Short abstract
Stem cell therapy has shown promise in treating a variety of pathologies, such as myocardial infarction, ischaemic stroke and organ transplantation. The stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor-4 (CXCR4) axis plays a key role in stem cell mobilization. This review describes the important role of SDF-1 in tissue injury and how it works in tissue revascularization and regeneration via CXCR4. Furthermore, factors influencing the SDF-1/CXCR4 axis and its clinical potential in ischaemia reperfusion injury, such as renal transplantation, are discussed. Exploring signalling pathways of the SDF-1/CXCR4 axis will contribute to the development of stem cell therapy so that more clinical problems can be solved. Controlling directional homing of stem cells through the SDF-1/CXCR4 axis is key to improving the efficacy of stem cell therapy for tissue injury. CXCR4 antagonists may also be effective in increasing circulating levels of adult stem cells, thereby exerting beneficial effects on damaged or inflamed tissues in diseases that are currently not treated by standard approaches.
Keywords: SDF-1, CXCR4, stem cell, injury repair, renal transplantation
Introduction
Stromal cell-derived factor-1 (SDF-1), also known as CXC motif chemokine 12 (CXCL12), is a member of the chemokine family. The SDF1 gene has a coding region of 267 bp that encodes an 89 amino-acid polypeptide residue, with the N-terminus of SDF-1 thought to be essential for receptor anchoring and activation. One of the currently recognized SDF-1 receptors is CXC chemokine receptor 4 (CXCR4), which consists of 352 amino acids.1 Upon activation, CXCR4 conveys various signals to control a variety of biological functions, such as cell chemotaxis, proliferation, apoptosis, survival, and differentiation.2 SDF-1 was originally thought to be one of the growth factors of B-lineage progenitor cells. Unlike chemokines induced by traditional inflammation, however, SDF-1 is continuously expressed in bone marrow stromal cells and bone marrow endothelial cells.3
The chemotaxis function of SDF-1 is mediated through interaction with its receptor, CXCR4, which initiates downstream signalling pathways. The CXCR4 receptor is expressed in several cell types, including blood cells (lymphocytes and monocytes), platelets, haematopoietic stem cells, embryonic stem cells, and mesenchymal stem cells.4 CXCR4 expression on the surface of mesenchymal and haematopoietic stem cells is of great clinical value due to the potential application in cell transplantation, and thus, CXCR4 has become a focus for scholars worldwide. CXCR4 is a seven transmembrane receptor that signals through the G protein cascade-mediated signal transduction pathway, and the activated receptor has been shown to increase intracellular calcium ion concentration and possess strong lymphocyte chemotaxis activity.5 The SDF-1/CXCR4 axis regulates the transport and chemotaxis of progenitor cells during embryonic development, playing an important role in embryonic development prior to birth. For example, studies in SDF-1 or CXCR4 knockout mice have shown impaired embryonic tissue development. After birth, the SDF-1/CXCR4 axis recruits postnatal cells to sites of injury, and is the regulatory centre for stem cell mobilization, migration, and homing.6
Factors influencing function of the SDF-1/CXCR4 axis
The CXCR4 blocker, AMD3100, has been shown to enhance the mobilization of bone marrow cells through SDF-1/CXCR4, resulting in a reliable source of haematopoietic stem cells for the treatment of haematological diseases.6 However, repair mechanisms involving mesenchymal stem cells are different from that of haematopoietic stem cells in the treatment of haematological diseases. Mesenchymal stem cells in peripheral blood circulation must reach the site of injury to exert their abilities, but only about 3–5% of the cells get to the injured region, where they repair the tissues through the vascular endothelium.7–9 Difficulty in reaching the injury site severely limits the efficacy of mesenchymal stem cells in the treatment of solid organ injuries, such as acute kidney injury.10–16 Therefore, improvement of the function of stem cells, especially mesenchymal stem cells, has become a main focus of stem cell research, summarised in Table 1.10–16
Table 1.
A summary of studies investigating stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) axis in stem cell preconditioning.
| Publication | Cell type | Preconditioning | Protocol | Effect (increase or upregulation) |
|---|---|---|---|---|
| Zemani et al., 200810 | EPC | SDF-1 | 100 ng/ml SDF-1 for 30 min | Angiogenic capacityRegenerative potential in ischaemic hind limb (CXCR4-mediated) |
| Pasha et al., 200811 | MSC | SDF-1 | 50 ng/ml SDF-1 for 60 min | CXCR4-mediated survivalRegenerative potential in ischaemic myocardium (CXCR4-mediated) |
| Hung et al., 200712 | MSC | Hypoxaemia | 1% O2 for 24 h | CXCR4 expressionSDF-1-induced migrationEngraftment into developing embryos |
| Liu et al.,201013 | MSC | Hypoxaemia | 3% O2 for 24 h | CXCR4 and CXCR7 expressionSDF-1-induced migration, adhesion, survival |
| Kubo et al., 200914 | PBMNC | Hypoxaemia | 2% O2 for 24 h | CXCR4 expressionAdhesionRetention in ischaemic limb |
| Tang et al., 200915 | CLK | Hypoxaemia | 0.1% O2 for 6 h | CXCR4 expressionRegenerative potential in ischaemic myocardium (CXCR4-mediated) |
| Cencioni et al., 201316 | BM-c-kit+ cells | Acidic preconditioning | pH 7.0 for 24 h | CXCR4 expressionSDF-1-induced migration, differentiation (CXCR4-mediated)Regenerative potential in ischaemic limb |
EPC, endothelial progenitor cells; MSC, mesenchymal stem cell; CLK, cardiosphere-derived Lin–/c-kit+ progenitor cells; PBMNC, peripheral blood mononuclear cells; BM, bone marrow.
Several studies have shown that treating endothelial progenitor cells, mesenchymal stem cells and smooth muscle cells with exogenous SDF-1, a hypoxic environment, or an acidosis environment, enhances the SDF-1/CXCR4 axis function and improves the therapeutic effect. SDF-1 overexpression enhances stem cell homing and produces more molecules to directly participate in the neogenesis of vascularization. At present, hypoxia and acidosis treatment are mainly used to enhance stem cell survival and adhesion.10–16
In animal models of different degrees of ischaemia, Ceradini et al., (2004)17 found that the densities of bone marrow cells are higher in tissues with more severe ischaemia and that neovascularization is also significantly increased in the ischaemic sites. When endothelial cells are cultured in 1% oxygen for 12 hours, the expression of SDF-1 is increased 9-fold, and the hypoxia condition promotes adhesion of endothelial progenitor cells to endothelial cells.14
Expression of SDF-1 has been shown to increase in kidney tissues during renal ischaemia, but decrease in bone marrow, leading to reduced adhesion of certain stem cells to bone marrow tissue, but increased adhesion to ischaemic tissue.2 Regulation of SDF-1 expression directs the migration of stem cells to ischaemic kidney tissues to initiate repair.18 Ceradini et al.17 also found that because SDF-1α is highly expressed in ischaemic tissue, CXCR4-positive cells always migrate against the concentration gradient of oxygen, suggesting that SDF-1 is an endocrine regulator that mediates the migration of endothelial progenitor cells to the ischaemic region.
In addition to AMD3100, other drugs also affect the SDF-1/CXCR4 axis function. Cobalt chloride treatment increases the number of haematopoietic stem cells in peripheral blood while upregulating SDF-1 expression to promote the transfer of stem cells to the ischaemic site.19 Both in vivo and in vitro animal studies have confirmed that cobalt chloride stimulates the production of erythropoietin, which promotes mitosis in cultured cells, and facilitates endothelial progenitor cell proliferation in bone marrow and endothelial progenitor cell migration to peripheral blood.19 In rat models of hindlimb ischaemia, increased erythropoietin is associated with increased blood flow in the ischaemic area compared with controls.20 In clinical trials, patients with acute coronary syndrome have significantly elevated erythropoietin levels compared with patients with stable angina. Multivariate analysis has revealed that erythropoietin levels are an independent predictor of endothelial progenitor cell number.2,17
Ceradini et al.21 found that hydralazine stabilizes hypoxia-inducible factor (HIF)-1 and mimics hypoxia in organisms, i.e., it creates chemical hypoxia. HIF-1 is a transcription factor that is widely involved in hypoxia-induced specific responses in mammalian cells and plays a key role in the regulation of hypoxia-induced gene expression. HIF-1 participates in regulating a series of genes by interacting with hypoxia response elements in target genes that are induced by hypoxia, namely, angiogenin and platelet-derived growth factor. Although chemical hypoxia does not cause direct tissue damage, it stabilizes hypoxia-related factors, such as HIF-1. Elevated concentrations of HIF-1 in tissues further increase the expression of SDF-1 and numerous cytokines.22 The SDF-1 enhancer contains a responsive element for hypoxia, suggesting that SDF-1 may be a gene located downstream of HIF-1.13 Therefore, the injury site-specific application of hydralazine establishes a localized chemical hypoxia condition and promotes the recruitment of endothelial progenitor cells to that location. Furthermore, systemic administration of hydralazine increases the total number and function of endothelial progenitor cells, thereby improving the health of the entire cardiovascular system. If the effect of hydralazine on the SDF-1/CXCR4 axis is specific and precise, the clinician would only need to manage the location of drug application to achieve directional control of the movement of endothelial progenitor cells. HIF-1α binds to the 3′ enhancer sequence of the erythropoietin loci and promotes its transcription, suggesting that the increase in erythropoietin expression is regulated by elevation of HIF-1α.23 All of the above data suggest that these drugs influence the SDF-1/CXCR4 axis through the regulation of HIF-1α.
Si et al. (2015)24 reported that transforming growth factor (TGF)-β1 antibody affected surface expression of CXCR4 in mesenchymal stem cells from tissue homogenate of a rat model of renal ischaemia-reperfusion injury, and reduced its tendency to bind with SDF-1. TGF-β1 antibody was also shown to inhibit CXCR4 expression in the renal tissues of rats, suggesting that TGF-β1 may promote the homing of mesenchymal stem cells by influencing CXCR4 expression.
Cytokines (such as vascular endothelial growth factor [VEGF]), hypoxia, drugs, and many other conditions effectively regulate the SDF-1/CXCR4 axis. Therefore, hypoxia preconditioning combined with cytokine/drug treatment has become an important part of many cell transplantation therapies.
Adverse functions of the SDF-1/CXCR4 axis
Many types of tumours secrete SDF-1 to promote tumour growth and metastasis. The intrinsic levels of SDF-1 in several tissues, such as lung, liver, and lymph nodes, are relatively high, which may explain why these tissues are common sites for tumour metastasis.25 In addition to regulating cell proliferation and apoptosis, SDF-1 promotes cancerous growth by stimulating angiogenesis, which establishes tumour blood supply.26,27
Although the positive roles of the SDF-1/CXCR4 axis in injury repair have been recognized, there is evidence to suggest that the SDF-1/CXCR4 axis can play a negative role in ischaemic repair of certain tissues. In animal models of ischaemic reperfusion, the SDF-1/CXCR4 axis may cause increased infarct size and extended scar length in cardiac ischaemia, leading to left ventricular dysfunction.28 This phenomenon may be a result of infiltration of inflammatory cells and aggregation of fibre cells mediated by the CXCR4 signalling pathway, and concurs with results from a model of pulmonary fibrosis.29 These data suggest that the therapeutic potential of the SDF-1/CXCR4 axis in ischaemia-reperfusion injury remains unclear and requires further investigation.
SDF-1/CXCR4 axis in ischaemic injury response
The SDF-1/CXCR4 axis is the initiating factor controlling the migration of stem cells, although SDF-1 was originally described as a cytokine released by plasma cells that maintains stem cells in a ‘hibernating state’ in bone marrow.30
Ischaemic injuries in tissues and organs are often accompanied by many other events, including cellular hypoxia, low pH/acidic cellular environment, inflammatory reactions and insufficient supply of nutrients, all of which initiate the process of revascularization and tissue regeneration. VEGF, fibroblast growth factor (FGF)-2, platelet-derived growth factor, and angiogenin all participate in tissue remodelling, whereas cytokines, such as SDF-1, interleukin (IL)-8, and monocyte chemoattractant protein-1, promote stem cell homing, enhance progenitor cell proliferation and activate the repair of mature endothelial cells and smooth muscle cells.31
Tissue ischaemia results in the formation of neovascular structures and vascular remodelling to ensure maximum tissue perfusion. Immediately after vascular remodelling, tissue regeneration initiates. The injured tissue releases VEGF, SDF-1, and granulocyte-colony stimulating factor (G-CSF) to promote the proliferation of endothelial cells and their migration along new capillaries. These factors also stimulate the recruitment of stem cells mobilized from bone marrow towards the damaged sites and promote stem cell differentiation into tissue cells.32,33 The SDF-1/CXCR4 axis plays a critical role in the revascularization process and in the regulation of endothelial/progenitor cell activities.
Once activated, CXCR4 induces several signalling cascades and initiates blood vessel formation. Through launching calcium mobilization and activating protein kinases, including protein kinase C, phosphatidylinositol 3-kinase, tyrosine kinase 2, and nuclear factor-kappa B, CXCR4 regulates cell migration, cytoskeletal reorganization, extracellular stromal cell adhesion, extracellular signal-regulated kinase-1/2 activation, AKT serine/threonine kinase activation, and cell growth, survival, and apoptosis.32
The core issue in ischaemic tissue damage is tissue hypoperfusion and cell hypoxia, which disrupts the cellular oxidative respiratory chain, ultimately causing metabolic and functional impairment in cells. Under hypoxic conditions, SDF-1 expression is upregulated in endothelial cells through HIF, and the degree of upregulation is proportional to the degree of hypoxia. In addition, hypoxia-ischaemia induces HIF-1-mediated upregulation and activation of CXCR4 expression, thereby further enhancing the chemotaxis effects of SDF-1.33 SDF-1 overexpression in plasma is an important prerequisite for bone marrow mobilization. SDF-1 regulates the adhesion of stem cells to endothelial cells and promotes stem cell transendothelial movement by activating integrin protein, which causes progenitor cells to be disseminated into ischaemic tissue after extravasation.34 Consequently, under the action of cytokines and mechanical stress at the injured site, progenitor cells begin to differentiate into damaged tissue cells and exert paracrine signalling effects to reduce tissue damage and accelerate repair. However, many high-quality studies indicate that progenitor cells rarely differentiate and mainly have paracrine effects on tissue repair.17,35,36
Cytokines, such as VEGF and FGF, have been confirmed to upregulate SDF-1 expression levels, and neutralizing these factors has been shown to block the angiogenic response induced by VEGF and FGF.12 A study involving a rat model of myocardial infarction revealed that two months after myocardial infarction, transplantation of SDF-1-overexpressing fibroblasts improved cardiac function and ventricular remodelling, providing evidence that regulating cytokines and the SDF-1/CXCR4 axis affects the number of stem cells in circulation and achieves better repair of tissue damage.37 The importance of SDF-1 in clinical diagnosis of ischaemic cardiovascular diseases has become increasingly prominent. In patients with myocardial infarction, the SDF-1 level is the only factor that can predict if CD34+ cells will increase after the use of cytokines.36 Moreover, the level of SDF-1 is notably higher in patients with unstable angina than in patients with stable angina.
AMD3100 and mechanism of activation
The role of AMD3100 in stem cell mobilization has generated strong interest among researchers. The major ligand-binding sites in the CXCR4 receptor are Aspl71 and Asp262 in the IV and VI transmembrane regions, as well as Glu288 in the VII transmembrane region. Among these sites, Glu288 is the principle site of action for AMD3100 where it blocks SDF-1 binding to CXCR4.38
CXCR4 is highly expressed in haematopoietic and mesenchymal stem cells, and SDF-1, which is continuously secreted by bone marrow stromal cells and endothelial cells, exerts a strong chemotactic pull on these cells. This interaction is the operating basis of the SDF-1/CXCR4 axis in the migration and homing of stem cells. During the stem cell homing process, translocation of vascular endothelial cells and extracellular matrix into the marrow pods is a prerequisite for further interaction with stromal cells.16 Adhesion molecules, SDF-1, and growth stimulatory factors all participate in this step to complete the entire process of homing and planting. First, E-selectin and P-selectin mediate the rolling of stem cells in the endothelial cell layer. Subsequent secretion of SDF-1 by endothelial cells activates CXCR4+ stem cells and induces the interaction between lymphocyte function antigen (LFA)-1/intercellular adhesion molecule (ICAM) and very late activation antigen (VLA)-4/vascular cell adhesion molecule (VCAM)-1 to initiate the attachment of stem cells to endothelial cells. VLA-4 and VLA-5 then interact with extracellular matrix fibronectin to allow stem cells to pass through the extracellular matrix and move to the bone marrow ridge by following the concentration gradient of SDF-1, thereby completing the homing process.33,39 In contrast, mobilization is the opposite process of homing. Under the action of cytokines and chemokines, the adhesion molecules that mediate haematopoietic stem cell anchoring in bone marrow stromal cells are destroyed, thereby releasing stem cells to mobilize into peripheral blood.
As a specific antagonist of CXCR4, AMD3100 has no activation effect on CXCR4 itself but can effectively block the interaction between SDF-1 and CXCR4. Thus, AMD3100 has great potential to be utilized as a stem cell mobilizer and a resourceful tool to provide a large amount of stem cells to treat certain related diseases. In December 2008, the US Food and Drug Administration approved the use of AMD3100 by injection (produced by Genzyme) as an atypical drug for diseases that require stem cell transplantation, such as multiple myeloma and non-Hodgkin's lymphoma.40
In addition to the above effects, AMD3100 synergistically downregulates the expression of adhesion molecules and prevents the chemotaxis effect of SDF-1 on stem cells, despite high SDF-1 expression levels in the bone marrow milieu. This effect further inhibits stem cell homing and promotes stem cells to enter the peripheral blood circulation. Subcutaneous injection of AMD3100 increases CD34+ cells in peripheral blood by more than 10-fold.41 Although AMD3100 blocks the SDF-1/CXCR4 axis, however, the homing ability of stem cells is reduced, and the cells lack proper tissue repair function.
In summary, AMD3100 is safe and effective in mobilizing stem cells and has great potential for application in the repair of damaged tissues.
Mechanism of injury repair with stem cells
Stem cell technology has improved considerably since the 1990s, and has become increasingly popular among scholars worldwide. In normal mouse models, the number of stem cells in the peripheral blood is low, and their functions are unnoticeable. However, in mouse models with various injuries, both the numbers and functions of stem cells in peripheral blood are significantly increased.42
To determine whether the SDF-1/CXCR4-axis is the driving force for recruitment of haematopoietic stem cells to the injured kidney, migration of exogenous haematopoietic stem cells was followed in a model of unilateral renal ischaemia/reperfusion. This model gives an opportunity to compare the migration of haematopoietic stem cells in injured and normal tissue within one mouse, and showed that injury is necessary for haematopoietic stem-cell migration and that SDF-1 alone is not sufficient for migration.43,44
At present, stem-cell repair of injury sites is commonly believed to consist mainly of the following two pathways:45–51 (1) direct differentiation of stem cells into damaged tissue cell types; and (2) acceleration of the repair process through paracrine actions that produce anti-inflammatory and anti-apoptotic molecules, and angiogenic factors, to reduce the apoptotic and inflammatory response of damaged cells. When damage to the body occurs, the activities of the above two pathways are significantly elevated.
Ischaemia-reperfusion injury and kidney transplantation
Ischaemia-reperfusion injury is a common pathophysiological process, and is generally found in key organs, such as the heart, brain, and kidney. For example, kidney transplantation, which involves kidney extraction, transplantation surgery, and transplantation of renal arteriovenous fistula, is the most typical ischaemia-reperfusion injury observed in the clinic.
In renal ischaemia-reperfusion injury, mitochondria-mediated oxidative burst is believed to be the primary cause of cell damage. Ischaemia-reperfusion injury is primarily caused by increased oxygen free radicals in kidney tissue cells, intracellular calcium overload, and disorders in energy and hormone metabolism.52 In this complicated pathophysiological process, the damage to renal tissues caused by reactive oxygen species is the main mechanism of producing renal tissue lesions, and is also the ultimate path for many factors to inflict damage to the kidney tissue. During ischaemia and hypoxia, the enzymatic scavenging system of free radicals is supressed, ATP consumption is elevated, and hypoxanthine and xanthine accumulate. When reperfusion begins to restore the oxygenated blood supply, the catalytic activities of xanthine and hypoxanthine oxidase increase, and leakage of univalent oxygen free radicals from the mitochondria also increases.53 Moreover, neutrophils are activated by complements, and metabolites are derived from arachidic acrylic acid.54 These resulting effects all cause an exponential increase in the production of oxygen free radicals.
In China, kidneys donated by the general public are gradually replacing the traditional kidney collections from death-row inmates,55 and the quality of donor kidneys is simultaneously reducing. The incidence of post-transplant delayed graft function has increased notably, which has a serious negative impact on the rate of graft survival. The physiological basis of delayed graft function is mostly a result of acute tubular necrosis caused by ischaemia-reperfusion injury.56 Although researchers have tried to improve ischaemia-reperfusion, using early biomarkers, new organ preservation technology and effective drugs, none can solve the problem completely. If renal ischaemia-reperfusion injury can be properly treated or prevented, the prognosis of patients will significantly improve. Thus, ischaemia-reperfusion injury remains an important topic in the field of organ transplantation.
With improvements in transplant surgery and immunosuppressive drugs, acute rejection is being well-controlled in most medical transplant centres, however, chronic rejection and chronic allograft nephropathy still threaten long-term graft survival, and are a major reason for graft loss. In 2014, Chen et al.57 reported that SDF-1/CXCR4 signalling could preserve microvascular integrity and decrease kidney fibrosis in chronic kidney disease, including renal transplant recipients. Through in vitro and in vivo evidence, they showed that augmentation of the SDF-1/CXCR4 axis, either purposefully or serendipitously with novel or existing therapies, may attenuate renal decline in chronic kidney disease so that renal transplant recipients may have a new way to prevent graft loss.57
Unfortunately, stem cell therapy has not yet proved effective in clinical practice, and barriers to effective kidney transplantation remain, however, stem cell therapy still shows great potential for the future.
Research progress in graft repair by stem cells
In a US study from Johns Hopkins, using rat models of skin-grafts, rats were divided into five groups as follows: normal saline group, FK506 group, AMD3100 group, AMD3100 plus FK506 (AF) group (7-day dosing regimen) and AF prolonged treatment group (7 day regimen repeated at the 1st, 2nd, and 3rd month). FK506, a calcineurin inhibitor, is an immunosuppressive agent widely used in kidney transplant recipients. The concentration used for low dose FK506 was 0.05 mg/kg/day, and the concentration used for AMD3100 was 1 mg/kg. Skin graft survival time in AF-group rats was found to be considerably longer than in the single-drug treated group or control group. Pathological immunohistochemistry showed that in the AF group, CD34+ and CD133+ cells increased significantly in the transplanted kidney tissues. However, in the AMD3100 single-drug group, CD34+ and CD133+ cells in the peripheral blood significantly increased, but these cells did not show significant changes in the transplanted kidney. These results suggest that AMD3100 mobilizes stem cells into the peripheral blood circulation, but these stem cells are retained in the liver, spleen, lung, and other organs. Only 3% of the mobilized cells will eventually pass through the vascular endothelium and reach the targeted recruitment site of the lesion. Nonetheless, after adding a small dose of FK506, the survival rate and pathological appearance of the rat skin grafts were significantly improved, suggesting that low dose FK506 plays an important protective role in ischaemia-reperfusion injury. The mechanism of action of FK506 on stem cell recruitment is currently unclear, but low dose FK506 may upregulate SDF-1 levels in the graft via FK-binding protein 12 and not through the traditional calcineurin pathway.58
Direct differentiation of stem cells
After performing bone marrow transplantation and AMD3100 plus FK506 pretreatment, a group of American researchers transplanted green fluorescent protein (GFP)-labelled Lewis rat bone marrow into Dark Agouti (DA) mice. The mice then received kidney transplants using organs from non-transgenic DA mouse donors. Immunofluorescence staining revealed that even though these donor kidneys should have not express fluorescent protein, green fluorescence signal was detected in the transplanted kidneys of DA mice at 30 days following transplant, demonstrating that the recipient bone marrow stem cells were involved in repair of the transplanted kidneys. GFP-positive cells were localized in the peritubular area of the graft, and CD34+ cells were from the recipient. To determine the extent of host-derived stem cell repair in the grafts, the researchers performed another set of experiments using female (XX) donors and male (XY) Lewis recipients for renal transplantation, followed by PCR to detect the Y chromosomal DNA. The results indicated the presence of Y chromosomal DNA in renal tubules, glomeruli, and around the capillaries of renal tubules from the transplant. After calculation, 20–30% of the cells were Y-positive at 1-month postsurgery, and 30–50% of the cells were positive for Y chromosome at 4 months following surgery, suggesting that recipient bone marrow stem cells contribute to the repair of the donor kidney.58 The same result is also shown in swine models.59 Importantly, stem cell mobilization driven by AF drugs appears to promote the formation of donor-recipient chimeras.
Paracrine effects of stem cells
In addition to the theory that stem cells directly differentiate into renal tubular epithelial cells for repair, some scholars believe that paracrine signalling of stem cells plays a major role during the injury repair process. Mesenchymal stem cells secrete anti-inflammatory molecules and inhibit the production of oxygen free radicals, restoring the enzymatic removal system for free radicals, resulting in equilibrium between free-radical generation and elimination.60 Thus, mesenchymal stem cells play an indirect role in anti-oxidation, suggesting that the inhibition of oxygen free radical production by bone marrow mesenchymal stem cells may be one of the protective mechanisms of bone marrow-derived stem cells for renal injury. It has been found that at 6 months following application of AMD3100 plus low dose FK506, the degree of nephritic cell infiltration and fibrosis is significantly lower versus controls. In addition, inflammatory factors, such as interferon-γ, IL-17, IL-10, and IL-6, are significantly reduced, while the CD8+/CD25+/Foxp3+ regulatory T cells increase.61,62 This observation may be related to a paracrine effect exerted by stem cells after migrating to the injured site.
Furthermore, mesenchymal stem cells promote the production of regulatory CD4+/CD25+ T cells to inhibit the proliferation of T lymphocytes and reduce inflammatory infiltration and kidney damage, thereby establishing a local immune-tolerant environment for transplanted kidneys.63
In summary, stem cells not only participate in the repair of transplanted kidneys, but also reduce apoptosis and tissue inflammation, prevent future fibrosis, and protect renal function. Infusion of bone marrow mesenchymal stem cells reduces ischaemia-reperfusion injury, and the mechanism may include mesenchymal stem cells secreting protective cytokines, such as mitogenic factors and the pro-survival factor insulin-like growth factor (IGF)-1, in a paracrine manner.64 These factors participate in regulating the production and clearance of free radicals and improving the balance of oxidative and antioxidant systems in the body. A microenvironment conducive to the repair of renal tubular epithelial cells is created, by inhibiting apoptosis of renal tubular epithelial cells and reducing the degree of tissue damage, as well as preventing kidney fibrosis and improving kidney function.
In renal ischaemia-reperfusion injury, the loss and dysfunction of renal tubular epithelial cells are the main changes that promote the regeneration and repair of renal tubular epithelial cells. Thus, maintaining the structural integrity of renal tubules has become a central focus in treating this type of injury. When acute tubular necrosis occurs, intrarenal cells provide the main source for cellular regeneration. Bone marrow mesenchymal stem cells can differentiate into intrinsic renal cells, such as mesangial cells, renal tubular epithelial cells, and podocytes.65,66
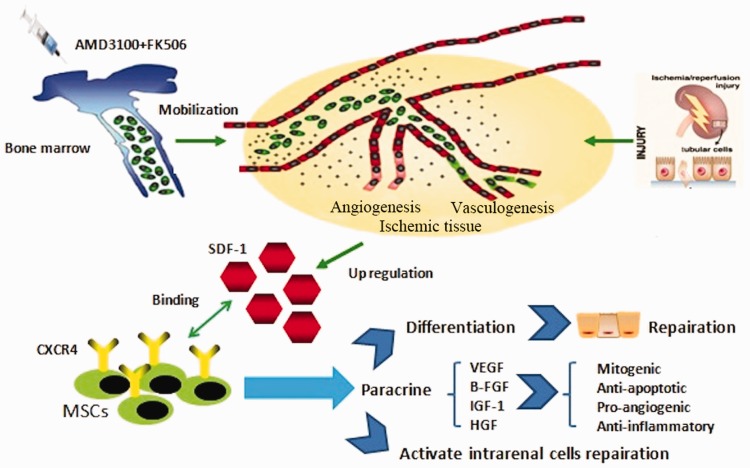
In stem-cell treatment of injuries in kidney transplants, bone marrow stem cells not only differentiate into renal tissue cells but also secrete growth factors to stimulate proliferation of renal tubular epithelial cells. The SDF-1/CXCR4 axis plays a key role in kidney ischaemia/reperfusion injury (summarised in Figure 1). Bone marrow stem cells secrete various substances, such as epidermal growth factor, IGF-1, and hepatocyte growth factor. These growth factors promote cell proliferation and upregulate HIF-1α expression. Under natural conditions, HIF-1α and its downstream gene products protect the kidney through many mechanisms, including increasing oxygen supply, improving energy metabolism, promoting angiogenesis and tissue remodelling, preventing free radical damage, reducing inflammatory damage, and preventing apoptosis. Moreover, HIF-1α expression also promotes the migration of bone marrow stem cells and facilitates tissue repair by enhancing activity of the SDF-1/CXCR4 axis. Both elevated local metabolism and intensified local oxygen supply and demand conflict increase HIF-1α expression.66,67 Some studies have shown that the bone marrow inducer, G-CSF, induces HIF-1α expression, even under normal conditions.67
Figure 1.
The stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) axis in kidney ischaemia/reperfusion injury. MSCs, mesenchymal stem cells; VEGF, vascular endothelial growth factor; B-FGF, fibroblast growth factor; IGF-1, insulin-like growth factor-1; HGF, hepatocyte growth factor.
Conclusions
With the application of immunosuppressive agents in clinical practice, the incidence of postoperative acute rejection has been drastically reduced, and the 1-year survival rate of kidney transplants has reached over 90%. However, delayed graft function and chronic rejection are still challenging issues for most clinicians. Studies on the application of stem cell technology in related fields have a broad perspective and significance. Once stem cells are successfully mobilized and recruited to the injured site, the ischaemic-reperfusion environment may promote formation of a chimeric kidney.68,69 Some scholars suggest that evidence of >40% recipient repopulation may be a starting point for weaning of conventional immunosuppression,59 but this is only a result based on basic research, and far removed from clinical practice. This treatment method not only protects the graft through bone marrow mobilization and repair, but also helps to establish immune tolerance in the recipient by inducing the formation of a graft chimera and increasing the number of local regulatory T cells. The detailed mechanism of the SDF-1/CXCR4 axis in kidney transplantation and its related signalling pathways in various types of injury repair will become the key point in stem cell therapy studies.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work is partly supported by National Natural Science Foundation of China (No.81771720) and Capital Clinical Features Research Foundation of China (Z171100001017055).
References
- 1.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol 2002; 42: 469–499. [DOI] [PubMed] [Google Scholar]
- 2.Tögel F, Isaac J, Hu Z, et al. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 2005; 67: 1772–1784. [DOI] [PubMed] [Google Scholar]
- 3.Cheng M, Huang K, Zhou J, et al. A critical role of Src family kinase in SDF-1/CXCR4- mediated bone-marrow progenitor cell recruitment to the ischemic heart. J Mol Cell Cardiol 2015; 81: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma M, Afrin F, Satija N, et al. Stromal-derived factor-1/ CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev 2011; 20: 933–946. [DOI] [PubMed] [Google Scholar]
- 5.Teixidó J, Martínez-Moreno M, Díaz-Martínez M, et al. The good and bad faces of the CXCR4 chemokine receptor. Int J Biochem Cell Biol 2018; 95: 121–131. [DOI] [PubMed] [Google Scholar]
- 6.Korbling M, Estrov Z. Adult stem cells for tissue repair–a new therapeutic concept? N Engl J Med 2003; 349: 570–582. [DOI] [PubMed] [Google Scholar]
- 7.Bosch-Marce M, Okuyama H, Wesley JB, et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res 2007; 101: 1310–1318. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues CO, Shehadeh LA, Hoosien M, et al. Heterogeneity in SDF-1 expression defines the vasculogenic potential of adult cardiac progenitor cells. PLoS One 2011; 6: e24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melchionna R, Di Carlo A, De Mori R, et al. Induction of myogenic differentiation by SDF-1 via CXCR4 and CXCR7 receptors. Muscle Nerve 2010; 41: 828–835. [DOI] [PubMed] [Google Scholar]
- 10.Zemani F, Silvestre JS, Fauvel-Lafeve F, et al. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol 2008; 28: 644–650. [DOI] [PubMed] [Google Scholar]
- 11.Pasha Z, Wang Y, Sheikh R, et al. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res 2008; 77: 134–142. [DOI] [PubMed] [Google Scholar]
- 12.Hung SC, Pochampally RR, Hsu SC, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One 2007; 2: e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Xue W, Ge G, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem Biophys Res Commun 2010; 401: 509–515. [DOI] [PubMed] [Google Scholar]
- 14.Kubo M, Li TS, Kamota T, et al. Increased expression of CXCR4 and integrin αM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency. J Cell Physiol 2009; 220: 508–514. [DOI] [PubMed] [Google Scholar]
- 15.Tang YL, Zhu W, Cheng M, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res 2009; 104: 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cencioni C, Melchionna R, Straino S, et al. Ex vivo acidic preconditioning enhances bone marrow ckit+ cell therapeutic potential via increased CXCR4 expression. Eur Heart J 2013; 34: 2007–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004; 10: 858–864. [DOI] [PubMed] [Google Scholar]
- 18.Si XY, Li JJ, Yao T, et al. Transforming growth factor-β1 in the microenvironment of ischemia reperfusion-injured kidney enhances the chemotaxis of mesenchymal stem cells to stromal cell-derived factor-1 through upregulation of surface chemokine (C-X-C motif) receptor 4. Mol Med Rep 2014; 9: 1794–1798. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Yu Q, Fu S, et al. CXCR4 antagonist AMD3100 promotes mesenchymal stem cell mobilization in rats preconditioned with the hypoxia-mimicking agent cobalt chloride. Stem Cells Dev 2018; 27: 466–478. [DOI] [PubMed] [Google Scholar]
- 20.Chen F, Liu Q, Zhang ZD, et al. Co-delivery of G-CSF and EPO released from fibrin gel for therapeutic neovascularization in rat hindlimb ischemia model. Microcirculation 2013; 20: 416–424. [DOI] [PubMed] [Google Scholar]
- 21.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med 2005; 15: 57–63. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest 2000; 106: 809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Falco E, Porcelli D, Torella AR, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 2004; 104: 3472–3482. [DOI] [PubMed] [Google Scholar]
- 24.Si X, Liu X, Li J, et al. Transforming growth factor-β1 promotes homing of bone marrow mesenchymal stem cells in renal ischemia-reperfusion injury. Int J Clin Exp Pathol 2015; 8: 12368–12378. [PMC free article] [PubMed] [Google Scholar]
- 25.Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol 2004; 35: 233–245. [DOI] [PubMed] [Google Scholar]
- 26.Meng W, Xue S, Chen Y. The role of CXCL12 in tumor microenvironment. Gene 2018; 641: 105–110. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Cao HB, Li WJ, et al. The CXCL12 (SDF-1)/CXCR4 chemokine axis: Oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin J Nat Med 2018; 16: 801–810. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Wolf PL, Escudero R, et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med 2003; 342: 626–633. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Yu X, He Y, et al. Activation of A2aR attenuates bleomycin-induced pulmonary fibrosis via the SDF-1/CXCR4 axis-related pathway. Am J Transl Res 2017; 9: 4125–4136. [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Hou J, Wu B, et al. Effects of platelet-rich plasma and cell coculture on angiogenesis in human dental pulp stem cells and endothelial progenitor cells. J Endod 2014; 40: 1810–1814. [DOI] [PubMed] [Google Scholar]
- 31.Jujo K, Hamada H, Iwakura A, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci USA 2010; 107: 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Wang J, Scott PG, et al. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen 2007; 15: S18–S26. [DOI] [PubMed] [Google Scholar]
- 33.Sun Z, Zhang X, Locke JE, et al. Recruitment of host progenitor cells in rat liver transplants. Hepatology 2009; 49: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Gu H, Zhang W, et al. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol 2011; 301: H1496–H1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heldman AW, Difede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA 2014; 311: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson JA, Little D, Toth AP, et al. Stem cell therapies for knee cartilage repair: the current status of preclinical and clinical studies. Am J Sports Med 2014; 42: 2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partanen A, Valtola J, Ropponen A, et al. Preemptive plerixafor injection added to pegfilgrastim after chemotherapy in non-Hodgkin lymphoma patients mobilizing poorly. Ann Hematol 2017; 96: 1897–1906. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Du C, Feng Z, et al. Hologram quantitative structure activity relationship, docking, and molecular dynamics studies of inhibitors for CXCR4. Chem Biol Drug Des 2015; 85: 119–136. [DOI] [PubMed] [Google Scholar]
- 39.Lin Q, Wesson RN, Maeda H, et al. Pharmacological mobilization of endogenous stem cells significantly promotes skin regeneration after full-thickness excision: the synergistic activity of AMD3100 and tacrolimus. J Invest Dermatol 2014; 134: 2458–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kean LS, Sen S, Onabajo O, et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood 2011; 118: 6580–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toupadakis CA, Wong A, Genetos DC, et al. Long-term administration of AMD3100, an antagonist of SDF-1/CXCR4 signaling, alters fracture repair. J Orthop Res 2012; 30: 1853–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardo-Saganta A, Law BM, Tata PR, et al. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 2015; 16: 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tajima F, Sato T, Laver JH, et al. CD34 expression by murine hematopoietic stem cells mobilized by granulocyte colony-stimulating factor. Blood 2000; 96: 1989–1993. [PubMed] [Google Scholar]
- 44.Stroo I, Stokman G, Teske GJ, et al. Haematopoietic stem cell migration to the ischemic damaged kidney is not altered by manipulating the SDF-1/CXCR4-axis. Nephrol Dial Transplant 2009; 24: 2082–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakai K, Yamamoto A, Matsubara K, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 2012; 122: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatch HM, Zheng D, Jorgensen ML, et al. SDF-1α/XCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells 2002; 4: 339–351. [DOI] [PubMed] [Google Scholar]
- 47.Ting AE, Mays RW, Frey MR, et al. Therapeutic pathways of adult stem cell repair. Crit Rev Oncol Hematol 2008; 65: 81–93. [DOI] [PubMed] [Google Scholar]
- 48.Duffield JS, Park KM, Hsiao LL, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 2005; 115: 1743–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008; 2: 284–291. [DOI] [PubMed] [Google Scholar]
- 50.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells 2007; 25: 2896–2902. [DOI] [PubMed] [Google Scholar]
- 51.Kuroda Y, Kitada M, Wakao S, et al. Bone marrow mesenchymal cells: how do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp 2011; 59: 369–378. [DOI] [PubMed] [Google Scholar]
- 52.Ikarashi K, Li B, Suwa M, et al. Bone marrow cells contribute to regeneration of damaged glomerular endothelial cells. Kidney Int 2005; 67: 1925–1933. [DOI] [PubMed] [Google Scholar]
- 53.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in post-ischemic kidney. J Clin Invest 2005; 115: 1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopkins C, Li J, Rae F, et al. Stem cell options for kidney disease. J Pathol 2009; 217: 265–281. [DOI] [PubMed] [Google Scholar]
- 55.Diflo T. Use of organs from executed Chinese prisoners. Lancet 2004; 364(Suppl 1): s30–31. [DOI] [PubMed] [Google Scholar]
- 56.Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet 2010; 375: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 57.Chen LH, Advani SL, Thai K, et al. SDF-1/CXCR4 signaling preserves microvascular integrity and renal function in chronic kidney disease. PLoS One 2014; 9: e92227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu X, Okabayashi T, Cameron AM, et al. Chimeric allografts induced by short-term treatment with stem cell mobilizing agents result in long-term kidney transplant survival without immunosuppression: a study in rats. Am J Transplant 2016; 16: 2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cameron AM, Wesson RN, Ahmadi AR. Chimeric allografts induced by short-term treatment with stem cell mobilizing agents result in long-term kidney transplant survival without immunosuppression: II, study in miniature swine. Am J Transplant 2016; 16: 2066–2076. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Zhang Y, Wang W, et al. Mesenchymal stem cells modified with heme oxygenase-1 have enhanced paracrine function and attenuate lipopolysaccharide-induced inflammatory and oxidative damage in pulmonary microvascular endothelial cells. Cell Physiol Biochem 2018; 49: 101–122. [DOI] [PubMed] [Google Scholar]
- 61.Crop MJ, Baan CC, Korevaar SS, et al. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation 2009; 87: 896–906. [DOI] [PubMed] [Google Scholar]
- 62.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 2004; 15: 1794–1804. [DOI] [PubMed] [Google Scholar]
- 63.Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 2008; 36: 309–318. [DOI] [PubMed] [Google Scholar]
- 64.Shabani Azandaryani Z, Davoodian N, Samiei A, et al. Insulin-like growth factor-I promotes hepatic differentiation of human adipose tissue-derived stem cells. Cell Biol Int 2019; 43: 476–485. [DOI] [PubMed] [Google Scholar]
- 65.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 2002; 62: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 66.Huls M, van den Heuvel JJ, Dijkman HB, et al. ABC transporter expression profiling after ischemic reperfusion injury in mouse kidney. Kidney Int 2006; 69: 2186–2193. [DOI] [PubMed] [Google Scholar]
- 67.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up- regulating CXCR4. Nat Immunol 2002; 3: 687–694. [DOI] [PubMed] [Google Scholar]
- 68.Fogt F, Beyser KH, Poremba C, et al. Recipient-derived hepatocytes in liver transplants: a rare event in sex-mismatched transplants. Hepatology 2002; 36: 173–176. [DOI] [PubMed] [Google Scholar]
- 69.Idilman R, Erden E, Kuzu I, et al. Recipient-derived hepatocytes in sex-mismatched liver allografts after Liver transplantation: early versus late transplant biopsies. Transplantation 2004; 78: 1647–1652. [DOI] [PubMed] [Google Scholar]