Short abstract
Oxygen deficiency in the plateau environment weakens aerobic metabolism and reduces the energy supply, leading to high-altitude diseases including decreased circulatory function, decreased nutrient and energy supply to tissues and organs, and decreased waste discharge. The involvement of many metabolic pathways is reflected in dramatic changes in levels of endogenous small molecule metabolites. Metabolomics represents a promising technique for mechanistic studies and drug screening, and metabonomics, or quantitative metabolomics, has been increasingly applied to the study of hypoxic diseases and their pathogenesis, as well as to pharmacodynamics at high altitudes. In this article, we review the recent literature on the pathogenesis of altitude hypoxia and the clinical and preclinical metabonomics of drug interventions. Endogenous metabolites and metabolic pathways change significantly under high-altitude hypoxia. Some drug interventions have also been shown to regulate pathway metabolism, and the problems of applying metabonomics to hypoxic diseases at high altitude and the prospects for its future application are summarized.
Keywords: High altitude, hypoxia, metabonomics, metabolic pathway, drug intervention, metabolite
Introduction
The plateau environment is an ecological environment characterized by low oxygen, low pressure, and high radiation.1 When humans enter the plateau environment from the plains, their tissues and organs are subject to physiological hypoxia. High altitude forces the body to reduce the arterial oxygen partial pressure and oxygen saturation, eventually leading to tissue hypoxia.2 In turn, hypoxia, including acute high-altitude hypoxia or mild altitude reactions, affects the metabolism of substances in the body and hinders normal body functions, potentially resulting in high-altitude pulmonary edema (HAPE)3 and high-altitude cerebral edema (HACE),4 while chronic high-altitude hypoxia can cause polycythemia and cardiovascular disease.5
Approximately 140 million people worldwide, accounting for 2% of the world’s total population, live in high-altitude regions.6 Furthermore, the tourism industry, the development of transportation, and the increased military demands of countries mean that the number of people living in the plateau environment has increased. Preventing high-altitude hypoxia and reducing resulting damage to the body has thus become a focus of attention.7
Metabonomics was first put forward in the middle and late 1990s and focuses on the changes in metabolites in biological systems.8,9 Metabonomics has recently been widely used to identify biomarkers of hypoxia, and in toxicology and pharmacology, as well as other fields.10,11 Notable studies demonstrated that high-altitude hypoxia could lead to a lack of reactive oxygen species and oxidative stress, with changes in the corresponding metabolites.12,13 The rise of drug metabonomics can also provide important information on the mechanisms of action of clinical drugs. In this paper, we review progress in metabonomics research into the mechanisms of hypoxia and of anti-hypoxia drugs at high altitude. By reviewing the changes in metabolites and the pathophysiological mechanisms involved in acclimatization to plateau hypoxia, as well as new drug developments in relation to plateau adaptation, this review will open a metabonomics window for future studies of plateau hypoxia.
Mechanism of hypoxia at high altitude based on metabonomics
Changes in metabolites in the body vary under different anoxic environments, and this review focuses mainly on the metabonomics of acute and chronic high-altitude hypoxia.
Metabonomics studies of acute plateau hypoxia
Metabonomic information on acute altitude sickness is currently based mainly on clinical samples. However, metabonomics analysis of changes in related endogenous metabolites can further reveal the mechanisms responsible for the occurrence and development of acute high-altitude hypoxia and provide a basis for its clinical diagnosis.
Hypoxia at high altitudes has a dramatic and comprehensive effect on metabolites, with significant changes in a variety of metabolites and key enzymes. Liao et al.14 found that metabolic pathways related to the inflammatory response, fatty acid transportation, bile acid metabolism, and heme metabolism changed at high altitude. O’Brien et al15 examined the first metabolomic response to progressive exposure to environmental hypoxia in healthy participants by metabolomic profiling of plasma from 198 healthy individuals before and during an ascent to Everest Base Camp (5,300 m) in 2019. They showed that the rate of glycolysis and fat-store mobilization increased, while isoleucine and glucose decreased, and lactic acid and circulating levels of free fatty acids (palmitic acid, linoleic acid, and oleic acid) increased.15 Zhu et al.16 found that plasma levels of hypoxanthine, cysteinyl glycine, D-arabinol, L-threonine, 2-ketobutyric acid, and succinic semialdehyde were significantly increased in patients with acute high-altitude hypoxia. These studies also confirmed the view of Serkova et al.,17 who showed that the physiological processes of acute and long-term hypobaric hypoxia exposure at high altitude included cardiac, pulmonary, and hematological changes, and that adaptation to hypoxia was often reflected in pathway regulation of related metabolites. Tissot van Patot et al.18 found that hypoxia induced factor-1 (HIF-1) DNA binding and HIF-1 α protein in leukocytes were significantly changed by hypoxia. HIF-1 has been suggested to increase the glycolysis energy supply by increasing the expression of pyruvate dehydrogenase kinase and other enzymes under hypoxia.19,20 In addition, hypoxia also led to a decrease in the level of circulating total glutathione and increases in the levels of lactic acid and succinic acid.18 They also found that urinary 15-F(2t)-isoprostanes were associated with markers of anoxic stress, thus revealing the mechanism of hypoxia at the cellular level.
Interpreting the changes that occur during plateau hypoxia is complicated and the changes are thus still not fully understood. However, there are several possible explanations, including inhibition of mitochondrial function by the hypoxic environment, resulting in increased anoxic glycolysis and inhibition of the mitochondrial tricarboxylic acid (TCA) cycle.21 The increases in lactic acid22 and 12,13-DiHOME support this view.23
Lou et al.24 studied systemic changes in the human body induced by acute hypoxia reflected in the urine. Expression levels of purine and adenosine metabolites were significantly increased after exposure to hypoxia, while energy and lipid metabolism were also affected. In addition, Luo et al.25 found that plasma levels of amino acids were significantly increased in patients with HAPE, while β-glucose, trimethylamine, and lipid metabolites were decreased. Liu et al.26 showed that adenosine monophosphate-activated protein kinase was highly expressed in erythrocytes after acute high-altitude hypoxia, and adenosine concentration and soluble CD73 activity were also increased sharply. They also used metabonomics to reveal the adaptation mechanism of the body under an anoxic environment.26
The combination of target and nontarget metabonomics is commonly used to study the effects of acute high-altitude hypoxia. Li et al.27 used both target and nontarget metabonomics to screen 14 differential metabolites in patients with HAPE, and identified C8-ceramide, sphingosine, and glutamine as candidate biomarkers for the diagnosis of HAPE. Using target metabonomics, Pichler et al.28 found that tetrahydrobiopterin and methionine sulfoxide levels increased significantly in patients with acute high-altitude hypoxia. These results imply that metabolic methods can be used to recognize small changes in other metabolites, and that these newly revealed metabolites have the potential to act as biomarkers of specific diseases and to provide a basis for their clinical diagnosis. The main metabolite spectra and metabolic pathways involved in metabonomics studies of clinical acute high-altitude hypoxia are summarized in Table 1. The metabonomics biomarkers identified encompassed a series of pathways related to energy metabolism (TCA cycle, glycolysis, amino acid metabolism, and fatty acid metabolism), inflammatory response-related metabolism (linoleic acid metabolism, arachidonic acid metabolism, phospholipid metabolism, and purine metabolism), heme metabolism, bile acid metabolism, and others, and may support the exploration of the currently unknown mechanism responsible for the changes and adaptations to high-altitude hypoxia.
Table 1.
Primary metabolic markers and pathways involved in clinical acute plateau hypoxia.
| Sample | Metabolic markers (changed compared with normal controls) | Metabolic pathways |
|---|---|---|
| Human plasma | Pentyl carnitine, octanoyl carnitine, decenyl carnitine, oleoyl carnitine, octadecenyl carnitine, linoleamide, palmitic amide (increased) | Fatty acid metabolism25,27 |
| Glutamic acid, methionine, glyceric acid, pyroglutamic acid, phenylpyruvic acid, phenylalanine, valine, leucine, cysteinylglycine, citrate, tyrosine, L-histidine, 1-methylhistidine, histamine, betaine, lysine, isoleucine, glycine, glutamine (increased); L-glutamine, L-glutamic acid, succinic acid, creatine, taurine, 3-indoleacetic acid, 2-oxobutyric acid (decreased) | Amino acid metabolism15,16,25,28 | |
| LysoPC (16:0), LysoPC (22:4), LysoPC (P18:0), LysoPC (38:5), LysoPC (20:2), LysoPC (38:5) (elevated); LysoPC (18:2), LysoPC (20:3), LysoPC (22:5) (decreased) | Phospholipid metabolism15,25,27 | |
| Sphingosine, sphingomyelin 1-phosphate, sphingomyelin (d18:1/16:0), palmitoylcarnitine, C8-ceramide (elevated) | Sphingolipid metabolism15,25,27 | |
| Bilirubin (elevated) | Heme metabolism25 | |
| Chenodeoxycholate-3-sulfuric acid, taurine ursodeoxycholic acid (increased) | Bile acid metabolism25 | |
| Lactic acid, succinic acid, D-arabitol,3-hydroxybutyric acid (increased); citric acid, α-glucose, β-glucose (decreased) | Glucose metabolism15,21,25 | |
| Hypoxanthine, inosine (elevated) | Purine metabolism27 | |
| Human urine | 1-Methyladenosine, 5-methylthioadenosine, cytosine, xanthine, hypoxanthine, uric acid (increased) 3-inodoleacetic acid, L-glutamic (decreased) | Purine metabolism24 |
| L-Carnitine, propionyl carnitine, butyryl carnitine, decanoyl carnitine (increased) | Carnitine metabolism24 |
LysoPC, lysophosphatidylcholine.
Metabonomics studies of chronic plateau hypoxia
Long-term exposure to high altitude environments is likely to result in high-altitude polycythemia (HAPC),29 hypertension, gastric mucosal lesions,30 and other chronic altitude diseases, with in vivo changes in metabolites and related metabolic pathways.31
The effects of high-altitude exposure on energy metabolism are generally believed to be related to ATP consumption and phosphate accumulation. Under hypoxic conditions, the body can increase the muscle energy supply by changing the potential metabolic pathway. D’Alessandro et al.32 found that the results of a metabonomics study of erythrocytes after exposure of healthy volunteers to hypoxia at high altitude for different periods of time were consistent with the results of basic studies in vitro,33 in that hypoxia promoted glycolysis and the pentose phosphate pathway and catabolism of purine and nitric oxide. In addition, D’Alessandro et al.32 also proved for the first time that purine, triose, pentose phosphate, and sphingosine 1-phosphate in red blood cells could be used as metabolic markers after long-term hypoxia at high altitude. At the same time, these results also showed that metabolic regulation can effectively improve the adaptive response to hypobaric hypoxia. A recent study from China34 showed that levels of serum metabolites, including fumaric acid, inosine, phytic acid, and ribose, were significantly higher in patients with chronic altitude sickness than in normal residents. In addition, they also found that isoleucine, fumaric acid, glucose 1-phosphate, and citrulline may be serum biomarkers of chronic altitude sickness. Combined with the recently discovered adenosine-A2B-AMPK/Sphk1/ENT1 signal transduction pathway,35 these results suggest possible treatment strategies for HAPC. The main metabolite spectra and metabolic pathways involved in metabonomics studies of clinical chronic altitude hypoxia are summarized in Table 2. We speculate that supplementation with the amino acids valine, alanine, and proline might improve energy metabolism and promote high-altitude adaptation.
Table 2.
Primary metabolic markers and pathways involved in clinical chronic plateau hypoxia.
| Sample | Metabolic markers (changed compared with normal controls) | Metabolic pathways |
|---|---|---|
| Human plasma | Propyl sugar, pentose phosphate, methyl phosphate (increased) | Glucose metabolism32–34 |
| Sphingomyelin 1-phosphate (elevated) | Sphingomyelin metabolism32 | |
| Glucose-6-phosphate (increased at first and then decreased); glyceraldehyde 3-phosphate, fumaric acid, pyruvic acid, ribose, glucose-1-inosine phosphate (increased); glyceraldehyde 3-phosphate, fumaric acid, pyruvate, ribose, glucose-1-phosphate (increased); phosphoglycerate, phosphoenol pyruvate, 6-phosphogluconolactone, 6-phosphogluconate, lyxose (reduced) | Glycolysis and pentose phosphate32,34 | |
| Glutathione, alanine, serine, aspartic acid, tyrosine, 5-oxyproline, glycine, trimethyl lysine, L-cysteine, citrulline, isoleucine (increased); glutathione synthase, glutamic acid, glutamine (decreased) | Metabolism and transamination of amino acids and glutamine32,34 | |
| Nitrite, adenine, adenosine, niacinamide, ornithine, asymmetric dimethyl arginine, niacinamide (increased); arginine, α-ketoglutaric acid, ADP, ATP (decreased); citrulline (increased and then decreased) | Nitrogen metabolism32,34 | |
| L-Homoserine (increased); creatine, creatine anhydride, creatine phosphate (first increased and then decreased); taurine/hypotaurine (decreased) | Arginine and sulfur metabolism32 |
Although endogenous markers found in animal models differ from those found in the clinic, the related pathways are similarly involved in energy metabolism, including glucose metabolism and amino acid metabolism. Redox homeostasis and carbohydrate, fat, and energy pathways were affected in animal studies of adaptation to the plateau environment. Cao et al.36 validated this theory by comparing the specific metabolic alterations between plateau pikas in the Kekexili Reserve (4630 m) and those at the foot of Laji Mountain (2600 m) on the Qinghai-Tibet Plateau, using gas chromatography time-of-flight mass spectrometry metabolomics. Furthermore, an experimental study of chronic hypoxia showed that the metabolic pathway in the model animals changed accordingly. Using metabonomics analysis of rat urine, Koundal et al.37 showed that hypoxia resulted in major disturbances in energy metabolism. Taurine metabolism and the TCA cycle are important pathways leading to the pathophysiological changes caused by hypobaric hypoxia, and the TCA cycle has been proven to play a role in erythrocyte metabolism under anoxic conditions.38 Interestingly, this experiment also found that the metabolic response of the intestinal microflora was significantly decreased after hypoxia, suggesting that a chronic high-altitude hypoxic environment could also affect the intestinal microflora. Together with a recent research report,39,40 these results further improve our understanding of the dialectical relationship between intestinal flora disorders and plateau hypoxia. In addition, hypoxia pretreatment can reduce hypoxia-induced damage to tissues, cells, organs, and systems. Zhou et al.41 accordingly examined the changes in metabolites and related pathways in mice. They noted that the sphingolipid metabolic pathway changed under hypoxic conditions, with increases in levels of long-chain fatty acid metabolites with alkene bonds and decreases in levels of sphingomyelin without alkene bonds. These interesting results demonstrate the potential of the discovered endogenous markers to protect the body from hypoxia. Maimaitiyimin et al.42 analyzed the differences between hypoxia model and normal rats and found that endogenous substances related to energy metabolism were disturbed and amino acid levels were significantly increased in the hypoxia group. At the same time, β-glucose and α-glucose levels were significantly increased, suggesting that blood glucose and amino acid metabolism disorders may represent a novel approach to the prevention and treatment of hypoxia. The main metabolite spectra and metabolic pathways involved in the metabonomics studies of chronic high-altitude hypoxia in animal models are summarized in Table 3.
Table 3.
Primary metabolic markers and pathways involved in chronic plateau hypoxia in animals.
| Sample | Metabolic markers (changed compared with normal controls) | Metabolic pathways |
|---|---|---|
| Mouse brain tissue | Estradiol, 20-dione, 19-hydroxytestosterone, estrone glucuronic acid (increased); 17β-estradiol-3-glucuronide, 2-methoxyestrone-3-glucuronide, 3α, 21-dihydroxy-5 β-progesterone-11, 16α-hydroxydehydroepiandrosterone, 11-deoxycortisol, corticosterone (decreased) | Steroid hormone biosynthesis41 |
| 4-Hydroxyretinoic acid, 18-hydroxyretinic acid, all-trans-5, 6-epoxy retinoic acid (increased); retinal ester (decreased) | Retinol metabolism41 | |
| Linoleic acid (reduced) | Linoleic acid metabolism41 | |
| Leukotriene C4 (increased); 5, 6-dihydroxyeicosapentaenoic acid, 14, 15-dihydroxyeicosapentaenoic acid, 8, 9-dihydroxyeicosapentaenoic acid, 11, 12-dihydroxyeicosapentaenoic acid, 12-keto-tetrahydro-leukotriene B4 (decreased) | Arachidonic acid metabolism41 | |
| Heme, phenolinyl ester a, c-diamide, biliverdin, D-urocholinogen (increased) | Porphyrin and chlorophyll metabolism41 | |
| D-Pantoyl-L-cysteine, pantothenic acid 4-phosphate (increased), α-ketoisovaleric acid (decreased) pantothenic acid and CoA biosynthesis phospholipid acid [16-0-18-1 (9Z)], lysophosphatidylcholine (22:0) (increased); lysophosphatidylcholine [20:3 (5Z, 8Z, 11Z)], glycerol-ethanolamine phosphate (decreased) | Glycerol phospholipid metabolism41 | |
| Rat plasma | Isoleucine, valine, glycine, tyrosine, phenylalanine, pyruvate, 1-methyl-histidine (increased) | Amino acid metabolism42 |
| Lactic acid, glycoprotein, shark-inositol, creatine, α-glucose, β-glucose (increased) | Glycolysis42 | |
| Plateau pikas | Lactic acid, trehalose-6-phosphate, succinic acid, fumaric acid (increased); 2′-deoxyadenosine 5′-monophosphate (decreased) | Glucose metabolism36 |
| Taurine, methionine, tyrosine, citrulline and glutathione (increased) | Amino acid and glutathione metabolism36 | |
| Rat urine | Glycine, serine, threonine, arginine, proline (increased) | Amino acid metabolism37 |
| Glycerol phospholipid (elevated) | Phospholipid metabolism37 |
The mechanisms responsible for chronic hypoxia adaptation and metabolic changes may be affected by genetic factors.43 Mitochondrial gene polymorphisms,44 heat shock protein 70 family gene polymorphisms,45 and the endothelial nitric oxide synthase gene (dbSNP number: rs1799983; protein polymorphism Glu298Asp)46 were deemed to be associated with promoting adaptation to hypoxia in long-term plateau residents. However, more research on the changes in and mechanisms of metabolites is needed, at both the basic and clinical levels.
Metabonomics of drug interventions in plateau hypoxia
Although few studies have investigated the metabonomics of drugs at high altitude, this approach can nevertheless provide a theoretical basis for the future prevention and treatment of plateau hypoxia. The metabonomics of drug interventions was a concept originally put forward by Clayton in 2006,47 and metabonomics analysis of hypoxia drugs at high altitude can be used to determine the changes in endogenous metabolites in hypoxia models after drug interventions.48 Revealing the metabolic pathways associated with drug interventions and the pharmacological mechanisms of high-altitude hypoxia drugs will improve effective drug treatments and provide new ideas for individualized treatment.
Maimaitiyimin et al.42 conducted a metabonomics study on the effect of acetyl-L-cysteine (Da) on chronic high-altitude hypoxia in rats by proton nuclear magnetic resonance methods. Da can improve glucose metabolism and amino acid metabolism by increasing the body’s oxygenation and decreasing the degree of anaerobic glycolysis. They found that intragastric administration of a Da suspension increased amino acid levels (valine, tyrosine, 1-methyl-histidine, leucine, phenylalanine, and methionine) and decreased β-glucose and α-glucose levels in the serum of rats with high-altitude hypoxia. Da can thus be used to improve the symptoms of hypoxia at high altitude from the point of view of energy metabolism. Similar conclusions were reached by Hung et al.,49 who showed that acetazolamide could be used to treat patients with acute mountain sickness; by increasing parasympathetic tone, acetazolamide can accelerate the acclimatization to ascents to the plateau environment.
In addition, traditional Tibetan medicine (TTM) has also been shown to be an effective pretreatment for preventing the occurrence of plateau hypoxia. Liu et al.50 developed the traditional Chinese medicine Fu Fang Jin Jing Oral Liquid (FJJOL), based on the TTM Rhodiola rosea. Following treatment with FJJOL, nuclear magnetic resonance-based metabolomics revealed that brain levels of ATP, lactate, malate, and fumarate recovered in hypoxia model mice. Notably, FJJOL significantly rescued the hypoxia-induced effects on energy metabolism. FJJOL may thus be an alternative therapy for the treatment of hypoxia. Previous studies also showed that supplements based on Rhodiola crenulata and Cordyceps sinensis accelerated physiological adaptation to anoxic environments at high altitudes and improved aerobic exercise ability by decreasing parasympathetic activity and balancing circulating hormones and hematological changes.51 Notably, erythrocyte, hematocrit, and hemoglobin levels were all elevated to varying degrees.
HAPC is a common type of chronic high-altitude hypoxia. Lu et al.52 found that the Tibetan medicine Zuo-Mu-A Decoction (ZMAD) had a beneficial protective effect on blood parameters and against myocardial injury in HAPC model rats. The main endogenous markers involved were erythropoietin and 8-hydroxy-2'-deoxyguanosine. Many TTM drugs may also be effective for the prevention and treatment of HAPC and high-altitude hypoxia. However, there are currently relatively few studies on the metabonomics of drug intervention in this field, and further studies are therefore needed.
Continuous developments in metabonomics technology are expected to promote research into additional mechanisms of hypoxia at high altitude and to support the development of reasonable and effective therapeutic drugs in the near future.
Conclusions and prospects
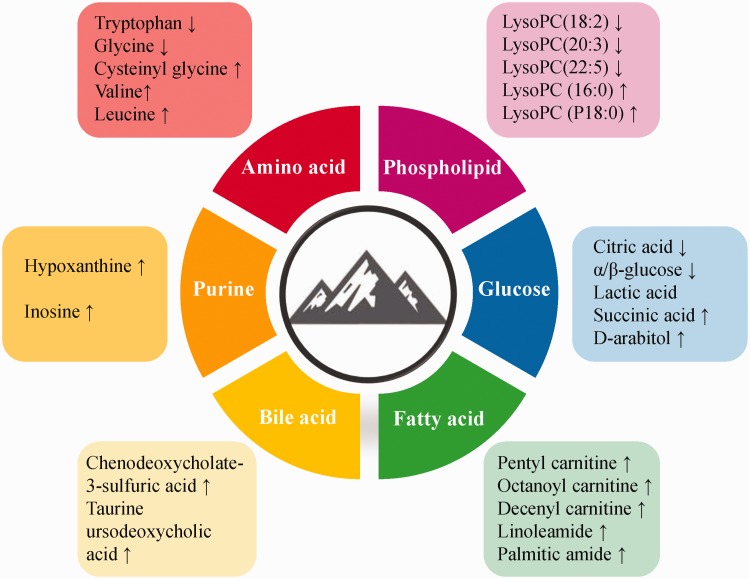
Metabonomics, as a new technology in systems biology, is playing an increasingly important role in the field of high-altitude hypoxia. Metabonomics provides a good starting point for studying the mechanisms of high-altitude hypoxia and for overcoming the deficiencies of more traditional studies examining single components and targets involved in traditional hypoxia mechanisms. Comparisons of circulatory system, organ, and urine metabonomics in acute and chronic plateau hypoxia clearly identified some metabolites in blood (Figure 1), but only small amounts of amino acids and lipids in urine. However, despite increasing attention to the treatment and research of altitude hypoxia, its pathogenesis is still not clearly explained. Fortunately, metabonomics can also provide the technical basis for clinical screening of anti-altitude hypoxia targets, by detecting changes in metabolites such as glucose, amino acids, and choline after pharmaceutical interventions, and can also be used to verify the effectiveness of a drug on the metabolic pathway, thus providing a favorable basis for the development and marketing of new drug indications.
Figure 1.
Changes in major metabolites in plateau hypoxia. LysoPC, lysophosphatidylcholine.
Metabonomics still has many problems and limitations, and the screening and identification of biomarkers remains both a focus and difficulty of metabonomics research. In addition, the complexity of analyses and data processing, as well as the deeper relationship between differential metabolites and the pathogenesis of altitude diseases, also present challenges. Furthermore, it remains difficult to fully interpret the overall comprehensive effect and mechanisms of high-altitude hypoxia by metabonomics alone. Nevertheless, this review opens a new metabonomics window for future studies of plateau hypoxia involving the combined application of multiple disciplines and technologies to clarify the mechanisms of altitude hypoxia and adaptation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Tianjin Science and Technology Project (15ZXLCSY00040) and National Major Science and Technology Projects in the 13th Five-Year Plan (2018ZX10732-202-004-005).
References
- 1.West JB. Recent advances in high altitude medicine and biology. High Alt Med Biol 2015; 16: 73. [DOI] [PubMed] [Google Scholar]
- 2.Horscroft JA, Kotwica AO, Laner V, et al. Metabolic basis to Sherpa altitude adaptation. Proc Natl Acad Sci U S A 2017; 114: 6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richalet JP, Larmignat P, Poitrine E, et al. Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am J Respir Crit Care Med 2012; 185: 192–198. [DOI] [PubMed] [Google Scholar]
- 4.Joyce KE, Lucas SJE, Imray CHE, et al. Advances in the available non-biological pharmacotherapy prevention and treatment of acute mountain sickness and high altitude cerebral and pulmonary oedema. Expert Opin Pharmacother 2018; 19: 1891–1902. [DOI] [PubMed] [Google Scholar]
- 5.Zheng C, Chen Z, Zhang L, et al. Metabolic risk factors and left ventricular diastolic function in middle-aged Chinese living in the Tibetan plateau. J Am Heart Assoc 2019; 8: e010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh LC. High altitude dermatology. Indian J Dermatol 2017; 62: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grocott MP, Martin DS, Levett DZ, et al. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med 2009; 360: 140–149. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 2016; 17: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson JK, Wilson ID. Understanding 'global' systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov 2003; 2: 668–676. [DOI] [PubMed] [Google Scholar]
- 10.Buzkova J, Nikkanen J, Ahola S, et al. Metabolomes of mitochondrial diseases and inclusion body myositis patients: treatment targets and biomarkers. EMBO Mol Med 2018; 10: e9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang C, Chen L, Rabinowitz JD. Metabolomics and isotope tracing. Cell 2018; 173: 822–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, Wang Y, Lu H, et al. ‘Ome’ on the range: update on high-altitude acclimatization/adaptation and disease. Mol Biosyst 2014; 10: 2748–2755. [DOI] [PubMed] [Google Scholar]
- 13.Tremblay JC, Hoiland RL, Howe CA, et al. Global reach 2018: high blood viscosity and hemoglobin concentration contribute to reduced flow-mediated dilation in high-altitude excessive erythrocytosis. Hypertension 2019; 6: 1327–1335. [DOI] [PubMed]
- 14.Liao WT, Liu B, Chen J, et al. Metabolite modulation in human plasma in the early phase of acclimatization to hypobaric hypoxia. Sci Rep 2016; 6: 22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien KA, Atkinson RA, Richardson L, et al. Metabolomic and lipidomic plasma profile changes in human participants ascending to Everest Base Camp. Sci Rep 2019; 9: 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu G, Yin C, Tian Z, et al. Metabolomic analysis of plasma from patients with acute mountain sickness using chromatography-mass spectrometry. Medicine (Baltimore) 2015; 94: e1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serkova NJ, Reisdorph NA, Tissot van Patot MC. Metabolic markers of hypoxia: systems biology application in biomedicine. Toxicol Mech Methods 2008; 18: 81–95. DOI: 10.1080/15376510701795769. [DOI] [PubMed] [Google Scholar]
- 18.Tissot van Patot MC, Serkova NJ, Haschke M, et al. Enhanced leukocyte HIF-1alpha and HIF-1 DNA binding in humans after rapid ascent to 4300 m. Free Radic Biol Med 2009; 46: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 19.McClain DA, Abuelgasim KA, Nouraie M, et al. Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism. J Mol Med (Berl) 2013; 91: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu CJ, Wang LY, Chodosh LA, et al. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol 2003; 23: 9361–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards LM, Lawler NG, Nikolic SB, et al. Metabolomics reveals increased isoleukotoxin diol (12,13-DHOME) in human plasma after acute Intralipid infusion. J Lipid Res 2012; 53: 1979–1986. DOI: 10.1194/jlr.P027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paddenberg R, Goldenberg A, Faulhammer P, et al. Mitochondrial complex II is essential for hypoxia-induced ROS generation and vasoconstriction in the pulmonary vasculature. Adv Exp Med Biol 2003; 536: 163–169. DOI: 10.1007/978-1-4419-9280-2_21. [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan S, Hammock BD, Newman JW, et al. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J Am Coll Nutr 2003; 22: 502–510. [DOI] [PubMed] [Google Scholar]
- 24.Lou BS, Wu PS, Liu Y, et al. Effects of acute systematic hypoxia on human urinary metabolites using LC-MS-based metabolomics. High Alt Med Biol 2014; 15: 192–202. [DOI] [PubMed] [Google Scholar]
- 25.Luo Y, Zhu J, Gao Y. Metabolomic analysis of the plasma of patients with high-altitude pulmonary edema (HAPE) using 1H NMR. Mol Biosyst 2012; 8: 1783–1788. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Zhang Y, Wu H, et al. Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation 2016; 134: 405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo L, Tan G, Liu P, et al. Three plasma metabolite signatures for diagnosing high altitude pulmonary edema. Sci Rep 2015; 5: 15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pichler HJ, Sonntag D, Hefti U, et al. Oxidative stress in hypobaric hypoxia and influence on vessel-tone modifying mediators. High Alt Med Biol 2013; 14: 273–279. [DOI] [PubMed] [Google Scholar]
- 29.Fan X, Ma L, Zhang Z, et al. Associations of high-altitude polycythemia with polymorphisms in PIK3CD and COL4A3 in Tibetan populations. Hum Genomics 2018; 12: 37. DOI: 10.1186/s40246-018-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K, He C. Gastric mucosal lesions in Tibetans with high-altitude polycythemia show increased HIF-1A expression and ROS production. Biomed Res Int 2019; 2019: 6317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chunhua J, Jian C, Fuyu L, et al. Chronic mountain sickness in Chinese Han males who migrated to the Qinghai-Tibetan plateau: application and evaluation of diagnostic criteria for chronic mountain sickness. BMC Public Health 2014; 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Alessandro A, Nemkov T, Sun K, et al. AltitudeOmics: Red Blood Cell metabolic adaptation to high altitude hypoxia. J Proteome Res 2016; 15: 3883–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subudhi AW, Bourdillon N, Bucher J, et al. AltitudeOmics: the integrative physiology of human acclimatization to hypobaric hypoxia and its retention upon reascent. PLoS One 2014; 9: e92191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xuefeng C, Zhenzhong B, Lan M, et al. Preliminary study of patients with chronic mountain sickness by GC-TOF-MS based serum metabolomics analysis. Chinese Journal of Pathophysiology 2017; 33: 1676–1682. DOI: 10.3969/j.issn.1000-4718.2017.09.023. [Google Scholar]
- 35.Sun K, Liu H, Song A, et al. Erythrocyte purinergic signaling components underlie hypoxia adaptation. J Appl Physiol (1985) 2017; 123: 951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao XF, Bai ZZ, Ma L, et al. Metabolic alterations of Qinghai-Tibet plateau pikas in adaptation to high altitude. High Alt Med Biol 2017; 18: 219–225. [DOI] [PubMed] [Google Scholar]
- 37.Koundal S, Gandhi S, Kaur T, et al. “Omics” of high altitude biology: a urinary metabolomics biomarker study of rats under hypobaric hypoxia. OMICS 2015; 19: 757–765. [DOI] [PubMed] [Google Scholar]
- 38.Nemkov T, Sun K, Reisz J, et al. Metabolism of citrate and other carboxylic acids in erythrocytes as a function of oxygen saturation and refrigerated storage. Front Med (Lausanne) 2017; 4: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Zhang J, Wang R, et al. Effects of gut microbiota on drug metabolism and guidance for rational drug use under hypoxic conditions at high altitudes. Curr Drug Metab 2019; 20: 155–165. [DOI] [PubMed] [Google Scholar]
- 40.Khanna K, Mishra KP, Ganju L, et al. High-altitude-induced alterations in gut-immune axis: a review. Int Rev Immunol 2018; 37: 119–126. [DOI] [PubMed] [Google Scholar]
- 41.Zhou T, Wang M, Cheng H, et al. UPLC-HRMS based metabolomics reveals the sphingolipids with long fatty chains and olefinic bonds up-regulated in metabolic pathway for hypoxia preconditioning. Chem Biol Interact 2015; 242: 145–152. [DOI] [PubMed] [Google Scholar]
- 42.Maimaitiyimin D, Aikemu A, Kamilijiang M, et al. Effects and mechanisms of acetyl-L-cysteine in rats with chronic mountain sickness with H1-NMR metabolomics methods. Med Sci Monit 2014; 20: 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macinnis MJ, Koehle MS, Rupert JL. Evidence for a genetic basis for altitude illness: 2010 update. High Alt Med Biol 2010; 11: 349–368. [DOI] [PubMed] [Google Scholar]
- 44.Qi Y, Sun J, Zhu T, et al. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with high-altitude pulmonary oedema: a meta-analysis. J Renin Angiotensin Aldosterone Syst 2011; 12: 617–623. [DOI] [PubMed] [Google Scholar]
- 45.Qi Y, Niu WQ, Zhu TC, et al. Genetic interaction of Hsp70 family genes polymorphisms with high-altitude pulmonary edema among Chinese railway constructors at altitudes exceeding 4000meters. Clinica Chimica Acta 2009; 405: 17–22. [DOI] [PubMed] [Google Scholar]
- 46.Wang P, Koehle MS, Rupert JL. Genotype at the missense G894T polymorphism (Glu298Asp) in the NOS3 gene is associated with susceptibility to acute mountain sickness. High Alt Med Biol 2009; 10: 261–267. [DOI] [PubMed] [Google Scholar]
- 47.Clayton TA, Lindon JC, Cloarec O, et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature 2006; 440: 1073–1077. [DOI] [PubMed] [Google Scholar]
- 48.Zhou X, Nian Y, Qiao Y, et al. Hypoxia plays a key role in the pharmacokinetic changes of drugs at high altitude. Curr Drug Metab 2018; 19: 960–969. [DOI] [PubMed] [Google Scholar]
- 49.Hung PH, Lin FC, Tsai HC, et al. The usefulness of prophylactic use of acetazolamide in subjects with acute mountain sickness. J Chin Med Assoc 2019; 82: 126–132. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Zhu W, Guan S, et al. Metabolomic analysis of anti-hypoxia and anti-anxiety effects of Fu Fang Jin Jing Oral Liquid. PLoS One 2013; 8: e78281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CY, Hou CW, Bernard JR, et al. Rhodiola crenulata- and Cordyceps sinensis-based supplement boosts aerobic exercise performance after short-term high altitude training. High Alt Med Biol 2014; 15: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu MQ, Tsring N, Yu TY, et al. Protective effects of traditional Tibetan medicine Zuo-Mu-A Decoction (ZMAD) on the blood parameters and myocardium of high altitude polycythemia model rats. Chin J Integr Med 2017; 23: 908–915. [DOI] [PubMed] [Google Scholar]