Short abstract
Objective
Probiotics may be efficacious in preventing ventilator-associated pneumonia (VAP). The aim of this network meta-analysis (NMA) was to clarify the efficacy of different types of probiotics for preventing VAP.
Methods
This systematic review and NMA was conducted according to the updated preferred reporting items for systematic review and meta-analysis. A systematic literature search of public databases from inception to 17 June 2018 was performed.
Results
NMA showed that “Bifidobacterium longum + Lactobacillus bulgaricus + Streptococcus thermophiles” was more efficacious than “Ergyphilus” in preventing VAP (odds ratio: 0.15, 95% confidence interval: 0.03–0.94). According to pairwise meta-analysis, “B. longum + L. bulgaricus + S. thermophiles” and “Lactobacillus rhamnosus” were superior to placebo in preventing VAP. Treatment rank based on surface under the cumulative ranking curves revealed that the most efficacious treatment for preventing VAP was “B. longum + L. bulgaricus + S. thermophiles” (66%). In terms of reducing hospital mortality and ICU mortality, the most efficacious treatment was Synbiotic 2000FORTE (34% and 46%, respectively).
Conclusions
Based on efficacy ranking, “B. longum + L. bulgaricus + S. thermophiles” should be the first choice for prevention of VAP, while Synbiotic 2000FORTE has the potential to reduce in-hospital mortality and ICU mortality.
Keywords: Network meta-analysis, probiotics, randomized controlled trial, ventilator-associated pneumonia, systematic review, mortality
Introduction
Ventilator-associated pneumonia (VAP) remains an important cause of morbidity and mortality in mechanically ventilated patients and is the most commonly occurring nosocomial bacterial infection in the intensive care unit (ICU). It has been estimated that VAP may be responsible for 27% to 47% of infections in patients receiving mechanical ventilation in the ICU.1 Although VAP increases the economic and clinical burden, the application of existing VAP prevention strategies has been variable, with inadequate outcomes.2
The pathogenesis of VAP is complex but mostly involves two important processes: bacterial colonization of the upper digestive tract and aspiration of contaminated secretions into the lower airway.3 The endogenous flora plays an important role in the development of VAP, given that translocation of and abnormal colonization of the upper digestive tract with potentially pathogenic bacteria is believed to be the prime mechanism responsible for VAP. Colonization of an endotracheal tube with biofilm-forming bacteria results in embolization into the alveoli at some stage during suctioning or bronchoscopy; however, inhalation of pathogens from infected aerosols and direct inoculation are also common.4,5
Numerous studies have assessed various strategies to prevent VAP, including non-pharmacological and pharmacological interventions.6,7 Current efficacious non-pharmacological interventions to prevent VAP target modifiable risk factors that are relevant to aspiration and colonization, including bed head elevation, subglottic secretion draining or silver-coated endotracheal tubes, intensive oral care, and shortening of the duration of mechanical ventilation.1 Pharmacological interventions to prevent VAP aim to attenuate the burden of bacterial colonization of the upper digestive tract. Several studies have reported that the incidence of VAP can be decreased by using non-absorbable antibiotics and systemic antibiotic prophylaxis, applied topically to the gastrointestinal tract.8,9 However, there are some limitations to the widespread use of selective decontamination of the digestive tract, such as the overgrowth of Gram-positive bacteria and the development of antibiotic resistance by both Gram-negative and Gram-positive bacteria.10
Given this background, probiotic therapy has emerged as an intriguing alternative to antibiotics. Probiotics are defined by the World Health Organization and the Food and Agriculture Organization as living non-pathogenic microorganisms that are able to tolerate the hostile gastrointestinal environment and have demonstrated well-documented beneficial health effects in the host. Their use may be beneficial in regaining the stability of the endogenous flora and in preventing VAP.
In recent years, several reports have suggested that oral probiotic therapy may indeed prevent VAP.11,12 However, the outcomes of such studies remain controversial.13–15 Accordingly, several meta-analyses have been published in this field, but have yielded different results. In 2010, Siempos et al.16 performed a meta-analysis that included five randomized controlled trials (RCTs) and concluded that the use of probiotics was associated with a lower incidence of VAP. This result was confirmed by a Cochrane systematic review of eight RCTs.17 However, two other meta-analyses, carried out by Gu et al. and Wang et al.,18,19 concluded that probiotics were not beneficial in patients undergoing mechanical ventilation. In all of these meta-analyses, the experimental treatment group was formed by pooling the extensive variety of varying probiotic strains that were used in the original clinical trials. However, this approach does not provide a meaningful answer to clinicians as to which specific probiotic strain or product has evidence-based efficacy in preventing VAP.
To resolve this issue, we used a network meta-analysis (NMA) to determine the efficacy of different probiotic strains for preventing VAP and their effects on in-hospital mortality, ICU mortality, ICU length of stay, and diarrhea rate. By using NMA of data from RCTs of probiotics for the prevention of VAP, we sought to develop a clinically meaningful and updated understanding of the relative efficacy of different probiotic product treatments.
Methods
Search strategy and study selection
A systematic review and NMA were conducted according to the updated preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines (electronic supplemental material [ESM] 1) and recommendations for NMA.18 We performed a systematic literature search in the PubMed (National Library of Medicine, Bethesda, USA), Web of Science, EMBASE (Elsevier, Amsterdam, the Netherlands), and Cochrane databases up to 17 June 2018. The following search terms were used in several logical combinations: “probiotic*”, “probiotics*”, “prebiotic*”, “prebiotics*”, “symbiotic*”, “synbiotics*”, “lactobacillus*”, “lactobacilli*”, “bifidobacterium*”, “VAP*”, “pneumonia*”, and “ventilator-associated pneumonia*”, with a restriction on “clinical trial”. In addition, reference lists of formerly published meta-analyses were screened in detail to identify additional eligible studies. The literature search was independently completed by two reviewers (Fan Qiongli and Yu Xiu-Mei). Disagreements on the inclusion of studies were resolved through discussion.
Selection criteria
Eligible studies were those in which comparative outcomes including VAP rate, in-hospital mortality rate, ICU mortality rate, ICU length of stay, and diarrhea rate were reported for patients undergoing mechanical ventilation who were treated with placebo or probiotics (including synbiotics, which contain both probiotics and prebiotics). The following inclusion criteria were used: (1) participants were patients who underwent mechanical ventilation and whose treatment procedure included probiotics, either alone or in combination with other interventions; (2) study design was restricted to RCTs; and (3) at least one of the following outcomes were included: VAP rate, in-hospital mortality rate, ICU mortality rate, ICU length of stay, or diarrhea rate. The following types of manuscript were excluded: letters to the editor, studies published in a book, reviews, and studies not published in Chinese or English. In the event of duplicate trials with accumulating numbers of patients or prolonged follow-up periods, the most informative manuscript for qualitative evaluation was included in the meta-analysis.
Data extraction and outcome measures
From the eligible studies, information on inclusion criteria, experimental groups, key features, and outcomes was extracted independently by the two reviewers using a standardized information collection sheet. Where data were not provided in the article, an attempt was made to contact the author via email. From the included studies, we extracted the first author, publication year, study design, number of patients, intervention (including type of probiotic agent, dose, and route and duration of administration), patient characteristics, and clinical outcomes. The primary outcome measure was the VAP rate. The secondary outcome measures were in-hospital mortality rate, ICU mortality rate, ICU length of stay, and diarrhea rate.
Assessment quality and publication bias
To assess the methodological quality of the included studies, quality assessment was performed by two authors independently using the risk of bias assessment tool described in the Cochrane Handbook for Systematic Reviews.21 The tool’s features of interest are adequacy of outcome assessment, personnel and outcome assessors, blinding of contributors, allocation concealment, selective outcome reporting, incomplete outcome data, and other biases. Funnel plots were used to evaluating publication bias for each outcome. The quality of all selected articles was ranked according to the Jadad composite scale.22 According to this scale, extremely high-quality research has a score of ≥3 and low-quality research has a score of ≤2.
Statistical analyses
Based on a Bayesian theorem, a comprehensive NMA was used to compare studies for every probiotic strain or combinations of strains.23 In addition, based on the extracted data, we also performed pairwise meta-analyses on comparative studies using RevMan 5.2.9 software (Cochrane Collaboration, Oxford, UK). The data extracted from the relevant trials were combined and dichotomous results were expressed as risk ratios (RRs) with their 95% confidence intervals (CIs), while continuous outcome measures were expressed as mean differences (MDs) with their 95% CIs. Statistical heterogeneity among trials was evaluated using Cochran’s Q statistic (χ2 test) and the Higgins I2 statistic to determine the percentage of total variation across studies resulting from heterogeneity. Heterogeneity was predefined as high, moderate, or low with I2 values above 75%, 50%, and 25%, respectively. A fixed effects model was used to pool studies where the I2 statistic was ≤50%; otherwise, a random effects model was used.
NMA was performed to compare the efficacy among treatments with different probiotics. Network graphs were constructed using STATA (version 13.0; StataCorp LP, College Station, TX, USA) for each outcome variable and were composed of nodes and edges. Nodes represented competing interventions, while edges between the nodes illustrated the comparison of interventions between the included studies. The number of participants receiving the intervention was represented by node size. The number of studies that were compared between the respective nodes was represented by edge thickness. The geometry of networks summarized how the evidence base was built up and whether different probiotic strains were compared directly or were only indirectly compared using network evidence. The analysis of network comparison was performed using ADDIS software v1.16.8, an online open-source application based on R statistical software (http://drugis.org/addis).24 The pooled estimates were obtained using the Markov chain Monte Carlo method.2 Markov chains were run simultaneously with different, arbitrarily chosen preliminary values.
To test for convergence, the Brooks–Gelman–Rubin method was used. A common heterogeneity parameter was assumed for all comparisons and global heterogeneity was assessed using the I2 statistic with the GeMTC R package (version 3.2.2; http://CRAN.R-project.org).25 To rank the treatments for all outcomes, surface under the cumulative ranking curves (SUCRAs) were generated to express the efficacy or safety of each treatment as a percentage relative to an imaginary treatment that is always optimal, without uncertainty.26
Results
Characteristics and risk of bias assessment of the included trials
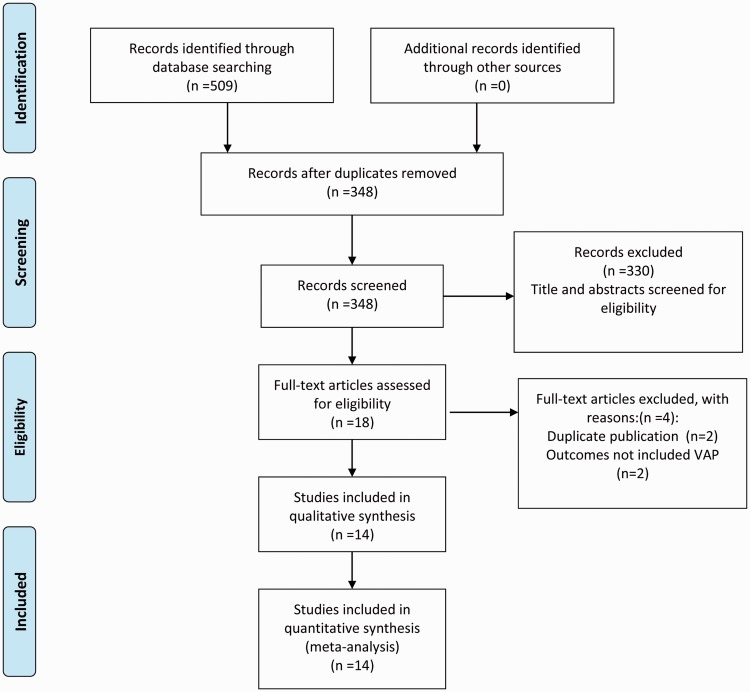
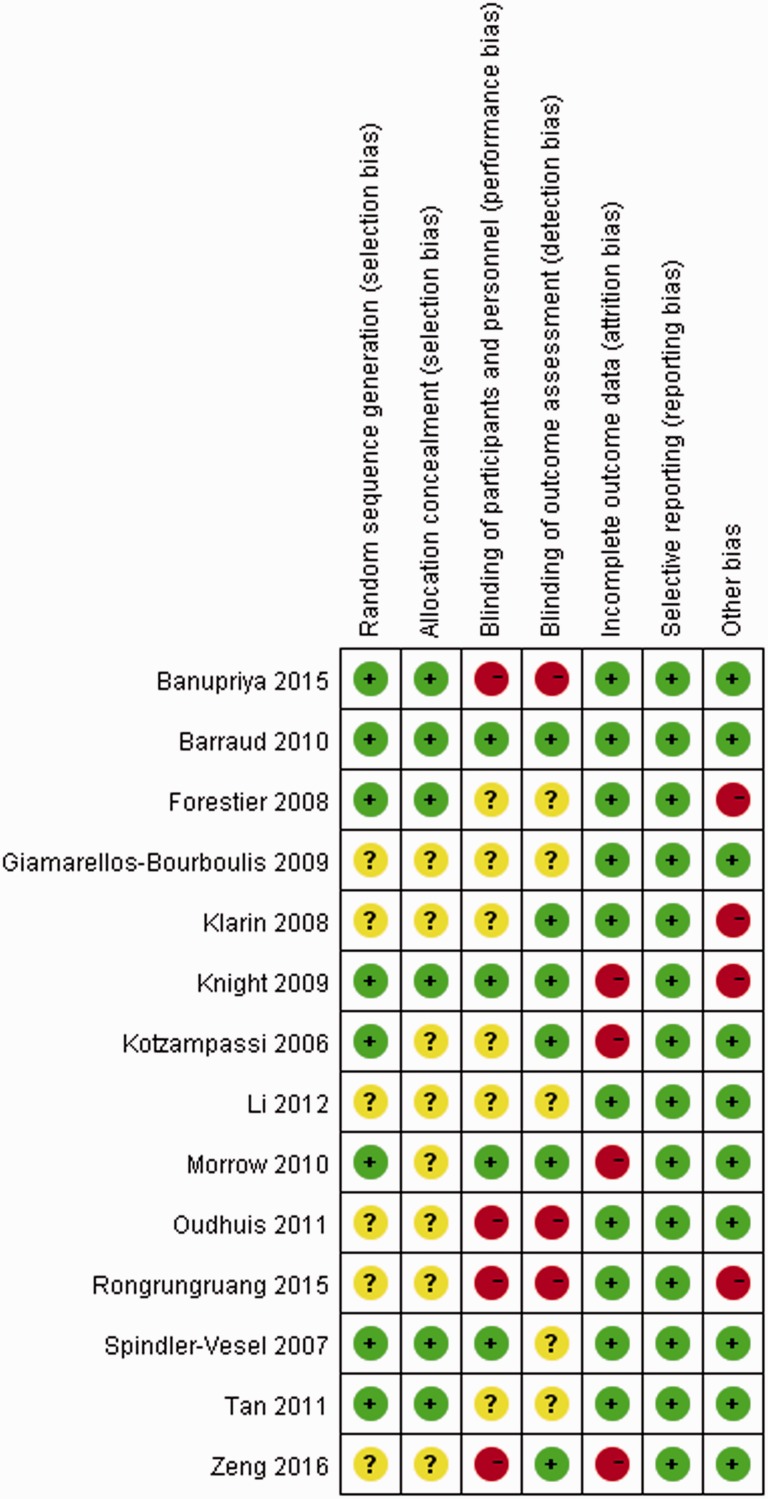
A total of 348 citations were identified in the literature search, and the full text of 18 potentially eligible articles was retrieved. Four reports were excluded because they were duplicates or did not include VAP as an outcome measure. Finally, 14 parallel RCTs (2036 patients), published between 2006 and 2016 and comparing eight types of placebo or probiotic strains, were included in this NMA. A flowchart of the literature search according to the PRISMA statement is shown in Figure 1.27 In this NMA, 990 participants were randomly assigned to a probiotic treatment group and 1046 to a placebo group. Table 1 shows the details for each study, including the baseline characteristics of patients, study publication year, strain of probiotics or intervention used, definition of VAP, and study design.13–15,28–38 In the majority of studies, the included patients presented with severe multiple organ injuries necessitating emergency tracheal intubation and ventilation support. Additionally, most patients were older than 18 years, with only one study including children. In the probiotic group, Synbiotic 2000 FORTE contained probiotics as well as the fibers beta-glucan, inulin, pectin, and resistant starch as prebiotics, which may have affected efficacy. Therefore “Synbiotic 2000 FORTE” was treated as an entire product and not a specific strain or multi-strain treatment. The results of risk of bias assessment of the included trials according to the Jadad composite scale are displayed in Figure 2.
Figure 1.
Flowchart of the literature search according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement.
Table 1.
Characteristics of included studies.
| Study/Year | Study country | Patient characteristics | Study design | Intervention probiotic strain(s) and control | Mode of administration/duration of probiotic treatment | Definition of VAP | Jadad Score |
|---|---|---|---|---|---|---|---|
| Kotzampassi 2006 | Greece | Trauma patients; severe multiple organ injuries necessitating emergency tracheal intubation and ventilation support and subsequent hospitalization in ICU; n = 72. | Double-blind, placebo-controlled, multi-center, ran = ndomized clinical trial. | Probiotic group: Synbiotic 2000 FORTE containing Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subsp paracasei, and Lactobacillus plantarum. Control group: placebo. |
Synbiotic 2000FORTE administered by a nasogastric tube or through gastrostomy once daily for 15 consecutive days. | New or persistent consolidation in lung X-ray; purulent tracheobronchial secretion; and clinical pulmonary infection score >6. | 4 |
| Spindler-Vesel 2007 | Slovenia | Multiple injured patients with an ISS (Injury Severity Score) of >18 and at least a 4-day ICU stay; n = 113. | Prospective, randomized, single-blind, multiple treatment arm study. | Probiotic group: Synbiotic 2000 FORTE containing Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subsp paracasei, and Lactobacillus plantarum. Control group: placebo. |
Once daily via nasogastric/orogastric tube until ICU discharge or death. | Microbiological specimens were collected and nosocomial infections were recorded as recommended by the Centers for Disease Control and Prevention and consensus conferences on ventilator-associated pneumonia. | 2 |
| Forestier 2008 | France | Patients aged 18 years or older with a stay >48 hours and a nasogastric feeding tube; n = 208. | Randomized, double-blind, placebo-controlled pilot study. | Probiotic group: Lactobacillus rhamnosus Control group: placebo (growth medium without bacteria) Control group: Peptide. |
L. casei rhamnosus (109 colony-forming units) twice daily through a double-lumen nasogastric suction tube or orally, after removal of the tube, from the third day after admission to the ICU until discharge or death. | At least 1 positive sample (protected specimen brush or plugged telescoping catheter for broncho-alveolar minilavage (>103 CFU/mL)) or endotracheal aspirate with (>105 CFU/mL and >25 leucocytes/high-power field); also required is the presence of 1 or several new abnormal radio graphical and progressive parenchymatous infiltrates and 1 of the following signs: purulent sputum production, fever (temperature > 38.5°C), pathogenic bacteria in blood culture without other infection source and bronchoalveolar minilavage with more than 5% cells with intracellular bacteria. | 4 |
| Klarin 2008 | Sweden | Patients aged 18 years or older and critically ill with an anticipated need for mechanical ventilation of at least 24 h; n = 44. | Randomized, controlled, open-labeled pilot study | Probiotic group: Lactobacillus plantarum. Control group: chlorhexidine solution. |
Lp299 was applied to the mucosal surface of the oral cavity as 10 mL of a solution containing a total 1010 CFU of Lp299. | New, persistent, or progressive infiltrate on chest radiograph combined with at least 3 of the following 4 criteria: purulent tracheal aspirate; positive culture of tracheal aspirates occurring after 48 h of mechanical ventilation; rectal or urine bladder temperature >38.0 °C or <35.5 °C; WBC count >12 or <3 × 109/L. | 2 |
| Knight 2009 | United Kingdom | Intubated adult patients under mechanical ventilation for a minimum of 48 hours and with no contraindications to enteral nutrition; n = 259. | Randomized, double-blind, placebo-controlled trial | Probiotic group: Synbiotic 2000 FORTE containing Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subsp paracasei, and Lactobacillus plantarum. Control group: crystalline cellulose-based placebo |
≥2 days (4 doses in 48 h) of Synbiotic 2000 FORTE. | VAP was suspected if there was new progressive or persistent (24-h) infiltration on chest radiograph plus at least 2 of the following: temperature >38.0°C; leukocytosis (white blood cell count >12 × 103 μL−1) or leukopenia (WBC count < 4 × 103 μL−1); purulent tracheobronchial secretions. All suspected cases were reviewed with appropriate clinical, radiological, and sequential microbiological data (tracheal aspirates and bronchoalveolar lavage). Diagnosis was made prospectively and only confirmed if a blinded microbiologist and intensive care physician agreed on the diagnosis. Pneumonia was classified as VAP when diagnosed 48 h after intubation. | 4 |
| Giamarellos-Bourboulis 2009 | Greece | Trauma patients; severe multiple organ injuries necessitating emergency tracheal intubation and ventilation support and subsequent hospitalization in ICU; n = 72. | Double-blind, randomized clinical trial. | Probiotic group: Synbiotic 2000 FORTE containing Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subsp paracasei, and Lactobacillus plantarum. Control group: placebo. |
Synbiotic 2000 FORTE was given in doses of 12 g (1 sachet) per day for a 15-day study period, diluted in 100 mL of tap water. | New or persistent consolidation in lung X-ray; purulent tracheobronchial secretion; and clinical pulmonary infection score >6. | 3 |
| Morrow 2010 | United States | Adults ≥19 years old were eligible for enrolment if the lead investigator and the treating physician agreed that there was a 95% likelihood that the patient would require mechanical ventilation with an endotracheal tube for at least 72 h; n = 138. | Prospective, randomized, double-blind, placebo-controlled trial. | Probiotic group: Lactobacillus rhamnosus. Control group: placebo. |
2 × 109 CFU of Lactobacillus rhamnosus GG, twice daily. | According to the American College of Chest Physicians (ACCP) clinical criteria, quantitative cultures of distal airways samples were obtained by non-bronchoscopic bronchoalveolar lavage using a protected catheter (Combicath; KOL Biomedical Instruments, Chantilly, VA, USA). ACCP clinical criteria require a new and persistent infiltrate on chest radiographs with 2 of 3 supporting findings: fever (>38.5°C or < 35.0°C); leukocytosis (WBC < 10,000/mm3 or < 3000/mm3); and purulent sputum. | 4 |
| Barraud 2010 | France | All intubated adult patients under mechanical ventilation for a predicted period of at least 2 days; n = 149. | A double-blind, concealed randomized, placebo-controlled trial | Probiotic group: Ergyphilus, consisted of a multi-species probiotic preparation containing revivable bacteria (mainly Lactobacillus rhamnosus GG but also Lactobacillus casei, Lactobacillus acidophilus, and Bifidobacterium bifidum). Control group: placebo capsules |
Treatment was administered daily through the enteral feeding tube for the entire period of mechanical ventilation (but for a duration not exceeding 28 days). After weaning from the ventilator, treatment was given for 2 additional days and then stopped in the case of successful extubation, or continued in the case of extubation failure. | New and persistent infiltrate on chest radiograph associated with at least 1 of the following: purulent tracheal secretions; temperature 38.3°C or higher and a leukocyte count of 10,000 μL−1 or higher; and positive quantitative cultures of distal pulmonary secretions obtained from bronchoalveolar lavage (significant threshold >104 CFU/ml. | 3 |
| Tan 2011 | China | Closed head injury alone; admission within 24 hours after trauma; Glasgow Coma Scale score between 5 and 8; aged 18–60 years; and able to be fed via nasogastric tube within 48 hours after admission; n = 35. | Prospective, randomized, single-blind, parallel-arm pilot study. | Probiotic group: Golden Bifid (Shuangqi Pharmaceutical Co., Ltd, Inner Mongolia, China): Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus. Control group: enteral nutrition. |
Golden Bifid dissolved in 20 mL sterilized, distilled water and administered through a nasogastric tube for 21 consecutive days; 7 sachets administered BID at 7 am, 3 pm, and 11 pm (total count number of 109). | Pneumonia occurring more than 48 h after endotracheal intubation and diagnosed by the presence of both a new or progressive radiographic infiltrate plus at least two of the following clinical features: fever >38.0°C; leukocytosis (WBC count > 12 × 109/L); leucopenia (WBC count < 4 × 109/L), or purulent tracheobronchial secretions and positive semiquantitative cultures of tracheobronchial secretions. | 2 |
| Oudhuis 2011 | The Netherlands | Patients >18 years with expected duration of mechanical ventilation of at least 48 h, expected length of ICU stay of at least 72 h, or both; n = 254. | Open label, crossover of units design, randomized clinical trial. | Probiotic group: Lactobacillus plantarum 299/299v. Control group: selective decontamination of the digestive tract. |
Dose of 5 × 109 CFU together with 6 g of rose-hip (Probi AB, Lund, Sweden). The manufactured freeze-dried powder was dissolved in 75 mL of water and administered twice daily through a nasogastric tube. | Confirmation of clinically suspected VAP required ≥2% cells containing intracellular organisms and/or a quantitative culture result of ≥104 CFU/mL in bronchoalveolar lavage fluid. | 4 |
| Li 2012 | China | Neonates with an anticipated need for mechanical ventilation of at least 48 h; n = 165. | Prospective, randomized clinical trial. | Probiotic group: bifidobacterium, lactobacillus, and enterococcus. Control group: routine treatment |
Probiotic group was administered oral probiotics in addition to routine treatment. Powderle viable (Xinyi Pharmaceutical Co., Ltd, Shanghai, China) 0.5 × 108 CFU Bifidobacterium longum, 0.5 × 107 CFU Lactobacillus bulgaricus, and 0.5 × 107 Enterococcus faecalis. | VAP was defined by the presence of: (1) purulent tracheobronchial secretion more than 48 hours after endotracheal intubation; (2) a new or progressive infiltrate on chest radiograph; (3) fever and leucocytosis (WBC count > 10 × 103 µL−1). | 2 |
| Rongrungruang 2015 | Thailand | Adult hospitalized patients expected to receive mechanical ventilation for at least 72 hours and with no VAP at enrollment; n= 147. | Prospective, randomized, open-label controlled trial. | Probiotic group: Lactobacillus casei. Control group: no additional product. |
Eighty ml of commercially available fermented dairy product containing 8 × 109 colony-forming units (cfu) of Lactobacilluscasei for oral care after the standard oral care once daily. In addition, this product was given via enteral feeding once daily for 28 days or when endotracheal tubes were removed. Probiotics were discontinued when diarrhea related to probiotics occurred. | A diagnosis of VAP was made if the patient had a new, persistent, or progressive infiltrate visible on a chest radiograph in combination with at least 3 of the following 4 criteria: (1) body temperature >38°C or <35.5°C; (2) leukocytosis (>10,000 leukocytes/mm3) or leukopenia (<3,000 leukocytes/mm3), (3) purulent tracheal aspirate; and (4) semi-quantitative culture of tracheal aspirate samples positive for pathogenic bacteria. | 4 |
| Banupriya 2015 | India | Children aged ≤12 years admitted to ICU and who were likely to need mechanical ventilation for >48 h; n = 150. | Open-label, randomized controlled trial | Probiotic group: Lactobacillus, Bifidobacterium, and Streptococcus thermophilus. Control group: standard care, no placebo. |
One probiotic capsule contained 3.3 billion CFU of probiotic organisms was administered twice daily with milk through a nasogastric tube for the initial 7 days or until discharge, whichever was earlier. | VAP was defined as a new (developing more than 48 hours after the start of mechanical ventilation or within 48 hours of extubation) or persisting radiographic infiltrate (persisting radiographically for at least 72 h) that developed in conjunction with one of the following: (1) radiographic evidence of pulmonary abscess formation; (2) two of the following: fever (increase in core temperature ≥1°C and core temperature >38.3°C); leukocytosis (25% increase in circulating leukocytes from baseline/leukocyte count >10,000/mm3); and purulent tracheal aspirate [Gram’s stain > 25 neutrophils per high-power field (×400 magnification)]; (3) positive blood or pleural fluid culture with microorganisms recovered from blood or pleural fluid cultures identical to the organisms recovered from cultures of respiratory secretions. | 3 |
| Zeng 2016 | China | Critically ill adult patients aged ≥18 years with an expected need of mechanical ventilation for at least 48 h; n = 235 | Open-label, randomized, controlled multicenter trial | Probiotic group: Bacillus subtilis and Enterococcus faecalis. Control group: standard preventive strategies only. |
Probiotics capsules were broken open and the contents diluted in 50–80 mL sterile water. This solution was administered as a bolus via a nasogastric tube. | A clinical diagnosis of VAP was based on the presence of a new, persistent, or progressive infiltrate on chest radiograph that persisted for at least 48 hours (as interpreted by radiologists blinded to the patients’ treatment assignments) combined with at least two of the following: criteria: (1) a temperature > 38.0°C or < 35.5°C; (2) blood leukocytosis count >12 × 103/mm3 or <3 × 103/mm3 and/or left shift; (3) purulent tracheal aspirates. All clinical diagnoses of VAP were evaluated and agreed upon by two of the authors. | 4 |
CFU, colony-forming unit; ICU, intensive care unit; VAP, ventilator-associated pneumonia; WBC, white blood cell.
Figure 2.
Risk of bias assessment for the included trials.
Primary outcome measures
VAP
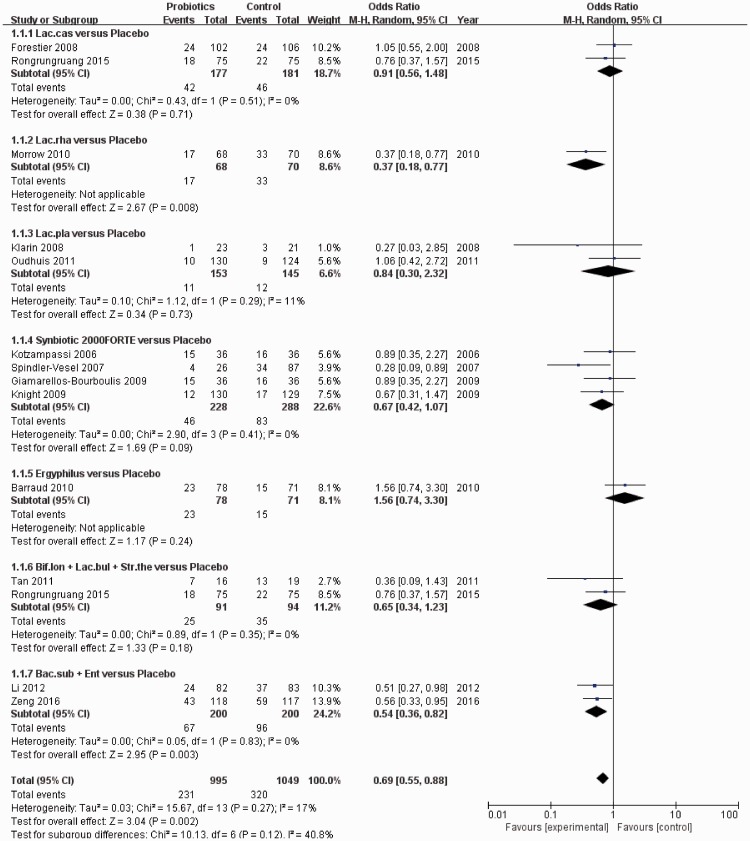
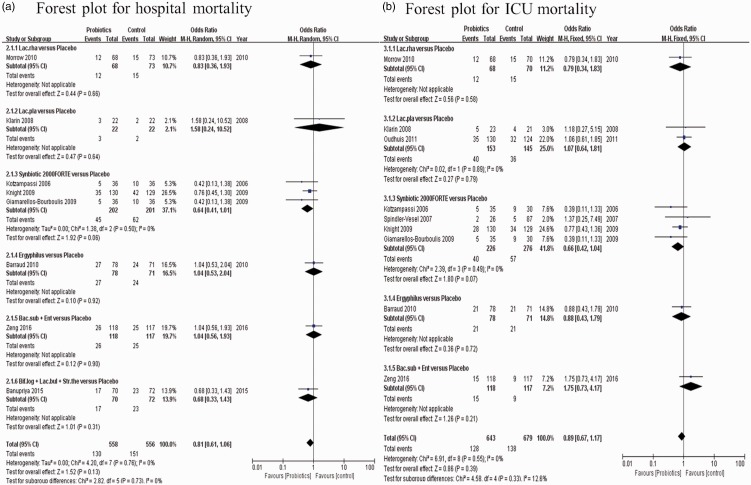
The risk of bias in studies that contributed to the primary outcomes was generally low (Figure 2). The network of the VAP rate included nine arms, 14 studies, and 2036 patients (Figure 3a). The actual number of patients in the probiotics and placebo groups with VAP is shown in Table 2. In pairwise comparisons between probiotics and placebo for the VAP rate, we analyzed subgroups based on strain type. Fourteen articles were included, and there were 995 patients in the probiotic group and 1049 patients in the placebo group. Overall, there was a clear benefit associated with intervention with probiotics compared with placebo in terms of preventing VAP (OR: 0.62, 95% CI: 0.46–0.84, P = 0.002) (Figure 4). Based on subgroup analysis, both the probiotic strain type “Lactobacillus rhamnosus” and “Bacillus subtilis + Enterococcus faecalis” were superior to placebo (OR: 0.37, 95% CI: 0.18–0.77, P = 0.008 and OR: 0.54, 95% CI: 0.36–0.82, P = 0.003, respectively). Only one study analyzed the effect of “L. rhamnosus” (probiotic group n = 68 and placebo group n = 70) and two studies compared “B. subtilis + E. faecalis” (n = 200) versus placebo (n = 200).
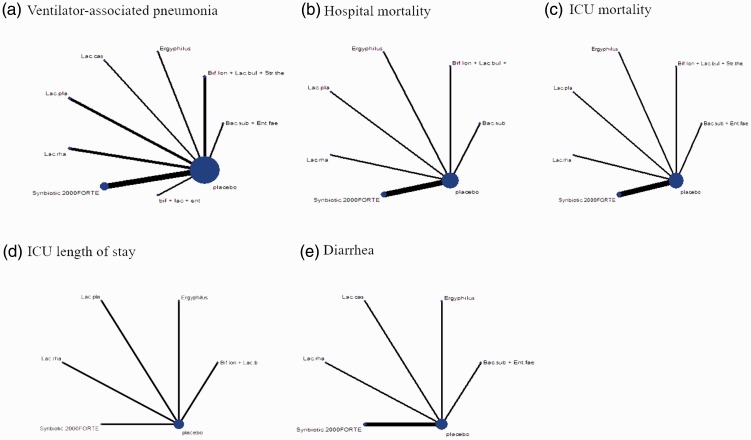
Figure 3.
a–e: Evidence network of eligible comparisons for network meta-analysis. Width of the lines is proportional to the number of trials, comparing every pair of treatments, and the size of each circle is proportional to the number of randomly assigned participants (sample size).
Table 2.
Outcome data of included studies in the meta-analysis of probiotics for VAP prevention (probiotics vs control).
| Study/Year | VAP (n/N) |
Hospital mortality (n/N) |
ICU mortality (n/N) |
ICU length of stay (mean(SD)/N) |
Diarrhea (n/N) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Probiotics | Control | Probiotics | Control | Probiotics | Control | Probiotics | Control | Probiotics | Control | |
| Forestier 2008 | 24/102 | 24/106 | NA | NA | NA | NA | NA | NA | NA | NA |
| Morrow 2010 | 17/68 | 33/70 | 12/68 | 15/73 | 12/68 | 15/70 | 14.8(11.8)/68 | 14.6(11.6)/70 | 46/68 | 57/70 |
| Klarin 2008 | 1/23 | 3/21 | 3/22 | 2/22 | 5/23 | 4/21 | 10.6(6.2)/23 | 7.6(3.7)/21 | NA | NA |
| Knight 2009 | 12/130 | 17/129 | 35/130 | 42/129 | 28/130 | 34/129 | NA | NA | 7/130 | 9/129 |
| Kotzampassi 2006 | 15/36 | 16/36 | 5/36 | 10/36 | 5/35 | 9/30 | 27.7(15.2)/35 | 41.3(20.5)/30 | 5/36 | 10/36 |
| Spindler-Vesel 2007 | 4/26 | 34/87 | NA | NA | 2/26 | 5/87 | NA | NA | NA | NA |
| Barraud 2010 | 23/78 | 15/71 | 27/78 | 24/71 | 21/78 | 21/71 | 18.7(12.4)/78 | 20.2(20.8)/71 | 48/78 | 42/71 |
| Tan 2011 | 7/16 | 13/19 | NA | NA | NA | NA | NA | NA | NA | NA |
| Rongrungruang 2015 | 18/75 | 22/75 | NA | NA | NA | NA | NA | NA | 19/75 | 14/75 |
| Zeng 2016 | 43/118 | 59/117 | 26/118 | 25/117 | 15/118 | 9/117 | NA | NA | 43/118 | 59/117 |
| Banupriya 2015 | 12/70 | 35/72 | 17/70 | 23/72 | NA | NA | 7.7(4.6)/70 | 12.54(9.91)/72 | NA | NA |
| Giamarellos-Bourboulis 2009 | 15/36 | 16/36 | 5/36 | 10/36 | 5/35 | 9/30 | NA | NA | NA | NA |
| Li 2012 | 24/82 | 37/83 | NA | NA | NA | NA | NA | NA | NA | NA |
| Oudhuis 2011 | 10/130 | 9/124 | NA | NA | 35/130 | 32/124 | NA | NA | NA | NA |
NA = not available; VAP = ventilator-associated pneumonia, ICU = intensive care unit.
Figure 4.
Forest plot for ventilator-associated pneumonia (VAP), including subgroup analysis of eight different probiotic strains. Fourteen studies were included.
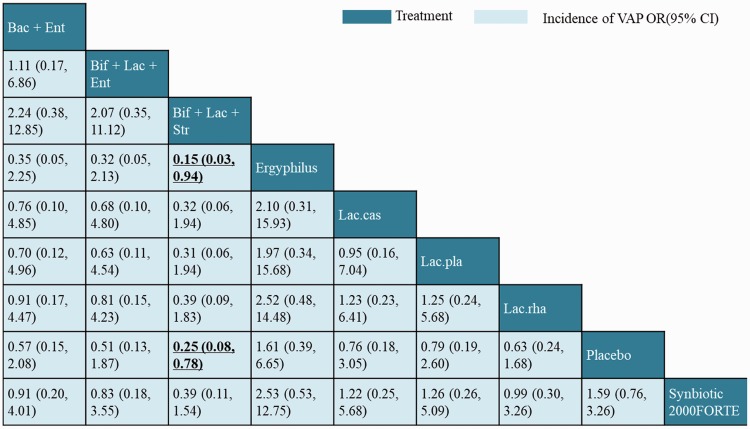
The NMA results for the primary outcome are illustrated in a league table in Figure 5. In terms of efficacy, the head-to-head comparison between different probiotic strain types showed that only the “Bifidobacterium longum + Lactobacillus bulgaricus + Streptococcus thermophiles” combination was superior to Ergyphilus (OR: 0.15, 95% CI: 0.03–0.94). In addition, we compared the estimated rank probabilities of different probiotics using SUCRAs. In terms of efficacy for preventing VAP, the most efficacious treatment was “B. longum + L. bulgaricus + S. thermophiles” (66%) and the least efficacious was Ergyphilus (60%). The top-ranking candidates for efficacious treatment in terms of different outcomes are listed in Table 3.
Figure 5.
Network meta-analysis of ventilator-associated pneumonia (VAP) outcome. Comparisons should be read from left to right. The efficacy estimate is located at the intersection of the column-defining treatment and the row-defining treatment. For efficacy, an odds ratio (OR) <1 favors the column-defining treatment.
Table 3.
Relative ranking of eight probiotic strains assessed using SUCRA values.
| Probiotic strains | VAP (%) | Hospital mortality (%) | ICU mortality (%) | ICU length of stay (%) | Diarrhea (%) |
|---|---|---|---|---|---|
| Synbiotic 2000 FORTE | 2 | 31 | 46 | 72 | 26 |
| Lac.pla | 4 | 14 | 5 | 3 | NA |
| Lac.rha | 3 | 17 | 28 | 5 | 45 |
| Lac.cas | 5 | NA | NA | NA | 2 |
| Ergyphilus | 1 | 7 | 18 | 7 | 3 |
| Bif + Lac + Str | 66 | 25 | NA | 12 | NA |
| Bac + Ent | 8 | 6 | 2 | NA | 24 |
| Bif + Lac + Ent | 11 | NA | NA | NA | NA |
P-values in bold and underlined are significant; Lac.pla = Lactobacillus plantarum, Lac.rha = Lactobacillus rhamnosus, Lac.cas = Lactobacillus casei, Bif + Lac + Str = Bifidobacterium longum + Lactobacillus bulgaricus + Streptococcus thermophilus, Bac + Ent = Bacillus subtilis + Enterococcus faecalis, Bif + Lac + Ent = Bifidobacterium + Lactobacillus + Enterococcus, NA = Not available.
Hospital and ICU mortality
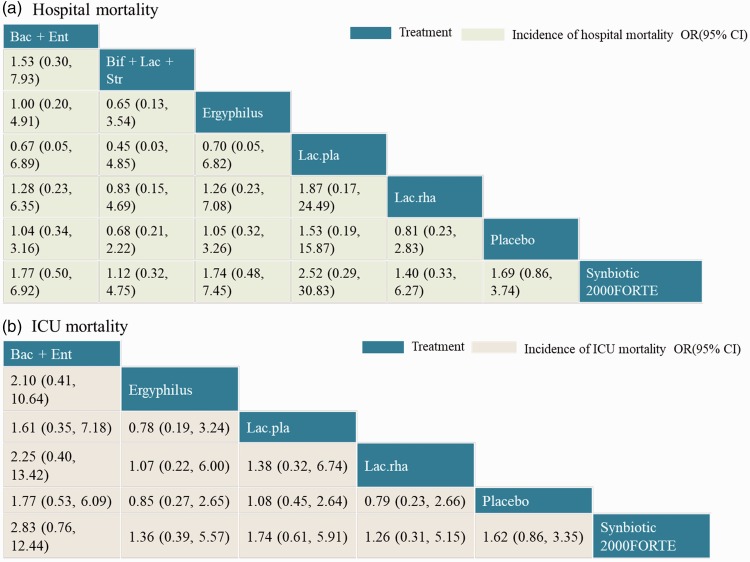
Using the available data in the existing literature, we also performed an NMA between probiotics and placebo to compare the outcomes of in-hospital mortality and ICU mortality. Detailed results of pairwise meta-analyses and subgroup analyses based on probiotic strains are shown in Figure 6a and b. There were eight studies included for the outcome of hospital mortality, with 558 patients in the probiotic group and 556 patients in the control group. Nine studies were included for the outcome of ICU mortality, with 643 patients in the probiotic group and 679 patients in the control group. In the pooled analysis, there was no significant difference in either in-hospital mortality or ICU mortality between the two groups (OR: 0.81, 95% CI: 0.61–1.06, P = 0.13; and OR: 0.89, 95% CI: 0.67–1.17, P = 0.39, respectively). This result was consistent with those from the pairwise subgroup comparisons. Figure 3b and c shows a comparison of probiotic strains or combinations of strains used in the original trials in terms of reduction of in-hospital mortality and ICU mortality, respectively. The network of in-hospital mortality rate (Figure 3b) included six arms, eight studies, and 1114 patients, while the network of ICU mortality (Figure 3c) included six arms, nine studies, and 1322 patients.
Figure 6.
a–b: Forest plot for in-hospital and intensive care unit (ICU) mortality. In subgroup analysis, six different probiotic strains were included for in-hospital mortality and five different probiotic strains for ICU mortality.
The NMA results for in-hospital mortality and ICU mortality outcomes are shown in Figure 7a and b. There was no significant difference in the head-to-head comparisons of different probiotic types. Treatments were also ranked based on SUCRAs and cumulative probability plots; the top-ranking candidate efficacious probiotics are presented in Table 3. In terms of reducing hospital mortality, the most efficacious probiotic type was Synbiotic 2000FORTE (34%) and the least efficacious probiotic strain was Lactobacillus plantarum (52%). Furthermore, for reducing ICU mortality, the most efficacious probiotic strain was Synbiotic 2000FORTE (46%) and the least efficacious probiotic type was “B. subtilis + E. faecalis” (61%).
Figure 7.
a–b: Network meta-analysis of hospital and intensive care unit (ICU) mortality outcome. Comparisons should be read from left to right. The efficacy estimate is located at the intersection of the column-defining treatment and the row-defining treatment. For efficacy, an odds ratio (OR) below 1 favors the column-defining treatment.
Secondary outcome measures
ICU length of stay
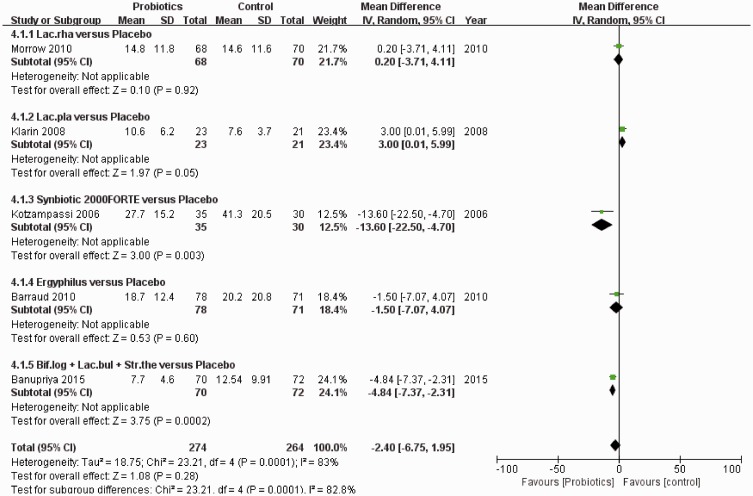
Data on ICU length of stay were reported in five studies (538 participants), with 274 patients in the probiotic group and 264 patients in the control group. The corresponding results of pairwise meta-analysis and subgroup analyses are shown in Figure 8. No significant difference was detected in ICU length of stay between probiotics and placebo interventions (MD: −3.89, 95% CI: −8.36–0.57, P = 0.09). Networks of eligible comparisons for ICU length of stay are presented in Figure 3d, showing five arms.
Figure 8.
Forest plot for intensive care unit (ICU) length of stay, including subgroup analysis of five probiotic strains. Five studies were included.
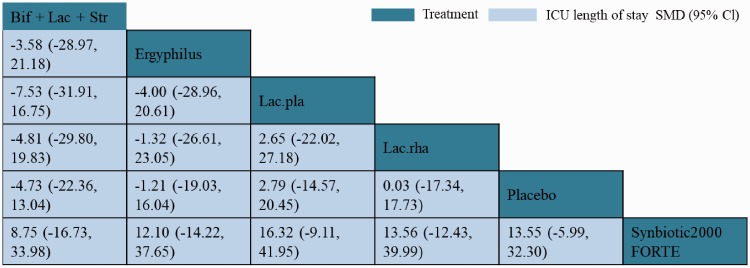
NMA results for the ICU length of stay are shown in Figure 9. There was no significant difference between different probiotics in reducing the length of ICU stay. However, Synbiotic 2000FORTE was shown to be significantly more efficacious than placebo in reducing the length of ICU stay (MD 13.70, 95% CI 2.03–24.88). Based on SUCRAs and cumulative probability plots, the ranking of probiotics by efficacy in reducing ICU length of stay revealed that the most efficacious probiotic type was Synbiotic 2000FORTE (72%) and the least efficacious was L. plantarum (48%).
Figure 9.
Network meta-analysis of intensive care unit (ICU) length of stay as outcome.
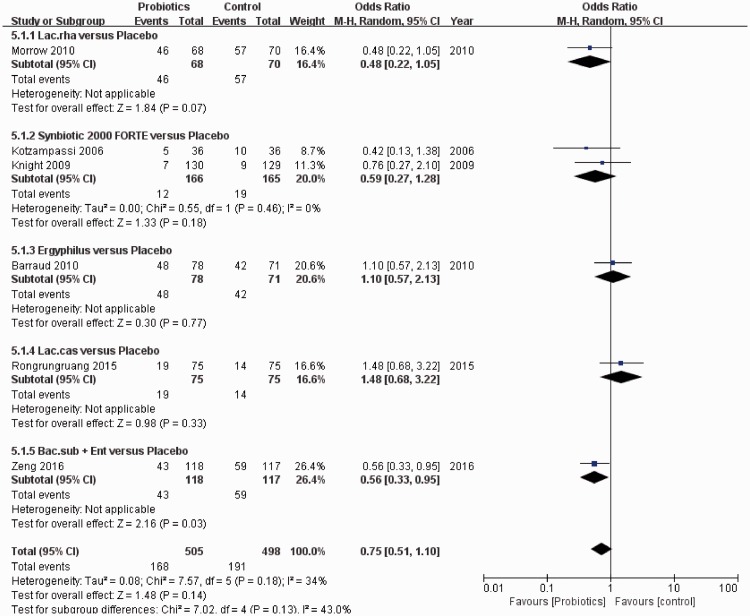
Diarrhea
Six studies reported the incidence of diarrhea for patients who received mechanical ventilation and either probiotics (505 participants) or placebo (498 participants). The results of pairwise meta-analyses are given in Figure 10. No significant difference was observed in the incidence of diarrhea following treatment with probiotics compared with placebo (OR: 0.75, 95% CI: 0.51–1.10, P = 0.14). However, subgroup analysis showed that “B. subtilis + E. faecalis” was significantly superior to placebo in terms of preventing diarrhea (OR: 0.56, 95% CI: 0.33–0.95, P = 0.03).
Figure 10.
Forest plot for diarrhea, including subgroup analysis of five probiotic strains. Six studies were included.
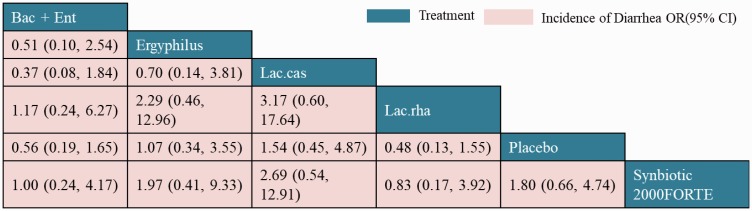
Networks of eligible comparisons for diarrhea prevention are shown in Figure 3e. NMA results for the incidence of diarrhea are shown in Figure 11. There was no significant difference between different interventions, including all types of probiotics and placebo. The ranking of treatments based on cumulative probability plots and SUCRAs showed that for preventing diarrhea, the most efficacious treatment was L. rhamnosus (45%) and the least efficacious was L. casei (55%).
Figure 11.
Network meta-analysis of diarrhea as outcome.
Discussion
Probiotic therapy may represent an effective strategy for preventing VAP, which is a costly, and currently the most prevalent, ICU-acquired infection worldwide.11,29,39 Probiotics have several important advantages over antibiotics, such as a good safety profile and few contraindications for clinical application. Nevertheless, previous meta-analyses have reported conflicting data on the use of probiotics for preventing VAP in mechanically ventilated patients.16–19 These previous meta-analyses pooled data related to all probiotic strains used in treatment across the included studies, without considering the different efficiencies of specific stains. In contrast, our comprehensive and up-to-date meta-analysis of 14 trials and 2036 patients is the first to use an NMA to compare the eight probiotic strains available for the prevention of VAP in mechanically ventilated patients. Based on pairwise analysis, our results can be considered conclusive and are consistent with the results of previous studies.17,39,40 As Weng et al.40 reported in their meta-analysis involving 1969 patients, probiotics may be effective compared with placebo in preventing VAP, but do not reduce the risk of hospital mortality, ICU mortality, or diarrhea. Instead of combining all probiotic strains, as in standard meta-analyses, different probiotics were compared head-to-head using NMA. We were therefore able to determine the most efficacious strains for preventing VAP in mechanically ventilated patients, based on the current literature. We found that only “B. longum + L. bulgaricus + S. thermophiles” was significantly more efficacious than “Ergyphilus” in preventing VAP. In pairwise meta-analysis, subgroup analysis was performed based on probiotic strain types. The results of this direct comparison between probiotics and placebo were similar to the NMA results, but there were also some inconsistences such as the finding that Synbiotic 2000FORTE was more efficacious than placebo in reducing ICU length of stay in NMA but not according to pairwise analysis. Although there were no significant differences in preventing VAP among different probiotic strains, ranking analyses were performed based on cumulative probability plots and cumulative ranking curves. The results showed that “B. longum + L. bulgaricus + S. thermophiles” was the most efficacious probiotic type for preventing VAP, while “Ergyphilus” was the least efficacious.
The present study had several strengths and limitations. First, there were inconsistencies in the included literature. As shown in Figure 2, although most of the trials adequately reported the methodology, several domains remained unclear because of insufficient information. Second, the wide range of daily doses and length of administration of probiotic therapy among the different trials may limit the ability to draw robust clinical conclusions and make recommendations. Third, considering the diversity in protocols of the included studies, significant heterogeneity was present. It is therefore arguable whether the consequences of special protocols should be merged for the calculation of pooled ORs. Fourth, because “Synbiotic 2000 FORTE” was not a specific strain or multi-strain but contained 4 fibers, the efficacy of this product cannot be attributed only to the probiotics. Despite these limitations, the results of this NMA provided important evidence about the efficacy of probiotics for preventing VAP, by comparing the outcomes of VAP between interventions involving different probiotics.
Conclusions
The present NMA disclosed three important findings. (1) The most efficacious probiotics for preventing VAP was “B. longum + L. bulgaricus + S. thermophiles”. (2) Accounting for the results of efficacy ranking based on cumulative probability plots and SUCRAs, Synbiotic 2000FORTE has the potential to be superior to other probiotics for reducing in-hospital mortality and ICU mortality. (3) Among the eight types of probiotics, L. rhamnosus was associated with the lowest diarrhea rate while L. casei was associated with the highest diarrhea rate. No report to date has used NMA to assess probiotic strain-specific effects on the development of VAP in mechanically ventilated patients. Our study may provide guidance to physicians regarding the selection of probiotics in the ICU. However, further rigorous clinical trials with direct comparisons between different types of probiotics are warranted.
Supplemental Material
Supplemental material, IMR876753 Supplemental Material for Synbiotics for prevention of ventilator-associated pneumonia: a probiotics strain-specific network meta-analysis by Qiong-Li Fan, Xiu-Mei Yu, Quan-Xing Liu, Wang Yang, Qin Chang and Yu-Ping Zhang in Journal of International Medical Research
Acknowledgements
The authors thank the study participants as well as current and past researchers and staff for their contribution to this research.
List of abbreviations
Bac + Ent = Bacillus subtilis + Enterococcus faecalis
Bif + Lac + Ent = Bifidobacterium + Lactobacillus + Enterococcus
Bif + Lac + Str = Bifidobacterium longum + Lactobacillus bulgaricus + Streptococcus thermophilus
ICU = intensive care unit
Lac.cas = Lactobacillus casei
Lac.pla = Lactobacillus plantarum
Lac.rha = Lactobacillus rhamnosus
NA = not available
VAP = ventilator-associated pneumonia.
Authors’ contributions
Qiong-Li Fan and Xiu-Mei Yu are co-first authors; they wrote the main manuscript text and prepared Figures 1–11 and Tables 1–3. Qin Chang and Yu-Ping Zhang contributed substantially to the study conception and the design, and gave their final approval of the manuscript version to be published. Wang Yang, Qiong-Li Fan, and Xiu-Mei Yu contributed to the analysis and interpretation of all data, and drafted the manuscript. Qin Chang and Yu-Ping Zhang critically revised the manuscript for important intellectual content.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The work was supported by the clinical research foundation of the Army Military Medical University (2018XLC3026 and 2014D307).
Role of the funding source
The funders had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; the preparation, review or approval of the manuscript; or the decision to submit the manuscript for peer review.
References
- 1.Grap MJ, Munro CL, Unoki T, et al. Ventilator-associated pneumonia: the potential critical role of emergency medicine in prevention. J Emerg Med 2012; 42: 353–362. [DOI] [PubMed] [Google Scholar]
- 2.Kallet RH. The vexing problem of ventilator-associated pneumonia: observations on pathophysiology, public policy, and clinical science. Respir Care 2015; 60: 1495–1508. [DOI] [PubMed] [Google Scholar]
- 3.Baselski V, Klutts JS, Baselski V, et al. Quantitative cultures of bronchoscopically obtained specimens should be performed for optimal management of ventilator-associated pneumonia. J Clin Microbiol 2013; 51: 740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baselski V, Klutts JS. Point-counterpoint: quantitative cultures of bronchoscopically obtained specimens should be performed for optimal management of ventilator-associated pneumonia. J Clin Microbiol 2013; 51: 740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kollef MH. What is ventilator-associated pneumonia and why is it important? Respiratory Care 2005; 50: 714–724. [PubMed] [Google Scholar]
- 6.Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med 2004; 32: 1396–1405. [DOI] [PubMed] [Google Scholar]
- 7.Craven DE. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest 2006; 130: 251–260. [DOI] [PubMed] [Google Scholar]
- 8.de Smet AM, Kluytmans JA, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 2009; 360: 20–31. [DOI] [PubMed] [Google Scholar]
- 9.de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003; 362: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 10.Ebner W, Kropec-Hubner A, Daschner FD. Bacterial resistance and overgrowth due to selective decontamination of the digestive tract. Eur J Clin Microbiol Infect Dis 2000; 19: 243–247. [DOI] [PubMed] [Google Scholar]
- 11.Theodorakopoulou M, Perros E, Giamarellos-Bourboulis EJ, et al. Controversies in the management of the critically ill: the role of probiotics. Int J Antimicrob Agents 2013; 42: S41–S44. [DOI] [PubMed] [Google Scholar]
- 12.Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care 2005; 50: 714–721; discussion 721–724. [PubMed] [Google Scholar]
- 13.Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care 2011; 15: R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rongrungruang Y, Krajangwittaya D, Pholtawornkulchai K, et al. Randomized controlled study of probiotics containing Lactobacillus casei (Shirota strain) for prevention of ventilator-associated pneumonia. J Med Assoc Thai 2015; 98: 253–259. [PubMed] [Google Scholar]
- 15.Klarin B, Molin G, Jeppsson B, et al. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomised controlled open pilot study. Crit Care 2008; 12: R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med 2010; 38: 954–962. [DOI] [PubMed] [Google Scholar]
- 17.Bo L, Li J, Tao T, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev 2014. Oct 25; (10): CD009066 doi: 10.1002/14651858.CD009066.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Liu KX, Ariani F, et al. Probiotics for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of high-quality randomized controlled trials. PLoS One 2013; 8: e83934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu WJ, Wei CY, Yin RX. Lack of efficacy of probiotics in preventing ventilator-associated pneumonia probiotics for ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. Chest 2012; 142: 859–868. [DOI] [PubMed] [Google Scholar]
- 20.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 2011; 14: 429–437. [DOI] [PubMed] [Google Scholar]
- 21.Kim KS, Jo JK, Chung JH, et al. Quality analysis of randomized controlled trials in the International Journal of Impotence Research: quality assessment and relevant clinical impact. Int J Impot Res 2017; 29: 65–69. [DOI] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 23.Efthimiou O, Debray TP, van Valkenhoef G, et al. GetReal in network meta-analysis: a review of the methodology. Res Synth Methods 2016; 7: 236–263. [DOI] [PubMed] [Google Scholar]
- 24.van Valkenhoef G, Lu G, de Brock B, et al. Automating network meta-analysis. Res Synth Methods 2012; 3: 285–299. [DOI] [PubMed] [Google Scholar]
- 24.Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64: 163–171. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 28.Forestier C, Guelon D, Cluytens V, et al. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care 2008; 12: R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med 2010; 182: 1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight DJ, Gardiner D, Banks A, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med 2009; 35: 854–861. [DOI] [PubMed] [Google Scholar]
- 31.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, et al. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically ill trauma patients: early results of a randomized controlled trial. World J Surg 2006; 30: 1848–1855. [DOI] [PubMed] [Google Scholar]
- 32.Spindler-Vesel A, Bengmark S, Vovk I, et al. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr 2007; 31: 119–126. [DOI] [PubMed] [Google Scholar]
- 33.Barraud D, Blard C, Hein F, et al. Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med 2010; 36: 1540–1547. [DOI] [PubMed] [Google Scholar]
- 34.Zeng J, Wang CT, Zhang FS, et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med 2016; 42: 1018–1028. [DOI] [PubMed] [Google Scholar]
- 35.Banupriya B, Biswal N, Srinivasaraghavan R, et al. Probiotic prophylaxis to prevent ventilator associated pneumonia (VAP) in children on mechanical ventilation: an open-label randomized controlled trial. Intensive Care Med 2015; 41: 677–685. [DOI] [PubMed] [Google Scholar]
- 36.Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, et al. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma 2009; 67: 815–821. [DOI] [PubMed] [Google Scholar]
- 37.Li XC, Wang JZ, Liu YH. [Effect of probiotics on respiratory tract pathogen colonization in neonates undergoing mechanical ventilation]. Zhongguo Dang Dai Er Ke Za Zhi 2012; 14: 406–408. [PubMed] [Google Scholar]
- 38.Oudhuis GJ, Bergmans DC, Dormans T, et al. Probiotics versus antibiotic decontamination of the digestive tract: infection and mortality. Intensive Care Med 2011; 37: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care 2012; 16: R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng H, Li JG, Mao Z, et al. Probiotics for preventing ventilator-associated pneumonia in mechanically ventilated patients: a meta-analysis with trial sequential analysis. Front Pharmacol 2017; 8: 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, IMR876753 Supplemental Material for Synbiotics for prevention of ventilator-associated pneumonia: a probiotics strain-specific network meta-analysis by Qiong-Li Fan, Xiu-Mei Yu, Quan-Xing Liu, Wang Yang, Qin Chang and Yu-Ping Zhang in Journal of International Medical Research