Short abstract
Objectives
Plasma free DNA is a promising new tumor biomarker, which may have applications in clinical diagnosis and treatment of non-small cell lung cancer (NSCLC).
Methods
Plasma free DNA was collected from 120 healthy controls and 116 patients with NSCLC before and after treatment.
Results
The mean plasma free DNA levels in 116 NSCLC patients (200.70 ± 88.54 ng/mL) were higher than those of 120 healthy controls (18.65 ± 6.30 ng/mL). Further analysis showed that the mean serum free DNA level in stage I/II NSCLC patients was 172.75 ± 72.87 ng/mL, significantly lower than that of stage III/IV patients (221.88 ± 93.86 ng/mL). Following surgery and effective chemotherapy, the plasma free DNA levels of NSCLC patients decreased significantly.
Conclusions
Through quantitation of plasma free DNA, this study established proof-of-concept for a highly sensitive and specific detection method, which can be used for diagnosis, prognosis and treatment monitoring in NSCLC patients.
Keywords: Non-small cell lung cancer, plasma free DNA, polymerase chain reaction (PCR), clinical diagnosis, therapy, biomarker
Introduction
At present, the incidence and mortality of lung cancer is increasing year over year. Although methods for diagnosis and treatment of lung cancer continue to improve, mortality rates have not decreased significantly. Tumors that are discovered and treated early (typically using surgery) have significantly improved cure rates resulting in longer patient survival. Therefore, early detection of lung cancer remains the most effective strategy to reduce mortality. Common methods for clinical diagnosis of lung cancer include detection of serum tumor markers, examination of exfoliative sputum cytology, fiberoptic bronchoscopy examination, lung puncture biopsy, and imaging. However, these techniques all have limitations in terms of specificity, sensitivity, and patient compliance. Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancers, and about 70% of NSCLC patients are diagnosed with advanced lung cancer,1 which generally carries a short survival rate. In 1948, scientists discovered the presence of free DNA in human blood and plasma. In 1997, a study found that plasma free DNA levels in cancer patients were significantly higher than those of healthy individuals. Seventeen years later, it was discovered that this DNA was derived from tumors and bore its mutation signatures. In recent years, the rapid development of biotechnology has greatly improved our ability to detect DNA, making it possible to quantitate plasma free DNA in the blood. Studies have shown that as tumors grow, they release DNA molecules into the peripheral blood whose genetic properties are identical to those of tumor cells.2 There is a large amount of free DNA in the sera of patients with malignant tumors, about 10 times more than that in healthy individuals. Some studies have achieved good clinical efficacy using individualized targeted therapies tailored to the specific mutations detected in the plasma free DNA of lung cancer patients.3 In this study, the plasma free DNA levels of NSCLC patients were measured before and after treatment to assess their potential value in clinical diagnosis and treatment of these patients.
Methods
Patients and controls
NSCLC patients treated from December 2015 to October 2017 in the Department of Respiratory Medicine, Shaanxi People's Hospital, were enrolled in this study. NSCLC was pathologically confirmed. Patients with hepatitis, autoimmune diseases and other infectious diseases were excluded. Simultaneously, healthy controls were enrolled. All participants provided written informed consent prior to enrollment. The study was approved by the Ethics Committee of Shaanxi Provincial People's Hospital (approval number 104).
Clinical efficacy was evaluated according to the objective World Health Organization criteria for solid tumors. Complete response (CR) was defined as the complete disappearance of all measurable tumor lesions. Partial response (PR) was defined as reduction in tumor volume (maximum diameter multiplied by vertical length) by >50%, with no new lesions found. Stable disease (SD) was defined as a change in tumor size of ≤25%, with no new lesions found. Progressive disease (PD) was defined as an increase in tumor size of >25%, and/or the appearance of new tumors. The occurrence of adverse reactions following chemotherapy was evaluated using the National Cancer Institute Common Toxicity Criteria (NCICTC). Grade 0 adverse reactions reflected normal lack of toxicity, grade I reactions reflected mild toxicity, grade II reactions reflected moderate toxicity, grade III reactions reflected severe toxicity, grade IV reactions reflected incapacitating or life-threatening toxicity, and grade V reactions reflected fatal toxicity.
Sample preparation and plasma DNA extraction
Peripheral blood samples were collected at room temperature and allowed to clot for 2 hours, then were centrifuged at 1509 ×g at 20°C for 10 minutes. The upper plasma layer (1 mL) was placed in a 2-mL test tube and stored at −80°C. Plasma free DNA was extracted using a QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Fifty microliters of protease, 500 μL of buffer Al and 500 μL of ethanol were added to each 500 μL of plasma sample, followed by 50 μL of buffer AE for elution. The extracted plasma free DNA was stored at −20°C.
Determination of plasma free DNA levels
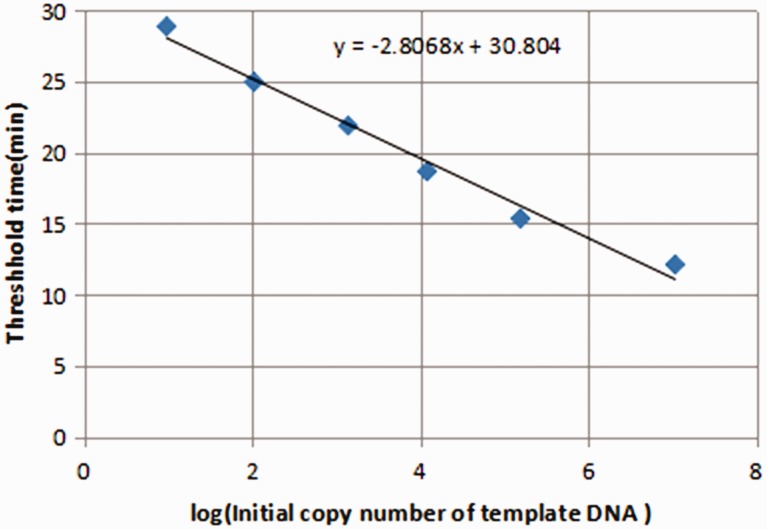
The β-actin primer sequences were as described by Yoon et al.4 The upstream (5'-ccacactgtgcccatctacg-3') and downstream (5'-aggatcttcatgaggtagtcagtcag-3') primers (Shanghai Engineering Bioengineering Technology Service Co., Shanghai, China) were used to amplify a 99-bp amplicon. Twenty-microliter RT-PCR reactions were prepared containing 11 μL of fluorescent PCR reaction fluid (SYBR premix Ex Taq, Takara Bio, Shiga, Japan), 1 μL each of upstream and downstream primers, 1 μL of DNA template, and 6 μL of ddH2O. The following cycling parameters were determined according to the primer sequence: 95°C for 1 minute; 40 cycles of 95°C for 20 seconds, 60°C for 30 seconds and 68°C for 45 seconds. A standard curve was constructed using the ct values derived from DNA standards of known concentrations. The ct value was linearly correlated with the logarithm of DNA content, and sample concentrations were determined using their ct values reported by the PCR Analyzer (Applied Biosystems, Foster City, CA, USA). Relationships between plasma free DNA levels, clinical parameters and histological types in NSCLC patients were analyzed (Figure 1).
Figure 1.
Standard curve of log computed tomography (ct) values of known concentrations of DNA standards. The logarithmic relationship between ct values and DNA copy number was linear.
Statistical analyses
Statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL). Descriptive statistics were expressed as means ± standard deviations (x ± s) and differences between groups were assessed using the paired t-test. Values of p < 0.05 were considered statistically significant.
Results
Comparison of plasma free DNA levels between NSCLC patients and healthy controls
One hundred and sixteen NSCLC patients (64 males and 52 females) with an average age of 58.6 ± 10.5 years (range: 41–78 years) were enrolled. The study cohort consisted of 50 patients with stage I/II NSCLC and 66 patients with stage III/IV NSCLC according to the Union for International Cancer Control (UICC) staging system for lung cancer. One hundred and twenty healthy controls (61 males and 59 females) with an average age of 57.4 ± 8.4 years (range: 38–79 years) were enrolled.
Plasma free DNA levels in NSCLC patients (200.70 ± 88.54 ng/mL) were significantly higher than those in healthy controls (18.65 ± 6.30 ng/mL) (p = 0.006).
Relationships between plasma free DNA levels and clinical parameters
Plasma free DNA levels in NSCLC patients were not significantly correlated with age, sex or tumor histological type, but were correlated with tumor size and clinical stage (p = 0.042) (Table 1). Levels of plasma free DNA in patients with tumor diameters >3 cm or with later stage tumors were significantly higher (p = 0.004) than in patients with smaller or earlier-stage tumors.
Table 1.
Relationships between plasma free DNA levels and clinical parameters in NSCLC patients.
| Parameters | Cases (n = 116) | Plasma free DNA level (ng/mL) | t | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 64 | 198.38 ± 80.98 | 1.9845 | 0.47 |
| Female | 52 | 203.56 ± 97.78 | ||
| Age | ||||
| ≤60 years | 49 | 194.32 ± 87.52 | 1.9830 | 0.56 |
| >60 years | 67 | 205.37 ± 89.64 | ||
| Tumor size | ||||
| <3 cm | 49 | 168.42 ± 68.17 | 1.9810 | 0.004 |
| >3 cm | 67 | 224.31 ± 94.56 | ||
| Pathology | ||||
| Adenocarcinoma | 60 | 207.90 ± 80.58 | 1.9824 | 0.36 |
| Squamous cell carcinoma | 56 | 192.98 ± 96.48 | ||
| Clinical stage | ||||
| I/II | 50 | 172.75 ± 72.87 | 1.9812 | 0.005 |
| III/IV | 66 | 221.88 ± 93.86 |
NSCLC, non-small cell lung cancer.
Relationships between plasma free DNA levels and response to therapy in NSCLC patients
We assessed levels of plasma free DNA in 50 NSCLC patients who underwent surgical interventions, and found that these were significantly lower compared with preoperative levels (p = 0.005). This reduction might be associated with reduced loads of tumor cells in the body following treatment. In addition, 66 NSCLC patients were treated with chemotherapy. Plasma free DNA levels decreased significantly in patients who responded to chemotherapy (p = 0.004). By contrast, levels of plasma free DNA in NSCLC patients who did not respond to chemotherapy decreased to some extent following chemotherapy, but this decrease was not significant. Further comparison of responders and non-responders to chemotherapy revealed that levels of plasma free DNA of NSCLC patients responding to chemotherapy were significantly lower than those of patients who did not respond to chemotherapy(p = 0.004). This finding suggested that changes in plasma free DNA levels induced by therapies can be used as indicators of clinical efficacy in NSCLC patients (Table 2).
Table 2.
Changes in plasma free DNA levels before and after NSCLC treatment.
| Group | Surgery group (n = 50) | Chemotherapy group (n = 66) |
|
|---|---|---|---|
| Responders (n = 39) | Non-responders (n = 27) | ||
| Before treatment | 190.73 ± 79.36 ng/mL | 207.68 ± 93.79 ng/mL | 209.74 ± 97.90 ng/mL |
| After treatment | 98.46 ± 45.66 ng/mL | 106.47 ± 58.88 ng/mL | 188.75 ± 76.69 |
| t | 1.9845 | 1.9917 | 2.0066 |
| p | <0.001 | <0.001 | 0.25 |
Comparison between responders and non-responders after chemotherapy, t = 1.9977, p < 0.001. NSCLC, non-small cell lung cancer.
Discussion
Lung cancer is a highly malignant tumor. According to the American Lung Cancer Association, the 5-year survival rate of patients with non-metastatic lung cancer was 55%, while survival of patients with distal metastases was only 4%.5 Because most solid tumors can be cured by surgery at early stages, the prognoses of patients receiving early diagnoses are better. At present, the main diagnostic methods used to detect lung cancer are imaging and pathology. Unfortunately, most tumors diagnosed by imaging and pathology are detected in the middle and late stages. Immunological detection of serum tumor biomarkers provides a simple method for the early diagnosis of lung cancer, but sensitivity and specificity remain inadequate. Detection of biomarkers within tumor tissue requires invasive sampling of tumor specimens, thus limiting its application in lung cancer screening.
The relationships between kinetic properties of plasma free DNA, tumor load and therapeutic response have been studied in other solid malignancies.6–9 Plasma free DNA carries comprehensive information of intrinsic high specificity and sensitivity, and thus has unique advantages over conventional protein markers. Studies of breast,6 ovarian10 and colon cancer11 have established the potential value of plasma free DNA as a biomarker for monitoring tumor loads during therapy. A large number of studies have shown that the sensitivity and specificity of plasma free DNA is much higher than currently-used clinical imaging methods. A 2012 study of colon cancer patients showed that 50 million malignant cells could release detectable levels of plasma free DNA into the bloodstream, while tumors of equivalent size could not be imaged.12 Levels of plasma free DNA increased primarily via three mechanisms: (i) circulation of tumor cells or micro-metastatic lesions; (ii) tumor cell apoptosis or necrosis; and (iii) tumor cell proliferation, with spontaneous release of nucleic acids.13
In recent years, some scholars have studied the molecular biology of lung cancer more deeply.14 Szpechcinski et al.15 compared plasma free DNA levels in patients with NSCLC, respiratory inflammatory disease as well as in healthy volunteers. They found that plasma free DNA levels in patients with NSCLC were much higher than those of the other two groups, confirming its clinical value as a diagnostic marker of NSCLC. Levels of plasma free DNA increased rapidly with disease progression, and declined following successful treatment. Quantitative evaluation of plasma free DNA levels can also be an important prognostic indicator.16 Plasma free DNA levels were compared with five tumor markers (SSC-Ag, CA125, CEA, NSE, and CYFRA21-1) in patients with lung cancer. Plasma free DNA level was a much more sensitive marker of lung cancer than any of these tumor markers alone, although the combination of all five tumor markers was similarly sensitive. Other scholars believe that quantitative measurement of plasma free DNA combined with tumor markers (CA125, CEA, CYFRA21-1, NSE, SSC-Ag) could significantly improve diagnostic efficiency in lung cancer.
In this study, plasma free DNA levels in patients with NSCLC were significantly higher than those of healthy controls (p = 0.006), suggesting that detection of plasma free DNA could be an important component of early NSCLC screening. Similar conclusions were reached in a study of serum free DNA and cancer in foreign countries.17 Further analyses showed that serum free DNA levels in NSCLC patients reflected a close relationship between tumor size and clinical staging. Levels of plasma free DNA in patients with larger or later stage tumors were significantly higher (p = 0.004 and p = 0.005, respectively) than in patients with smaller or earlier-stage tumors. In NSCLC patients who responded to chemotherapy, levels of plasma free DNA decreased significantly (p < 0.001). This finding suggested that plasma free DNA levels were positively correlated with the staging and tumor load of NSCLC patients, and could potentially be used as an important index of curative effect. This hypothesis is consistent with the findings of Madhavan et al.18 Their results suggested that plasma free DNA levels were significantly higher in patients with stage III/IV NSCLC compared with those with stage I/II NSCLC, and were also associated with clinical stage. Patients with stage III/IV NSCLC may have a larger number of tumor cells, a higher incidence of apoptosis and necrosis and thus increased levels of DNA release, leading to a significant increase in plasma free DNA levels in patients with advanced NSCLC. Thus, the patient's plasma free DNA level may be an effective marker to evaluate survival and prognosis. Analysis of plasma free DNA levels in NSCLC patients with tumors of different pathological types showed no significant difference in plasma free DNA levels in patients with adenocarcinoma and squamous cell carcinoma. This finding was consistent with the results of Szpechcinski et al.15 but inconsistent with those of Deng et al.19 The data collectively suggests that plasma free DNA levels in patients with adenocarcinoma are significantly higher than those in patients with squamous cell carcinoma, and that inconsistencies between measurements may be related to small samples sizes and/or the absence of stochastic principles. Further research and discussion is needed in this area.
Conclusion
Levels of serum free DNA are closely related to the occurrence and metastasis of NSCLC. Thus, serum free DNA levels may have clinical significance and in the early diagnosis, staging and prognosis of lung cancer. The non-invasive nature of obtaining plasma free DNA samples results in high compliance. Plasma free DNA levels can be used for real-time and dynamic monitoring of patients with NSCLC, and as an effective tool for the diagnosis of NSCLC. These levels may also provide guidance for individualized therapies and prognostic evaluation. This method has the potential to improve NSCLC screening, enabling detection of previously difficult to cure NSCLCs in time to effectively treat these tumors and save the lives of patients.
Declaration of conflicting interests
The authors have no conflicts of interest to declare.
Funding
This work was supported by the Shaanxi Provincial Science and Technology Department Social Development Project (2019SF-151) and the Shaanxi Health Research Project (2018D091).
References
- 1.Maione P, Rossi A, Sacco PC, et al. Advance in chemotherapy in advanced non-small-cell lung cancer. Expert Opin Pharmacother 2010; 11: 2997–3007. [DOI] [PubMed] [Google Scholar]
- 2.Diaz LA, Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 2014; 32: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinzani P, Salvianti F, Orlando C, et al. Circulating cell-free DNA in cancer. Methods Mol Biol 2014; 1160: 133–145. [DOI] [PubMed] [Google Scholar]
- 4.Yoon KA, Park S, Lee SH, et al. Comparison of circulating plasma DNA levels between lung cancer patients and healthy controls. J Mol Diagn 2009; 11: 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 6.Dawson SJ, Rosenfeld N, Caldas C. Circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013; 368: 1199–1209. [DOI] [PubMed] [Google Scholar]
- 7.McBride D, Orpana A, Sotiriou C, et al. Use of cancer-specific genomic rearrangements to quantify disease burden in plasma from patients with solid tumors. Genes Chromosomes Cancer 2010; 49: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leary RJ, Kinde I, Diehl F, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med 2010; 2: 20ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipson EJ, Velculescu VE, Pritchard TS, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer 2014; 2: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forshew T, Murtaza M, Parkinson C, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med 2012; 4: 136ra68. [DOI] [PubMed] [Google Scholar]
- 11.Tie J, Kinde I, Wang Y, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 2015; 26: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz LA, Jr, Williams RT, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012; 486: 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakao M, Muramatsu H, Sone K, et al. Epidermal growth factor receptor-tyrosine kinase inhibitors for non-small-cell lung cancer patients aged 80 years or older: a retrospective analysis. Mol Clin Oncol 2015; 3: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ying M, Zhu XX, Zhao Y, et al. KRAS mutation as a biomarker for survival in patients with non-small cell lung cancer, a meta-analysis of 12 randomized trials. Asian Pac J Cancer Prev 2015; 16: 4439–4445. [DOI] [PubMed] [Google Scholar]
- 15.Szpechcinski A, Chorostowska-Wynimko J, Struniawski R, et al. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br J Cancer 2015; 113: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson E, Winter C, George A, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med 2015; 7: 1034–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulivi P, Mercatali L, Casoni GL, et al. Multiple marker detection in peripheral blood for NSCLC diagnosis. PLoS One 2013; 8: e57401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madhavan D, Wallwiener M, Bents K, et al. Plasma DNA integrity as a biomarker for primary and metastatic breast cancer and potential marker for early diagnosis. Breast Cancer Res Treat 2014; 146: 163–174. [DOI] [PubMed] [Google Scholar]
- 19.Shuiqiu D, Xuennong OY, Zongyang Y, et al. Changes and significance of serum free DNA level in advanced NSCLC. Shandong Medicine 2013; 53: 6–8. [Google Scholar]