Short abstract
Objective
This study compared the incidence of cerebrospinal fluid (CSF) leakage and residual tumors between functional and nonfunctional pituitary adenomas treated with the endoscopic endonasal transsphenoidal approach (EETA).
Methods
All patients underwent endocrine examinations and brain magnetic resonance imaging before and after surgery. The length of admission, incidence of central diabetes insipidus, incidence of CSF leakage, symptom relief, and presence of residual tumors were compared between patients with functional and nonfunctional pituitary adenomas.
Results
Thirty-eight patients were enrolled, among whom 12 and 26 had functional and nonfunctional pituitary adenomas, respectively. The incidence of CSF leakage was significantly higher in patients with nonfunctional adenomas; a hard or elastic tumor character accounted for the difference. A large tumor size and cavernous sinus invasion were risk factors for residual tumors. No significant differences were found in sex, length of admission, operative times, incidence of diabetes insipidus, or number of residual tumors between the two groups. The hormone levels were lower postoperatively than preoperatively in patients with nonfunctional adenomas.
Conclusion
Nonfunctional pituitary macroadenomas resulted in more CSF leakage. Use of the rescue nasoseptal flap reduced unnecessary nasal destruction. Cooperation between a neurosurgeon and otolaryngologist was safer and more effective when using the EETA.
Keywords: Pituitary adenoma, endoscopic endonasal transsphenoidal approach (EETA), transcranial approach, transsphenoidal approach, magnetic resonance imaging (MRI), cerebrospinal fluid (CSF)
Introduction
Pituitary adenomas, which are tumors that occur in the pituitary gland, represent approximately 10% to 15% of all intracranial tumors.1 They arise from epithelial cells in the adenohypophysis of the pituitary gland and rarely metastasize.2–5 According to their size, pituitary adenomas can be classified as microadenomas (<1 cm in diameter) or macroadenomas (>1 cm in diameter). They can also be classified as functional or nonfunctional adenomas. Functional pituitary adenomas often cause hormonal effects by oversecreting hormones. Nonfunctional pituitary adenomas (or null cell adenomas) do not secrete hormones but often cause mass effects, such as optic nerve compression.6
The two main surgical approaches for the treatment of pituitary adenomas are the transcranial approach, which includes the frontal craniotomy method and the subfrontal method, and the transsphenoidal approach, which includes the microscopic method and the endoscopic method. The endoscopic endonasal transsphenoidal approach (EETA) has increasingly been employed for the removal of pituitary adenomas. In this study, we compared the surgical outcomes, incidence of cerebrospinal fluid (CSF) leakage, and incidence of residual tumors between patients with functional and nonfunctional pituitary adenomas treated with the EETA.
Materials and methods
Ethics statement
This was a retrospective study. The data were collected by reviews of charts and images of patients with pituitary adenomas undergoing endoscopic transsphenoidal tumor excision via the bilateral nostrils. The protocol was reviewed and approved by the institutional review board (IRB) of Mackay Memorial Hospital (IRB approval number: 17MMHIS162e). According to the IRB, patient consent was not required for this retrospective study because the patients’ data were de-identified.
Surgical indications
The surgical indications were uncontrolled hormonal abnormalities and a compressive mass effect. For prolactinomas, our first choice of treatment was dopamine agonist therapy. Surgery was suggested only for patients with acute neurological deterioration or those intolerant of medical treatment. All patients underwent brain magnetic resonance imaging (MRI) before and after surgery. The preoperative and 1-year postoperative MRI findings were collected to analyze the tumor size, cavernous sinus invasion, and any residual tumors. All surgical procedures were performed by a neurosurgeon and otolaryngologist via the bilateral nostrils.
Data collection and review
The data analyzed in this study were collected from January 2010 to January 2017. If a bed-rest order was recorded in the chart because of watery rhinorrhea or the sensation of fluid in the pharynx combined with a positive glucose oxidase strip test, the patient was considered to have had CSF leakage. Significant CSF leakage was defined as rhinorrhea that did not resolve after bed rest and that required a second surgical intervention. The presence of a residual tumor was determined by the radiologist’s report and included suspected residual tumors. Endocrine data were reviewed before surgery, and at 1 day, 3 months, and 1 year after surgery. These data included the concentrations of growth hormone (GH), insulin-like growth factor-1 (IGF-1), prolactin (PRL), thyroid-stimulating hormone (TSH), free thyroxin, adrenocorticotropic hormone (ACTH), free cortisol, luteinizing hormone, follicle-stimulating hormone, estradiol, and testosterone. Except in patients with prolactinoma, all cases of acromegaly and Cushing disease were diagnosed by endocrinologists. If the GH and ACTH levels were controversial, a hormone suppression test such as the glucose tolerance test or dexamethasone suppression test was performed.
Surgical technique
The patients were placed in the supine position with the head immobilized in a Mayfield head holder. All patients underwent endoscopic endonasal surgery with a transsphenoidal approach with the assistance of a navigation system. During surgery, the sphenoid ostium was located and the middle turbinate was avoided, especially the anterior two-thirds, to preserve the patient’s sense of smell as much as possible. Septectomy and sphenoidotomy were then performed to the sellar region. After opening the sellar region, the tumor was removed piece by piece inside the capsule if the tumor character was soft. If the tumor character was hard, we performed extracapsular dissection. After internal decompression, we dissected the tumor along the planes of the cavernous sinus and its capsule. The arachnoid membrane was usually torn when dissecting the suprasellar portion, and high-flow CSF leakage was frequently encountered after extracapsular tumor removal. We used bipolar endoscopic suction and commercial hemostats (Floseal; Baxter, Deerfield, IL, USA) to stop intraoperative bleeding. We then used an artificial dural graft (DuraGen; Integra LifeSciences, Plainsboro, NJ, USA) and a fibrin sealant (Tissucol; Baxter) for multilayer closure if minor CSF leakage was suspected. If high-flow CSF leakage was noted during surgery, we immediately applied a nasoseptal flap to reduce the rate of postoperative CSF leakage.
Case illustration
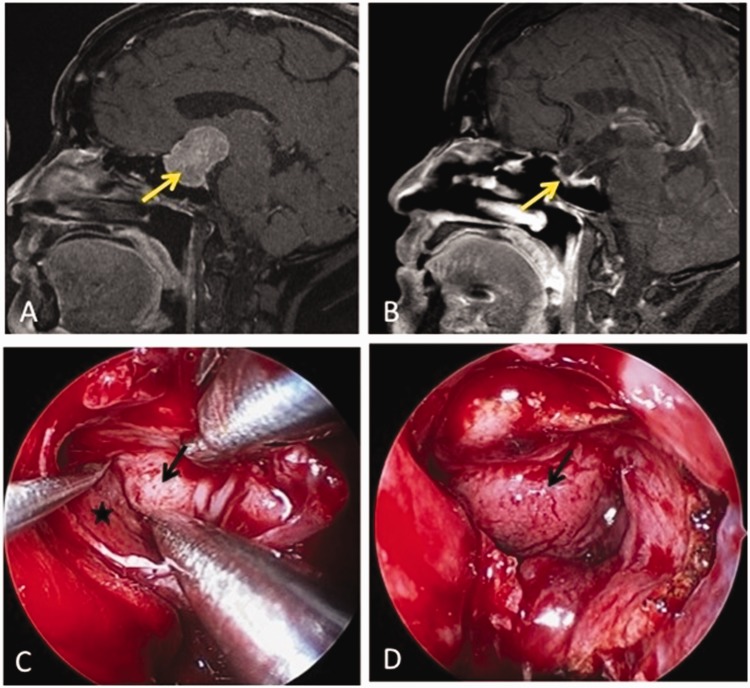
A 51-year-old woman presented with a 4-month history of bitemporal hemianopsia. Preoperative brain MRI showed a pituitary tumor with suprasellar extension. The size of the tumor was 2.3 × 2.7 × 3.5 cm. Because the tumor was quite hard, we performed endoscopic endonasal transsphenoidal tumor removal by extracapsular dissection. High-flow CSF leakage occurred after removing the tumor. A nasoseptal flap was used to seal the CSF leaks immediately. No CSF leakage occurred after surgery, and no residual tumors were found on postoperative brain MRI (Figure 1).
Figure 1.
A patient with a nonfunctional pituitary macroadenoma underwent adenomectomy by extracapsular dissection. (a) Preoperative brain MRI (the signal T1 is sagittal with contrast): The tumor was 4 cm in diameter with suprasellar extension (yellow arrow). (b) Postoperative MRI: No residual tumor was present at the 1-year follow-up (yellow arrow). (c) After central debulking, the tumor was dissected between the capsule (black arrow) and cavernous sinus (asterisk). (d) Because of high-flow cerebrospinal fluid leakage after tumor removal, a nasoseptal flap (black arrow) was harvested to cover the arachnoid and dura defect. Abbreviation: MRI, magnetic resonance imaging.
Statistical analysis
We compared the demographics and outcomes between patients with and without functional pituitary adenomas using Student’s t test for continuous variables and Fisher’s exact test for categorical variables. The endocrine data are presented as mean ± standard deviation. The changes in endocrine data throughout the follow-up period were examined using a generalized estimating equation with an exchangeable working correlation matrix. A P value of <0.05 was considered statistically significant. Data analyses were conducted using Statistical Product and Service Solutions (SPSS) 22 (IBM Corp., Armonk, NY, USA).
Results
Demographics and surgical outcomes
In total, 38 patients were enrolled in the study. The mean patient age was 54.3 ± 11.2 years with a range of 31 to 72 years. Twenty-one patients were women (55%) and 17 were men (45%). Of the 38 patients, 12 had functional pituitary adenomas and 26 had nonfunctional pituitary adenomas. Of the 12 functional adenomas, 7 secreted GH, 2 secreted ACTH, 1 secreted TSH, and 2 secreted PRL.
Patients with functional adenomas were significantly younger (P = 0.025) and had tumors with a softer character (P = 0.027) and smaller size (P = 0.009) than patients with nonfunctional adenomas. Six of 12 functional tumors and all 26 nonfunctional tumors (total of 32 tumors) were macroadenomas, with a significant difference between the two groups (P < 0.001). The surgical outcome assessment included perioperative CSF leakage, diabetes insipidus, residual tumors, length of admission, and operation time. With the exception of perioperative CSF leakage (P = 0.012), there were no significantly different outcomes between the two groups. The perioperative CSF leaks were less prevalent in patients with functional than nonfunctional adenomas. According to the postoperative MRI reports, there were three residual tumors in the functional group (one in a patient with acromegaly and two in patients with prolactinomas) and nine residual tumors in the nonfunctional group (Table 1).
Table 1.
Demographics and surgical outcomes of patients.
|
Pituitary adenoma |
||||
|---|---|---|---|---|
| PatientVariable | Total (n = 38) | Functional (n = 12) | Nonfunctional (n = 26) | P value |
| Demographics | ||||
| Sex | 0.161 | |||
| Female | 21 | 9 | 12 | |
| Male | 17 | 3 | 14 | |
| Age (years) | 54.3 ± 11.2 | 48.4 ± 9.7 | 57.1 ± 10.9 | 0.025 |
| Tumor size (cm) | 2.2 ± 0.9 | 1.6 ± 1.0 | 2.5 ± 0.8 | 0.009 |
| Tumor type | <0.001 | |||
| Microadenoma | 6 | 6 | 0 | |
| Macroadenoma | 32 | 6 | 26 | |
| Tumor character | 0.027 | |||
| Soft or fragile | 24 | 11 | 13 | |
| Hard or elastic | 14 | 1 | 13 | |
| Surgical outcomes | ||||
| Perioperative CSF leakage | 0.012 | |||
| No | 23 | 11 | 12 | |
| Yes | 15 | 1 | 14 | |
| Diabetes insipidus | 0.481 | |||
| No | 15 | 6 | 9 | |
| Yes | 23 | 6 | 17 | |
| Residual tumor | 0.714 | |||
| No | 26 | 9 | 17 | |
| Yes | 12 | 3 | 9 | |
| Length of admission (days) | 11.9 ± 7.7 | 12.3 ± 11.2 | 11.8 ± 5.7 | 0.836 |
| Operation time (minutes) | 165.6 ± 61.8 | 169.8 ± 56.5 | 163.6 ± 65.1 | 0.780 |
Data are presented as number of patients or mean ± standard deviation.
CSF, cerebrospinal fluid.
Risk factors for residual tumors
Tumor size, adenoma type, tumor character, and cavernous sinus invasion were considered risk factors for residual tumors. According to the P value calculated in the data analysis, the incidence of a residual tumor was associated with tumor size (P < 0.001) and cavernous sinus invasion (P = 0.0001). The tumor character and adenoma type were not predictors of a residual tumor in our series (Table 2).
Table 2.
Risk factors for residual tumors.
|
Residual tumor |
|||
|---|---|---|---|
| Factor | Yes (n = 12) | No (n = 26) | P value |
| Tumor size (cm) | 3.0 ± 0.9 | 1.8 ± 0.7 | <0.001 |
| Adenoma type | 0.714 | ||
| Functional | 3 | 9 | |
| Nonfunctional | 9 | 17 | |
| Tumor character | 0.296 | ||
| Soft or fragile | 6 | 18 | |
| Hard or elastic | 6 | 8 | |
| Cavernous sinus invasion | 0.0001 | ||
| Yes | 7 | 0 | |
| No | 5 | 26 | |
Data are presented as number of patients or mean ± standard deviation.
Biological cure and image-based cure of functional pituitary tumors
For functional tumors, biological cure is more important than imaging evidence of total excision. Among the patients with functional pituitary tumors, two of seven patients with acromegaly did not achieve biological cure after the surgery. One 34-year-old patient had a preoperative GH level of 136 ng/mL. Her preoperative MRI showed a 2.5-cm-diameter macroadenoma with cavernous sinus invasion. Her GH level was 19.6 ng/mL 1 day after the surgery. She received treatment with octreotide (Sandostatin LAR; Novartis, Basel, Switzerland) and had good hormone control (GH level of 1.75 ng/mL) at the last follow-up. Another 54-year-old patient with a 1.5-cm macroadenoma had a decrease in GH from 24.7 to 6.54 ng/mL 1 day after surgery. This patient also did not achieve biological cure. However, with an IGF-1 level of 567 ng/mL, her GH level decreased to 3.26 ng/mL at the 1-year follow-up. Her symptoms were largely resolved. In addition, her two follow-up MRI examinations showed no residual tumor, and she was still undergoing follow-up at the time of this writing. Two patients with PRL-secreting macroadenomas underwent surgery for sudden headache and visual defects due to apoplexy. Their vision improved after surgery. The postoperative MRI reports showed a residual tumor within the cavernous sinus, and the PRL level remained abnormal in both patients. These patients were treated with cabergoline until the 3- and 4-year follow-up, respectively, and their PRL levels finally decreased to the normal range. One patient with a thyrotropinoma (TSHoma) and two patients with Cushing disease showed normal hormone levels after surgery. They were considered to have achieved both biological cure and image-based cure.
Endocrine data of nonfunctional pituitary adenomas
Both neurological symptom relief and hormone function preservation are important for patients undergoing pituitary adenoma surgery. For all 26 patients with nonfunctional pituitary adenomas, the endocrine data were checked repeatedly during the follow-up period. The levels of GH and IGF-1 were decreased after surgery and became significantly low at the last follow-up (P < 0.05). The levels of cortisol and PRL were elevated transiently on postoperative day 1 (P < 0.05). The cortisol returned to the preoperative level, but the PRL level continued to decrease during the long-term follow-up (P < 0.05) (Table 3).
Table 3.
Endocrine outcomes in patients with nonfunctional pituitary adenomas (n = 26).
|
Hormone levels |
||||||
|---|---|---|---|---|---|---|
| GH (ng/mL) | IGF-1 (ng/mL) | ACTH (pg/mL) | Cortisol (μg/dL) | PRL (ng/mL) | TSH (μIU/mL) | |
| Preoperative | 0.34 ± 0.43 | 124.1 ± 74.8 | 30.4 ± 20.6 | 13.4 ± 18.2 | 12.8 ± 15.6 | 1.31 ± 1.07 |
| Postoperative day 1 | 0.20 ± 0.22 | 88.5 ± 59.4 | 34.4 ± 16.2 | 16.2 ± 30.8a | 30.8 ± 27.8a | 1.22 ± 1.13 |
| Last follow-up | 0.05 ± 0.03a | 45.8 ± 29.7a | 28.3 ± 10.6 | 10.6 ± 7.2 | 7.2 ± 7.8a | 1.52 ± 1.47 |
aStatistically significant (P < 0.05) in comparison of postoperative day 1 and last follow-up versus preoperative values.
Abbreviations: GH, growth hormone; IGF-1, insulin-like growth factor-1; PRL, prolactin; TSH, thyroid-stimulating hormone; ACTH, adrenocorticotropic hormone.
Discussion
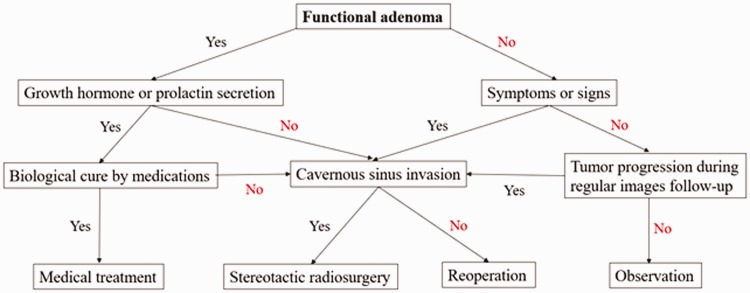
The pituitary gland is located in the sella turcica of the sphenoid bone and is lateral to the wall of the cavernous sinus.7 Over the years, surgery for pituitary tumors has undergone multiple evolutions in technique and technology.8 Endoscopic endonasal transsphenoidal surgery for pituitary tumors is increasingly used and is considered safe and effective according to the current literature.9 Karki et al.10 reported that resection of large and giant pituitary adenomas by transsphenoidal surgery was safe and efficient and had low morbidity and mortality. Gaillard11 stated that the use of an endoscope created a much larger workspace and better visualization compared with a microscope. Consistent with this, the use of an endoscope in the present study provided wide surgical visualization through angled telescopes, allowing us to easily identify the anatomy and tumor. Therefore, we were able to perform tumor resection under better vision than with a microscope. According to the 1-year follow-up MRI reports in our cases, the incidence of residual tumors was 34% (13/38). Moreover, the residual tumors were either inside the cavernous sinus or within the sella turcica without a mass effect. Roelfsema et al.12 claimed that the highest incidence of recurrence occurred between 1 and 5 years after surgery. Bodhinayake et al.13 showed that a larger tumor size (diameter of >10 mm) was associated with a higher risk of recurrence. Tumor size and cavernous sinus invasion were risk factors for residual tumors. In our patients, although 6 of the 12 functional adenomas and all 26 nonfunctional tumors were macroadenomas, there was no statistical significance in residual tumors between the two groups. Most of the residual tumors in our patients were stable. Our treatment strategies for residual pituitary tumors are illustrated in Figure 2. Two patients in the nonfunctional group had evidence of radiologic enlargement on MRI at the second follow-up, and they underwent stereotactic radiosurgery. Although patients with CSF leakage had longer hospital stays, and although the nonfunctional group had a higher incidence of CSF leakage, the hospital stay was not significantly different between the two groups. The reason might be that one of the patients with Cushing disease remained in the hospital for 46 days because of CSF leakage, diabetes insipidus, and electrolyte imbalance. This patient was the only patient with CSF leakage in the functional adenoma group. If she had been excluded from the study, the functional group would have had a shorter hospital stay than the nonfunctional group.
Figure 2.
Treatment strategies for residual pituitary tumors.
Linsler et al.14 reported that the hormonal function of the pituitary gland could be preserved in endoscopic endonasal transsphenoidal surgery. For functional evaluation, we compared the preoperative and postoperative endocrine data in both the functional and nonfunctional groups. In the functional group, the postoperative target hormone level was lower than the preoperative level. One patient with acromegaly and two patients with prolactinomas received adjuvant medical treatment (mentioned in the Results section). The endocrine data were finally within the normal range. With respect to the endocrine outcome in the nonfunctional group, we found that the GH and IGF-1 levels decreased immediately after surgery and became significantly low during the last follow-up. The cortisol and PRL levels increased immediately after surgery but decreased at the last follow-up. All patients routinely received hydrocortisone substitution postoperatively until adequate endogenous steroid levels were established, which explains why the cortisol level was higher on postoperative day 1 than preoperatively (Table 3). The early PRL increase might have resulted from loss of inhibition and/or pituitary stalk dysfunction. The long-term decrease in the GH, ICF-1, and PRL levels might have resulted from destruction of the normal pituitary gland and/or its blood supply during surgery. Some patients developed symptoms of pan-hypopituitarism secondary to cortisol and thyroxin hormone therapy, and this affected our data. This could explain why the cortisol and thyroxin levels did not decrease in our series.
The most common complication of the endoscopic transsphenoidal adenomectomy is CSF leakage. The higher incidence of CSF leakage in our series might have resulted from our wide inclusion criteria. Any patient with episodes of watery rhinorrhea is required to be on bed rest, regardless of the amount and frequency. Zhou et al.15 reported that the tumor consistency and size were independently associated with intraoperative CSF leakage. Intraoperative CSF leakage more readily occurs in patients with fibrous or large tumors.15 In the present study, the functional group had a significantly higher number of patients with perioperative CSF leakage than the nonfunctional group. This might have occurred because the functional tumors in this study were softer or more fragile than the nonfunctional tumors. Hard or elastic tumors were more likely to sustain arachnoid injury during dissection, and some of them required extracapsular dissection. These factors accounted for the higher incidence of CSF leakage in the nonfunctional group in our series. Current therapeutic strategies for CSF leakage include bed rest, nasal packing, autologous fat grafts, nasoseptal flaps, or lumbar drainage. Most of our CSF leaks were minor and resolved after 3 to 7 days of bed rest. Three patients had persistent CSF leakage after conservative treatment and underwent a second surgery to stop the leakage. Among them, two patients were treated with a nasoseptal flap and one was treated with lumbar drainage. All of these patients were in the nonfunctional group. The overall rate of clinically significant postoperative CSF leakage in our patients was 7% (3/38). In recent studies, postoperative CSF leakage reportedly occurred in 0.6% to 8.5% of patients.11,16–19 Paluzzi et al.18 stated that the rate of postoperative CSF leakage decreased from 5.0% to 2.9% after introduction of the nasoseptal flap. Horridge et al.20 suggested that the use of the nasoseptal flap can minimize postoperative CSF leakage. Rivera-Serrano et al.21 introduced a rescue nasoseptal flap technique without disruption of the sphenopalatine artery. They suggested removing the tumor first and then applying a nasoseptal flap depending on the degree of CSF leakage.21 In our cases, we did not routinely apply the nasoseptal flap at the beginning of the procedure. We did not preserve the posterior pedicle (the sphenopalatine artery) during the operation and harvest the nasoseptal flap until we encountered high-flow CSF leaks. No patients in the present study developed postoperative meningitis. However, two patients developed postoperative sinusitis and were admitted to the hospital for antibiotic therapy. Some authors have suggested that lumbar CSF drainage during surgery can reduce the rate of intraoperative CSF leakage.22 In our series, lumbar CSF drainage was performed only for treatment of postoperative CSF leakage, and we obtained good results for the remission of CSF leakage.
Conclusion
After surgery, the target hormone levels decreased in the functional group. During the long-term follow-up, the GH, IGF-1, and PRL levels also decreased in the nonfunctional group. All patients in both groups finally achieved symptom relief. CSF leakage was more frequent in the nonfunctional group because of the harder tumor character. Residual tumors were associated with the initial tumor size and cavernous sinus invasion. A rescue nasoseptal flap was applied according to the degree of CSF leakage. Endoscopic transsphenoidal tumor excision was safer and more effective when performed under cooperation between a neurosurgeon and an otolaryngologist.
Acknowledgement
We are grateful for the grant support from Mackay Memorial Hospital in publishing this article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Jesser J, Schlamp K, Bendszus M. [Pituitary gland tumors]. Radiologe 2014; 54: 981–988. [DOI] [PubMed] [Google Scholar]
- 2.Al-Brahim NY, Asa SL. My approach to pathology of the pituitary gland. J Clin Pathol 2006; 59: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev 1998; 19: 798–827. [DOI] [PubMed] [Google Scholar]
- 4.Asa SL, Ezzat S. The pathogenesis of pituitary tumours. Nat Rev Cancer 2002; 2: 836–849. [DOI] [PubMed] [Google Scholar]
- 5.Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol 2011; 7: 257–266. [DOI] [PubMed] [Google Scholar]
- 6.Theodros D, Patel M, Ruzevick J, et al. Pituitary adenomas: historical perspective, surgical management and future directions. CNS Oncol 2015; 4: 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin S, Ansorge O. Development and microscopic anatomy of the pituitary gland In: De Groot LJ, Chrousos G, Dungan K, et al. (eds) Endotext. South Dartmouth (MA): MDText.com, Inc, 2000. [PubMed] [Google Scholar]
- 8.Gandhi CD, Christiano LD, Eloy JA, et al. The historical evolution of transsphenoidal surgery: facilitation by technological advances. Neurosurg Focus 2009; 27: E8. [DOI] [PubMed] [Google Scholar]
- 9.Charalampaki P, Ayyad A, Kockro RA, et al. Surgical complications after endoscopic transsphenoidal pituitary surgery. J Clin Neurosci 2009; 16: 786–789. [DOI] [PubMed] [Google Scholar]
- 10.Karki M, Sun J, Yadav CP, et al. Large and giant pituitary adenoma resection by microscopic trans-sphenoidal surgery: surgical outcomes and complications in 123 consecutive patients. J Clin Neurosci 2017; 44: 310–314. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard S. The transition from microscopic to endoscopic transsphenoidal surgery in high-caseload neurosurgical centers: the experience of Foch Hospital. World Neurosurg 2014; 82: S116–S120. [DOI] [PubMed] [Google Scholar]
- 12.Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 2012; 15: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodhinayake I, Ottenhausen M, Mooney MA, et al. Results and risk factors for recurrence following endoscopic endonasal transsphenoidal surgery for pituitary adenoma. Clin Neurol Neurosurg 2014; 119: 75–79. [DOI] [PubMed] [Google Scholar]
- 14.Linsler S, Hero-Gross R, Friesenhahn-Ochs B, et al. Preservation of hormonal function by identifying pituitary gland at endoscopic surgery. J Clin Neurosci 2017; 43: 240–246. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Yang Z, Wang X, et al. Risk factors and management of intraoperative cerebrospinal fluid leaks in endoscopic treatment of pituitary adenoma: analysis of 492 patients. World Neurosurg 2017; 101: 390–395. [DOI] [PubMed] [Google Scholar]
- 16.Gondim JA, Almeida JP, Albuquerque LA, et al. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary 2011; 14: 174–183. [DOI] [PubMed] [Google Scholar]
- 17.Jho HD. Endoscopic transsphenoidal surgery. J Neurooncol 2001; 54: 187–195. [DOI] [PubMed] [Google Scholar]
- 18.Paluzzi A, Fernandez-Miranda JC, Tonya Stefko S, et al. Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary 2014; 17: 307–319. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Zhou T, Wei S, et al. Endoscopic endonasal transsphenoidal surgery of 1,166 pituitary adenomas. Surg Endosc 2015; 29: 1270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horridge M, Jesurasa A, Olubajo F, et al. The use of the nasoseptal flap to reduce the rate of post-operative cerebrospinal fluid leaks following endoscopic trans-sphenoidal surgery for pituitary disease. Br J Neurosurg 2013; 27: 739–741. [DOI] [PubMed] [Google Scholar]
- 21.Rivera-Serrano CM, Snyderman CH, Gardner P, et al. Nasoseptal “rescue” flap: a novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope 2011; 121: 990–993. [DOI] [PubMed] [Google Scholar]
- 22.Mehta GU, Oldfield EH. Prevention of intraoperative cerebrospinal fluid leaks by lumbar cerebrospinal fluid drainage during surgery for pituitary macroadenomas. J Neurosurg 2012; 116: 1299–303. [DOI] [PubMed] [Google Scholar]