Short abstract
Objective
To assess the potential relationship between benign prostate hyperplasia (BPH) and metabolic syndrome in men under 60 years old.
Methods
We searched the Medline, Embase, and Web of Science databases for studies of patients with metabolic syndrome and BPH using the key words ‘metabolic syndrome’, ‘benign prostatic hyperplasia’, and ‘BPH’. The odds ratios (ORs) and 95% confidence intervals (95%CIs) were extracted from the included studies and the role of metabolic syndrome in BPH and its characteristics (International Prostate Symptom Score (IPSS), total prostate volume (TPV), postvoid residual (PVR)) were evaluated by meta-analysis.
Results
Six comparative studies comprising 61,826 individuals were identified and included in this meta-analysis. There were significant correlations between metabolic syndrome and BPH (OR = 1.24, 95%CI = 1.19–1.29), clinical BPH (OR = 1.37, 95%CI = 1.03–1.70), and TPV (OR = 2.34, 95%CI = 1.25–3.42). However, there was no significant association between metabolic syndrome and IPSS (OR = 1.19, 95%CI = 0.35–2.04) or PVR (OR = 2.15, 95% CI = 0.95–3.34).
Conclusions
These results indicate that metabolic syndrome is significantly and positively correlated with the incidence of BPH in younger men aged <60 years. However, there was no significant relationship between metabolic syndrome and BPH-related symptoms.
Keywords: Benign prostate hyperplasia, metabolic syndrome, young population, meta-analysis, prostate volume, prevalence
Introduction
Benign prostate hyperplasia (BPH) is characterized by increased prostate volume, a relatively narrow urethra, recurrent urinary tract infections, and lower urinary tract symptoms (LUTS). BPH is common among older men, with potentially significant impacts on their daily life.1 The prevalence of BPH increases with age, with an increasing incidence of pathological BPH from 8% to 80% between men in their 40s and 90s, respectively.2 However, social economic lifestyle changes have been reflected by a significant increase in the age-adjusted prevalence of BPH in past decades.3 In addition to age, emerging evidence has suggested that other factors may also be involved in the development of BPH, including metabolic syndrome, androgen disorders, and ethnicity.3–5 Thus although BPH has traditionally been regarded as an age-dependent disease, its incidence in the younger male population has increased in recent years, and it is therefore imperative to identify the risk factors of BPH in this young population of men under 60 years old.
Like BPH, metabolic syndrome, manifesting as obesity, low high-density lipoprotein cholesterol (HDL-C), hypertension, and elevated triglycerides, is also an age-dependent condition. Numerous recent studies have indicated that metabolic syndrome may be involved in the development of BPH.4,5 However, the components of metabolic syndrome could be positive or negative predictors or BPH,6,7 and the mechanism of interaction between metabolic syndrome and BPH still needs to be elucidated.
Although some studies have examined the association between metabolic syndrome and BPH based on the overall worldwide population, regardless of age,8,9 both age and region contribute to the incidences of metabolic syndrome and BPH, leading to potentially unreliable results in non-adjusted analyses. BPH is an age-dependent disease, and the risk factors for BPH in the young male population under 60 are still unclear.10 We therefore performed a meta-analysis to identify the association between metabolic syndrome and BPH among men under 60.
Materials and methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA).11
Literature search
We searched the Medline, Embase, and Web of Science databases up to 20 October 2018 for studies involving metabolic syndrome and BPH using the following keywords: ‘metabolic syndrome’, ‘benign prostatic hyperplasia’, and ‘BPH’. Related references in the identified records were also reviewed for further potential inclusions. Duplicate records were removed using Mendeley Desktop Software (Elsevier, Amsterdam, The Netherlands).
Inclusion and exclusion criteria
Studies were included if the diagnosis of metabolic syndrome was based on meeting three of the following five criteria: waist circumference ≥85 cm; systolic blood pressure ≥130 mmHg and/or diastolic ≥85 mmHg and/or receiving antihypertensive treatment; HDL-C <40 mg/dL and/or receiving treatment for reduced HDL-C; elevated fasting blood sugar ≥100 mg/dL and/or drug treatment for elevated FBS; and elevated triglycerides ≥150 mg/dL and/or receiving antihypercholesterolemic treatment. Included studies were also required to provide detailed extractable information about the association between metabolic syndrome and BPH or BPH characteristics (International Prostate Symptom Score (IPSS), total prostate volume (TPV), postvoid residual (PVR)), and to be published in English. Studies were excluded if they met any of the following criteria: studies not carried out in humans; odds ratios (ORs) and 95% confidence intervals (95%CIs) could not be extracted or calculated; not original studies (e.g., reviews, letters, editorials, case reports); and records not relevant to the association between metabolic syndrome and BPH or BPH-related characteristics.
Data synthesis and analysis
The following items were extracted using a predefined table: first author (year of publication), country, study design, definition of metabolic syndrome, group (with or without metabolic syndrome), case number, mean age, body mass index (kg/m2), IPSS, and mean prostate volume (MPV; mL). The methodological quality of each study was evaluated using the Newcastle–Ottawa Scale (NOS), including the general aspects of subject selection, comparability of groups, and clinical outcomes. The total NOS score ranged from 0 to 9, with a higher score indicating better methodological quality. Both the data extraction and quality assessment of the included studies were checked by two reviewers independently, and any disagreement was addressed through discussion with a third reviewer.
Statistical analysis
The ORs and 95% CIs for individuals with and without metabolic syndrome were extracted to evaluate the potential role of metabolic syndrome in the development of BPH and its characteristics (TPV ≥30 mL, PVR ≥39 mL, and IPSS >7). The ORs were further pooled and analyzed using Stata software, version 12.0 (StatCorp, College Station, TX, USA). Heterogeneity among the included studies was assessed using the I2 statistic and P-value. If the I2 value was >50% or P < 0.05, indicating the presence of significant heterogeneity, we preferred to calculate the pooled ORs using a random-effects model; otherwise, the analyses were performed using a fixed-effects model. A pooled result with an OR >1, with 1 not included in its 95%CI, indicated that metabolic syndrome was significantly associated with BPH and its characteristics. We used the ‘trim and fill’ method to detect potential unpublished studies and validate our primary conclusions. A value of P<0.05 was considered significant.
Results
Literature search and study selection
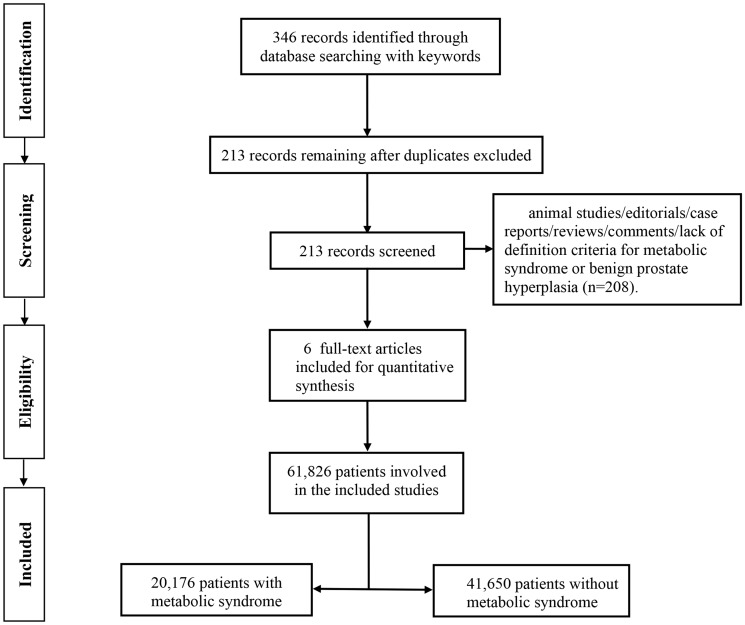
A total of 346 records were initially identified using the search strategy described above, of which 213 studies remained after the deletion of duplicates. After checking the titles and abstracts, 208 studies were removed because they were animal studies, editorials, case reports, reviews, or lacked data or definition criteria for metabolic syndrome or BPH. Six studies were therefore included for further analysis,12–17 comprising 61,826 individuals (20,176 individuals with metabolic syndrome, 41,650 individuals without metabolic syndrome). The flow chart for the study is depicted in Figure 1.
Figure 1.
Flow chart for selection of included studies.
Characteristics of the included studies
The population sizes of the included studies ranged from 778 to 57,790. All the study subjects were from Asian countries (China, Korea) and all were aged under 60 years. The included studies were published from 2012 to 2018 in English. One study was prospectively designed, four studies were retrospectively designed, and the design of the remaining study was not reported. Four of the six studies used the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATTIII),18 one used the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLB) and International Diabetes Federation (IDF),19 and the remaining study used the Abdominal Obesity and Metabolic Syndrome (AOMS) definition criteria for metabolic syndrome.20 The ORs and 95%CIs were extracted for individuals with metabolic syndrome who satisfied at least three of the required components versus individuals without metabolic syndrome. Two of the six studies were published by Park et al.14,15 in 2012 and 2013, focusing on the relationship between metabolic syndrome and BPH and its components, respectively. The value of metabolic syndrome in BPH or clinical BPH (cBPH) was examined in two cohorts in Zhao et al.’s study,12 respectively. The qualities of the included studies according to the NOS scores21 ranged from 7 to 9 (mean score, 8). The characteristics are detailed in Table 1.
Table 1.
Characteristics of included studies.
| Reference | Country | Design | Definition of metabolic syndrome | Measurement of prostate volume | Group | Case number | Mean age, years (range) | Checkpoints | Mean IPSS (range) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Park et al.14 | Korea | Retrospective | NCEP-ATTIII | Transrectal ultrasound | With Mets | 355 | 54 (52,56) | BPH | 10 (5–15) | 8 |
| Without Mets | 869 | 54 (52,56) | 10 (5–15) | |||||||
| Kwon et al.17 | Korea | NA | NCEP-ATTIII | Trans- rectal ultrasound | With Mets | 208 | 54 (52–56) | TPV, PVR | 14 (10,19) | 7 |
| Without Mets | 570 | 54 (52–56) | 14 (10,19) | |||||||
| Park et al.15 | Korea | Retrospective | NCEP-ATTIII | Transrectal ultrasound | With Mets | 355 | 54 (52,56) | TPV, PVR, IPSS | 10 (5–15) | 8 |
| Without Mets | 869 | 54 (52,56) | 10 (5–15) | |||||||
| Yin et al.16 | China | Retrospective | Abdominal obesity and metabolic syndrome | Transrectal ultrasound | With Mets | 253 | 50–59 | TPV, PVR, IPSS | NA | 9 |
| Without Mets | 651 | 50–59 | NA | |||||||
| Zhao et al.12 | China | Prospective | AHA/NHLB, IDF | Suprapubic ultrasound | With Mets | 258 | 54 (51,58) | BPH, cBPH | 2 (1,4) | 8 |
| Without Mets | 872 | 52 (49,57) | 2 (1,4) | |||||||
| Yoo et al.13 | Korea | Retrospective | NCEP-ATTIII | NA | With Mets | 19102 | 50 (50–60) | cBPH | NA | 8 |
| Without Mets | 38688 | 50 (50–60) | NA |
IPSS, International Prostate Symptom Score; Mets, metabolic syndrome; NCEP-ATIII, National Cholesterol Education Program-Adult Treatment Panel III; BPH, benign prostate hyperplasia; TPV, total prostate volume; PVR, postvoid residual; NA, not assessed; AHA/NHL B, American Heart Association/National Heart, Lung, and Blood Institute; IDF, International Diabetes Federation; cBPH, clinical benign prostate hyperplasia.
Relationship between BPH and metabolic syndrome
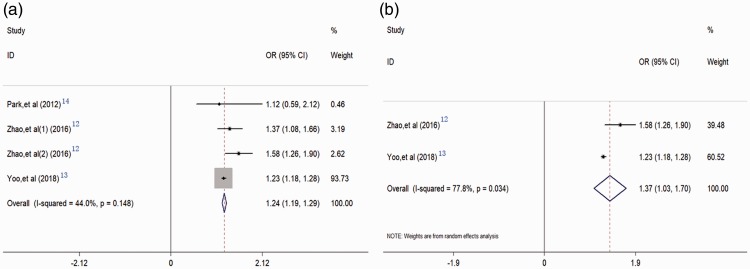
Three studies including four cohorts and comprising 60,144 individuals reported the relationship between BPH and metabolic syndrome.12–14 The pooled results indicated that metabolic syndrome was a risk factor for the development of BPH (OR = 1.24, 95%CI = 1.19–1.29), based on multivariate hazard ratios with a fixed-effects model without significant heterogeneity (I2 = 44%, P = 0.148) (Figure 2a). Moreover, two of the four cohorts focused on the relationship between metabolic syndrome and cBPH, defined as patients with BPH requiring treatment or with a history of medication. The pooled results derived from the random-effects model also showed that metabolic syndrome was significantly correlated with cBPH (OR = 1.37, 95%CI = 1.03–1.70) (Figure 2b).
Figure 2.
Forest plots evaluating the associations between benign prostatic hyperplasia (BPH) and metabolic syndrome. BPH and metabolic syndrome (a); clinical BPH and metabolic syndrome (b). OR, odds ratio; CI, confidence interval.
Relationship between metabolic syndrome and TPV
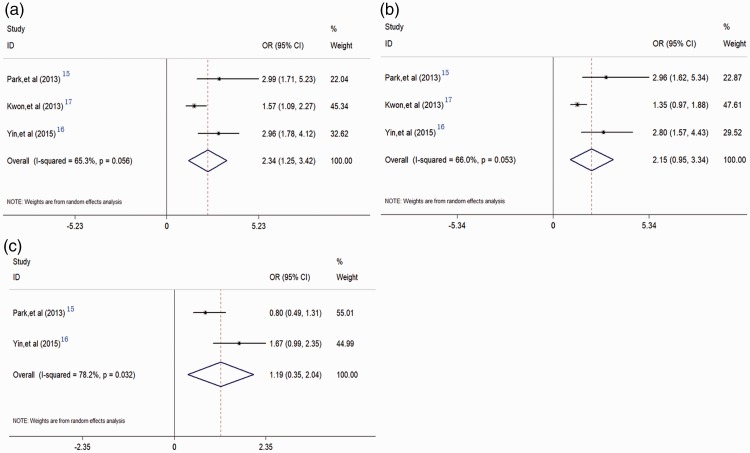
Three studies including 2,906 individuals presented data on the relationship between metabolic syndrome and TPV.15–17 The pooled results showed that metabolic syndrome was significantly correlated with higher TPV (TPV ≥30 mL vs. TPV >30 mL) (OR = 2.34, 95% CI = 1.25–3.42), based on random-effects analysis (Figure 3a).
Figure 3.
Forest plots evaluating the relationships between metabolic syndrome and benign prostatic hyperplasia (BPH)-related characteristics. Metabolic syndrome and total prostate volume (a); metabolic syndrome and postvoid residual (b); and metabolic syndrome and International Prostate Symptom Score (c).
Correlation between metabolic syndrome and PVR
The correlation between metabolic syndrome and PVR was reported in three studies involving 2,906 individuals.15–17 The combined analysis indicated that metabolic syndrome was significantly correlated with PVR (OR = 2.15, 95% CI = 0.95–3.34), derived from random-effects analysis (Figure 3b).
Relationship between metabolic syndrome and IPSS
Two studies including 2,128 individuals provided data to assess the relationship between metabolic syndrome and IPSS.15,17 However, the pooled results suggested there was no significant relationship between metabolic syndrome and IPSS (IPSS >7 vs. IPSS ≤7) (OR = 1.19, 95% CI = 0.35–2.04) (Figure 3c).
Evaluation of publication bias
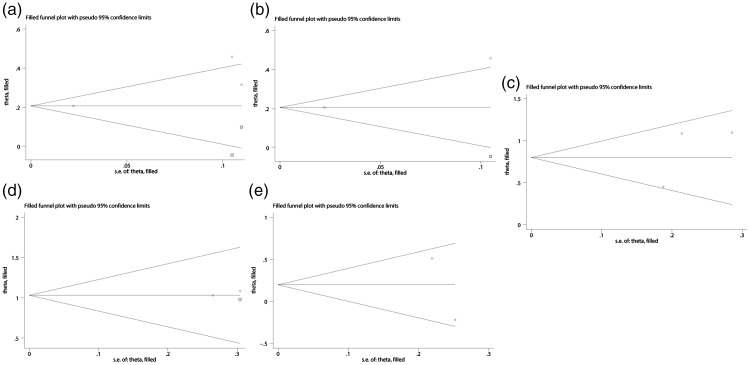
We used the trim and fill method to detect potential publication bias, which indicated that there were two, one, zero, one, and zero unpublished studies regarding the associations between metabolic syndrome and BPH, cBPH, TPV, PVR, and IPSS, respectively (Figure 4). However, the results derived from the trim and fill method were consistent with our previous conclusions. The results are detailed in Table 2.
Figure 4.
‘Trim and fill’ results identifying potential unpublished studies for benign prostatic hyperplasia (BPH) and metabolic syndrome (a), clinical BPH and metabolic syndrome (b), total prostate volume and metabolic syndrome (c), postvoid residual and metabolic syndrome (d), and International Prostate Symptom Score and metabolic syndrome (e). Circles indicate included studies and squares indicate possible unpublished studies. s.e., standard error.
Table 2.
Trim and fill results derived from fixed- and random-effects models.
| Groups | Fixed model (OR, 95%CI) | P value | Random model (OR, 95%CI) | P value |
|---|---|---|---|---|
| Mets vs. BPH | 1.229 (1.181, 1.279) | <0.001 | 1.229 (1.076, 1.404) | 0.002 |
| Mets vs. cBPH | 1.229 (1.179, 1.281) | <0.001 | 1.229 (0.996, 1.516) | 0.054 |
| Mets vs. TPV | 2.223 (1.734, 2.848) | <0.001 | 2.343 (1.491, 3.684) | <0.001 |
| Mets vs. PVR | 2.804 (2.021, 3.890) | <0.001 | 2.804 (2.021, 3.890) | <0.001 |
| Mets vs. IPSS | 1.216 (0.879, 1.683) | 0.237 | 1.169 (0.570, 2.395) | 0.670 |
Mets, metabolic syndrome; BPH, benign prostate hyperplasia; cBPH, clinical benign prostate hyperplasia; TPV, total prostate volume; PVR, postvoid residual; IPSS, International Prostate Symptom Score; OR, odds ratio; CI; confidence interval.
Discussion
BPH has become one of the most common benign diseases affecting men worldwide. The incidence of BPH increases with age and imposes a great economic burden. BPH-related LUTS can also have significant effects on quality of life.22 However, the specific mechanism responsible for the development of BPH remains unclear, and identifying the potential risk factors is thus an imperative issue. Recent epidemiological studies have suggested a possible association between BPH and metabolic syndrome conditions;22,23 however, this relationship is still controversial due to heterogeneity among other factors, such as age and geographic region.23,24 We therefore evaluated the role of metabolic syndrome in BPH in young men under 60, taking account of age and population.
This meta-analysis evaluated six high-quality studies regarding the role of metabolic syndrome in the development of BPH and its related characteristics (TPV ≥ 30 mL, PVR ≥39 mL, and IPSS >7). The pooled results indicated that metabolic syndrome could be a potential risk factor for the development of BPH or cBPH. We also performed meta-analyses to assess the influence of metabolic syndrome on BPH-related characteristics, and showed that patients with metabolic syndrome were more likely to have a TPV ≥30 mL. However, the pooled results showed no significant association between metabolic syndrome and the BPH-related symptoms IPSS >7 and PVR ≥39 mL. Indeed, not all men with BPH present with clinical symptoms. The current results were based on primary data for men under 60, and represent the first meta-analysis to evaluate the association between metabolic syndrome and BPH in this young male population. Although the risk factors for the initiation of BPH in young people remain unclear, these results may provide a new direction for managing BPH in younger men.
Although numerous previous studies have reported the potential association between metabolic syndrome and BPH,25 the mechanisms underlying this relationship still need to be identified. Hyperinsulinemia and insulin resistance induced by metabolic syndrome may promote the initiation and progression of BPH,26 and recent studies27 indicated that prostatic inflammation induced by metabolic syndrome may contribute to the development and progression of BPH. Although there are various potential mechanisms, Vignozzi et al.28 proposed a ‘3-hit’ hypothesis to interpret the relationship between BPH and metabolic syndrome: metabolic syndrome conditions can induce prostatic inflammation (1-hit), sustain the inflammation (2-hits), and cause sex steroid abnormalities (3-hits), thus promoting remodeling and enlargement of the prostate.
Other studies that investigated the role of metabolic syndrome in BPH did not meet the criteria for inclusion in the current meta-analysis. Most of these related studies29 also indicated that metabolic syndrome was a significant risk for BPH or its characteristics. However, Yang et al.30 demonstrated that metabolic syndrome may have favorable effects on BPH-related symptoms in healthy middle-aged men. Two previous meta-analyses9,31 evaluated the association between BPH and metabolic syndrome based on the overall population, and both concluded that metabolic syndrome was a key link in the development and progression of BPH. BPH is an age-dependent disease, and metabolic syndrome conditions differ among populations with different lifestyles and diet habits. Nevertheless, the previous meta-analyses did not account for the impact of age, and did not specifically investigate the association between BPH and metabolic syndrome in a young population. In contrast, the current meta-analysis included the most recent studies of the relationship between BPH and metabolic syndrome in men under 60, and demonstrated a role for metabolic syndrome in the development of BPH in this population.
There were some unavoidable limitations in this meta-analysis. First, although we adopted strict inclusion and exclusion criteria, there was still significant heterogeneity among the studies, especially with regard to the associations between metabolic syndrome and cBPH, TPV, and IPSS, which were pooled analyses using a random-effects model. Moreover, most of the included studies were retrospective, and may thus have included patient-selection bias. Finally, few studies focused on the association between metabolic syndrome and BPH in young Asian populations, and the number of relevant studies included in this meta-analysis was therefore limited, and publication bias was also unavoidable. However, we performed a trim and fill analysis to identify potential unpublished studies, and further analyzed the results with both fixed- and random-effects models to validate our conclusions.
In conclusion, this study represents the first meta-analysis to evaluate the relationship between BPH and metabolic syndrome among men aged under 60. Metabolic syndrome was significantly correlated with the development of BPH, cBPH, and TPV, but not with BPH-related symptoms such as IPSS and PVR. Further large-scale randomized controlled trials are required to validate the association between metabolic syndrome and BPH in younger men.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by a grant from the Finance Department of Hunan Province (no. 2016-129). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Woodard TJ, Manigault KR, McBurrows NN, et al. Management of benign prostatic hyperplasia in older adults. Consult Pharm 2016; 31: 412–424. [DOI] [PubMed] [Google Scholar]
- 2.Barry MJ, Fowler FJ, Jr, Bin L, et al. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol 1997; 157: 10–14. [PubMed] [Google Scholar]
- 3.Stroup SP, Palazzi-Churas K, Kopp RP, et al. Trends in adverse events of benign prostatic hyperplasia (BPH) in the USA, 1998 to 2008. BJU Int 2012; 109: 84–87. [DOI] [PubMed] [Google Scholar]
- 4.De Nunzio C, Aronson W, Freedland SJ, et al. The correlation between metabolic syndrome and prostatic diseases. Eur Urol 2012; 61: 560e70. [DOI] [PubMed] [Google Scholar]
- 5.Kopp W. Diet-induced hyperinsulinemia as a key factor in the etiology of both benign prostatic hyperplasia and essential hypertension? Nutr Metab Insights 2018; 11: 1178638818773072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung JH, Ahn SV, Song JM, et al. Obesity as a risk factor for prostatic enlargement: a retrospective cohort study in Korea. Int Neurourol J 2016; 20: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Yahya E, Mohammad MT, Muhaidat J, et al. Functional balance and gait characteristics in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. Am J Mens Health 2019; 13: 1557988319839879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo S, Oh S, Park J, et al. Effects of metabolic syndrome on the prevalence of prostate cancer: historical cohort study using the national health insurance service database. J Cancer Res Clin Oncol 2019; 145: 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gacci M, Corona G, Vignozzi L, et al. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int 2015; 115: 24–31. [DOI] [PubMed] [Google Scholar]
- 10.Son H, Park J, Song SH, et al. Rapid increase of health care utilization and cost due to benign prostatic hyperplasia in Korean men: retrospective population based analysis using the health insurance review and assessment service data. J Korean Med Sci 2015; 30: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao SC, Xia M, Tang JC, et al. Associations between metabolic syndrome and clinical benign prostatic hyperplasia in a northern urban Han Chinese population: a prospective cohort study. Sci Rep 2016; 6: 33933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo S, Oh S, Park J, et al. The impacts of metabolic syndrome and lifestyle on the prevalence of benign prostatic hyperplasia requiring treatment: historical cohort study of 130454 men. BJU Int 2019; 123: 140–148. [DOI] [PubMed] [Google Scholar]
- 14.Park YW, Min SK, Lee JH. Relationship between lower urinary tract symptoms/benign prostatic hyperplasia and metabolic syndrome in Korean men. World J Mens Health 2012; 30: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park YW, Kim SB, Kwon H, et al. The relationship between lower urinary tract symptoms/benign prostatic hyperplasia and the number of components of metabolic syndrome. Urology 2013; 82: 674–679. [DOI] [PubMed] [Google Scholar]
- 16.Yin Z, Yang JR, Rao JM, et al. Association between benign prostatic hyperplasia, body mass index and metabolic syndrome in Chinese men. Asian J Androl 2015; 17: 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon H, Kang HC, Lee JH. Relationship between predictors of the risk of clinical progression of benign prostatic hyperplasia and metabolic syndrome in men with moderate to severe lower urinary tract symptoms. Urology 2013; 81: 1325–1329. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 20.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887. [DOI] [PubMed] [Google Scholar]
- 21.Wells G, Shea B, O’Connell D, et al. Newcastle -Ottawa quality assessment scale case control studies Ottawa Hospital Research Institute, 2013; http: //www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (2013, accessed 5 April 2019).
- 22.Speakman M, Kirby R, Doyle S, et al. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int 2015; 115: 508–519. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Zeng X, Liu Y, et al. Impact of metabolic syndrome on benign prostatic hyperplasia in elderly Chinese men. Urol Int 2014; 93: 214–219. [DOI] [PubMed] [Google Scholar]
- 24.Zhao S, Chen C, Chen Z, et al. Relationship between metabolic syndrome and predictors for clinical benign prostatic hyperplasia progression and international prostate symptom score in patients with moderate to severe lower urinary tract symptoms. Urol J 2016; 13: 2717–2726. [PubMed] [Google Scholar]
- 25.Sebastianelli A, Gacci M. Current status of the relationship between metabolic syndrome and lower urinary tract symptoms. Eur Urol Focus 2018; 4: 25–27. [DOI] [PubMed] [Google Scholar]
- 26.Dahle SE, Chokkalingam AP, Gao YT, et al. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol 2002; 168: 599–604. [PubMed] [Google Scholar]
- 27.Russo GI, Cimino S, Castelli T, et al. Molecular correlates in urine for the obesity and prostatic inflammation of BPH/LUTS patients. Prostate. 2018; 78: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vignozzi L, Gacci M, Maggi M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nat Rev Urol 2016; 13: 108e19. [DOI] [PubMed] [Google Scholar]
- 29.Seo DH, Yoon S, Choi JH, et al. The correlation between body mass index and routine parameters in men over fifty. World J Mens Health 2017; 35: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang TK, Hsieh JT, Chen SC, et al. Metabolic syndrome associated with reduced lower urinary tract symptoms in middle-aged men receiving health checkup. Urology 2012; 80: 1093–1097. [DOI] [PubMed] [Google Scholar]
- 31.Wang JY, Fu YY, Kang DY. The association between metabolic syndrome and characteristics of benign prostatic hyperplasia. Medicine (Baltimore) 2016; 95: e3243. [DOI] [PMC free article] [PubMed] [Google Scholar]