Short abstract
Objective
To ascertain plasma levels of heat shock protein 90α (HSP90α) and squamous cell carcinoma antigen (SCC-Ag) and their diagnostic potential in cervical cancer.
Methods
In a cross-sectional study, patients’ cervical tissue samples were screened for high risk (HR) human papilloma virus (HPV) DNA and underwent a thinprep-liquid based cytology test (TCT). Plasma samples were analysed by enzyme-linked immunosorbent assay (ELISA) for HSP90 α and SCC-Ag levels.
Results
Of the 295 women who underwent screening, 75 were healthy controls 75 (HR-HPV−ve TCT−ve), 110 were HR-HPV+ve, TCT−ve and 110 were HR-HPV+ve TCT+ve. There were significant differences between levels of HSP90α and SCC-Ag proteins across the patient groups with those positive for cervical cancer having the greatest levels of proteins compared with other groups. For patients with high grade SCC there was a significant correlation between levels of HSP90α and SCC-Ag. The area under the ROC curve for combined HSP90α*SCC-Ag was the largest compared with the single proteins. Using a cut-off value of 16.4 ng/ml to delineate cervical cancer diagnosis, the sensitivity and specificity of HSP90α*SCC-Ag were 90.3% and 95.1% respectively.
Conclusion
Plasma HSP90α protein levels correlated well with SCC-Ag levels in patients with cervical cancer and the combination of HSP90α*SCC-Ag may be a useful diagnostic biomarker.
Keywords: Cervical lesions, heat shock protein 90α, squamous cell carcinoma antigen, high-risk HPV, biomarkers
Introduction
Cervical cancer is one of the most common gynaecological malignancies and is the fourth leading cause of all cancer deaths in women.1,2 An epidemiological survey conducted in China over the period from 1998 to 2008, suggested that the incidence cervical cancer is increasing.3 Although there are several potential causes of cervical cancer, human papilloma virus (HPV) infection is the main underlying cause,4 and up to 14 high risk (HR) HPV genotypes have been implicated5
Precancerous lesions usually take 10–20 years to develop into cervical cancer.6 Accordingly, women who have received inadequate or no screening for cervical cancer may present with advanced stage disease.2 Therefore, early diagnosis is critical for a good prognosis. Commonly used screening methods for cervical lesions include cervical cytology tests, human papillomavirus (HPV) detection and colposcopy examination.1 However, the current screening techniques and strategies have many deficiencies.1,7 Consequently, there has been an interest in identifying markers that can complement standard pathological evaluations to identify the presence of cancer cells in cervical tissues.7,8 Among the different histological subtypes of cervical cancer, squamous cell carcinoma (SCC) accounts for 85-90% of all cervical cancer cases.7 Studies have reported that elevated pre-treatment SCC-antigen (Ag) levels correlate with the stage of the disease, size of the tumour and depth of cervical stromal invasion.7,9 However, the detection of SCC-Ag alone has certain limitations. While serum levels of SCC-Ag may reflect response to therapy,10 some studies have reported that the tumour marker has no prognostic value.10,11
Heat shock proteins (HSPs) are vital in the synthesis and stability of several signal transduction proteins and so have an important role in cell survival.12 HSPs are classified according to their size and HSP90 has been investigated as a potential target in cancer therapy.12 Two forms of HSP90 (HSP90α and HSP90β) are located in the cytoplasm.13 HSP90α appears to respond to stress14 whereas Hsp90β is involved in processes that maintain viability.15 Studies have shown that expression level of HSP90α was associated with the occurrence and metastasis of cancer.16,17 Other studies have shown that HSP90α can act as a tumour marker in liver cancer18 and lung cancer.19,20
This study was designed to assess and compare levels of HSP90α and SCC-Ag in plasma samples from patients who underwent screening for cervical cancer and explore if HSP90α had diagnostic applications.
Methods
For this cross-sectional study, tissue samples were obtained from women who underwent cervical cancer screening in the gynaecological outpatient department of the Third Clinical Medical College (Affiliated Tumour Hospital) of Xinjiang Medical University between April 2017 and May 2018.
HR-HPV DNA detection in combination with a thinprep-liquid based cytology test (TCT) was used as the screening method.20 HPV DNA testing by the second-generation hybridization capture test (HC2) assay method was performed with the automated HC2 assay system according to the manufacturer’s protocol. The samples were analysed for the presence of HR HPV types (i.e., 16, 18, 31,33, 35, 39, 45, 51, 52, 56, 58, 59, 68. Samples were classified as HR HPV DNA positive if the ratio of the relative light unit (RLU) reading obtained from the luminometer to the mean value for the positive cutoff value (PC) was ≥1. For the TCT test, section preparation, and staining were performed according to the manufacturer’s instructions. TCT positive samples were graded as follows: atypical squamous cells of undetermined significance (ASC-US); low-grade squamous intraepithelial lesion (LSIL); high-grade squamous intraepithelial lesion (HSIL) or carcinoma in situ; squamous cell carcinoma (SCC).
In accordance with the test results, patients were separated into three groups: a healthy control group (HR-HPV−ve, TCT−ve); an infected group (HR-HPV+ve, TCT−ve); a cervical lesion group (HR-HPV+ve TCT+ve). Colposcopic biopsy was performed in patients with HR-HPV+ve and TCT (≥ASC-US).
Blood samples from the patients were obtained in the morning after an overnight fast. The samples (2ml) were placed into tubes containing EDTA-K2 and centrifuged at 3000r/min for 10 minutes at room temperature. Plasma samples were stored until use at -20 degrees for less than 1 month, or frozen at -80 degrees for no more than 6 months.
Plasma levels of HSP90α were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Yantai Protgen Biotechnology Development Co., Ltd, Yantai, China), according to the manufacturer’s protocols. Optical density was measured using a spectrophotometer at 450 nm. The amount of protein in each sample was calculated according to a standard curve of optical density values and Curve Expert 1.3 software was used to analyse the data.
SCC Ag concentrations were determined using an i2000SR full-automatic chemiluminescence immunoassay analyzer (Abbott Laboratories, Abbott Park, IL, USA). All analyses were performed according to the manufacturers’ instructions.
All patients provided written informed consent before participating in the study and the study protocol was approved by the Ethics Committee of the Third Clinical Medical College (Affiliated Tumor Hospital) of Xinjiang Medical University.
Statistical analyses
Data were analysed using the IBM Statistical Package for Social Sciences (SPSS®) for Windows® release 21.0 (IBM Corp., released 2012; Armonk, NY, USA) and a P-value <0.05 was considered to indicate statistical significance.
According to results from the Kolmogorov-Smirnov (K-S) test, the data were skewed and so nonparametric tests (Wilcoxon, rank sum test) were used to compare differences. ROCKIT software (ROCKIT 0.9B Beta Version, Charles E. Metz, University of Chicago) was used to generate Receiver operating characteristic (ROC) curves to compare predictive sensitivity, specificity, and the area under the curve (AUC) with 95% CI.
The correlation between SCC-Ag and HSP90α according to TCT results was examined using Spearman’s correlation test. Measures of diagnostic accuracy (i.e., Youden index, Likelihood ratio (LR), positive predictive value [PPV] and negative predictive value [NPV]) were calculated.21
Results
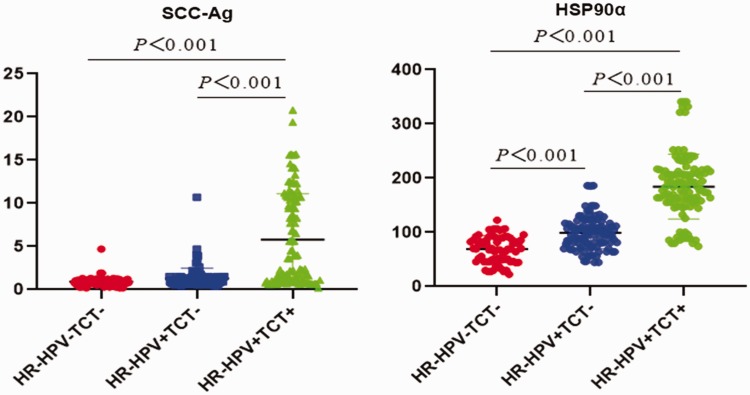
Between April 2017 and May 2018, 295 women (median age 52 years, range 25 to 80 years) were screened for cervical lesions in our department. According to test results, 75 women were in the HR-HPV−ve TCT−ve group (i.e., heathy controls), 110 were in the HR-HPV+ve, TCT−ve group and 110 were in the HR-HPV+ve TCT+ve group (Table 1). Therefore, 110 patients had a colposcopic biopsy to confirm positive results. Patients’ plasma levels of SCC-Ag and HSP90α were statistically significantly different across the three patient groups (Table 1; Figure 1) In addition, HSP90α levels were statistically significantly different between patient groups.
Table 1.
Plasma levels of squamous cell carcinoma antigen (SCC-Ag) and heat shock protein (HSP)90α according to results of HR-HPV DNA and TCT methods.
| HR-HPV−ve, TCT−ve(Controls)n=75 | HR-HPV+ve, TCT−ven=110 | HR-HPV+ve TCT+ven=110 | Statistical Significance | |
|---|---|---|---|---|
| SCC-Ag (ng/ml) | 0.8 (0.7,1.2) | 1.1 (0.8, 1.5)# | 3.6 (1.0, 10.7)* | P=0.001 |
| HSP90α (ng/ml) | 70.1 (48.6, 89.6) | 101.1 (80.6, 112.6)# | 183.6 (155.5, 212.8)*γ | P=0.001 |
Values shown as median and interquartile range
#P < 0.05 compared with control group
*P < 0.05 compared with control group
γP < 0.05 compared with HR-HPV+ve, TCT−ve group
HR-HPV, high risk types of human papilloma virus; TCT, thinprep-liquid based cytology test; SCC-Ag, squamous cell carcinoma antigen; HSP, heat shock protein.
Figure 1.
Enzyme-linked immunosorbent assay (ELISA) detection of plasma levels of squamous cell carcinoma antigen (SCC-Ag) and heat shock protein (HSP)90α according to high risk human papilloma virus (HR-HPV) DNA and thinprep-liquid based cytology test (TCT) results.
According to TCT results, 29 patients had ASC-US, 60 patients had LSIL, 21 patients had HSIL and none had SCC (Table 2). Patients in the ASC-US group, showed no correlation between HSP90α and SCC-Ag results. However, for patients in the LSIL and HSIL groups, statistically significant correlations were detected for plasma HSP90α and SCC-Ag levels; the most significant correlation between proteins was observed in the HSIL group (Table 2).
Table 2.
Correlation between squamous cell carcinoma antigen (SCC-Ag) and heat shock protein (HSP)90α according to results of the thinprep-liquid based cytology test (TCT).
| TCT results | SCC-Agn/N | HSP90αn/N | Correlation coefficient | Statistical Significance |
|---|---|---|---|---|
| ASC-US | 16/29 | 25/29 | 0.27 | ns |
| LSIL | 39/60 | 50/60 | 0.51 | P = 0.002 |
| HSIL | 17/21 | 16/21 | 0.71 | P < 0.001 |
| SCC | 0/0 | 0/0 | – | – |
ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion or carcinoma in situ; SCC, squamous cell carcinoma,; ns, not signifiant.
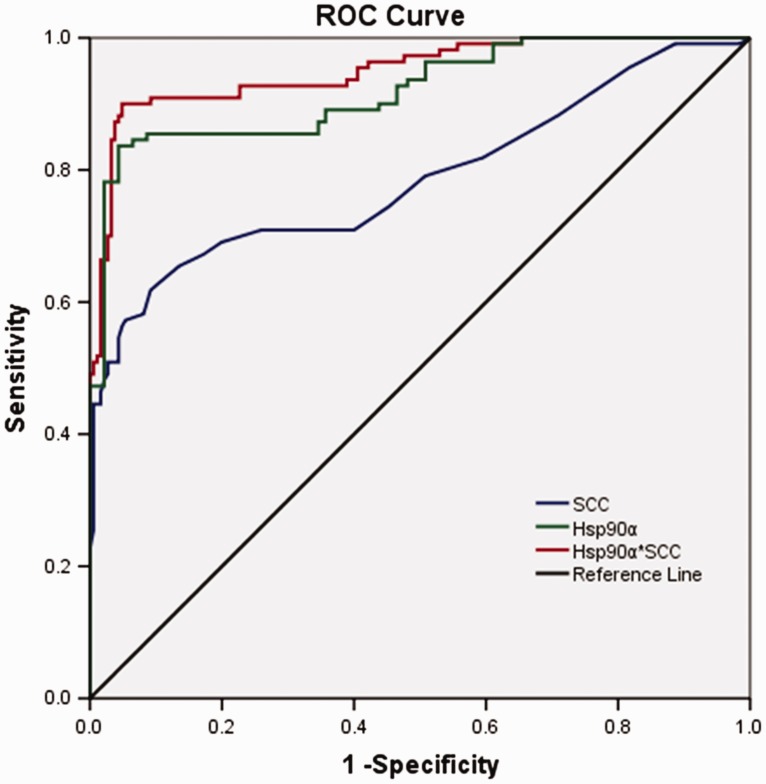
Area under the ROC curves, cut off values for cervical cancer diagnosis, sensitivities and specificities for plasma SCC-Ag, HSP90α and HSP90α*SCCAg were determined (Figure 2, Table 3). Using a cut-off value of 16.4 ng/ml to delineate cervical cancer diagnosis, compared with the other groups, the largest AUC and highest sensitivity and specificity were for the combined HSP90α*SCC-Ag group. Therefore, this combination of proteins may have more advantages as a diagnostic indicator of cervical lesions over the proteins alone.
Figure 2.
Receiver operating characteristic (ROC) curves were generated for plasma heat shock protein (HSP)90α, squamous cell carcinoma antigen (SCC-Ag) and HSP90α*SCCAg.
Table 3.
ROC curve analysis showing values for squamous cell carcinoma antigen (SCC-Ag) and heat shock protein (HSP)90α alone and together in the diagnosis of cervical cancer.
| AUC (95%CI) | Cut-off value(ng/ml) | Sensitivity(%) | Specificity(%) | |
|---|---|---|---|---|
| SCC-Ag | 0.79 (0.73–0.85) | 1.75 | 62.4 | 91.1 |
| HSP90α | 0.92 (0.89–0.95) | 142.3 | 84.7 | 96.4 |
| HSP90α*SCC | 0.95 (0.92–0.98) | 16.4 | 90.3 | 95.1 |
AUC, area under curve.
Combining data from the ROC curve analysis with other measures of diagnostic accuracy, HSP90α*SCCAg appears to be more discriminative than the proteins alone (Table 4).
Table 4.
Diagnostic accuracy tests of squamous cell carcinoma antigen (SCC-Ag) and heat shock protein (HSP)90α alone or together in the diagnosis of cervical cancer.
| Youden’s index* | Likelihood ratio# |
Predictive Valueγ |
|||
|---|---|---|---|---|---|
| LR+ | LR− | PPV (%) | NPV (%) | ||
| SCC-Ag | 0.57 | 12.4 | 0.40 | 83.2 | 79.8 |
| HSP90α | 0.80 | 20.7 | 0.18 | 93.5 | 89.9 |
| HSP90α*SCC | 0.85 | 18.5 | 0.11 | 92.7 | 94.1 |
LR+, Likelihood ratio for positive test results; LR-, Likelihood ratio for negative test results; PPV, Positive predictive value, NPV, Negative predictive value;
*The greater the number the greater the accuracy
#Ratio of the probability that a test result is correct to the probability that the test result is incorrect.
γProportions of true positive and true negative results
Discussion
Although cervical cancer is preventable, over the past decade, the incidence and mortality rates associated with the disease have increased in China.3 Therefore, there is a need to establish a comprehensive prevention screening program. Currently, cervical smear tests, liquid-based cytology and colposcopy examinations are commonly used to diagnose the disease. However, due to inadequate screening programs, a diagnosis of cervical cancer may be missed.2 Therefore, finding new and easy methods for early detection of the cancer is imperative.
Tumour markers are proteins or enzymes secreted by tumour cells or host cells during the process of carcinogenesis. Indeed, detecting tumour markers in the blood can be a critical method for the early tumour detection.7 Our study found that there were significant differences between levels of HSP90α and SCC-Ag across the patient groups with those positive for cervical cancer having the greatest levels of proteins compared with other patient groups and controls. In addition, we found that there was a significant correlation between SCC-Ag and HSP90α in patients with cervical cancer and the association between the two proteins increased as the severity of the cancer increased. Using ROC analysis, combined HSP90α*SCC-Ag had the largest AUC compared with the single proteins. Other measures of diagnostic accuracy (i.e., Youden’s; index, likelihood ratios and predictive values) tended to confirm the discriminatory potential of HSP90α*SCC-Ag. These results suggest that HSP90α*SCC-Ag has more advantages and clinical implications in the diagnosis of cervical cancer lesions than either SCC-Ag or HSP90α alone. Limitations of the study included the small sample size and the cross-sectional and single centre design. Further, large scale, prospective multicentre studies are required to confirm our results.
In summary, this study found that by comparison with controls, plasma samples from women with cervical cancer contained significantly high levels of HSP90α. Furthermore, compared with HSP90α or SCC-Ag, the combination of HSP90α*SCC-Ag had a better diagnostic potential as a biomarker for cervical lesions. The addition of HSP90α*SCC-Ag assessment to HR-HPV DNA detection and cytology could possibly result in a more robust diagnostic test for early detection of cervical cancer and lead to reductions in missed diagnosis and mortality from the disease.
Acknowledgements
We would like to express our gratitude to Professor Huang Yanchun for her help with the study and manuscript. We are grateful for the help of the department of pathology, the Third Clinical Medical College of Xinjiang Medical University (Affiliated Tumor Hospital) and would like to thank our colleagues who assisted us with the study.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This work was financed by Grants from the Xinjiang Natural Science Foundation of China (No. 2018D01C261).
References
- 1.Sun H, Shen K, Cao D. Progress in immunocytochemical staining for cervical cancer screening. Cancer Manag Res 2019; 11:1817–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YJ, Munsell MF, Park JC, et al. A retrospective review of symptoms and palliative care interventions in women with advanced cervical cancer. Gynecol Oncol 2015; 139: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu SY, Zheng RS, Zhao FH, et al. Trend analysis of cervical cancer incidence and mortality rates in Chinese women during 1989-2008. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2014; 36: 119–125. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Fact sheets. Human papillomavirus (HPV) and cervical cancer. 24 January 2019. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
- 5.Persano S, Valentini P, Kim JH, et al. Colorimetric detection of human papillomavirus by double isothermal amplification. Chem Commun (Camb) 2013; 49: 10605–10607. [DOI] [PubMed] [Google Scholar]
- 6.Castle PE, Murokora D, Perez C, et al. Treatment of cervical intraepithelial lesions. Int J Gynaecol Obstet 2017; 138 Suppl 1:20–25. [DOI] [PubMed] [Google Scholar]
- 7.Dasari S, Wudayagiri R, Valluru L. Cervical cancer: biomarkers for diagnosis and treatment. Clinica Chimica Acta 2015; 445: 7–11. [DOI] [PubMed] [Google Scholar]
- 8.Hellberg D, Tot T. Tumor marker score for prognostication of early-stage squamous cell cervical cancer. Anticancer Res 2014; 34: 887–892. [PubMed] [Google Scholar]
- 9.Farzaneh F, Shahghassempour S, No-shine B, et al. Application of tumor markers SCC-Ag, CEA, and TPA in patients with cervical precancerous lesions. Asian Pac J Cancer Prev 2014; 15: 3911–3914. [DOI] [PubMed] [Google Scholar]
- 10.Salvatici M, Achilarre MT, Sandri MT, et al. Squamous cell carcinoma antigen (SCC-Ag) during follow-up of cervical cancer patients: role in the early diagnosis of recurrence. Gynecol Oncol 2016; 142: 115–119. [DOI] [PubMed] [Google Scholar]
- 11.Bolger BS, Dabbas M, Lopes A, et al. Prognostic value of preoperative squamous cell carcinoma antigen level in patients surgically treated for cervical carcinoma. Gynecol Oncol 1997; 65:309–313. [DOI] [PubMed] [Google Scholar]
- 12.Lianos GD, Alexiou GA, Mangano A, et al. The role of heat shock proteins in cancer. Cancer Lett 2015; 360: 114–118. [DOI] [PubMed] [Google Scholar]
- 13.Hoter A, El-Sabban ME, Naim HY. The HSP90 family: structure, regulation, function, and implications in health and disease. Int J Mol Sci 2018; 19 pii E2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayaprakash P, Dong H, Zou M, et al. Hsp90α and Hsp90β together operate a hypoxia and nutrient paucity stress-response mechanism during wound healing. J Cell Sci 2015; 128: 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altieri DC. Mitochondrial HSP90s and tumor cell metabolism. Autophagy 2013; 9: 244–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Song X, Zhuo W, et al. The regulatory mechanism of Hsp90α secretion and its function in tumor malignancy. Proc Natl Acad Sci U S A 2009; 106: 21288–21293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Song X, Chen Y, et al. PLCγ1-PKCγ signaling-mediated Hsp90α plasma membrane translocation facilitates tumor metastasis. Traffic 2014; 15: 861–878. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, Xu X, Huang D, et al. Plasma heat shock protein 90alpha as a biomarker for the diagnosis of liver cancer: an office, large-scale, and multicenter clinical trial. EBioMedicine 2017; 24: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Liu X, Lou J, et al. Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin Cancer Res 2014; 20: 6016–6022. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Sun W, Hao X, et al. Down-regulation of cellular FLICE-inhibitory protein (Long Form) contributes to apoptosis induced by Hsp90 inhibition in human lung cancer cells. Cancer Cell Int 2012; 12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Šimundić AM. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC. 2009. Jan 20; 19:203–211. [PMC free article] [PubMed] [Google Scholar]