Short abstract
Objective
This study was performed to assess the predictive value of the anti-Müllerian hormone (AMH) serum level for spontaneous pregnancy in women after endometriosis surgery.
Methods
In total, 124 patients with suspected ovarian endometrioma planning to undergo laparoscopic ovarian cystectomy were divided into a high AMH group (AMH > 2 ng/mL) and low AMH group (AMH ≤ 2 ng/mL) according to their preoperative AMH levels. The postoperative AMH levels were also measured, and pregnancy outcomes were followed up.
Results
Twenty-one patients were excluded, and 52 pregnancies were registered in the remaining 103 patients diagnosed with endometriosis. The pregnancy rate was significantly greater in the high than low AMH group. Receiver operator characteristics analysis of preoperative AMH, postoperative AMH, and the AMH decline rate showed that preoperative AMH was associated with the greatest area under the curve. Kaplan–Meier curves showed that women in the high AMH group had a significantly higher cumulative pregnancy rate than those in the low AMH group.
Conclusion
The preoperative AMH level might be a useful marker to predict the occurrence of natural pregnancy and could be offered as part of the fertility strategy to women who desire pregnancy after endometriosis surgery.
Keywords: Anti-Müllerian hormone, endometriosis, cumulative pregnancy rate, fertility, ovarian cystectomy, pregnancy outcome
Introduction
Laparoscopic surgical removal of endometriosis is an effective approach to treating endometriosis.1 However, endometriosis is an enigmatic disease associated with reduced ovarian reserve, decreased pregnancy rates, and even infertility. The ovarian reserve was historically assessed by the ultrasound sinus follicle count and endocrine markers such as statin B, follicle-stimulating hormone, and estradiol.2 However, these markers are not ideal representations of ovarian reserve and menstrual cycle variation. Anti-Müllerian hormone (AMH) was recently introduced as a novel and valuable marker of ovarian reserve.3 AMH, also known as Müllerian inhibiting substance, is produced by the primary, secondary, and antral follicles to prevent depletion of the primordial follicle pool.4
The role of serum AMH as a predictor of assisted reproductive techniques (ART) outcomes has been extensively debated. AMH has been shown to accurately predict the potential success of ART prior to treatment initiation.5,6 However, it is not necessary to perform ART for all patients who undergo surgery for endometriomas because some women can still develop a natural pregnancy.7 Therefore, one of the most difficult aspects of postoperative fertility management is precise prediction of the chance of spontaneous pregnancy in patients with endometriosis.
To the best of our knowledge, few reports have focused on the correlation between the serum AMH concentration and natural fecundability outcomes in patients with endometriosis. Therefore, we performed the present study to assess the predictability of the serum AMH level for spontaneous pregnancy in women undergoing endometriosis surgery.
Patients and methods
This prospective cohort study was approved by the Ethics Review Board of Women’s Hospital Zhejiang University School of Medicine. This study included consecutive patients suspected of having ovarian endometrioma at their initial evaluation who presented to the hospital from July 2015 to February 2017. The inclusion criteria were an age of 20 to 35 years and a plan to conceive after surgery. The exclusion criteria were any suspicious findings of malignant disease, recurrent endometriosis, and hormone therapy within 3 months before surgery. Written informed consent was obtained from all patients.
Blood samples were obtained from all patients to determine their preoperative AMH levels from the third to fifth days of their spontaneous menstrual cycle before surgery. The serum AMH concentrations were measured using an AMH/Müllerian inhibiting substance enzyme immunoassay kit (Immunotech version; Beckman Coulter, Marseille, France). The lowest amount of AMH in a sample that can be detected with 95% probability is 0.06 ng/mL. The threshold value of 2.0 ng/mL in women ≤35 years of age was used in the present study based on a previous report.8 The women were divided into two groups according to their preoperative AMH level: the high AMH group (AMH > 2 ng/mL) and the low AMH group (AMH ≤ 2 ng/mL). All patients underwent cyst enucleation and suture hemostasis in ovarian endometrioma stripping surgery.
Blood samples were obtained again to measure the postoperative AMH levels from the third to fifth days of the first menstruation after endometrioma stripping surgery. On the basis of the change in the AMH levels, the rate of AMH decline was calculated using the following formula9: rate of AMH decline (%) = 100 × (preoperative AMH level − postoperative AMH level)/preoperative AMH level.
After surgery, women who planned to conceive underwent a 2-year follow-up period of pregnancy outcomes. Patients were withdrawn from the study for the following reasons: no wish to conceive after surgery, unable to be followed up, tubal infertility, male infertility, and achievement of pregnancy by ART.
The statistical analysis was conducted using GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA, USA). The data analysis was based on Student’s t test. A receiver operator characteristic (ROC) curve was plotted to analyze the predictive value of AMH, and Kaplan–Meier (K-M) analysis was used to assess the cumulative pregnancy rate (CPR). For all analyses, values of P < 0.05 were considered statistically significant.
Results
Of 124 patients with a pathological diagnosis of endometriosis, we excluded 16 patients who were lost to follow-up and 5 patients who became pregnant through ART. Hence, 103 women who attempted to conceive spontaneously were included in the study, and their clinical characteristics are shown in Table 1. The patients in the high AMH group (n = 61) and low AMH group (n = 42) showed no statistically significant differences in age at surgery, body mass index, revised American Fertility Society stage, size of ovarian endometrioma, or unilaterality/bilaterality. The AMH level in both groups significantly decreased after surgery (P < 0.001 for both), and the rate of decline of AMH was significantly greater in the low AMH group than in the high AMH group (P < 0.001). Among the 103 patients, 52 pregnancies were registered in 52 women; the remaining 51 patients did not get pregnant.
Table 1.
Clinical characteristics of the study population (n = 103).
| Variables | High AMH group(n = 61) | Low AMH group(n = 42) | P/X2 value |
|---|---|---|---|
| Age at surgery, years | 30.36 ± 0.47 | 31.42 ± 0.57 | 0.156 |
| BMI, kg/m2 | 20.93 ± 0.29 | 20.45 ± 0.32 | 0.284 |
| rAFS classification | |||
| Stage III | 21 (34.4) | 13 (30.9) | 0.136 |
| Stage IV | 40 (65.6) | 29 (69.1) | |
| Diameter of ovarian | 7.46 ± 2.43 | 8.37 ± 2.89 | 0.434 |
| endometrioma, mm | |||
| Bilateral | 23 (37.7) | 18 (42.9) | 0.600 |
| Unilateral | 38 (62.3) | 24 (57.1) | |
| Preoperative AMH, ng/mL | 4.46 ± 1.20 | 1.29 ± 0.35 | <0.001 |
| Postoperative AMH, ng/mL | 3.31 ± 0.90 | 0.85 ± 0.26 | <0.001 |
| Decline rate of AMH, % | 0.25 ± 0.04 | 0.34 ± 0.02 | <0.001 |
| Pregnancy | 38 (62.3) | 14 (33.3) | 0.007 |
Data are presented as mean ± standard deviation or n (%).
AMH, anti-Müllerian hormone; BMI, body mass index; rAFS, revised American Fertility Society.
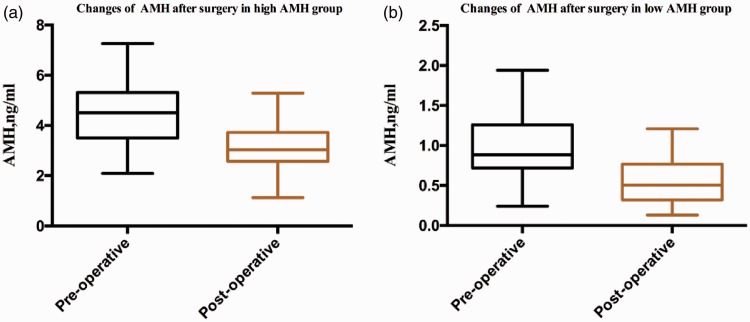
The AMH level was significantly higher before than after surgery in the high AMH group (4.51 ± 1.20 vs. 3.04 ± 0.90 ng/mL, respectively; P < 0.001). The same phenomenon was observed in the low AMH group (0.89 ± 0.36 vs. 0.51 ± 0.27 ng/mL, respectively; P < 0.001) (Figure 1).
Figure 1.
Comparison of AMH changes after surgery in different groups. AMH, anti-Müllerian hormone.
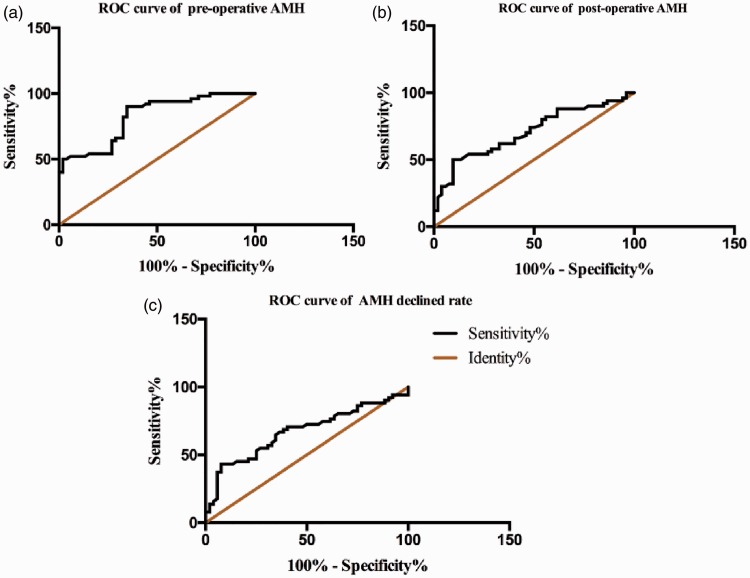
As shown by the ROC analysis in Figure 2, the area under the curve (AUC) of the preoperative AMH level was 0.812 [95% confidence interval (CI), 0.730–0.893; Std. P < 0.001], the AUC of the postoperative AMH was 0.706 (95% CI, 0.604–0.808; P < 0.001), and the AUC of the AMH decline rate was 0.671 (95% CI, 0.565–0.777; P = 0.003). The preoperative AMH level was associated with the highest AUC, suggesting that the preoperative AMH level is highly associated with pregnancy. The best cut-off point of the preoperative AMH was 3.545 (sensitivity, 80.39%; specificity, 69.23%), and the Youden index was 0.496 (Youden index = sensitivity + specificity − 1).
Figure 2.
(a) Area under the curve of preoperative AMH. (b) Area under the curve of postoperative AMH. (c) Area under the curve of AMH decline rate. AMH, anti-Müllerian hormone; ROC, receiver operating characteristic.
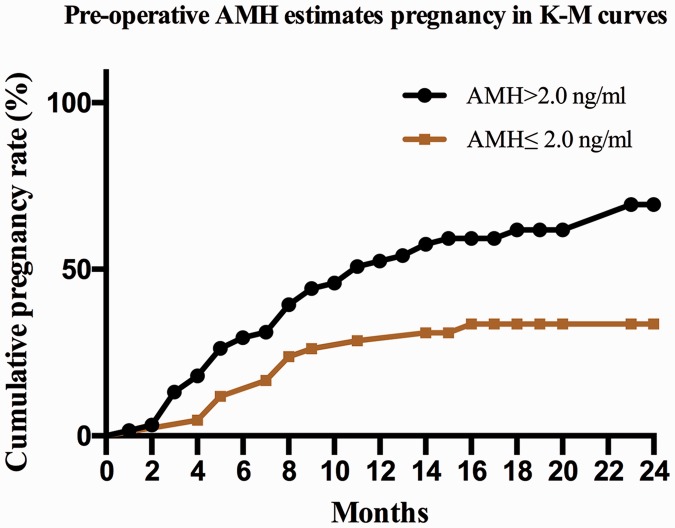
K-M curves were used to estimate the correlation of the CPR with the preoperative AMH level. The women in the high AMH group had a significantly higher CPR than those in the low AMH group (P < 0.001) (Figure 3). The probability of conceiving during the first 12 and 24 months postoperatively was 50.82% and 69.44%, respectively, in the high AMH group. However, the CPR was 28.57% in the first year after surgery in the low AMH group, and there was only slight growth in the subsequent year (CPR of 33.61%).
Figure 3.
Cumulative pregnancy rate in different groups. AMH, anti-Müllerian hormone; K-M, Kaplan–Meier.
Discussion
This study showed that the preoperative AMH was associated with the highest AUC, suggesting that the preoperative AMH level is highly associated with pregnancy. The K-M curves to estimate the association of the CPR with the preoperative AMH showed that women in the high AMH group had a higher CPR than those in the low AMH group.
Numerous studies have confirmed the negative impact of ovarian cystectomy for endometrioma on ovarian reserve.10,11 This negative impact is regarded to have several underlying mechanisms. First, tissue damage may precede surgery. Fibrosis of the cyst may have negative effects on ovulatory function, disturb folliculogenesis, and decrease follicular density.12 Second, a portion of the ovarian cortex containing follicles may be inadvertently removed during cystectomy.13 Finally, ovarian reserve may be negatively impacted by surgery-related local inflammation and electrosurgical coagulation.14 Consistent with previous studies,15,16 we demonstrated that the postoperative serum AMH levels were significantly lower than the preoperative levels in both the high and low AMH groups. Our results also suggest that ovarian cystectomy for endometriomas affects the ovarian reserve and that AMH could be a marker capable of identifying ovarian depletion due to ovarian damage.
Studies of patients who have undergone endometrioma stripping surgery have shown that fertility can be maintained but that pregnancy rates are reduced.17 In one meta-analysis, the chance of pregnancy after ovarian surgery for endometrioma ranged from 30% to 67%.18 Our study confirms these results with a total pregnancy rate of 50.49% in women who were trying to conceive during a 24-month follow-up. A more acceptable fecundability rate was observed among women in the high than low AMH group, which implies that the AMH level is an important predictor of pregnancy after laparoscopic endometriosis surgery.
The data obtained from the present study are the first to demonstrate that AMH is the strongest parameter for estimating pregnancy in women with endometriosis. According to the ROC curves, the preoperative level AMH was associated with the highest AUC, suggesting that the preoperative AMH level is the most promising contributor to the prediction of pregnancy. Furthermore, the CPR was significantly higher in the high than low AMH group in the K-M analysis. This result suggests that chance of spontaneous pregnancy is strongly correlated with the preoperative AMH level and that a higher preoperative AMH level might be associated with a higher pregnancy rate. Hence, our data may assist physicians in encouraging patients with endometriosis who present with high AMH levels to continue to pursue spontaneous pregnancy during the first 2 years after surgery. Optimal fertility management would help to avoid costly and invasive fertility protocols, particularly in poorly resourced areas in which ART are difficult to access or women cannot afford it. However, for women with low AMH levels who are counseled regarding their substantially reduced pregnancy chances, providing an ART recommendation as soon as possible would seem crucial to optimize their chance of pregnancy.
On the basis of the results of our study, we conclude that the preoperative AMH level can be used as a unique tool to predict the occurrence of natural pregnancy after endometriosis surgery. Preoperative AMH level measurements can be used as part of a fertility strategy for women undergoing endometriosis surgery and seeking fertility assistance. However, the main limitation of the present study is its small sample size. Therefore, a large-scale study should be performed in the future to confirm the results of this study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by National Natural Science Foundation of China (No. 81170547), Natural Science Foundation of Zhejiang Province (LY18H040004 and Youth Foundation Project LQ19H040012) and granted from Gynecology Innovation Branch of Zhejiang Province.
References
- 1.Johnson NP, Hummelshoj L, Abrao MS, et al. Consensus on current management of endometriosis. Hum Reprod 2013; 28: 1552–1568. [DOI] [PubMed] [Google Scholar]
- 2.David M, Chel HL, Angela B. Interrelationships among reproductive hormones and antral follicle count in human menstrual cycles. Endocr Connect 2016; 5: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stracquadanio M, Ciotta L, Palumbo MA. Relationship between serum anti-Mullerian hormone and intrafollicular AMH levels in PCOS women. Gynecol Endocrinol 2018; 34: 223–228. [DOI] [PubMed] [Google Scholar]
- 4.Broer SL, Broekmans FJM, Laven JSE, et al. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014; 20: 688–701. [DOI] [PubMed] [Google Scholar]
- 5.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 2010; 16: 113–130. [DOI] [PubMed] [Google Scholar]
- 6.Heidar Z, Bakhtiyari M, Mirzamoradi M, et al. Prediction of different ovarian responses using anti-Müllerian hormone following a long agonist treatment protocol for IVF. J Endocrinol Investig 2005; 38: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 7.Leone Roberti Maggiore U, Ferrero S, Mangili G, et al. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update 2016; 22: 70–103. [DOI] [PubMed] [Google Scholar]
- 8.Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimullerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril 2014; 101: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 9.Kim YJ, Cha SW, Kim HO. Serum anti-Müllerian hormone levels decrease after endometriosis surgery. J Obstet Gynaecol 2017; 37: 342–346. [DOI] [PubMed] [Google Scholar]
- 10.Falconer H, Sundqvist J, Gemzell-Danielsson K, et al. IVF outcome in women with endometriosis in relation to tumour necrosis factor and anti-mullerian hormone. Reprod Biomed Online 2009; 18: 582–588. [DOI] [PubMed] [Google Scholar]
- 11.Turkcuoglu I, Melekoglu R. The long-term effects of endometrioma surgery on ovarian reserve: a prospective case–control study. Gynecol Endocrinol 2018; 34: 612–615. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SH, Khalaj K, Young SL, et al. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril 2016; 106: 1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asgari Z, Rouholamin S, Hosseini R, et al. Comparing ovarian reserve after laparoscopic excision of endometriotic cysts and hemostasis achieved either by bipolar coagulation or suturing: a randomized clinical trial. Arch Gynecol Obstet 2016; 293: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 14.Pergialiotis V, Prodromidou A, Frountzas M, et al. The effect of bipolar electrocoagulation during ovarian cystectomy on ovarian reserve: a systematic review. Am J Obstet Gynecol 2015; 213: 620–628. [DOI] [PubMed] [Google Scholar]
- 15.Campos CS, Vaamonde D, Andreoli C, et al. Follicular-fluid anti-Müllerian hormone concentration is similar in patients with endometriosis compared with nonendometriotic patients. Reprod Biomed Online 2010; 21: 470–473. [DOI] [PubMed] [Google Scholar]
- 16.Nadiane AL, Elisangela A, Renata S, et al. Decreased anti-Müllerian hormone and altered ovarian follicular cohort in infertile patients with mild/minimal endometriosis. Fertil Steril 2008; 89: 1064–1068. [DOI] [PubMed] [Google Scholar]
- 17.Carmona F, Martinez-Zamora MA, Rabanal A, et al. Ovarian cystectomy versus laser vaporization in the treatment of ovarian endometriomas: a randomized clinical trial with a five-year followup. Fertil Steril 2011; 96: 251–254. [DOI] [PubMed] [Google Scholar]
- 18.Vercellini P, Somigliana E, Vigano P, et al. Surgery for endometriosis-associated infertility: a pragmatic approach. Hum Reprod 2009; 24: 254–269. [DOI] [PubMed] [Google Scholar]