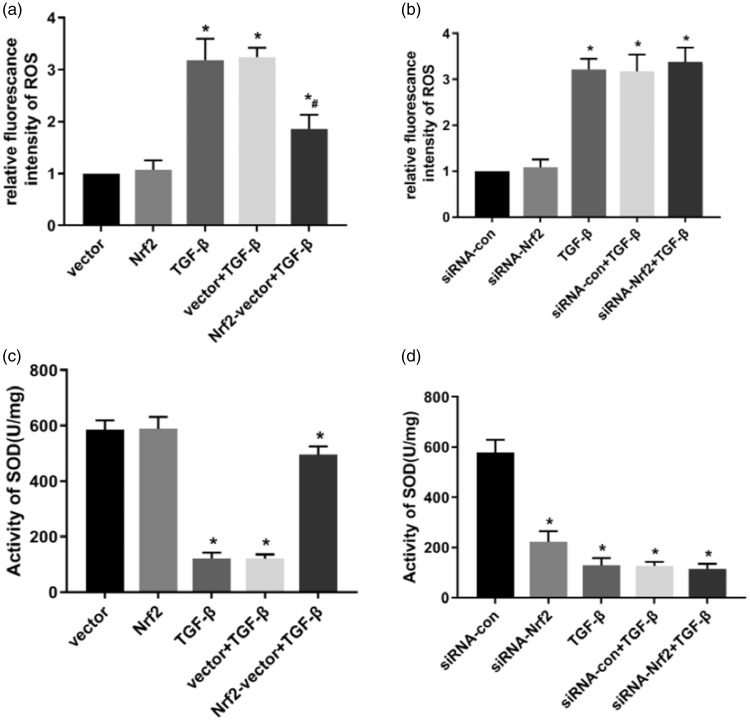
Nrf2 protected against TGF-β1-induced oxidative damage in NRK-52E cells
To determine whether Nrf2-regulated ROS production and whether SOD activity was associated with TGF-β1-induced oxidative damage, changes of ROS production and SOD activity were determined following Nrf2 overexpression and silencing. ROS production was significantly increased, whereas SOD activity remained unchanged in the Nrf2 siRNA-transfected cells (both P<0.05) (Figure 4b, d). Treating cells with 5 ng/mL TGF-β1 for 15 minutes resulted in a significant increase in ROS production and an evident decrease in SOD activity in NRK-52E cells (both P<0.05). In contrast, ROS levels were significantly decreased and SOD activity was significantly increased following Nrf2 overexpression and TGF-β1 treatment (both P<0.05) (Figure 4a, c), suggesting that an inhibition of ROS production was correlated with the protective effect of Nrf2 upon TGF-β1-induced oxidative damage.
Figure 4.
Effect of Nrf2 on NRK-52E cellular ROS. (a) ROS level following treatment with 5 ng/mL TGF-β1 for 15 minutes in Nrf2-overexpressing cells; (b) ROS level following Nrf2 knockdown in NRK-52E cells; (c) SOD activity in Nrf2-overexpressing cells after treatment with 10 ng/mL TGF-β1 for 15 minutes; (d) SOD activity following Nrf2 knockdown in NRK-52E cells. Results are presented as the mean ± standard error of five independent experiments. *P<0.05 vs. vector or siRNA-com, #P<0.05 vs. vector + TGF-β1 or siRNA-com + TGF-β1.
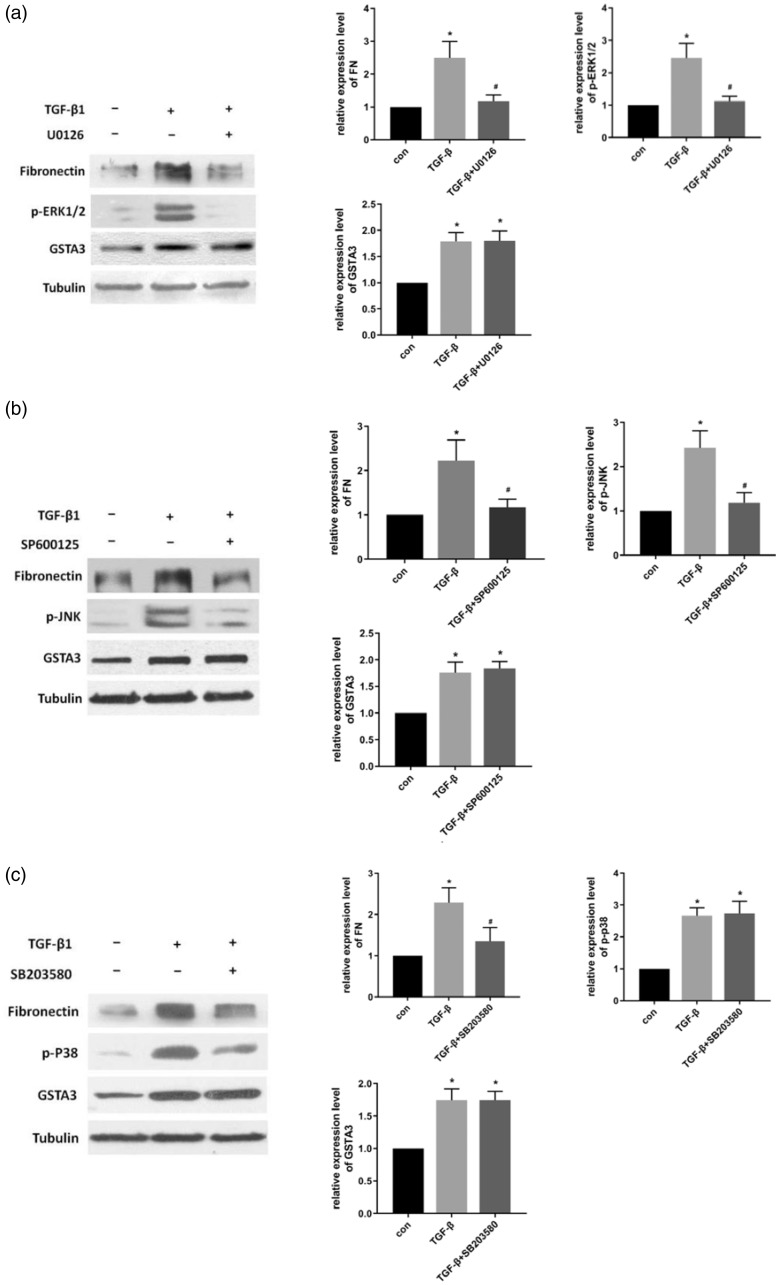
Effect of blocking P38 MAPK, JNK, and ERK signaling on GSTA3 expression and RIF
To investigate the link between GSTA3 and the MAPK pathways, MAPK inhibitors were used to individually block the P38, JNK, and ERK pathways. NRK-52E cells treated with MAPK inhibitors and TGF-β1 were harvested for western blot. The results demonstrated that TGF-β1 activated the MAPK signaling pathway and increased the levels of p-P38, p-JNK, and p-ERK1/2 in NRK-52E cells. Moreover, after blocking the P38, JNK, and ERK pathways, levels of FN, a RIF biomarker, were clearly downregulated. However, GSTA3 expression in the MAPK inhibitor group did not significantly differ from that in the TGF-β1 stimulation group (Figure 5).
Figure 5.
Effect of blocking MAPK signaling in NRK-52E cells. (a) Effect of blocking ERK signaling: NRK-52e cells were pretreated with ERK inhibitor (U0126, 25 mM) for 1 hour, and then induced with 5 ng/mL TGF-β1 for 48 hours; (b) Effect of inhibiting the JNK pathway: cells were pretreated with the JNK inhibitor SP600125 (20 µM) for 1 hour, and then incubated with 5 ng/mL TGF-β1 for 48 hours; (c) Effect of blocking the P38 pathway: after pretreatment with SB203580 (10 µM) for 1 hour, the cells were treated with 5 ng/mL TGF-β1 for 48 hours.
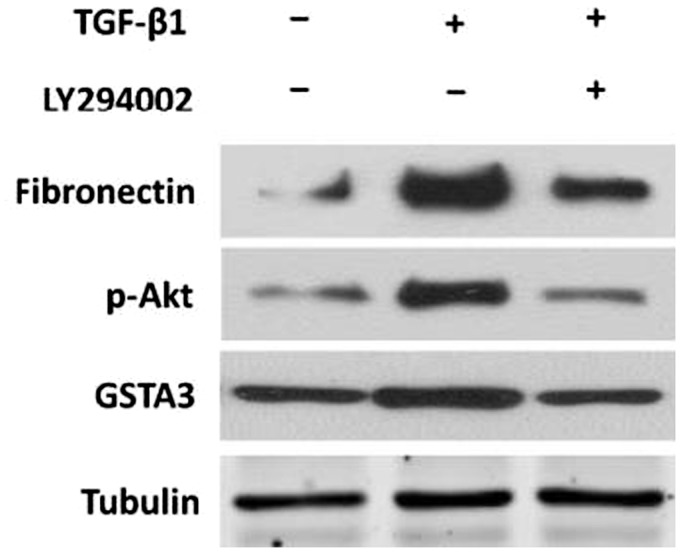
Effect of blocking PI3K/Akt signaling on GSTA3 expression and RIF
To confirm whether the PI3K/Akt pathway was associated with the inhibitory effect of GSTA3 on RIF, the PI3k/Akt inhibitor LY294002 was used for subsequent experiments. Western blot was performed to detect the levels of target proteins in RIF models and normal NRK-52E cells. In the TGF-β1-stimulated cells with PI3K inhibitor treatment, the levels of both FN and GSTA3 were significantly decreased compared with TGF-β1-stimulated cells without PI3K inhibitor (Figure 6).
Figure 6.
Effect of blocking PI3K/Akt signaling. LY294002 (20 µmol/L) pretreatment for 1 hour was followed by incubating the cells with 5 ng/mL TGF-β1 and 20 µmol/L LY294002 for 48 hours.
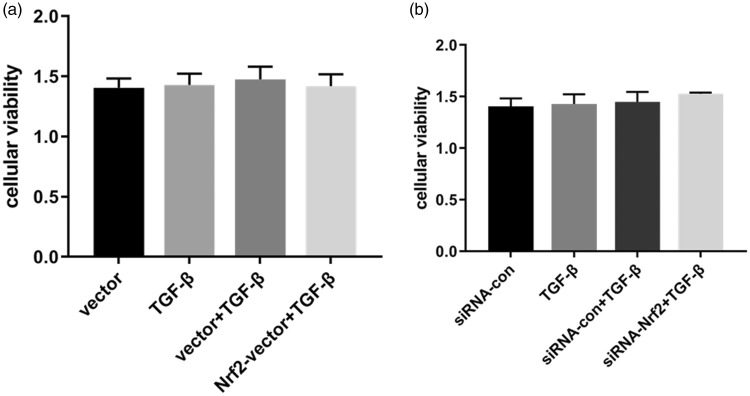
Cell viability
As illustrated in Figure 7, NRK-52E cells were successfully transiently transfected with pcDNA3.1(+)-Nrf2 or pcDNA3.1(+) using Lipofectamine 2000. Next, the transfected cells were treated with TGF-β1 for 24 hours, and cell viability was measured by CCK-8. siRNAs against Nrf2 and negative controls were also transfected into NRK-52E cells with Lipofectamine 2000. No statistical significance was observed in terms of cell viability among the different groups.
Figure 7.
CCK-8 measurements of cell viability. (a) NRK-52E cells were transiently transfected with pcDNA3.1(+)-Nrf2 or pcDNA3.1(+) using Lipofectamine 2000. The transfected cells were treated with TGF-β1 for 24 hours, and cell viability was measured by the CCK-8 assay. (b) siRNAs against Nrf2 and negative controls were transfected into NRK-52E cells with Lipofectamine 2000. Results are presented as the mean ± standard error of four independent experiments.
Discussion
RIF is the end-stage pathological feature related to the progression of advanced renal diseases.20 It has been confirmed that oxidative stress is significantly associated with the incidence of RIF.21 Recently, increasing evidence has suggested that cell signaling pathways that regulate oxidative stress play a pivotal role in the occurrence and progression of RIF through multiple cytokines.22 GSTA3 plays a critical role in various diseases associated with oxidation-regulating proteins. In a previous study, we demonstrated that GSTA3 attenuated TGF-β1-induced RIF, suggesting GSTA3 is a potential therapeutic target for RIF treatment. Nevertheless, the underlying molecular mechanism that regulates GSTA3 remains largely unknown. Consequently, this study was designed to explore the underlying molecular mechanism of GSTA3 in RIF.
In this study, we first observed changes in GSTA3 expression in NRK-52E cells stimulated with TGF-β1, and found that GSTA3 expression was rapidly increased to a peak level at 6 hours after TGF-β1 stimulation. GSTA3 was then gradually downregulated, and eventually was not expressed. Therefore, we assume that oxidative stress occurs in renal tubules at the early stage of RIF, and GSTA3 expression rises rapidly to protect renal tubular epithelial cells from oxidative stress. However, GSTA3 production does not compensate for the large amount of antioxidant stress over a prolonged stimulation time, leading to its gradual decline, which is one of the processes of GSTA3 in protecting renal tubules from oxidative stress-induced damage.
Nrf2 is a pivotal protein during oxidative stress reactions that also regulates downstream phase II metabolic enzymes and antioxidant protein/enzymes, which play vital roles in cellular protection.23 Our results confirmed that Nrf2 was upregulated and that its nuclear translocation was observed in TGF-β1-treated NRK-52E cells. More importantly, we found that increased expression of Nrf2 promoted the expression of GSTA3, while Nrf2 downregulation decreased GSTA3 levels, indicating that Nrf2 regulates GSTA3. MAPK signaling, including the P38, JNK, and ERK pathways, and the PI3K/Akt pathway are closely correlated with the occurrence and development of RIF induced by oxidative stress.24,25 It has been widely accepted that Nrf2 activation in renal tubular epithelial cells of mice and humans is associated with these signaling pathways. According to our results, GSTA3 expression was not significantly associated with the MAPK signaling pathway, whereas it was regulated by the PI3K/Akt signaling pathway. The PI3K/Akt–Nrf2–GSTA3 signaling pathway is involved in the pathogenesis of RIF, which provides a theoretical basis for the clinical treatment of RIF.
Additionally, we observed that GSTA3 expression was initially upregulated and then downregulated in NRK-52E cells following TGF-β1. GSTA3 protein levels were significantly increased during the first 12 hours after TGF-β1 stimulation, which led to a gradual increase in GSTA3 expression. We also found that GSTA3 protein expression in the TGF-β1-induced group was significantly lower than in control cells. We assumed that GSTA3 consumption coincided with the generation of GSTA3 when RIF occurred, and that GSTA3 consumption was increased accompanied by the decrease of GSTA3 production. The depletion of GSTA3 acted as one of the anti-oxidative stress processes, and the production of GSTA3 was due to TGF-β1-induced oxidative stress. We hypothesize that both were caused by the need to resist oxidative stress and biological self-regulation. Nevertheless, the consumption and generation of GSTA3 were not quantified owing to methods and equipment. The underlying mechanism between the generation and consumption of GSTA3 remains largely unknown. It should be noted that the in vitro RIF model was adopted alone, and data from animal experiments and clinical trials are still urgently required.
Conclusion
Taken together, GSTA3 can suppress and alleviate TGF-β1-induced RIF via the PI3K/Akt–Keap1/Nrf2 signaling pathway in NRK-52E cells. The PI3K/Akt–Keap1/Nrf2–GSTA3 signaling pathway plays a critical role in the incidence and progression of RIF. These experimental finding may provide novel ideas and targets for the clinical treatment of chronic kidney diseases.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
National Natural Science Foundation of China (Grant No. 81600538).
References
- 1.Perperopoulou F, Pouliou F, Labrou NE. Recent advances in protein engineering and biotechnological applications of glutathione transferases. Crit Rev Biotechnol 2017; 38: 1–18. [DOI] [PubMed] [Google Scholar]
- 2.Crawford DR, Ilic Z, Guest I, et al. Characterization of liver injury, oval cell proliferation and cholangiocarcinogenesis in glutathione S-transferase A3 knockout mice. Carcinogenesis 2017; 38: 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao Y, Liu J, Peng Y, et al. GSTA3 attenuates renal interstitial fibrosis by inhibiting TGF-Beta-induced tubular epithelial-mesenchymal transition and fibronectin expression. PLoS One 2016; 11: e0160855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tebay LE, Robertson H, Durant ST, et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med 2015; 88: 108–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki T, Yamamoto M. Molecular basis of the Keap1–Nrf2 system. Free Radic Biol Med 2015; 88: 93–100. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, Yu S, Zhang C, et al. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med 2015; 88: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Li W, Su ZY, et al. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem 2015; 26: 1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kensler KH, Slocum SL, Chartoumpekis DV, et al. Genetic or pharmacologic activation of Nrf2 signaling fails to protect against aflatoxin genotoxicity in hypersensitive GSTA3 knockout mice. Toxicol Sci 2014; 139: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djordjevic J, Djordjevic A, Adzic M, et al. Alterations in the Nrf2-Keap1 signaling pathway and its downstream target genes in rat brain under stress. Brain Res 2015; 1602: 20–31. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz S, Pergola PE, Zager RA, et al. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 2013; 83: 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci 2011; 7: 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama N, Kohno M, Yokoyama T. Inhibition of the p38 MAPK pathway ameliorates renal fibrosis in an NPHP2 mouse model. Nephrol Dial Transplant 2012; 27: 1351–1358. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Wu G, Gu X, et al. Kruppel-like factor 15 modulates renal interstitial fibrosis by ERK/MAPK and JNK/MAPK pathways regulation. Kidney Blood Press Res 2013; 37: 631–640. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Cao Y, Zhang Y, et al. Cyclic helix B peptide inhibits ischemia reperfusion-induced renal fibrosis via the PI3K/Akt/FoxO3a pathway. J Transl Med 2015; 13: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Chen Z, Zou Y, et al. Atorvastatin represses the angiotensin 2-induced oxidative stress and inflammatory response in dendritic cells via the PI3K/Akt/Nrf2 pathway. Oxid Med Cell Longev 2014; 2014: 148798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeayeng S, Wongkajornsilp A, Slominski AT, et al. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic Biol Med 2017; 108: 918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Zhang H, Li X, et al. Miltirone protects human EA.hy926 endothelial cells from oxidized low-density lipoprotein-derived oxidative stress via a heme oxygenase-1 and MAPK/Nrf2 dependent pathway. Phytomedicine 2016; 23: 1806–1813. [DOI] [PubMed] [Google Scholar]
- 18.Zhao M, Zhu P, Fujino M, et al. 5-Aminolevulinic acid with sodium ferrous citrate induces autophagy and protects cardiomyocytes from hypoxia-induced cellular injury through MAPK-Nrf-2-HO-1 signaling cascade. Biochem Biophys Res Commun 2016; 479: 663–669. [DOI] [PubMed] [Google Scholar]
- 19.Ali T, Kim T, Rehman SU, et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer's disease. Mol Neurobiol 2018; 55: 6076–6093. [DOI] [PubMed] [Google Scholar]
- 20.Xiao H, Shen HY, Liu W, et al. Adenosine A2A receptor: a target for regulating renal interstitial fibrosis in obstructive nephropathy. PLoS One 2013; 8: e60173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamura DM, Bahrami NM, Ren S, et al. Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD. J Am Soc Nephrol 2014; 25: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aminzadeh MA, Nicholas SB, Norris KC, et al. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant 2013; 28: 2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, An C, Gao Y, et al. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 2013; 100: 30–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Liu X, Wang B, et al. Pirfenidone suppresses MAPK signaling pathway to reverse epithelial-mesenchymal transition and renal fibrosis. Nephrology (Carlton) 2017; 22: 589–597. [DOI] [PubMed] [Google Scholar]
- 25.Qin J, Xie YY, Huang L, et al. Fluorofenidone inhibits nicotinamide adeninedinucleotide phosphate oxidase via PI3K/Akt pathway in the pathogenesis of renal interstitial fibrosis. Nephrology (Carlton) 2013; 18: 690–699. [DOI] [PubMed] [Google Scholar]